Abstract
Despite the recognition that some species might quickly adapt to new conditions under climate change, demonstrating and predicting such a fundamental response is challenging. Morphological variations in response to climate may be caused by evolutionary changes or phenotypic plasticity, or both, but teasing apart these processes is difficult. Here we built on the number of thoracic vertebrae (NTV) in ectothermic vertebrates, a known genetically-based feature, to establish a link with body size and evaluate how climate change might affect the future morphological response of this group of species. First we show that in old-world salamanders, NTV variation is strongly related to changes in body size. Secondly, using 22 salamander species as a case study, we found support for relationships between the spatial variation in selected bioclimatic variables and NTV for most of species. For 44% of species, precipitation and aridity were the predominant drivers of geographical variation of the NTV. Temperature features were dominant for 31% of species, while for 19% temperature and precipitation played a comparable role. This two-step analysis demonstrates that ectothermic vertebrates may evolve in response to climate change by modifying the number of thoracic vertebrae. These findings allow to develop scenarios for potential morphological evolution under future climate change, and to identify areas and species in which the most marked evolutionary responses are expected. Resistance to climate change estimated from species distribution models was positively related to present-day species morphological response, suggesting that the ability of morphological evolution may play a role for species’ persistence under climate change. The possibility that present-day capacity for local adaptation might help the resistance response to climate change can be integrated into analyses of the impact of global changes, and should also be considered when planning management actions favouring species persistence.
Keywords: amphibians, Bergmann’s rule, ectothermic vertebrates, local adaptation, morphological evolution, NDVI, precipitation, temperature
Introduction
One of the biggest challenges of global change ecology is to explain and predict the evolution of complex traits in nature and in response to environmental stressors. Body size is known to be an integrative trait linked to individual fitness, highly heritable, and influenced by natural selection (Blanckenhorn, 2000). For instance, the body size of endotherms appears to increase in areas with lower temperature (and at higher latitudes, a phenomenon known as "Bergmann’s Rule") reflecting the advantage of large body size in terms of thermoregulation (Meiri & Dayan, 2003). Nevertheless, it remains difficult to predict why a specific animal attains a specific size in a given habitat, or how body size might evolve, since multiple evolutionary forces determine body size-climate relationships (Ficetola et al., 2010, Rypel, 2014). The situation is even more complex for ectotherms since most of them have a limited capacity for thermoregulation and they have been shown to exhibit wide interspecific variation in terms of body size-climate relationships. As a consequence, only part of ectotherms showed patterns consistent with the Bergmann’s rule (Ashton & Feldmann, 2003, Adams & Church, 2008, Berke et al., 2013, Rypel, 2014). Other evolutionary forces are likely to be at play, such as selection for desiccation tolerance or starvation resistance, and may explain the complex relationships between body size variation and climate in ectotherms (Adams & Church, 2008, Olalla-Tárraga et al., 2009, Ficetola et al., 2010; Table 1). Furthermore, demonstrating evolutionary relationships between individual body size and climate is particularly challenging in nature since body size variation among populations may also reflect plasticity, differences in age, and food availability (Caruso et al., 2014, Merilä & Hendry, 2014, Rypel, 2014, Teplitsky & Millien, 2014, Connette et al., 2015).
Table 1.
Proposed evolutionary processes linking body size evolution to environmental variables variation in amphibians. Multiple processes can also interact, thus leading to complex and nonlinear relationships. See also Ficetola et al. (2010) for additional details.
| Hypotheses | Proposed process | Implicated bioclimatic variable(s) considered in this study |
|---|---|---|
| Heat balance | Large body size advantageous for thermoregulation in cold environments because increases thermal inertia (Olalla-Tárraga & Rodríguez, 2007) | Mean temperature |
| Endurance | Large body size favoured in areas with high thermal excursion, because it is associated to more fat reserves (Ashton, 2002) | Thermal excursion |
| Seasonality | Large body size favoured in areas with long growing season (Mousseau, 1997, Schutze & Clarke, 2008) | Temperature, thermal excursion, precipitation seasonality* |
| Starvation resistance | Large individuals have more reserves and can better survive during periods of food shortage, thus large body size is favoured in seasonal/cold environments where animals are inactive for long periods (Arnett & Gotelli, 2003, Ashton & Feldmann, 2003) | Temperature, thermal excursion, precipitation seasonality* |
| Water availability | Large body size is favoured in dry climates because it reduces desiccation tolerance (Ashton, 2002) | Precipitation, aridity |
| Primary productivity | Evolution of large body size favoured in more productive environments, where food supply is higher (Olalla-Tárraga & Rodríguez, 2007, Ficetola et al., 2010) | Normalized Difference Vegetation Index (NDVI) |
*amphibian activity depends on both temperature and water availability
In this study we explore evolutionary responses to climatic variation in ectothermic vertebrates through the analysis of the number of thoracic vertebrae (NTV). Despite the existence of plastic responses, NTV shows a high level of genetic determinism in ectotherms (Itazawa, 1959, Dohm & Garland, 1993, Jockusch, 1997) and affects growth rate and body size evolution (Lindell, 1996, Head & Polly, 2007, Reece & Mehta, 2013), making it a perfect integrative trait. Intraspecific patterns for NTV are therefore expected to reflect genuine evolutionary variation (Jockusch, 1997). In multiple groups of ectothermic vertebrates, including salamanders, the evolution of body size was strongly related to the number of vertebrae (Wake, 1991, Head & Polly, 2007, Reece & Mehta, 2013, Arntzen et al., 2015; see results section for an analysis on European salamanders), suggesting that the variation in the number of vertebrae is a key process in determining body size evolution. In other words, studying the relationships between climate and NTV, a trait with a strong genetic determinism that also determines body size, makes it possible to investigate the links with another trait, body size, which according to evolutionary theory may significantly evolve in response to climate change (Table 1). NTV is thus used here as a crucial link between climate and body size. Even though some analyses on fish have assessed the role of climate on variation of NTV (McDowall, 2008, Shikano & Merila, 2011), studies have neither explicitly tested multiple hypotheses on processes determining the intraspecific variation for this trait, nor they have explored the consequences of NTV variation for species evolution and persistence in the context of global changes.
Using a comprehensive dataset of urodelan amphibians (salamanders) from Europe and the Middle East (supplementary material, Fig. S1), we analysed the relationships between present-day climate and intraspecific variation for NTV across species’ ranges that potentially represent current local adaptations in response to climatic heterogeneity within their ranges. The observed relationships between NTV and climate were then used to explore the potential impact of climate change on morphological evolution, and to assess whether morphological responses to climate can help species to withstand climate change. Consensus projections extracted from multiple species distribution models (Thuiller et al., 2009) and global climate models forced by the latest emission pathways scenarios (Moss et al., 2010) were used to build reliable future distributions for all species. These projections were then related to the variation in NTV, assessing whether a morphological response would help the species to better withstand climate changes. Finally, we identified the areas where the most marked morphological responses are required in order to keep up with the pace of climatic variation.
Materials and methods
Data
Data on the number of thoracic vertebrae in Urodela species of Europe and the Middle East were collected from the literature (Veith, 1992, Veith et al., 1992, Crnobrnja-Isailovic et al., 1997, Lanza et al., 2009). Only individuals for which data on collection locality were available were considered and those from contact zones of hybridizing species were excluded. Furthermore, only species with data for at least nine populations were considered. Overall, our datasets comprised 6,090 individuals, representing 462 populations and 22 species (Fig. S1). Taxonomy followed recent checklists of European amphibians (Sillero et al., 2014).
