Abstract
Oligonucleotides (ONs) are short fragments of nucleic acids, currently being investigated as therapeutic agents. When administered subcutaneously (sc), ONs cause a specific local reaction originating around the injection site, such as erythema, itching, discomfort and pain, including more severe manifestations such as ulceration or necrosis. These injection site reactions (ISRs) are common, but rather poorly described in the literature. With this review, we aim to provide an overview on the extent of the problem of ISRs, based on reported incidence. A structured literature search was performed to identify reported incidence and clinical features of ISRs which yielded 70 manuscripts that contained information regarding ISRs. The data from literature was combined with data on file available at our institution. All sc administered ONs described in the literature lead to the occurrence of ISRs. The percentage of trial subjects that developed ISRs ranged from 22 to 100% depending on ON. The majority of ONs caused ISRs in more than 70% of the trial subjects. The severity of the observed reactions varied between different ONs. Occurrence rate as well as severity of ISRs increases with higher doses. For chemistry and target of the compounds, no clear association regarding ISR incidence or severity was identified. All ONs developed to date are associated with ISRs. Overcoming the problem of ISRs might add greatly to the potential success of sc‐administered ONs. Knowledge of these skin reactions and their specific immunostimulatory properties should be increased in order to obtain ONs that are more suitable for long‐term use and clinically applicable in a broader patient population.
Keywords: antisense, injection site reaction, oligonucleotide, subcutaneous
What is Already Known about this Subject
Oligonucleotides are promising drug candidates that are being thoroughly investigated for therapeutic effects for various indications.
To date, only very few data are available on injection site reactions after subcutaneous oligonucleotide administration.
What this Study Adds
A structured literature search of injection site reactions combined with data on file available at the Centre for Human Drug Research reveal clearly the clinical importance of understanding this within‐class adverse effect.
Introduction
Oligonucleotides are fragments of 12–24 nucleic acids in a target‐specific sequence 1. These compounds are designed either to inhibit mRNA of the targeted protein using the antisense principle, altering the reading frame by exon‐skipping and directly inhibiting the targeted protein (antagonist) or to bind as an agonist on the receptor (Figure 1). The latter ONs are under investigation for their immunostimulatory properties, such as C‐phosphate‐Guanine ONs (CpGs) 2. ONs are an attractive class of compounds as the synthesis and production of the drug has become fully automated, rapid and inexpensive, whereby every desired nucleic acid sequence can be generated. Over the years, different ON subclasses with distinct molecular structures have been generated. Initial ONs, dating from the early 1990s, were unmodified deoxyoligonucleotides. Around the year 2000, a phosphorothioate backbone was added to many ONs. This led to major improvement as phosphorothioate ONs are highly soluble in water, have increased nuclease stability and show excellent biologic activity 1.
Figure 1.
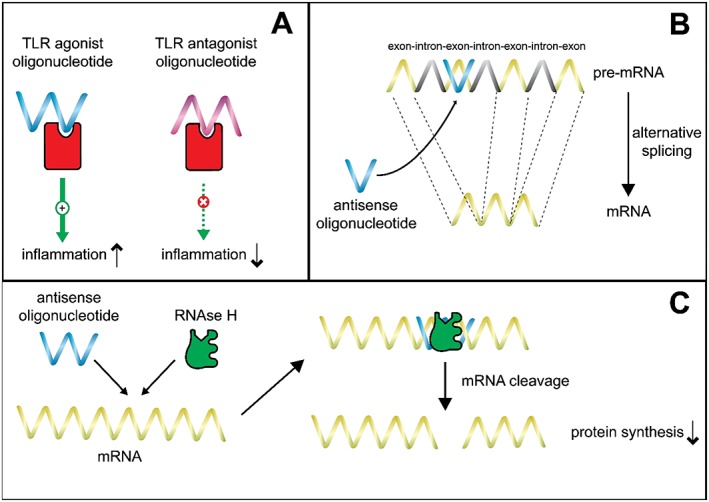
The different modes of action for therapeutic oligonucleotides. A) Activation or inhibition of the target protein to induce or inhibit immune activation. B) Exon skipping to induce alternative splicing. C) Inhibition of mRNA with antisense ON inhibits production of the protein
Despite the numerous ON drug candidates identified and studied over the last 20 years, up to the highest clinical trial phases, only two ONs achieved marketing approval by the FDA, mipomersen (in 2013) and fomivirsen (in 1998, for the treatment of CMV retinitis). This discrepancy may be explained by frequently untoward effects induced by ONs, including nephrotoxicity, hepatotoxicity, thrombocytopenia and inflammatory responses 3, 4, 5, 6. Subcutaneous (sc) administration of ONs also results in the occurrence of injection site reactions (ISRs), specific local skin reactions originating around the injection site manifesting itself as erythema, induration, itching, discomfort and pain, or more severely as ulceration or necrosis. ON‐induced ISRs are considered to be common, nonetheless detailed information in the literature regarding these skin effects is limited.
For example, mipomersen (Kynamro®; previously ISIS 301012), the only FDA‐approved oligonucleotide (ON) currently available, is known to cause ISRs. Mipomersen targets the mRNA for apolipoprotein B to treat homozygous familial hypercholesterolemia. Mipomersen carries a boxed warning for the serious risk of liver toxicity, which is considered to be an off‐target effect 7. Although it is known that in phase 3 trials, 5% of all treated subjects discontinued mipomersen treatment due to ISRs 8, detailed public information on ISR severity, incidence and causal mechanism is scarce. The full prescribing information of mipomersen states that the local reactions typically consist of erythema, pain, tenderness, pruritus and/or local swelling. However, no notification is made that reactions may be more severe, and/or lead to irreversible skin changes. The occurrence of ISRs is unlikely to be a specific feature of mipomersen, but a class effect of oligonucleotides.
With this review we aim to provide a comprehensive and detailed overview of the incidence, severity, clinical manifestations and pathophysiology of ON‐induced ISRs after sc administration.
