ABSTRACT
ERBB2 (v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2) amplification is associated with invasive breast cancer. We discovered that TOM1L1 (target of myb1-like 1) and ERBB2 co-amplification defines a novel mechanism involved in breast cancer metastatic progression. Upregulation of the vesicular trafficking protein TOM1L1 enhances plasma membrane delivery of membrane-type 1 matrix metalloprotease (MT1-MMP) for efficient extracellular matrix degradation and tumor cell dissemination.
KEYWORDS: Breast cancer, metastasis, tumor cell signaling, tyrosine kinase, vesicular trafficking
The v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 gene (ERBB2) encodes a receptor tyrosine kinase (TK) of the epidermal growth factor receptor family and is amplified or overexpressed in approximately 20% of human breast tumors. Aberrant ERBB2 levels at the plasma membrane result in constitutive TK activity that promotes cell transformation, leading to invasive breast carcinoma.1 ERBB2 is now considered an important therapeutic target in this cancer and several inhibitors have been developed for the clinic. For example, the anti-ERRB2 antibody trastuzumab (Herceptin®) shows a significant clinical response and is now used as a first-line treatment in ERBB2+ breast cancer. However, patients frequently develop tumor resistance and there is a clear need to better understand how ERBB2 drives metastatic progression in order to improve ERBB2-based therapy. The invasive properties of breast tumor cells are strongly coupled to their ability to induce extracellular matrix proteolysis for cell dissemination. Mechanistically, this process involves the secretion of matrix metalloproteases (MMPs). Specifically, the membrane-type 1 MMP (MT1-MMP) plays a key role in rupturing the basal extracellular matrix (ECM), allowing cell migration through type I collagen-rich interstitial tissues.2 Interestingly, MT1-MMP function is tightly regulated through its delivery from the trans-Golgi network to specialized F-actin-enriched plasma membrane domains called invadopodia that contact the ECM and mediate its proteolytic degradation.2 Whether this mechanism contributes to ERBB2-invasive carcinoma remains largely unknown.
Target of myb1-like 1 protein (TOM1L1) was originally identified as a novel SRC substrate and signaling protein of the adaptor family by Stein et al. and by our laboratory.3,4 TOM1L1 negatively regulates TK-induced cell proliferation and transformation.4,5 Moreover, it contains N-terminal VPS27/HRS/STAM (VHS) and GGA and TOM1 (GAT) homology domains that are found in many vesicular trafficking proteins. Surprisingly, our collaborators Orsetti et al. found that TOM1L1 (locus 17q22) is co-amplified with ERBB2 (locus 17q12) in breast cancer,6 suggesting an unexpected protumoral function of TOM1L1 in ERBB2+ tumors.
In our recent study published in Nature Communications,7 we confirmed that TOM1L1 is frequently (∼50%) co-amplified with ERBB2 using a larger set of breast tumor biopsies. We also found that TOM1L1 amplification is associated with bad prognosis in patients with ERBB2+/estrogen receptor (ER)+ breast tumors because it reduces metastasis-free survival by ∼25%. We then manipulated TOM1L1 levels in ERBB2+/ER2+ breast cancer cells to determine whether ERBB2 and TOM1L1 cooperate. Although growth and migratory properties were not affected, TOM1L1 overexpression strongly promoted invasion of these cells when plated on Matrigel or embedded in collagen matrix. In vivo, TOM1L1 upregulation increased brain and leg colonization by these tumor cells when injected into the heart left ventricle of nude mice. Mechanistically, this novel TOM1L1 activity involves a vesicular trafficking process that requires TOM1L1 association, via its GAT domain, with Toll-interacting protein (TOLLIP), another endosomal sorting protein that is upregulated in some breast cancer cell lines. We then established a link between TOM1L1-TOLLIP signaling and MT1-MMP–dependent invadopodia activity by showing that TOM1L1 promotes the long-range trafficking of MT1-MMP–positive endosomes from intracellular compartments to invadopodia for ECM degradation. On the basis of imaging data we propose a model where TOLLIP mediates TOM1L1 docking on RAB7+ late-endosomal compartments that contain MT1-MMP. Intriguingly, this novel TOM1L1 function requires ERBB2 activity, possibly via a phosphorylation-dependent mechanism. Although TOM1L1 is not tyrosine-phosphorylated in ERBB2-transformed cells, a phosphoproteomic analysis revealed that ERBB2 induces TOM1L1 phosphorylation at serine 321. This is critical for interaction with TOLLIP, MT1-MMP trafficking, and cell invasion (Fig. 1).