Eight bioclimatic variables were considered representing thermal environment, water availability, desiccation risk and primary productivity: mean annual temperature, mean temperature diurnal range, temperature annual range, mean annual summed precipitation, precipitation seasonality, summer precipitation, winter precipitation (caclulated from the period 1950-2000 and obtained from the worldclim dataset; Hijmans et al., 2005); the aridity index (Trabucco & Zomer, 2009) and the Normalized Difference Vegetation Index (NDVI), a proxy of primary productivity, obtained from the NOAA and the VEGETATION datasets (Gutman et al., 1997 and http://www.vgt.vito.be). Cave salamanders (genus Hydromantes) generally remain underground during the summer (Lanza et al., 2006, Lunghi et al., 2015), so we only considered summed precipitation during autumn, winter and spring, instead of annual, winter and summer precipitations. Bioclimatic parameters might be related to body size evolution through multiple processes, as they affect thermoregulation, water balance, activity length, endurance, starvation resistance and food availability (Table 1; reviewed in Ficetola et al., 2010). Environmental parameters were extracted at a resolution of 30'' for species in which all localities were defined with accuracy of less than 1 km (Table S1). For the remaining species, environmental parameters were extracted at a resolution of 2.5'.
Statistical analyses
Body size evolution and number of vertebrae
Phylogenetic generalized least squares (PGLS) were used to evaluate whether, at the interspecific level, the variation of body size was related to the number of vertebrae in European Urodela (see Wake, 1991 for examples on salamanders from other geographic areas). Data on the average number of vertebrae in the 22 study species (Table 2), plus eleven additional species for which data were available were used as the independent variable (Fig. S1). Data on the average body size of females (snout-vent length) were obtained from the literature (Lanza et al., 1995, Colleoni et al., 2014); we controlled for phylogeny by combining the trees used by previous comparative studies (Ficetola et al., 2013a, Colleoni et al., 2014). Available information was insufficient to test the relationships between the number of vertebrae and average body size at the intraspecific level.
Table 2.
Study species and variables selected by the best-AIC models. See Tab. S1 for a complete list of candidate models and their weights. N: number of populations / individuals for each species; (+): positive relationships; (-): negative relationships; (Q): quadratic relationships. Mean and range of the number of vertebrae for each species are reported in Tab. S6.
| Species | N | Range area (km2) | R2m | R2c | Variables in the best AICc model |
|---|---|---|---|---|---|
| Hydromantes ambrosii | 14/84 | 920 | 0.15 | 0.23 | Precipitation seasonality (+),Precip. Sept-May (Q) |
| H. flavus | 10/42 | 616 | 0 | 0 | |
| H. genei | 15/123 | 2155 | 0 | 0 | |
| H. imperialis | 13/116 | 2391 | 0.05 | 0.10 | Precipit. Sept-May (-), Temp. annual range (+) |
| H. italicus | 19/100 | 17675 | 0.03 | 0.03 | Precipit. Sept-May (+) |
| H. strinatii | 42/315 | 8836 | 0 | 0.14 | |
| H. supramontis | 12/87 | 616 | 0 | 0.02 | |
| Salamandra atra | 9/45 | 111164 | 0.30 | 0.40 | Temp. diurnal range (-), Winter precipit. (-) |
| S. corsica | 11/43 | 4413 | 0.14 | 0.14 | Temp. mean (-), Temp. diurnal range(+) |
| S. infraimmaculata | 14/599 | 80949 | 0.19 | 0.33 | Temp. annual range (Q) |
| S. salamandra | 89/2679 | 2162890 | 0.03 | 0.51 | Precip. Seasonality (Q) |
| Lissotriton helveticus | 9/55 | 1082200 | 0 | 0.30 | |
| L. italicus | 13/114 | 75324 | 0.16 | 0.24 | Winter precip. (+), NDVI (Q) |
| L. vulgaris | 40/276 | 7340650 | 0.43 | 0.71 | Aridity (+), Temp. mean (-) |
| Ichthyosaura alpestris | 40/326 | 1364076 | 0.15 | 0.53 | Precip. seasonality (+), NDVI (Q) |
| Ommatotriton ophryticus | 9/80 | 268433 | 0 | 0.71 | |
| O. vittatus | 13/49 | 53232 | 0.21 | 0.22 | NDVI (-) |
| Triturus carnifex | 28/300 | 337089 | 0.43 | 0.77 | Summer precip. (Q), Winter precip. (+) |
| T. cristatus | 18/142 | 4366886 | 0.57 | 0.59 | Temp. annual range (+), Winter precipit. (+) |
| T. dobrogicus | 14/127 | 272023 | 0.24 | 0.41 | Temp. mean (+), Temp. diurnal range (+) |
| T. macedonicus | 17/341 | 163791 | 0.15 | 0.30 | Aridity (Q), NDVI (+) |
| T. marmoratus | 13/47 | 474709 | 0.12 | 0.12 | Aridity (-) |
Relationships between the number of vertebrae and bioclimatic variables
An information-theoretic approach was used to identify the combination of environmental variables most likely to influence intraspecific variation for the number of vertebrae (Burnham & Anderson, 2002). First, mixed models relating the number of vertebrae to all combinations of environmental variables were built. For each species, mixed models were estimated using maximum likelihood, and included population identity as a random effect. Strong intraspecific genetic structure can obscure relationships between morphology and climate (Romano & Ficetola, 2010). Therefore, for species comprising multiple subspecies, or when available genetic data indicated the existence of a strong intraspecific genetic structure, we included clade / subspecies identity as an additional random effect to take into account the potential effect of long- term evolutionary isolation (Steinfartz et al., 2000, Babik et al., 2005, Sotiropoulos et al., 2007, Wielstra et al., 2010, Canestrelli et al., 2012). The models were then ranked using Akaike’s Information Criterion corrected for small sample size (AICc) (Burnham & Anderson, 2002). AICc may select overly complex models, we therefore considered a complex model as a candidate only if it had an AICc that was lower than the AICc of all of its simpler nested models (Symonds & Moussalli, 2011). Furthermore, we allowed a maximum of two environmental variables per model for species with less than 20 populations, and a maximum of three variables for the remaining species. We excluded models with pairwise correlation among variables ≥ 0.7; in all candidate models variance inflation factor was well below five, indicating lack of collinearity issues (Dormann et al., 2013). We used Moran’s I to test whether spatial autocorrelation may influence the results of our models. For all best-AICc models, residual autocorrelation was weak (P > 0.05 for all species), suggesting that autocorrelation did not bias our models (Dormann et al., 2007). In our results, we first present the best-AICc models, which are particularly relevant when they have strong support. Subsequently, we also performed model averaging, which may be more suitable when then there is not a single best model but rather a range of models that show good AICc values (Burnham & Anderson, 2002, Richards et al., 2011). For model averaging, we calculated the Akaike weight (w) of all candidate models including the intercept model, which is a measure of the support of the model, given the data (Burnham & Anderson, 2002). Model averaging was then used to obtain spatial projections of models under both present-day and future climatic conditions. If needed, environmental variables were transformed using square-root (annual, summer and winter precipitation) or logarithm (aridity) to improve normality and reduce skewness.
We used marginal and conditional R2 (R2m and R2c, respectively) to assess the fit of the mixed-effect models (Nakagawa & Schielzeth, 2013). Both R2m and R2c convey unique information. Specifically, R2m represents the pure effect of fixed factors, while R2c is the variance explained by the entire model (Nakagawa & Schielzeth, 2013). R2m and R2c of best models were then used to define the present-day morphological response to bioclimatic variables. More specifically, we defined the present marginal response as R2m. The marginal response corresponded to the present-day morphological response to bioclimatic variables, after taking into account population- and clade-level effects. We defined the present global response as R2c, i.e. the response integrating the effects bioclimatic variables, population- and clade-level variation on morphology, and also including eventual joint effects between fixed and random variables. The global morphological response also aims at including eventual variation from local ecological factors not explicitly investigated here.