Materials and methods
A structured literature search was performed to identify reported incidence and clinical features of ISRs in clinical trials up to and including February 2015. The following databases were used: PubMed, MEDLINE, Embase, Embase meeting abstracts, Web of Science, Web of Science meeting abstracts, COCHRANE, CENTRAL, CINAHL, Academic Search Premier (free text), ScienceDirect (free text), Wiley, SAGE (free text), HighWire (free text) and LWW (free text). Search terms were injection site reactions or related terms and oligonucleotides or related terms. We found a total of 514 hits, and only original trials reporting on phosphorothioate ONs were included. By screening the manuscript titles, 255 papers were excluded (animal/preclinical data, oligonucleotide used as adjuvant, no oligonucleotide therapy, compounds with different chemistry or no subcutaneous administration), by abstract screening another 189 results were excluded (review articles, for example on mipomersen and CpG‐structures, and above‐mentioned reasons for exclusion). Of the 70 remaining results the complete papers (when available) were studied and information regarding ISRs was extracted and is reported here. A cross‐check was performed by a search for ONs using the Integrity database 9. This yielded information on seven additional ONs that had been clinically tested, but not (at that time) reported on in the public domain. Further, publicly available documents from manufacturers of ONs and regulatory agencies were screened for relevant information on ISRs.
These data were combined with safety data on file available at the Centre for Human Drug Research in Leiden, the Netherlands, where a total of 204 subjects participated in trials with four different ONs. These studies were conducted in accordance with good clinical practice guidelines, after approval by the Central Committee on Research Involving Human Subjects (CCMO) of the Netherlands. Two of these compounds were made anonymous by naming them ON_CHDR to protect intellectual property.
Results
Incidence
The literature search yielded no review papers on ISRs. Twenty‐four different sc‐administered ONs were identified in the papers found by the literature search and the information from the Integrity database 9. For 19 compounds reporting was available, and for all these compounds ISRs occurred. For the other five compounds identified (PRO044, PRO045, PRO053, IsisGCCRRx and IsisTTRRx), no reporting was (yet) available. The data found in the search was supplemented with CHDR data on file regarding four other ONs for which also ISRs were invariably noted. An overview of incidence of ISRs associated with these ONs is provided in Figure 2. The percentage of trial subjects that develops ISRs differs with ON and ranged from 22 to 100%. The majority of ONs cause ISRs in more than 70% of the trial subjects and for two ONs it was reported that all treated individuals developed ISRs. For almost all ONs the incidence of ISRs is clearly dose‐dependent. This is illustrated by the incidence for mipomersen 10, ON_CHDR2, IMO‐8400 and ISIS325566 (Figure 3). For these four ONs, higher doses are associated with higher incidence of ISRs. Although the shape of the curve differs, the trend towards higher incidence with higher dose is clear and a plateau at 100% seems to occur from a certain dose level onwards. The only ON that did not show direct dose‐dependency was ISIS14803 with a 100% occurrence rate at all dose levels tested, which may reflect that the doses studied were too high to detect dose‐dependency.
Figure 2.
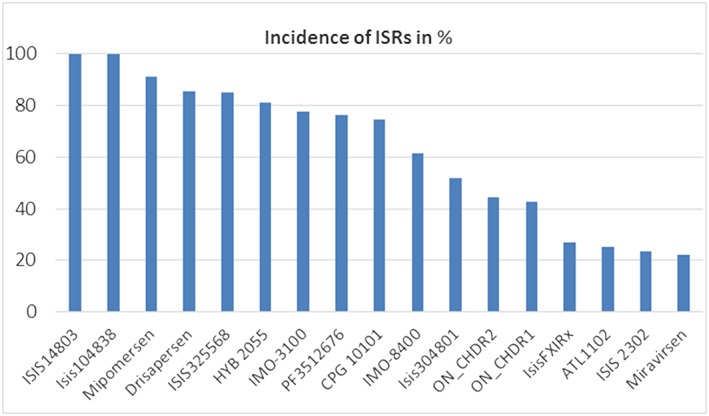
All 21 sc‐administered ONs resulted in ISRs. Incidence ranged from 22 to 100%. For four ONs, no incidence numbers were reported, namely IsisApo(a)Rx, Isis113715, ATL‐03 and IsisGCGR‐Rx. For ONs that were studies at different dose levels and/or multiple trials, an average ISR occurrence was calculated
Figure 3.
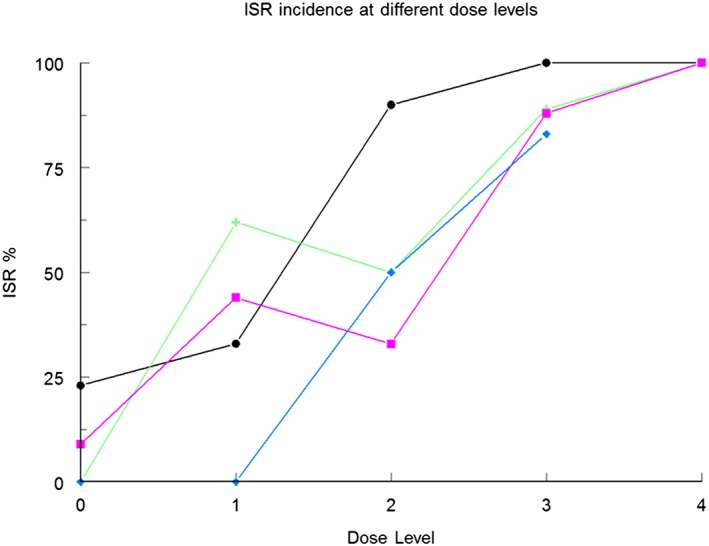
Dose‐dependent occurrence of ISRs after administration of four different ONs. Higher dose levels result in increased incidence of ISRs up to 100% at the highest dose level. The dose levels tested for ( ) ISIS32566 and (
) ISIS32566 and ( ) mipomersen 10 were: placebo (0), 50, 100, 200 and 400 mg. For (
) mipomersen 10 were: placebo (0), 50, 100, 200 and 400 mg. For ( ) IMO‐8400 dose levels were: placebo (0), 0.075, 0.15, 0.3 and 0.6 mg kg−1, and for (
) IMO‐8400 dose levels were: placebo (0), 0.075, 0.15, 0.3 and 0.6 mg kg−1, and for ( ) ON_CHDR2 dose levels were: placebo (0), 0.5, 1.5 and 5 mg kg−1
) ON_CHDR2 dose levels were: placebo (0), 0.5, 1.5 and 5 mg kg−1
ISRs following sc injection of ONs are characterized by a symmetrical erythemous skin lesion around the injection site, with a diameter ranging from 4 to 15 cm. The erythema may be accompanied by discomfort, pain, itch, induration and/or ulceration (Figure 4), but is usually not accompanied by lymphadenopathy. After the injection, the erythema generally appears after 24–96 h. It often reaches a maximal intensity around 48 h after injection. These data appear to be corroborated by publicly available sources reporting that the most common injection site reactions (incidence between parentheses) for mipomersen consisted of erythema (59%), pain (56%), haematoma (32%), pruritus (29%), swelling (18%) and discoloration (17%) 7. Information on severity and duration of ISRs is not readily available, but it appears that the resolution of a skin lesion differs greatly among individuals. A total of 204 subjects were actively treated with one of four different ONs at our centre. ISRs were reported in 122 (60%) of the subjects and complete resolution occurred in this group in over 80% of the cases. The duration to resolution ranged from 14 to 90 days. Approximately 20% of the participants developed a (semi)‐permanent discoloration of the skin. This manifested itself as persistent mild erythema or hypo/hyperpigmentation of the skin, which was usually smaller in diameter than the original erythemous lesion (Figure 5). Interestingly, the pooled phase III trials with mipomersen report that 7.7% (20/261) of individuals experienced reactions at a previous injection site when subsequent injections were administered at a different site; a so‐called injection site recall reaction.