Figure 1.
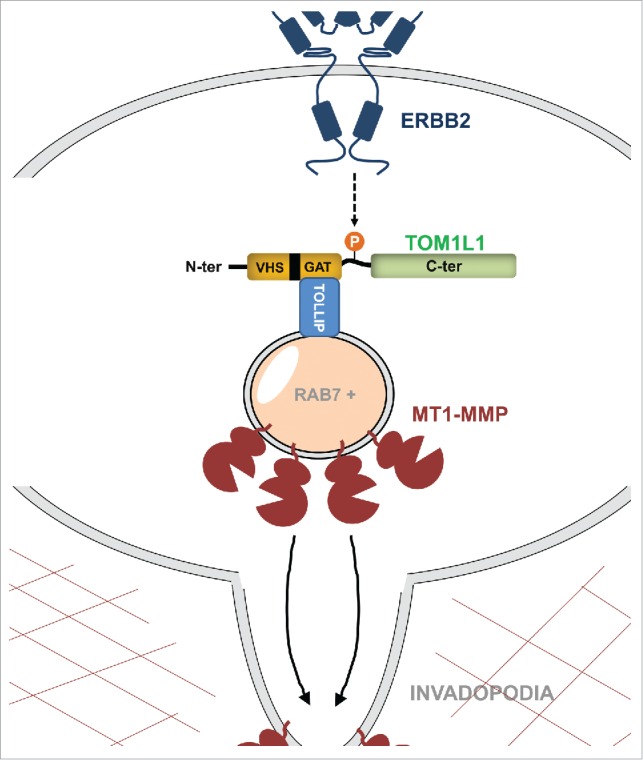
Mechanisms whereby ERBB2 and TOM1L1 cooperate to promote invasiveness in breast cancer. V-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2) induces phosphorylation of upregulated target of myb1-like 1 protein (TOM1L1) on Ser321, which promotes its association, via its GGA and TOM1 (GAT) homology domain, with Toll-interacting protein (TOLLIP) in late-endosomal RAB7+ compartments and membrane-type 1 matrix metalloprotease (MT1-MMP) trafficking to invadopodia for cell invasion. The VPS27/HRS/STAM homology domain (VHS) and the C-terminal sequence (C-ter) of TOM1L1 are highlighted.
Vesicular trafficking proteins play a central role in cell activity by, for example, regulating cell surface receptor levels, signaling, and degradation. Consequently, deregulation of this endocytic machinery might contribute to cell transformation.1 In agreement with this notion, our work shows that TOM1L1 upregulation defines a novel mechanism involved in the metastatic progression of ERBB2+ breast cancers. A similar mechanism has been recently described for the vesicular proteins RAB4 and RAB5 in breast cancer.8 Likewise, the GAT-containing protein GGA3 is involved in the promotion of cell migration.9 Interestingly, the genes encoding these trafficking proteins are amplified in several solid tumors7,8 (http://www.cbioportal.org) suggesting that this could be a general mechanism for deregulating trafficking activities in human cancer. Our work also shows that TOM1L1 might exert this novel function through the trafficking of important components needed for extracellular matrix degradation and cell dissemination. Moreover, our in vivo results suggest additional roles for TOM1L1 during later steps of metastasis formation, possibly during tumor cellular extravasation and/or colonization. Additional work is needed to determine whether and how TOM1L1 exerts these metastatic functions. One important question raised by our results concerns the apparent opposite functions of TOM1L1 during TK signaling that may involve TOM1L1 phosphorylation on specific residues. Indeed, TOM1L1 tumor inhibitory functions have been linked to tyrosine phosphorylation and association with clathrin for receptor endocytosis.5 Conversely, its protumoral activity is dependent on serine phosphorylation and association with TOLLIP for MT1-MMP plasma membrane delivery.6 Finally, these data suggest that this signaling cascade could represent a therapeutic target in these cancers. We have not identified the molecular motor involved in TOM1L1-dependent MT1-MMP vesicular trafficking or the Ser/Thr kinase responsible for TOM1L1 activation; nevertheless, inhibition of these enzymatic activities might represent an interesting strategy for improving trastuzumab-based therapy in patients with ERBB2+ breast cancer and TOM1L1 co-amplification. In conclusion, our findings highlight a novel role for GAT-containing vesicular trafficking proteins in metastatic induction driven by oncogenic TKs.