The models relating the number of vertebrae to bioclimatic variables were projected under multiple climate change scenarios on the basis of model-averaged models to obtain scenarios of how species might morphologically respond to climate change through rapid evolution in NTV and body size. We used the Hadley Centre Global Environment Model version 2–Earth System global circulation model (GCM) and four emission scenarios from the IPPC fifth assessment report, which are distinct “representative concentration pathways” (rcp): rcp2.6, rcp4.5, rcp6.0, rcp8.5. The four emission scenarios represent increasing emission pathways, leading to radiative forcing in 2100 of 2.6, 4.5, 6.0 and 8.5 Wm2 (hereafter named: HE2.6, HE4.5, HE6.0 and HE8.5; Moss et al., 2010). In order to explore variability among GCMs, three additional models were also considered [Geophysical Fluid Dynamics Laboratory Coupled Physical Model CM3 (GFDL-CM3); the Hadley Centre Global Environment Model version 2 – atmosphere (HadGEM2-AO); and the fifth version of the low resolution climate model of the Institut Pierre Simon Laplace (IPSL-CM5A-LR)], using one given rcp4.5 (hereafter named: GF4.5, HD4.5, IP4.5). These projections were available for all the climatic variables used in this study. However, NDVI was not projected over time but was kept constant. Data on temperature and precipitation under climate change scenarios were downloaded from http://www.worldclim.org. The aridity index under climate change scenarios was developed from these data, following Trabucco and Zomer (2009).
The present relationships between climate and NTV were used to predict the mean number of vertebrae in each grid cell under the present and future climatic scenarios. We then calculated the percentage of morphological variation for each map cell. Spatial projections were limited to areas within the range and, for species with large ranges not fully covered by sampled populations, to less than 500 km from sampled populations.
Variation in suitability: species distribution models
Correlative species distribution models (SDM) were used to assess relationships between the distribution of the study species and climate, and to evaluate potential changes in suitability as a consequence of climate change. Within Europe, the SDM were calibrated assuming presence in cells where the species is present in the European Herpetological Atlas (Sillero et al., 2014), refined at a 2.5' resolution by removing the cells outside the altitudinal limit of the species, or with unsuitable habitat (Ficetola et al., 2015). Outside of Europe, the models were calibrated using the refined IUCN range maps (Ficetola et al., 2015). For each species we used 5000 absence points, selected within the study area outside the cells where the species is recorded to be present. An ensemble of SDM forecasts (Araujo & New, 2007) was obtained for each species on the basis of the bioclimatic variables. The ensemble included projections with Generalized Additive Models, Boosting Regression Trees, Classification Trees, Multiple Adaptive Regression Splines and Random Forests. Models were developed using biomod2 (Thuiller et al., 2009), and the probability of occurrence was projected under both current and future conditions as described in Thuiller et al. (2014). In order to obtain the remaining proportion of suitable species range in the future, probabilities of occurrence were transformed into binary maps (presence/absence) using the value maximising the true skill statistics as a threshold (Thuiller et al., 2014). Future projections were limited to within the species range, due to the very limited dispersal capability of amphibians, particularly within the human-dominated landscapes of the study area (Araujo et al., 2006, Early & Sax, 2011).
Species’ resistance to projected climate change was estimated as the proportion of currently suitable range, that remains suitable under climate change scenarios. Species exposure was the mean absolute difference in environmental suitability between current and future conditions.
Relationship between morphological variation, changes in climatic suitability, and range size
Random-slope mixed models were used to measure the relationships between morphological variation, species resistance to climate change, exposure to climate change and species range (Table 2), under the seven climate change scenarios. First, we tested the relationship between exposure to climate change and future morphological response. Second, we evaluated whether species resistance to climate change was related to range size or present-day morphological variation. AICc was used to evaluate the support of potential predictors of resistance to climate change. In all models, species identity and scenario were considered as random effects. The results remained identical if the scenarios were considered as fixed factors. Uncertainty of variables was considerable, particularly given the differences among emission scenarios and GCMs. Therefore, as a dependent variable we did not use one single value per species (e.g. the mean across the seven climate change scenarios). Instead, for each species we used the projections under the seven scenarios, considering scenario and species identity as random effects, as we were interested in taking into account the heterogeneity across potential scenarios. In these mixed models, we used R2m as a measure of the pure effect of fixed factors. Some independent variables in these models (morphological variation, resistance to climate change and exposure to climate change) were not controlled by the researcher, with errors associated with their measurement. In this case, model II regression may be more appropriate (Legendre & Legendre, 2012), but unfortunately model II regressions are not easily implemented within mixed models. We therefore re-analysed all the significant relationships with ranging major axis regression (Legendre & Legendre, 2012), using 999 permutations to obtain 95% confidence intervals of regression coefficients. All statistical analyses were performed using the R statistical environment (R Development Core Team, 2014), using the packages caper, lmodel2, nlme, MuMIn, SpatialPack, raster and rgdal (Orme et al., 2013, Bivand et al., 2014, Hijmans, 2014, Pinheiro et al., 2014, Barton, 2015).
Results
Body size evolution and number of vertebrae
Across 33 species of salamanders from Europe and Asia, the variation of body size was strongly related to the number of vertebrae (PGLS: F1,31 = 33.7, B ± SE = 1.37 ± 0.24, P < 0.0001, R2 = 0.52; Fig. S2). This result confirmed observations on other ectothermic vertebrates (Head & Polly, 2007, Reece & Mehta, 2013), and suggests that in salamanders the variation in the number of vertebrae is a key process determining body size evolution.
Relationships between climate and present-day number of vertebrae
The NTV showed variability in all the species with one exception (Hydromantes genei), even though this discrete parameter only has a limited number of values for each species (Table S2). Intraspecific variability, measured as coefficient of variation, clearly increased with geographic range (partial correlation taking into account sample size: r = 0.67, N = 22, P < 0.001).
For 16/22 species (73%), our information-theoretic approach detected support for relationships between environmental features and variation in the number of vertebrae; the strength of relationships was highly variable among species, being very low (R2m < 0.1) in three species, moderate (0.1 < R2m < 0.3) in nine species, and good (R2m ≥ 0.3) in four species (Table 2, Figs. S3-S4). The best set of models explaining the variation in the number of vertebrae included variables representing precipitation, temperature and primary productivity (Table 2). The best models differed between species, and there was some uncertainty regarding the selection of the best models for several species (Table S1). For 44% of the 16 species with models, the weight of evidence for precipitation and aridity was predominant, temperature features were dominant for 31% of species, while for 19% temperature and precipitation variables played a comparable role (Table S3). Precipitation during the outdoor activity period (autumn to spring) was consistently included in the models for cave salamanders (Hydromantes), while patterns were more heterogeneous in the other genera.
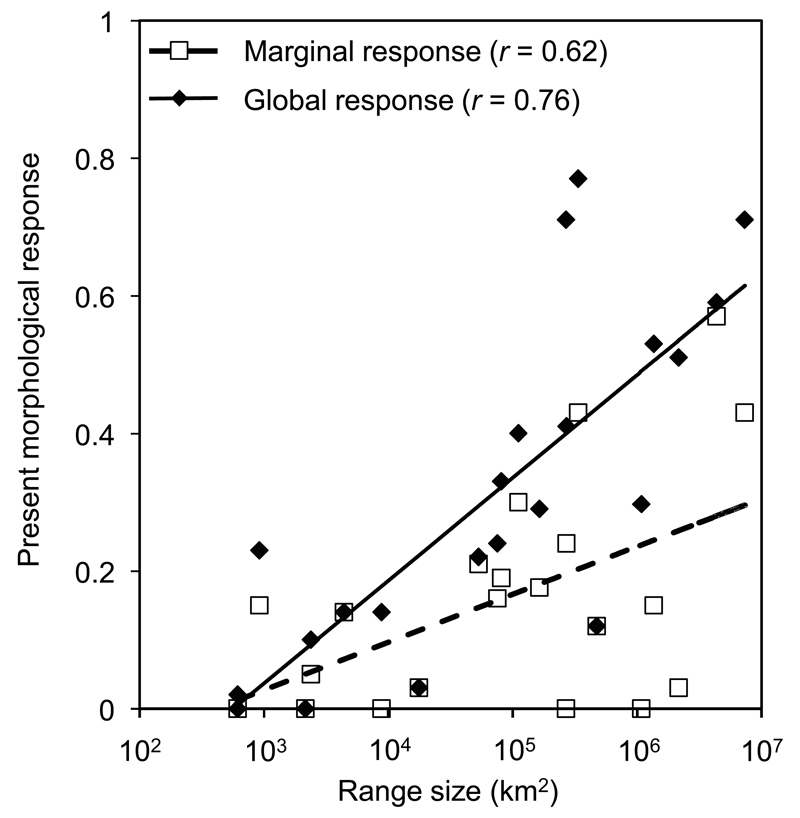
The few species for which we did not detect any relationship with bioclimatic variables tended to be those with the smallest ranges, although we still detected clear relationships in some of the highly localised species (Table 2). Both marginal and global present responses were more marked in species with large ranges (partial correlations: range area vs. marginal response: r = 0.62, P < 0.001; range area vs. global response: r = 0.76, P < 0.001, Fig. 1). For two species (L. helveticus and O. ophryticus) R2m was zero, while R2c was ≥ 0.3, suggesting that morphological variation might be caused either by historical factors, or by environmental factors not explicitly accounted here.