Figure 4.
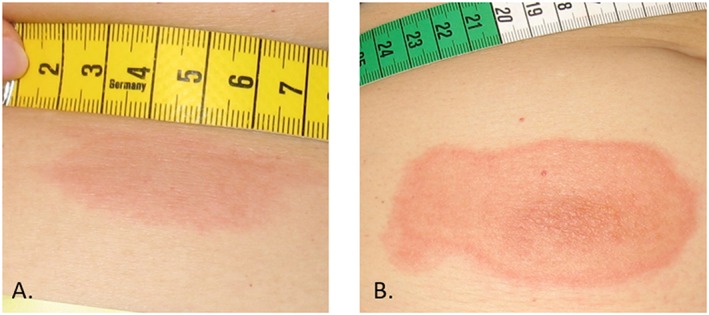
Examples of ISRs, ranging from mild erythema measuring several centimetres (A) to pronounced erythema of >10 cm with central ulceration (B)
Figure 5.
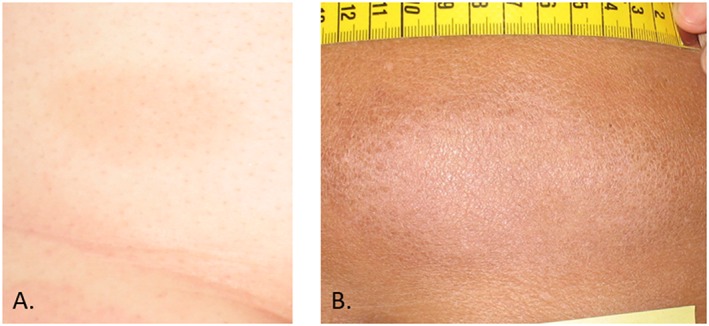
Examples of persisting discoloration of the skin. Mild hyperpigmentation (A) and hypopigmentation (B)
The severity of ISRs is generally described in the literature as ‘mild to moderate’. However, in most papers the definitions of the concepts ‘mild’ and ‘moderate’ are not specified and usually no information is provided on how many of each were reported. A notable exception was the reporting on the severity of the ISRs occurring for PF3512676 for which a grading system was used (see Table 1) 11. The majority of patients were reported to have an ISR of Grade 2 or lower (mild to moderate), up to 10% of patients reported an ISR of Grade 3 or greater, which was defined as severe and requiring dose modification. In the combined phase III six‐month trials, 5% of all mipomersen‐treated individuals discontinued owing to ISRs 8. Other patient drop‐outs as a result of ISRs were reported for PF 3512679 and ISIS2302 12. The severity profile may differ between compounds and between dose levels; however, this is difficult to assess as no grading system is consistently used throughout different studies.
Table 1.
An example of an ISR grading system to score the severity used for PF3512676 11
| Grade 1 | mild (does not interfere with daily life) |
| Grade 2 | moderate (interferes with daily life but no dose modification) |
| Grade 3 | severe (requires dose modification) |
| Grade 4 | disabling (requires drug discontinuation) |
Histology
Little is known of the histopathology of ISRs. The largest series currently available is from a dedicated ISR study performed with mipomersen. In this study, 32 individuals had post‐treatment skin biopsies of injection sites. Histological analyses of these injection sites showed that 9 of the 32 individuals had findings consistent with leukocytoclastic vasculitis (e.g. infiltrating neutrophils, prominent nuclear dust, lymphocytes and eosinophils with local macrophage infiltration 7). The histology of a biopsy of an erythematous ISR observed in our centre showed a non‐specific spongiotic appearance with few eosinophils (Figure 6A–C). The inflammatory influx was mainly perivascular and to a small extent also present in pre‐existing collagen and the basal layer of the epidermis. The subcutaneous fat tissue demonstrated necrosis and infiltration with eosinophils (Figure 6D).
Figure 6.