Disclosure of potential conflict of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by INCA, La Ligue Contre le Cancer (Labellisation Ligue 2014), CNRS and the University of Montpellier.
References
- 1.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 2000; 19:6102-14; PMID:11156523; http://dx.doi.org/ 10.1038/sj.onc.1203973 [DOI] [PubMed] [Google Scholar]
- 2.Poincloux R, Lizárraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci 2009; 122:3015-24; PMID:19692588; http://dx.doi.org/ 10.1242/jcs.034561 [DOI] [PubMed] [Google Scholar]
- 3.Seykora JT, Mei L, Dotto GP, Stein PL. 'Srcasm: a novel Src activating and signaling molecule. J Biol Chem 2002; 277:2812-22; PMID:11711534; http://dx.doi.org/ 10.1074/jbc.M106813200 [DOI] [PubMed] [Google Scholar]
- 4.Franco M, Furstoss O, Simon V, Benistant C, Hong WJ, Roche S. The adaptor protein Tom1L1 is a negative regulator of Src mitogenic signaling induced by growth factors. Mol Cell Biol 2006; 26:1932-47; PMID:16479011; http://dx.doi.org/ 10.1128/MCB.26.5.1932-1947.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T, Liu NS, Seet LF, Hong W. The emerging role of VHS domain-containing Tom1, Tom1L1 and Tom1L2 in membrane trafficking. Traffic 2010; 11:1119-28; PMID:20604899; http://dx.doi.org/ 10.1111/j.1600-0854.2010.01098.x [DOI] [PubMed] [Google Scholar]
- 6.Orsetti S, Nugoli M, Cervera N, Lasorsa L, Chuchana P, Ursule L, Nguyen C, Redon R, du Manoir S, Rodriguez C, et al.. Genomic and expression profiling of chromosome 17 in breast cancer reveals complex patterns of alterations and novel candidate genes. Cancer Res 2004; 64:6453-60; PMID:15374954; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-0756 [DOI] [PubMed] [Google Scholar]
- 7.Chevalier C, Collin G, Descamps S, Touaitahuata H, Simon V, Reymond N, Fernandez L, Milhiet PE, Georget V, Urbach S, et al.. TOM1L1 drives membrane delivery of MT1-MMP to promote ERBB2-induced breast cancer cell invasion. Nat Commun. 2016 Feb 22; 7:10765; http://dx.doi.org/ 10.1038/ncomms10765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frittoli E, Palamidessi A, Marighetti P, Confalonieri S, Bianchi F, Malinverno C, Mazzarol G, Viale G, Martin-Padura I, Garré M, et al.. A RAB5/RAB4 recycling circuitry induces a proteolytic invasive program and promotes tumor dissemination. J Cell Biol 2014; 206:307-28; PMID:25049275; http://dx.doi.org/ 10.1083/jcb.201403127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parachoniak CA, Luo Y, Abella JV, Keen JH, Park M. GGA3 functions as a switch to promote Met receptor recycling, essential for sustained ERK and cell migration. Dev Cell 2011; 20:751-63; PMID:21664574; http://dx.doi.org/ 10.1016/j.devcel.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]