Figure 1.
Current morphological response (R2) in 22 species of salamanders. Relationship between range size, marginal and global present-day morphological responses.
Interspecific variation in morphological response and resistance to climate change
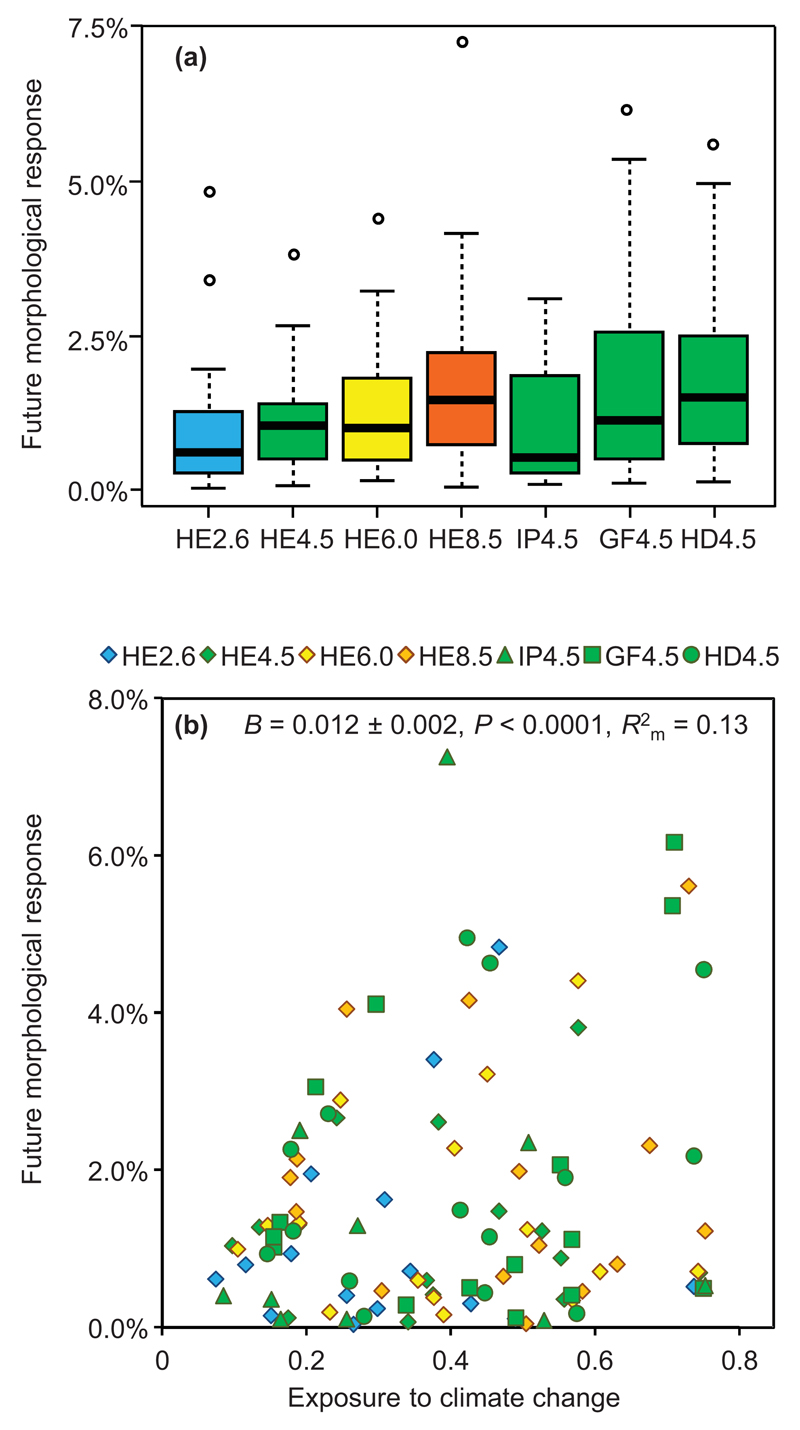
Each species’ morphological response to climate change was estimated as the mean relative change in the predicted number of vertebrae between current and future conditions. The future morphological response was highly variable among species and scenarios, ranging between 0.04% and 7.3% (Fig. 2a, Table S4). The future morphological response tended to increase in the most severe emissions scenarios (Fig. 2). Despite a high level of variability in the response (Fig. 2), the overall pattern was highly consistent across the different scenarios, as the species expected to have the most marked future response were consistently identified in all the scenarios (Spearman’s correlations of future morphological response between scenarios always ≥ 0.75).
Figure 2.
(a) Future morphological response expected under seven scenarios of climate change. (b) Relationship between exposure by climate change (i.e. the average variation in climatic suitability across the range) and future morphological response, under seven climate change scenarios. Different colours and symbols represent distinct emission scenarios and global circulation models, as depicted above the (a) panel.
Ensemble forecasting of species distribution models was used to evaluate potential changes in the species’ environmental suitability. The quality of the ensemble models was very good to excellent (Fig. S5). Exposure to climate change was a good predictor of the future morphological response. The species subjected to the highest levels of exposure (i.e., the ones suffering the strongest suitability changes under the climate change scenarios) were those which showed the most pronounced projected response in terms of morphology (F1,89 = 18.2, P < 0.0001, R2m = 0.13, Fig. 2b). For this analysis, a random intercept model was used because random slope models showed convergence problems, and the variance of random slope was 2.2 × 10-12.
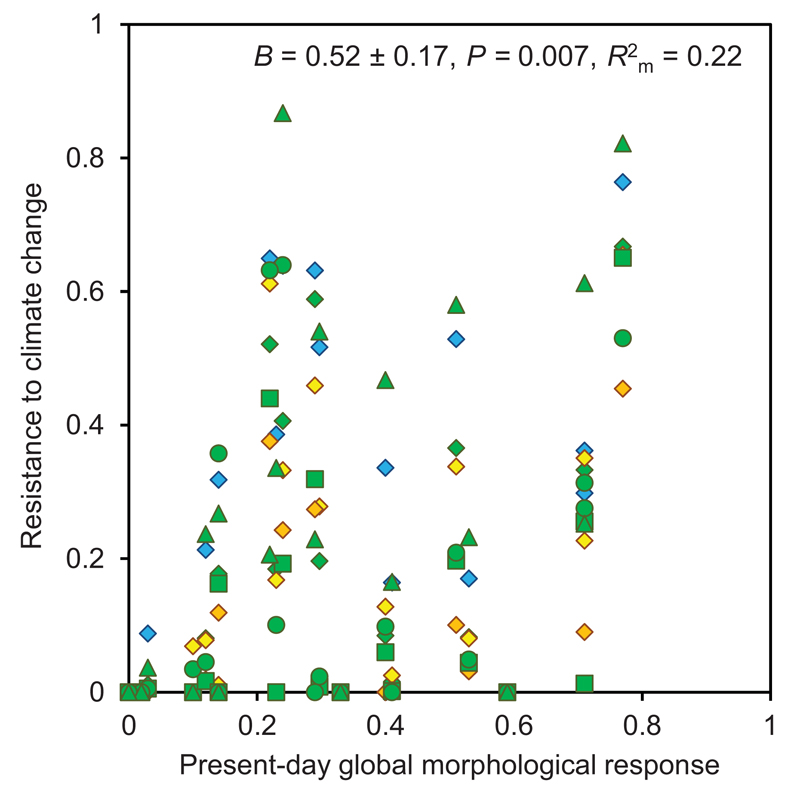
We tested the support of different predictors of resistance to climate change: geographical range (as widespread species often have broad ecological niches, and are thus hypothesised to better withstand climate change; Slatyer et al., 2013), and the extent of present-day morphological response (either marginal or global). Present global response was the best predictor of resistance to climate change (Fig. 3), while the relationships between resistance and range size or marginal responses were weaker (Table 3). Major axis regression confirmed the results obtained using standard mixed models (Table S5).