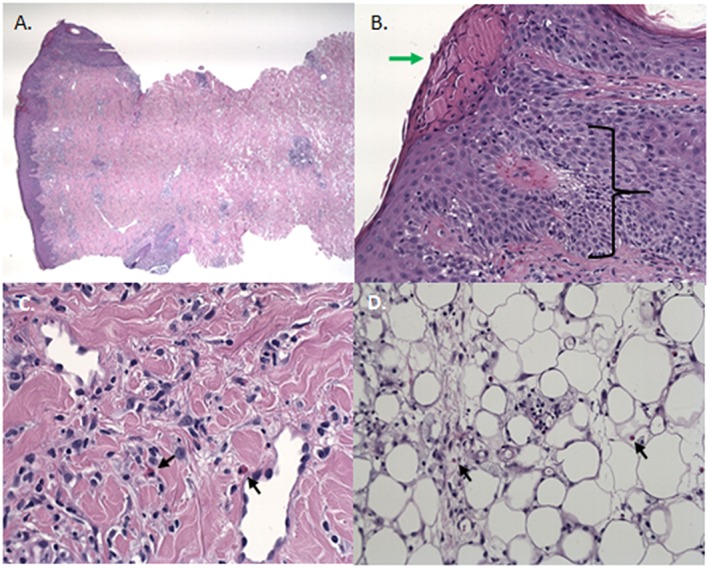
The histology of a biopsy of an erythematous ISR. A) Overview of biopsy. B) Spongiosis with exocytosis of lymphocytes and parakeratosis with serumcrustae. C) Infiltration with eosinophilic granulocytes. D) Necrosis of the subcutaneous fat tissue and infiltration with eosinophilic granulocytes
Discussion/Conclusion
Since detailed information on oligonucleotide‐induced ISRs is currently lacking, we conducted a systematic review of all data available in the public domain, and supported the findings with relevant data collected in clinical studies with subcutaneously administered ONs performed at the CHDR. All sc‐administered ONs described in the literature and studied at the CHDR resulted in the induction of ISRs. ON‐induced ISRs appear as symmetrical erythematous skin lesions, often accompanied by discomfort, pain, itch, induration and/or ulceration, variable in size, and with variable resolution times between compounds and individuals. This local immune response is in line with the general pro‐inflammatory potential of ONs in humans, since flu‐like symptoms and elevated CRP are common after sc administration of ONs 7. Also systemic adverse reactions directly following IV infusion (fever, nausea, malaise) are commonly observed in clinical trials with ONs 13, 14. Based on the data available, it is concluded that ON dose level is an important determinant for the induction of ISRs as occurrence rate and severity increase with higher dose levels. ONs differed mutually with respect to the incidence and severity of the induced ISRs, although no clear association with a specific ON subclass was observed. Although many efforts have been made to design ONs lacking immune‐stimulating effects, none of the established chemical modifications did result in the desired effect. The 5‐methyl‐cytosine substitution is claimed to reduce immune stimulation 7, 15. Locked nucleic acid (LNA) modifications are intended to enhance the antisense binding affinity to the mRNA target and increase its biological half‐life, reducing the probability of off‐target effects 16. However, the observed incidence and severity of the skin reactions induced by these newer compounds does not differ from older ON subclasses (Table 2). It is remarkable that the ONs with the highest ISR rates, ISIS 14803 and mipomersen, both include a 5‐methyl‐cytosine substitution. No other potential correlation between ON subclass or length and ISR incidence or severity was observed (Table 2). These findings demonstrate that it is at least uncertain that further chemical modification of ONs may result in the desired lack of immune‐stimulating activity. Nevertheless, it appears that chemical modifications are pursued. Examples of this approach are to introduce a so‐called steric bulk at the 5′‐position of the sugar‐phosphate backbone 17, to conjugate the oligonucleotide with peptides, proteins, carbohydrates, aptamers and small molecules 18, or introduce receptor‐binding molecules such as folate, anisamide or N‐acetyl galactosamine (GalNac) or dynamic polyconjugates to the oligonucleotide 19. An alternative strategy may be to alter the delivery of ONs in a manner which may bypass local immune responses. Altered delivery can be obtained using chelation 20 or the use of nanoparticles or liposomes 21. Moreover, avoiding ISRs could be achieved by oral administration of ONs, a concept that is currently being investigated 22. Whether these strategies are safe and efficacious in humans remains to be determined.
Table 2.
Listing of clinically tested oligonucleotides
| Name | Structure | Length | MOA | Indication | No. of studies found in the literature | Reference | ISR % |
|---|---|---|---|---|---|---|---|
| ISIS 14803 | PhON 5MCS | 20 units | Inhibits HCV RNA synthesis | Chronic HCV infection | 1 | 15 | 100 |
| ISIS 104838 | PhON, 2MOE | 20 units | Inhibits TNFα | Rheumatoid arthritis, Crohn's disease and psoriasis | 1 | 55 | 100 |
| Mipomersen | PhON, 2MOE 5MCS | 20 units | ApoB synthesis inhibitor | Hypercholesterolemia | 19 | 10, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 | 91.2 |
| Drisapersen | PhON, 2MOE | 20 units | Induces Exon 51 skipping in DMD | Duchenne's muscular dystrophy | 6 | 74, 75, 76, 77, 78, 79 | 85.5 |
| ISIS325568 | PhON, 2MOE | 20 units | Inhibits GCGR | Diabetes mellitus type 2 | 1 | 13 | 85 |
| HYB 2055 | Not reported | Not reported | Activates TLR 9 | Cancer | 1 | 80, 81 | 81.3 |
| IMO‐3100 | PhON | 18 units | Inhibits TLR 7,9 activation | Psoriasis | 1 | 82 | 65 |
| PF 3512676 | PhON, CpG | 24 units | Activates TLR 9 | Adjuvant of vaccin/chemo | 5 | 83, 84, 85, 86, 87 | 76.3 |
| CpG 10101 | PhON, CpG | 22 units | Activates TLR 9 | HCV infection | 3 | 88, 89, 90 | 74.5 |
| ON_CHDR2 | PhON, LNA | 14 units | Undisclosed | Undisclosed | – | – | 72.2 |
| IMO‐8400 | PhON, 2MOE | 18 units | Inhibits TLR 7, 8, 9 activation | Psoriasis | 1 | 91 | 61.5 |
| Isis304801 | PhON, 2MOE | 20 units | Inhibits Apolipoprotein C‐III | Dyslipidemia | 1 | 92, 93 | 52 |
| ON_CHDR1 | PhON, 2MOE | 12 units | Undisclosed | Undisclosed | – | – | 42.7 |
| IsisFIXRx (Isis416858) | PhON, 2MOE | 20 units | Reduces human factor XI | Prevention of thrombosis | 2 | 94, 95 | 33.3 |
| ATL1102 | PhON, 2MOE | 20 units | Inhibits CD49d | Relapsing‐remitting multiple sclerosis | 1 | 96 | 25 |
| ISIS 2302 | PhON | 20 units | Inhibits ICAM‐1 expression | Crohn's disease | 1 | 12 | 23.3 |
| Miravirsen | PhON, LNA | 15 units | Inhibits miR‐122 | HCV Infection | 2 | 97, 98 | 22.2 |
| IsisApo(a)Rx | Not reported | Not reported | Inhibits apolipoprotein (a) protein | Coronary artery disease | 1 | 99 | Not reported |
| Isis113715 | PhON, 2MOE | 20 units | Inhibits PTP‐1B protein | Diabetes mellitus type 2 | 2 | 100, 101 | Not reported |
| ATL‐03 | PhON, 2MOE | 20 units | Inhibits GHR Expression | Acromegaly | 1 | 9, 102 | Not reported |
| IsisGCGR‐Rx | Not reported | Not reported | Inhibits GCGR | Diabetes mellitus type 2 | 1 | 103 | Not reported |
2MOE = 2′‐O‐MOE structure, 5MCS = 5‐methyl‐cytosine substitution, CpG = Cytosine triphosphate deoxynucleotide‐Guanine triphosphate deoxynucleotide, LNA = locked nucleid acid structure, MOA = mode of action, PhON = Phosphorothioate oligonucleotide
Our review demonstrates that ISRs after sc administration of ONs are a serious problem. Obviously, severe and long‐lasting local inflammatory responses upon sc use of ONs are debilitating for potential future patient populations. In addition, relatively mild discomfort such as itch and cosmetic aspects like erythema and altered pigmentation may jeopardize adherence to therapy, particularly upon chronic use. This is illustrated by the relatively high percentage of participants in phase III mipomersen trials discontinuing therapy early owing to the occurrence of ISRs 8. Ultimately, the potential value of sc ON therapy is dependent on the severity of a particular disease and availability of alternative therapies. For example, ISR‐inducing sc ON therapy may be acceptable for patients suffering from an otherwise untreatable malignancy or lethal muscular dystrophy, whereas hypercholesterolemic patients would not readily consider the use of such a therapy.