Figure 3.
Relationship between present-day global morphological response (R2c) and resistance to climate change (i.e. the proportion of species range that remains suitable). Different colours and symbols represent distinct emission scenarios and global circulation models, as depicted above the panel.
Table 3.
Comparison of potential predictors of resistance to climate change across salamander species. All models are mixed models, including species identity and climatic scenarios as random factors.
| Model rank | Predictor | F | d.f. | P | R2m | ΔAICc |
|---|---|---|---|---|---|---|
| 1 | Present global response | 9.15 | 1,20 | 0.007 | 0.22 | 0 |
| 2 | Geographical range | 4.32 | 1,20 | 0.051 | 0.17 | 5.62 |
| 3 | Present marginal response | 3.38 | 1,20 | 0.081 | 0.10 | 10.56 |
| 4 | Null model | - | - | - | - | 40.66 |
Morphological response to climate change: intraspecific variation
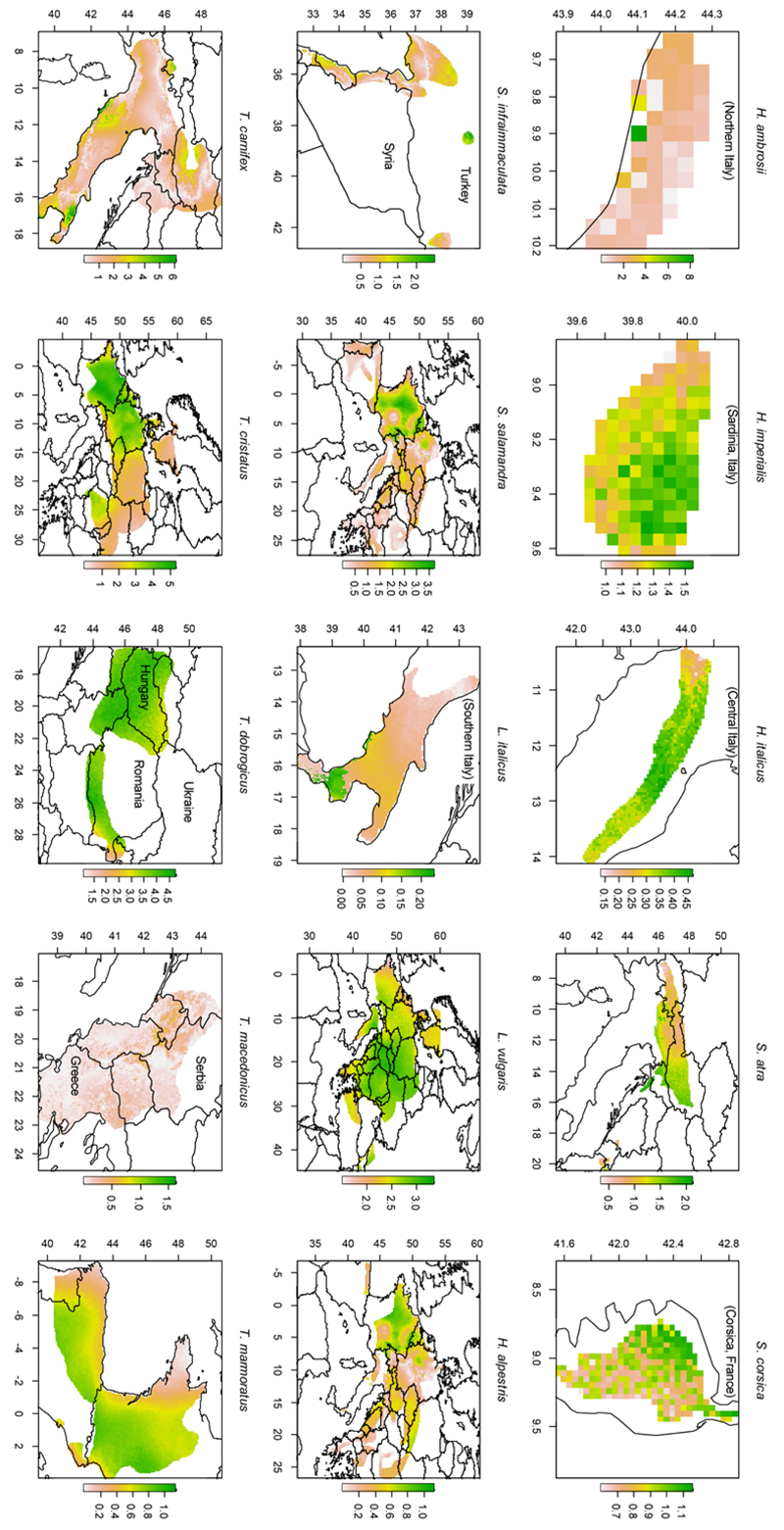
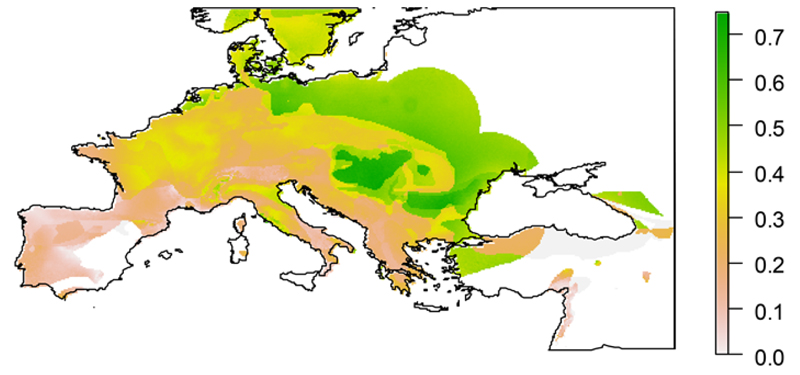
For each species, we identified the areas where the most marked morphological response could be expected. Within the species’ ranges the future morphological response was highly heterogeneous (Fig. 4, Fig. S6). For the 15 species with significant relationships between climate and the number of vertebrae (Table 2), we assessed whether the areas with the most marked future morphological response corresponded to those with the greatest variation in suitability, under the seven climate change scenarios. Only 22/105 correlations between morphological response and variation in suitability were significant, and just three of these remained significant after Bonferroni’s correction (Table S6). This suggests that these two responses to climate change might be unlinked within the species’ ranges. Overall, the most marked future morphological responses are expected to occur in the north-eastern portions of the study area (Fig. 5, Fig. S7), with consistent results across climate change scenarios (Table S7).
Figure 4.
Intraspecific variation in morphological response (%) expected in 15 salamander species under the climate change scenario HE4.5. See Fig. S6 for patterns under different climate change scenarios. Panels represent different areas and have very heterogeneous scales; the figures on the margins of each panel represent the geographic coordinates (lat-long) of each area.
Figure 5.
Intraspecific variation in morphological response averaged across multiple salamander species, and across seven climate change scenarios. The map is the average of the expected morphological responses under seven GCM and emission scenarios, each standardized between 0 and 1. See Fig. S7 for the expected responses under each of the seven scenarios. For each scenario, expected morphological responses are the average of all the species showing a relationship between number of vertebrae and ecogeographical variables, plus the species not showing a relationship with eco-geographical variables, but with range > 100 000 km2.
Discussion
The possibility of evolutionary responses to climate change is attracting growing interest, although evidence of such adaptive evolution remains limited (Holt, 1990, Carroll et al., 2014, Merilä & Hendry, 2014, Teplitsky & Millien, 2014). Phenotype-environment correlations, investigating multiple potential drivers over broad scales, may provide important insights, but can only focus on traits with a strong genetic basis (Merilä & Hendry, 2014). Our study represents one of the first large-scale evaluations of potential evolutionary changes in response to climate change. This analysis was possible due to the genetic basis of among-population differences in NTV (Jockusch, 1997), the strong evolutionary link between NTV and body size (Fig. S2), and to the close relationships between NTV and climatic parameters in some salamander species (Table 2).
Species showing strong morphological responses to the climatic variation currently occurring within their range may better resist climate change (Fig. 3). The possibility that present-day capacity for local adaptation might help the resistance to climate change is an intriguing new hypothesis that can be integrated into analyses of the impact of global changes. Dispersal may be essential to spread favourable adaptations across populations (Bell & Gonzalez, 2011). However, if there are high levels of heterogeneity regarding adaptations the arrival of maladapted individuals may reduce fitness and impede rapid evolutionary changes to adapt to new conditions (Schiffers et al., 2013). Our approach highlights the complexity of making evolutionary predictions, given the heterogeneity of interspecific responses and the complex spatial patterns of variation (Fig. 4).
Correlative analyses, such as phenotype-environment correlations and species distribution models have their own limitations. First, demonstrating a relationship with present-day data does not necessarily mean that the same effect will hold in the future (Merilä & Hendry, 2014). Second, the strength of relationships between present-day climate and morphological variation was highly heterogeneous among species, with some showing strong relationships, and others showing weaker or no effects. Relationships were often weak in species with very small geographical ranges, where populations experience less geographical variation for climatic conditions (Fig.1, Table 2). The robustness of our conclusions might thus be variable among species. Third, the GCM and emission scenarios show high variability, and this determines variation in the expected morphological responses. For instance, the strongest future morphological responses are expected to occur in the north-eastern portions of the study area, but this trend is weaker in the less severe emission scenarios (Fig. S7). Model averaging and ensemble forecasting are some of the best approaches to account for the multiple uncertainties among models and future scenarios (Burnham & Anderson, 2002, Araujo & New, 2007), and our overall conclusions remain robust to multiple scenarios. Fourth, our analyses tested simple linear or quadratic relationships between present-day morphology and climate, but responses may be more complex, and extrapolations may be particularly uncertain when climate is projected beyond the current range of variability. Fifth, our models were built at rather fine spatial scales (if possible, 1-km resolution), but many amphibians select microhabitats at even finer scales (e.g. Ficetola et al., 2013b). These microhabitats may have environmental conditions different from the ones estimated from broad-scale bioclimatic layers, such as mean temperature (Scheffers et al., 2014), and might act as micro-refugia, buffering against climate change effect (Dobrowski, 2011). More research is needed to assess how microhabitat selection may influence species resistance to global changes. Finally, NTV variation is only one piece of the evolutionary response puzzle, and species can employ other strategies to cope with change (e.g. behavioural changes, microhabitat shifts, evolution of other physiological or morphological parameters, etc.). Testing the reliability of our predictions would be challenging. Comparisons with fossils from periods with different climatic conditions may be an option (e.g. Fouquet et al., 2010, Maiorano et al., 2013), even though the number of fossil remains of urodeles is limited, and their taxonomic assignment often difficult (D'Orazi Porchetti et al., 2012). Although we cannot state with certainty that our set of species will evolve as predicted, we do provide a new path for jointly exploring evolutionary and distributional responses to climate change.
Measuring the amount of variation explained by mixed models is not an intuitive task, and it is only recently that generalizable and consistent methods have been developed (Nakagawa & Schielzeth, 2013, Johnson, 2014). Given the structure of mixed models, the present-day morphological response should be quantified by jointly using two parameters (R2m and R2m), as both convey unique information (Nakagawa & Schielzeth, 2013). Marginal morphological response (i.e., R2m) was the amount of morphological variation solely determined by the analysed climatic parameters, after taking into account the population- and clade-level variation. Conversely, the global morphological response (R2C) was the overall amount of explained variation, and also taking into account population- and clade-level effects. Differences between marginal and global morphological response were variable among species. In some case, marginal and global responses were nearly identical, but in nearly half of species the global response showed considerably higher values (Table 2). This might occur because of multiple processes, such as genetic variation determined by historical or stochastic processes, or adaptation to parameters not considered in this study (e.g. features of aquatic or underground habitats). Species with broad geographical range generally exploit broader niches and are expected to better withstand climate change (Slatyer et al., 2013).
However, resistance to climate change was more strongly related to the global morphological response than to the extent of species range (Table 3). This suggests that the species showing the strongest morphological response also exploit the broadest niches, and this may improve their persistence under climate change.
Morphological response to bioclimatic variables was highly heterogeneous among species. Such heterogeneous responses were somewhat expected. Previous studies on the evolution of body size in amphibians have reported highly contrasting patterns, as some closely related species sometimes show opposite responses to the same climatic variables (Adams & Church, 2008): such heterogeneity in the responses may arise because each species is subjected to different evolutionary pressures (Ficetola et al., 2010). However, identifying evolutionary processes determining body size variation is challenging, as body size is a highly plastic trait (Merilä & Hendry, 2014, Teplitsky & Millien, 2014). The pattern obtained through the analysis of the number of trunk vertebrae, a trait that is mostly genetically determined, was highly heterogeneous among species, and this result was consistent with body size analyses (Adams & Church, 2008). For instance, some newt species showed a positive relationship between NTV and temperature, while others showed negative or no relationships (Table 2). This supports the idea that no general rules of body size evolution exist for ectotherms, as multiple evolutionary processes are at play (e.g. Table 1), and the importance of such processes is highly heterogeneous across species.
Species may persist under dramatic environmental changes by migrating towards newly suitable areas or through the evolution of new adaptations. However, species persistence requires a rate of adaptation that outpaces that of environmental change, and fast adaptation is only possible if populations harbour enough standing genetic variation (Holt, 1990). Morphological variability among individuals was frequent within the study populations, suggesting the existence of some genetic variation, but the within-population variability was still consistently lower than the overall variability across populations (Fig. S8). In practice, both evolutionary adaptations and migration would require a relevant flow of individuals, either across populations or towards new areas. Unfortunately, successful range shifts are unlikely for small vertebrates with limited dispersal due to the extreme fragmentation of habitats and gaps in climate paths (Araujo et al., 2006, Early & Sax, 2011), thus more active management actions may be needed. Conservation biologists stress the importance of “evolutionary significant units” that should be independently managed to preserve intraspecific genetic variability and the evolutionary future of species (Latta, 2008). Our study offers new perspectives on how species with more variability might better withstand the challenge of climate change, but also poses conservation dilemmas. Species management might require the introduction of individuals showing adaptations to future climate conditions (Carroll et al., 2014), but in turn this could lead to genetic homogenisation among populations and a loss of variability. Since the genetic variability underpinning local adaptations is often underappreciated, translocations may lead to unwanted loss of adaptations (Ficetola & De Bernardi, 2005, Latta, 2008). Managers may be faced with the trade-off between the risk of species extirpation, and the struggle to maintain intraspecific variation. Providing detailed evolutionary information to answer this dilemma will be a major challenge in the coming years.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Acknowledgments
We thank V. Popescu, D. Moore, I. Auger and three reviewers for insightful comments on previous versions of the manuscript. GFF was partially funded by a grant of Univ. Milano-Bicocca. WT and JR received funding from the European Research Council under the European Community’s Seven Framework Programme FP7/2007- 2013 Grant Agreement no. 281422 (TEEMBIO). The LECA is part of Labex OSUG@2020, which supported this work (Investissements d'avenir – ANR10 LABX56). This paper is dedicated to Beatrice.