For the future clinical application of sc ONs, detailed understanding of the mechanisms causing the skin reactions is essential. It is unlikely that the occurrence of ISRs can be explained by the ON target or the target distribution. For example, ISIS 14803 and miravirsen both target RNA replication of the hepatitis C virus (HCV); however, the compounds have the highest and second lowest ISR rates (Table 2). It does not seem that the dermal localization of the ON target predisposes to a higher ISR occurrence rate: the target of the ON with the highest incidence is expressed hepatically (ISIS 14803), whereas CPG10101 that has a target in the skin shows a remarkably low incidence in ISRs. This is interesting given the fact CpG ONs are intended to act in a immunostimulatory fashion. Contrary to earlier assumptions, however, it was shown that TLR9 activation is not confined to the compounds containing unmethylated CPG, but depends on backbone structure 23, 24. In conclusion, the induction of ISRs by sc administration of ONs may be a class effect inherent to the physical‐chemical nature of the compounds, which can potentially be circumvented by further chemical modification, although published evidence is currently lacking. Further, we argue that rational development of tailored ON subclasses could benefit from in‐depth knowledge on the relationship between chemical modifications and the molecular pathways involved in the immune responses causing the ISRs.
Molecular targets
Which molecular targets may be implied in the observed ON‐induced immune responses? The skin functions as a mechanical barrier, and also as a first‐line immune defense, comprising innate and adaptive immune mechanisms 25. The skin contains a mixture of immune cells: keratinocytes releasing cytokines and chemokines in response to injury, resident dendritic cells, macrophages, innate, NK and helper T lymphocytes, and mast cells in the dermis 26. ON‐mediated activation of the immune system is likely to start in the dermis. Unfortunately, relatively limited information is available on the specific pathophysiology of ISRs. In general, drug‐induced skin reactions are non‐specific hypersensitivity‐like responses, characterized by dermal oedema with perivascular and interstitial acute and chronic inflammation with involvement of neutrophils, eosinophils and lymphocytes 27, 28, 29. This is compatible with the findings from biopsies performed upon occurrence of ISRs after sc administration of mipomersen 7. Similar histological responses were observed in a clinical study with a ribozyme construct, chemically resembling an ON 30. Specific features of ON‐induced ISRs include the involvement of immunological memory, as demonstrated by the injection site recall reactions related to previous exposure to the ON 7. Also, hyper‐ and hypopigmentation is incidentally observed after administration of mipomersen. They should be considered non‐specific post‐inflammatory clinical sequellae, also occurring in skin diseases including acne vulgaris, atopic dermatitis, skin infections and allergic reactions 31. The response is known to result from the activation of melanocytes with overproduction of melanin or an irregular dispersion of pigment, but the exact mechanism underlying post‐inflammatory hyperpigmentation is unknown 32. Some scattered histological information is available on immune responses in ON‐induced ISRs, but this information does not reveal which specific molecular pathways are driving the initial immune response.
The innate immune system, being the first line of defense, comprises different subsystems for the protection of the body against pathogens. The most likely initial drivers of the ON‐induced immune response are innate cytosolic sensors and Toll‐like receptors (TLRs) and the complement system, two innate immune pathways that may act synergistically 33. The TLR system comprises different pattern recognition receptors sensing a variety of pathogens ranging from bacteria to fungi, protozoa and viruses 34. More particularly, the cell‐membrane bound TLR2 and TLR4 can be activated by viruses and viral components, as are the endosomal TLR3, TLR7/TLR8 and TLR9, which sense double‐strand RNA, single‐strand RNA and CpG DNA, respectively. Since ONs are short nucleic acid sequences, which may mimic viral sequences, they could theoretically trigger innate cytosolic sensors such as retinoic acid‐inducible gene I (RIG‐I), melanoma differentiation‐associated gene 5 (MDA‐5), TLR3, TLR7/8, resulting in MyD88 (type I IFN) and NF‐kappa‐B and the expression of pro‐inflammatory cytokines by dendritic cells and macrophages 35, 36, 37, 38, 39. Complement activation is known to play a key role in dermatological inflammatory conditions 40. Leucocytoclastic vasculitis has been demonstrated in ISR biopsies of mipomersen‐treated human subjects 7, which implies the potential involvement of complement, as complement complexes and perivascular complement deposits are commonly observed in this type of vasculitis 41. Leukocytoclastic vasculitis (also known as hypersensitivity vasculitis/angiitis) is commonly confined to the skin and is caused by vascular damage due to nuclear debris from infiltrating neutrophils. The most common cause is secondary to medications. The common clinical observations fit the findings for ON‐associated ISRs, as the majority of the lesions are acute and show resolution in weeks to months, but 10% turn into a chronic condition characterized by persistent lesions or intermittent recurrence. These chronic lesions eventually develop into morphea (also known as localized scleroderma or circumscribed scleroderma), a condition consisting of patches of hardened skin with no internal organ involvement. Interestingly, the treatment for leukocytoclastic vasculitis is to stop the causative agent and to avoid steroids. The latter would explain why attempts to reduce ISR using steroids have failed. However, based on the data available in the public domain it remains difficult to draw firm conclusions on potential TLR and complement activation in the skin upon sc ON treatment.