References
- Adams DC, Church JO. Amphibians do not follow Bergmann's rule. Evolution. 2008;62:413–420. doi: 10.1111/j.1558-5646.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- Araujo MB, New M. Ensemble forecasting of species distributions. Trends in Ecology & Evolution. 2007;22:42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Araujo MB, Thuiller W, Pearson RG. Climate warming and the decline of amphibians and reptiles in Europe. Journal of Biogeography. 2006;33:1712–1728. [Google Scholar]
- Arnett AE, Gotelli NJ. Bergmann’s rule in larval ant lions: testing the starvation resistance hypothesis. Ecological Entomology. 2003;28:645–650. [Google Scholar]
- Arntzen JW, Beukema W, Galis F, Ivanovic A. Vertebral number is highly evolvable in salamanders and newts (family Salamandridae) and variably associated with climatic parameters. Contributions to Zoology. 2015;84:85–113. [Google Scholar]
- Ashton KG. Do amphibians follow Bergmann's rule? Canadian Journal of Zoology. 2002;80:708–716. [Google Scholar]
- Ashton KG, Feldmann CR. Bergmann's rule in nonavian reptiles: Turtles follow it, lizards and snakes reverse it. Evolution. 2003;57:1151–1163. doi: 10.1111/j.0014-3820.2003.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Babik W, Branicki W, Crnobrnja-Isailovic J, et al. Phylogeography of two European newt species — discordance between mtDNA and morphology. Molecular Ecology. 2005;14:2475–2491. doi: 10.1111/j.1365-294X.2005.02605.x. [DOI] [PubMed] [Google Scholar]
- Barton K. MuMIn: Multi-model inference. R package version 1.15.1. 2015 http://CRAN.R-project.org/package=MuMIn
- Bell G, Gonzalez A. Adaptation and Evolutionary Rescue in Metapopulations Experiencing Environmental Deterioration. Science. 2011;332:1327–1330. doi: 10.1126/science.1203105. [DOI] [PubMed] [Google Scholar]
- Berke SK, Jablonski D, Krug AZ, Roy K, Tomasovych A. Beyond Bergmann's rule: size–latitude relationships in marine Bivalvia world-wide. Global Ecology and Biogeography. 2013;22:173–183. [Google Scholar]
- Bivand R, Keitt T, Rowlingson B. rgdal: Bindings for the Geospatial Data Abstraction Library. R package version 0.8-16. 2014 http://CRAN.R-project.org/package=rgdal
- Blanckenhorn WU. The evolution of body size: What keeps organisms small? Quarterly Review of Biology. 2000;75:385–407. doi: 10.1086/393620. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer Verlag; 2002. [Google Scholar]
- Canestrelli D, Sacco F, Nascetti G. On glacial refugia, genetic diversity, and microevolutionary processes: deep phylogeographical structure in the endemic newt Lissotriton italicus. Biological Journal of the Linnean Society. 2012;105:42–55. [Google Scholar]
- Carroll SP, Jørgensen PS, Kinnison MT, et al. Applying evolutionary biology to address global challenges. Science. 2014;346 doi: 10.1126/science.1245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso NM, Sears MW, Adams DC, Lips KR. Widespread rapid reductions in body size of adult salamanders in response to climate change. Global Change Biology. 2014;20:1751–1759. doi: 10.1111/gcb.12550. [DOI] [PubMed] [Google Scholar]
- Colleoni E, Denoël M, Padoa Schioppa E, Scali S, Ficetola GF. Rensch’s rule and sexual dimorphism in salamanders: patterns and potential processes. Journal of Zoology. 2014;293:143–151. [Google Scholar]
- Connette GM, Crawford JA, Peterman WE. Climate change and shrinking salamanders: alternative mechanisms for changes in plethodontid salamander body size. Global Change Biology. 2015;21:2834–2843. doi: 10.1111/gcb.12883. [DOI] [PubMed] [Google Scholar]
- Crnobrnja-Isailovic J, Dzukic G, Krstic N, Kalezic ML. Evolutionary and paleogeographical effects on the distribution of the Triturus cristatus superspecies in the central Balkans. Amphibia-Reptilia. 1997;18:321–332. [Google Scholar]
- D'Orazi Porchetti S, Manni R, Sottili G. A salamandrid from the middle Pleistocene of northern Latium (Fosso di San Martino, Rome, Italy) Bollettino della Società Paleontologica Italiana. 2012;51:7–13. [Google Scholar]
- Dobrowski SZ. A climatic basis for microrefugia: the influence of terrain on climate. Global Change Biology. 2011;17:1022–1035. [Google Scholar]
- Dohm MR, Garland T. Quantitative genetics of scale counts in the Garter Snake Thamnophis sirtalis. Copeia. 1993;1993:987–1002. [Google Scholar]
- Dormann CF, Elith J, Bacher S, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:27–46. [Google Scholar]
- Dormann CF, McPherson JM, Araújo MB, et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography. 2007;30:609–628. [Google Scholar]
- Early R, Sax DF. Analysis of climate paths reveals potential limitations on species range shifts. Ecology Letters. 2011;14:1125–1133. doi: 10.1111/j.1461-0248.2011.01681.x. [DOI] [PubMed] [Google Scholar]
- Ficetola GF, Bonardi A, Colleoni E, Padoa-Schioppa E, Scali S. Evolution of sexual dimorphism in the number of tail vertebrae in salamanders: comparing multiple hypotheses. Evolutionary Biology. 2013a;40:220–227. [Google Scholar]
- Ficetola GF, De Bernardi F. Supplementation or in situ conservation? Evidence of local adaptation in the Italian agile frog Rana latastei and consequences for the management of populations. Animal Conservation. 2005;8:33–40. [Google Scholar]
- Ficetola GF, Pennati R, Manenti R. Spatial segregation among age classes in cave salamanders: habitat selection or social interactions? Population Ecology. 2013b;55:217–226. [Google Scholar]
- Ficetola GF, Rondinini C, Bonardi A, Baisero D, Padoa-Schioppa E. Habitat availability for amphibians and extinction threat: A global analysis. Diversity and Distributions. 2015;21:302–311. [Google Scholar]
- Ficetola GF, Scali S, Denoël M, Montinaro G, Vukov TD, Zuffi MAL, Padoa-Schioppa E. Ecogeographical variation of body size in the newt Triturus carnifex: comparing the hypotheses using an information-theoretic approach. Global Ecology and Biogeography. 2010;19:485–495. [Google Scholar]
- Fouquet A, Ficetola GF, Haigh A, Gemmell N. Using ecological niche modelling to infer past, present and future environmental suitability for Leiopelma hochstetteri, an endangered New Zealand native frog. Biological Conservation. 2010;143:1375–1384. [Google Scholar]
- Gutman G, Tarpley D, Ignatov A, Olson S. Global monthly AVHRR climatology over land clear-sky top-of-the-atmosphere variables. Boulder, Colorado: NOAA/NESDIS National Geophysical Data Center; 1997. http://www.ngdc.noaa.gov/ecosys/cdroms/AVHRR97_d1/aareadme.htm. [Google Scholar]
- Head JJ, Polly PD. Dissociation of somatic growth from segmentation drives gigantism in snakes. Biology Letters. 2007;3:296–298. doi: 10.1098/rsbl.2007.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ. raster: Geographic data analysis and modeling. R package version 2.2-31. 2014 http://CRAN.R-project.org/package=raster
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. High resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Holt RD. The microevolutionary consequences of climate change. Trends in Ecology & Evolution. 1990;5:311–315. doi: 10.1016/0169-5347(90)90088-U. [DOI] [PubMed] [Google Scholar]
- Itazawa Y. Influence of the environment on the number of vertebrae in fish. Nature. 1959;183:1408–1409. doi: 10.1038/1831408b0. [DOI] [PubMed] [Google Scholar]
- Jockusch EL. Geographic variation and phenotypic plasticity of number of trunk vertebrae in slender salamanders, Batrachoseps (Caudata : Plethodontidae) Evolution. 1997;51:1966–1982. doi: 10.1111/j.1558-5646.1997.tb05118.x. [DOI] [PubMed] [Google Scholar]
- Johnson PCD. Extension of Nakagawa & Schielzeth's R2GLMM to random slopes models. Methods in Ecology and Evolution. 2014;5:944–946. doi: 10.1111/2041-210X.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza B, Arntzen JW, Gentile E. Vertebral numbers in the Caudata of the Western Palaeartic (Amphibia) Atti del Museo Civico di Storia Naturale di Trieste. 2009;54:3–114. [Google Scholar]