The occurrence of ISRs appears to be species‐dependent, which is not surprising given the large differences in the immune system response between species 42. For example, monkeys are more sensitive to oligonucleotide‐induced complement activation than humans 43, 44. However, dedicated animal studies may shed light on the mechanisms underlying ISRs: in mice and non‐human primates, immunostimulatory effects of ONs were observed 2, 45, 46, 47, which demonstrates the potential relevance of these animal models for mechanistic studies. Subcutaneous administration of phosphorothioate ONs to rodents resulted in local swelling and induration at the injection site, with mononuclear cell infiltrates 46, 48, lymphoid hyperplasia and multiorgan lymphohistiocytic cell infiltrates 43, 49, 50. Administration of CpG‐containing ONs to mice resulted in TLR9 activation 35. Furthermore, experiments in non‐human primates have shown that phosphorothioate ONs may activate the alternative complement pathway 51, 52, possibly by interaction with Factor H 52, 53.
It is uncertain how these preclinical data translate to humans, but when combined with tailored preclinical studies applying human cell cultures or mouse models with a humanized immune system, potentially involved mechanisms in ON‐induced ISR development in man may be identified. The involvement of specific TLR pathways and complement in ON‐induced ISRs could be systematically explored in a clinical trial with mipomersen, a commercially available oligonucleotide that is considered to be safe, but does induce ISRs. Thorough investigation of biopsies from skin lesions with dedicated immunohistochemical staining may shed light on the exact immunological mechanisms involved. In addition, we would advocate standardized quantitative and qualitative assessments of skin reactions in all future clinical studies with sc administration of ONs including immunohistochemistry and electronmicroscopy. This would provide more insight into the course of the development of the lesions, and allow a more structured comparison between different compounds.
In summary, all ONs tested in clinical studies have been reported to induce ISRs, reflecting a drug class effect. Detailed information on ISRs in the experimental setting is currently lacking. It is recommended to perform a uniform and standardized assessment of the skin reactions for all future studies with ONs, to gain more insight and to allow comparison between different compounds. This assessment should include a standardized way of reporting the clinical features, scoring severity and reporting duration (Table 3). Also performing standardized medical photography and biopsies from affected skin could add greatly to the current knowledge.
Table 3.
Suggested uniform standardized ISR scoring system
| 0 = No | 1 = Mild | 2 = Moderate | 3 = Severe and undesirable | |
|---|---|---|---|---|
| Injection site reaction | None | Erythema OR tenderness OR itching | As 1 and pain OR swelling OR signs of inflammation | Ulceration or necrosis |
| Maximal diameter ISR | NA | Max 5 cm | Max 10 cm | Max 15 cm OR any diameter and systemic reaction OR flare‐up previous IS |
| Duration of symptoms | ≤1 day | 2–14 days | 2–6 weeks, reversible | Permanent |
| Sequelae | None | Minimal and tolerated by patient | Hardly tolerated OR wish for treatment by patient | Permanent despite treatment OR no treatment options |
| Likely impact on next dose | None | Injection site can be used in rotation AND no dose adaptation | Injection site should be avoided in rotation OR change dose regimen | Injection site cannot no longer be used OR discontinuation |
| ADL limitations | None | Minimal | Functional | Self‐care limitations |
ADL = ‘Activities of daily living’ and are defined as bathing, dressing and undressing, feeding self, using the toilet, taking medications, preparing meals, shopping for groceries or clothes, using the telephone, etc.
More recent ON subclasses with specific chemical modifications aiming to avoid immunological skin responses have at present not been successful to completely prevent occurrence of ISRs. The pathophysiology underlying the ISRs and the causative immune pathways remain speculative. The initial immunological activation is likely to be driven by specific TLRs and complement. However, the exact involvement of these pathways has not been studied in detail, or alternatively not reported upon in the public domain. We therefore advocate a systematic approach to elucidate the immunostimulatory effects of oligonucleotides, by performing dedicated clinical and preclinical studies. In‐depth knowledge on the exact mechanisms underlying these skin reactions will be of importance for the future of all ONs, not only the ones administered subcutaneously. In parallel, strategies to diminish or limit the skin response induced by ONs should be considered. It appears that neither systemic or locally applied corticosteroid treatment prevents development of ISRs 74, but other treatments that have been explored for leukocytoclastic vasculitis may be considered 54. Further, local ON exposure should be limited by restricting the dose to the minimal level exerting the desired clinical effect, and possibly by spreading an effective total dose over multiple administrations, an approach demonstrated to be effective for mipomersen 56.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
The authors would like to thank Jan Schoones, librarian at the Leiden University Medical Center, for guidance in performing a structured search of available literature, and Anne‐Roos Schrader of the Department of Pathology of Leiden University Medical Center for guidance in the histological interpretation.
van Meer, L. , Moerland, M. , Gallagher, J. , van Doorn, M. B. A. , Prens, E. P. , Cohen, A. F. , Rissmann, R. , and Burggraaf, J. (2016) Injection site reactions after subcutaneous oligonucleotide therapy. Br J Clin Pharmacol, 82: 340–351. doi: 10.1111/bcp.12961.
References
- 1. Dias N, Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther 2002; 1: 347–55. [PubMed] [Google Scholar]
- 2. Krieg AM. From A to Z on CpG. Trends Immunol 2002; 23: 64–5. [DOI] [PubMed] [Google Scholar]
- 3. Marquis JK, Grindel JM. Toxicological evaluation of oligonucleotide therapeutics. Curr Opin Mol Ther 2000; 2: 258–63. [PubMed] [Google Scholar]
- 4. Monteith DK, Levin AA. Synthetic oligonucleotides: the development of antisense therapeutics. Toxicol Pathol 1999; 27: 8–13. [DOI] [PubMed] [Google Scholar]
- 5. Cavagnaro JA, Levin AA, Henry SP. Toxicology of oligonucleotide therapeutics and understanding the relevance of toxicities In: Preclinical Safety Evaluation of Biopharmaceuticals: A Science‐based Approach to Facilitating Clinical Trials, ed Cavagnaro JA. Hoboken, NJ: Wiley, 2008; 538–74. [Google Scholar]
- 6. Crooke ST, Scott H, Kim TW, Kramer‐Stickland K, Zanardi T, Fey RA, et al. Toxicologic properties of 2'‐methoxyethyl chimeric antisense inhibitors in animals and man In: Antisense Drug Technology: Principles, Strategies and Applications, ed Crooke ST. Boca Raton, FL: CRC Press, 2008; 327–63. [Google Scholar]
- 7. Mipomersen FDA Briefing Document NDA 203568. Endocrinologic and Metabolic Drugs Advisory Committee Meeting, 18 October 2012.
- 8. Genzyme Corporation . KYNAMRO: Highlights of prescribing information. Cambridge, MA: Genzyme Corporation, 2013.
- 9. Reuters T. Integrity. Available at: http://thomsonreuters.com/en/products‐services/pharma‐life‐sciences/pharmaceutical‐research/integrity.html (last accessed 18 February 2015).
- 10. Kastelein JJ, Wedel MK, Baker BF, Su J, Bradley JD, Yu RZ, et al. Potent reduction of apolipoprotein B and low‐density lipoprotein cholesterol by short‐term administration of an antisense inhibitor of apolipoprotein B. Circulation 2006; 114: 1729–35. [DOI] [PubMed] [Google Scholar]
- 11. Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JS, Blasinska‐Morawiec M, et al. A phase III randomized study of gemcitabine and cisplatin with or without PF‐3512676 (TLR9 agonist) as first‐line treatment of advanced non‐small‐cell lung cancer. Ann Oncol 2012; 23: 72–7. [DOI] [PubMed] [Google Scholar]
- 12. Schreiber S, Nikolaus S, Malchow H, Kruis W, Lochs H, Raedler A, et al. Absence of efficacy of subcutaneous antisense ICAM‐1 treatment of chronic active Crohn's disease. Gastroenterology 2001; 120: 1339–46. [DOI] [PubMed] [Google Scholar]
- 13. van Dongen MGJ, Geerts BF, Morgan ES, Brandt TA, de Kam ML, Romijn JA, et al. First proof of pharmacology in humans of a novel glucagon receptor antisense drug. J Clin Pharmacol 2015; 55: 298–306. [DOI] [PubMed] [Google Scholar]
- 14. Jansen B, Wacheck V, Heere‐Ress E, Schlagbauer‐Wadl H, Hoeller C, Lucas T, et al. Chemosensitisation of malignant melanoma by BCL2 antisense therapy. Lancet 2000; 356: 1728–33. [DOI] [PubMed] [Google Scholar]
- 15. McHutchison JG, Patel K, Pockros P, Nyberg L, Pianko S, Yu RZ, et al. A phase I trial of an antisense inhibitor of hepatitis C virus (ISIS 14803), administered to chronic hepatitis C patients. J Hepatol 2006; 44: 88–96. [DOI] [PubMed] [Google Scholar]
- 16. Veedu RN, Wengel J. Locked nucleic acids: promising nucleic acid analogs for therapeutic applications. Chem Biodivers 2010; 7: 536–42. [DOI] [PubMed] [Google Scholar]
- 17. Seth PP, Jazayeri A, Yu J, Allerson CR, Bhat B, Swayze EE. Structure activity relationships of alpha‐L‐LNA modified phosphorothioate gapmer antisense oligonucleotides in animals. Mol Ther Nucleic Acids 2012; 1: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winkler J. Oligonucleotide conjugates for therapeutic applications. Ther Deliv 2013; 4: 791–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gish RG, Yuen MF, Chan HL, Given BD, Lai CL, Locarnini SA, et al. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antiviral Res 2015; 121: 97–108. [DOI] [PubMed] [Google Scholar]
- 20. Vaillant A, Bazinet M. Oligonucleotide chelate complexes. International Patent Application No. PCT/CA2011/000956, 2012.
- 21. Yu B, Mao Y, Bai LY, Herman SE, Wang X, Ramanunni A, et al. Targeted nanoparticle delivery overcomes off‐target immunostimulatory effects of oligonucleotides and improves therapeutic efficacy in chronic lymphocytic leukemia. Blood 2013; 121: 136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Putten M, Young C, van den Berg S, Pronk A, Hulsker M, Karnaoukh TG, et al. Preclinical studies on intestinal administration of antisense oligonucleotides as a model for oral delivery for treatment of duchenne muscular dystrophy. Mol Ther Nucleic Acids 2014; 3: e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haas T, Metzger J, Schmitz F, Heit A, Muller T, Latz E, et al. The DNA sugar backbone 2' deoxyribose determines toll‐like receptor 9 activation. Immunity 2008; 28: 315–23. [DOI] [PubMed] [Google Scholar]
- 24. Yasuda K, Rutz M, Schlatter B, Metzger J, Luppa PB, Schmitz F, et al. CpG motif‐independent activation of TLR9 upon endosomal translocation of ‘natural’ phosphodiester DNA. Eur J Immunol 2006; 36: 431–6. [DOI] [PubMed] [Google Scholar]
- 25. Greaves P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation. London: Academic Press, 2012. [Google Scholar]
- 26. Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol 2004; 4: 211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med 2003; 139: 683–93. [DOI] [PubMed] [Google Scholar]
- 28. Thyssen JP, Maibach HI. Drug‐elicited systemic allergic (contact) dermatitis – update and possible pathomechanisms. Contact Dermatitis 2008; 59: 195–202. [DOI] [PubMed] [Google Scholar]
- 29. Ramdial PK, Naidoo DK. Drug‐induced cutaneous pathology. J Clin Pathol 2009; 62: 493–504. [DOI] [PubMed] [Google Scholar]
- 30. Weng DE, Masci PA, Radka SF, Jackson TE, Weiss PA, Ganapathi R, et al. A phase I clinical trial of a ribozyme‐based angiogenesis inhibitor targeting vascular endothelial growth factor receptor‐1 for patients with refractory solid tumors. Mol Cancer Ther 2005; 4: 948–55. [DOI] [PubMed] [Google Scholar]
- 31. Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol 2010; 3: 20–31. [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor S, Grimes P, Lim J, Im S, Lui H. Postinflammatory hyperpigmentation. J Cutan Med Surg 2009; 13: 183–91. [DOI] [PubMed] [Google Scholar]
- 33. Hajishengallis G, Lambris JD. Crosstalk pathways between Toll‐like receptors and the complement system. Trends Immunol 2010; 31: 154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bauer S, Hartmann G. Toll‐Like Receptors (TLRs) and Innate Immunity. Berlin: Springer Verlag, 2004. [Google Scholar]
- 35. Senn JJ, Burel S, Henry SP. Non‐CpG‐containing antisense 2'‐methoxyethyl oligonucleotides activate a proinflammatory response independent of Toll‐like receptor 9 or myeloid differentiation factor 88. J Pharmacol Exp Ther 2005; 314: 972–9. [DOI] [PubMed] [Google Scholar]