- Lanza B, Caputo V, Nascetti G, Bullini L. Morphologic and genetic studies of the European plethodontid salamanders: taxonomic inferences (genus Hydromantes) Torino: Monografie XVI. Museo Regionale di Scienze Naturali; 1995. [Google Scholar]
- Lanza B, Pastorelli C, Laghi P, Cimmaruta R. A review of systematics, taxonomy, genetics, biogeography and natural history of the genus Speleomantes Dubois, 1984 (Amphibia Caudata Plethodontidae) Atti del Museo Civico di Storia Naturale di Trieste. 2006;52(Suppl.):5–135. [Google Scholar]
- Latta RG. Conservation genetics as applied evolution: from genetic pattern to evolutionary process. Evolutionary Applications. 2008;1:84–94. doi: 10.1111/j.1752-4571.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Legendre L. Numerical Ecology. Amsterdam: Elsevier; 2012. [Google Scholar]
- Lindell LE. Vertebral number in adders, Vipera berus: direct and indirect effects on growth. Biological Journal of the Linnean Society. 1996;59:69–85. [Google Scholar]
- Lunghi E, Manenti R, Ficetola GF. Seasonal variation in microhabitat of salamanders: environmental variation or shift of habitat selection? PeerJ. 2015;3:e1122. doi: 10.7717/peerj.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorano L, Cheddadi R, Zimmermann NE, et al. Building the niche through time: using 13,000 years of data to predict the effects of climate change on three tree species in Europe. Global Ecology and Biogeography. 2013;22:302–317. [Google Scholar]
- McDowall RM. Jordan's and other ecogeographical rules, and the vertebral number in fishes. Journal of Biogeography. 2008;35:501–508. [Google Scholar]
- Meiri S, Dayan T. On the validity of Bergmann’s rule. Journal of Biogeography. 2003;30:331–351. [Google Scholar]
- Merilä J, Hendry AP. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evolutionary Applications. 2014;7:1–14. doi: 10.1111/eva.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RH, Edmonds JA, Hibbard KA, et al. The next generation of scenarios for climate change research and assessment. Nature. 2010;463:747–756. doi: 10.1038/nature08823. [DOI] [PubMed] [Google Scholar]
- Mousseau TA. Ectotherms follow the converse to Bergmann's Rule. Evolution. 1997;51:630–632. doi: 10.1111/j.1558-5646.1997.tb02453.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution. 2013;4:133–142. [Google Scholar]
- Olalla-Tárraga MÁ, Diniz-Filho JAF, Bastos RPB, Rodríguez MÁ. Geographic body size gradients in tropical regions: water deficit and anuran body size in the Brazilian Cerrado. Ecography. 2009;32:581–590. [Google Scholar]
- Olalla-Tárraga MÁ, Rodríguez MÁ. Energy and interspecific body size patterns of amphibian faunas in Europe and North America: anurans follow Bergmann’s rule, urodeles its converse. Global Ecology and Biogeography. 2007;16:606–617. [Google Scholar]
- Orme D, Freckleton RP, Thomas GH, Petzoldt T, Fritz S, Isaac N, Pearse W. caper: Comparative Analyses of Phylogenetics and Evolution in R. R package version 0.5.2. 2013 http://CRAN.R-project.org/package=caper
- Pinheiro P, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. R package version 3.1-117. 2014 http://cran.r-project.org/web/packages/nlme
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Reece JS, Mehta RS. Evolutionary history of elongation and maximum body length in moray eels (Anguilliformes: Muraenidae) Biological Journal of the Linnean Society. 2013;109:861–875. [Google Scholar]
- Richards SA, Whittingham MJ, Stephens PA. Model selection and model averaging in behavioural ecology: the utility of the IT-AIC framework. Behavioral Ecology and Sociobiology. 2011;65:77–89. [Google Scholar]
- Romano A, Ficetola GF. Ecogeographic variation of body size in the spectacled salamanders (Salamandrina): influence of genetic structure and local factors. Journal of Biogeography. 2010;37:2358–2370. [Google Scholar]
- Rypel AL. Cold-water connection: Bergmann’s rule in North American freshwater fishes. American Naturalist. 2014;183:147–156. doi: 10.1086/674094. [DOI] [PubMed] [Google Scholar]
- Scheffers BR, Edwards DP, Diesmos A, Williams SE, Evans TA. Microhabitats reduce animal's exposure to climate extremes. Global Change Biology. 2014;20:495–503. doi: 10.1111/gcb.12439. [DOI] [PubMed] [Google Scholar]
- Schiffers K, Bourne EC, Lavergne S, Thuiller W, Travis JMJ. Limited evolutionary rescue of locally adapted populations facing climate change. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368:20120083. doi: 10.1098/rstb.2012.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze MK, Clarke AR. Converse Bergmann cline in a Eucalyptus herbivore, Paropsis atomaria Olivier (Coleoptera: Chrysomelidae): phenotypic plasticity or local adaptation? Global Ecology and Biogeography. 2008;17:424–431. [Google Scholar]
- Shikano T, Merila J. Body size and the number of vertebrae in the nine-spined stickleback (Pungitius pungitius) Biological Journal of the Linnean Society. 2011;104:378–385. [Google Scholar]
- Sillero N, Campos J, Bonardi A, et al. Updated distribution and biogeography of amphibians and reptiles of Europe based on a compilation of countrywide mapping studies. Amphibia-Reptilia. 2014;35:1–31. [Google Scholar]
- Slatyer RA, Hirst M, Sexton JP. Niche breadth predicts geographical range size: a general ecological pattern. Ecology Letters. 2013;16:1104–1114. doi: 10.1111/ele.12140. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos K, Eleftherakos K, Dzukic G, Kaiezic ML, Legakis A, Polymeni RM. Phylogeny and biogeography of the alpine newt Mesotriton alpestris (Salamandridae, Caudata), inferred from mtDNA sequences. Molecular Phylogenetics and Evolution. 2007;45:211–226. doi: 10.1016/j.ympev.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Steinfartz S, Veith M, Tautz D. Mitochondrial sequence analysis of Salamandra taxa suggests old splits of major lineages and postglacial recolonizations of Central Europe from distinct source populations of Salamandra salamandra. Molecular Ecology. 2000;9:397–410. doi: 10.1046/j.1365-294x.2000.00870.x. [DOI] [PubMed] [Google Scholar]
- Symonds MRE, Moussalli A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology. 2011;65:13–21. [Google Scholar]
- Teplitsky C, Millien V. Climate warming and Bergmann's rule through time: is there any evidence? Evolutionary Applications. 2014;7:156–168. doi: 10.1111/eva.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W, Lafourcade B, Engler R, Araujo MB. BIOMOD - a platform for ensemble forecasting of species distributions. Ecography. 2009;32:369–373. [Google Scholar]
- Thuiller W, Pironon S, Psomas A, et al. The European functional tree of bird life in the face of global change. Nature Communications. 2014;5:3118. doi: 10.1038/ncomms4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucco A, Zomer RJ. Global Potential Evapo-Transpiration (Global-PET) and Global Aridity Index (Global-Aridity) Geo-Database. Washington, DC: CGIAR Consortium for Spatial Information; 2009. Available online from the CGIAR-CSI GeoPortal at: http://www.csi.cgiar.org. [Google Scholar]
- Veith M. The fire salamander, Salamandra salamandra L. in central Europe: subspecies distribution and intergradation. Amphibia-Reptilia. 1992;13:297–313. [Google Scholar]
- Veith M, Degani G, Seitz A. Discordance of genetic and morphological variation of Salamandra salamandra (L) in Israel. Zoologischer Anzeiger. 1992;229:63–72. [Google Scholar]
- Wake DB. Homoplasy: the results of natural selection, or evidence of design limitations? American Naturalist. 1991;138:543–567. [Google Scholar]
- Wielstra B, Themudo GE, Guclu O, Olgun K, Poyarkov NA, Arntzen JW. Cryptic crested newt diversity at the Eurasian transition: The mitochondrial DNA phylogeography of Near Eastern Triturus newts. Molecular Phylogenetics and Evolution. 2010;56:888–896. doi: 10.1016/j.ympev.2010.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.