- 36. Agrawal S, Kandimalla ER. Role of Toll‐like receptors in antisense and siRNA [corrected]. Nat Biotechnol 2004; 22: 1533–7. [DOI] [PubMed] [Google Scholar]
- 37. Richardt‐Pargmann D, Vollmer J. Stimulation of the immune system by therapeutic antisense oligodeoxynucleotides and small interfering RNAs via nucleic acid receptors. Ann N Y Acad Sci 2009; 1175: 40–54. [DOI] [PubMed] [Google Scholar]
- 38. Burel SA, Han SR, Lee HS, Norris DA, Lee BS, Machemer T, et al. Preclinical evaluation of the toxicological effects of a novel constrained ethyl modified antisense compound targeting signal transducer and activator of transcription three in mice and cynomolgus monkeys. Nucleic Acid Ther 2013; 23: 213–27. [DOI] [PubMed] [Google Scholar]
- 39. Burel SA, Machemer T, Ragone FL, Kato H, Cauntay P, Greenlee S, et al. Unique O‐methoxyethyl ribose‐DNA chimeric oligonucleotide induces an atypical melanoma differentiation‐associated gene 5‐dependent induction of type I interferon response. J Pharmacol Exp Ther 2012; 342: 150–62. [DOI] [PubMed] [Google Scholar]
- 40. Panelius J, Meri S. Complement system in dermatological diseases – fire under the skin. Front Med (Lausanne) 2015; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dauchel H, Joly P, Delpech A, Thomine E, Sauger F, Le Loet X, et al. Local and systemic activation of the whole complement cascade in human leukocytoclastic cutaneous vasculitis; C3d,g and terminal complement complex as sensitive markers. Clin Exp Immunol 1993; 92: 274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barreiro LB, Marioni JC, Blekhman R, Stephens M, Gilad Y. Functional comparison of innate immune signaling pathways in primates. PLoS Genet 2010; 6: e1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Henry S, Kim TW, Kramer‐Stickland K, Zanardi TA, Fey RA, Levin AA. Toxicologic properties of 20‐methoxyethyl chimeric antisense inhibitors in animals and man In: Antisense Drug Technology: Principles, Strategies and Applications, 2nd edn, ed Crooke ST. Boca Raton, FL: CRC Press, 2008; 327–63. [Google Scholar]
- 44. Kwoh JT. An overview of the clinical safety experience of first‐ and second‐generation antisense oligonucleotides In: Antisense Drug Technology: Principles, Strategies and Applications, 2nd edn, ed Crooke ST. Boca Raton, FL: CRC Press, 2008; 365–99. [Google Scholar]
- 45. Kandimalla ER, Struthers M, Bett AJ, Wisniewski T, Dubey SA, Jiang W, et al. Synthesis and immunological activities of novel Toll‐like receptor 7 and 8 agonists. Cell Immunol 2011; 270: 126–34. [DOI] [PubMed] [Google Scholar]
- 46. Monteith DK, Henry SP, Howard RB, Flournoy S, Levin AA, Bennett CF, et al. Immune stimulation – a class effect of phosphorothioate oligodeoxynucleotides in rodents. Anticancer Drug Des 1997; 12: 421–32. [PubMed] [Google Scholar]
- 47. Ravindran C, Cheng YC, Liang SM. CpG‐ODNs induces up‐regulated expression of chemokine CCL9 in mouse macrophages and microglia. Cell Immunol 2010; 260: 113–8. [DOI] [PubMed] [Google Scholar]
- 48. Engelhardt JA. Predictivity of animal studies for human injection site reactions with parenteral drug products. Exp Toxicol Pathol 2008; 60: 323–7. [DOI] [PubMed] [Google Scholar]
- 49. Henry SP, Giclas PC, Leeds J, Pangburn M, Auletta C, Levin AA, et al. Activation of the alternative pathway of complement by a phosphorothioate oligonucleotide: potential mechanism of action. J Pharmacol Exp Ther 1997; 281: 810–6. [PubMed] [Google Scholar]
- 50. Choi SS, Chung E, Jung YJ. Newly identified CpG ODNs, M5‐30 and M6‐395, stimulate mouse immune cells to secrete TNF‐alpha and enhance Th1‐mediated immunity. J Microbiol 2010; 48: 512–7. [DOI] [PubMed] [Google Scholar]
- 51. Farman CA, Kornbrust DJ. Oligodeoxynucleotide studies in primates: antisense and immune stimulatory indications. Toxicol Pathol 2003; 31 (Suppl): 119–22. [DOI] [PubMed] [Google Scholar]
- 52. Henry SP, Beattie G, Yeh G, Chappel A, Giclas P, Mortari A, et al. Complement activation is responsible for acute toxicities in rhesus monkeys treated with a phosphorothioate oligodeoxynucleotide. Int Immunopharmacol 2002; 2: 1657–66. [DOI] [PubMed] [Google Scholar]
- 53. Alexander NJ, Clarkson TB, Fulgham DL. Circulating immune complexes in cynomolgus macaques. Lab Anim Sci 1985; 35: 465–8. [PubMed] [Google Scholar]
- 54. Fredenberg MF, Malkinson FD. Sulfone therapy in the treatment of leukocytoclastic vasculitis. Report of three cases. J Am Acad Dermatol 1987; 16: 772–8. [DOI] [PubMed] [Google Scholar]
- 55. Sewell KL, Geary RS, Baker BF, Glover JM, Mant TG, Yu RZ, Tami JA, Dorr FA. Phase I trial of ISIS 104838, a 2´‐methoxyethyl modified antisense oligonucleotide targeting tumor necrosis factor‐alpha. J Pharmacol Exp Ther 2002; 303: 1334–43. [DOI] [PubMed] [Google Scholar]
- 56. Flaim JD, Grundy JS, Baker BF, McGowan MP, Kastelein JJ. Changes in mipomersen dosing regimen provide similar exposure with improved tolerability in randomized placebo‐controlled study of healthy volunteers. J Am Heart Assoc 2014; 3: e000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Visser ME, Akdim F, Tribble DL, Nederveen AJ, Kwoh TJ, Kastelein JJP, et al. Effect of apolipoprotein‐B synthesis inhibition on liver triglyceride content in patients with familial hypercholesterolemia. J Lipid Res 2010; 51: 1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Selvey S, Raal FJ. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces LDL‐C in HoFH pediatric patients. J Clin Lipidol 2014; 8: 330. [Google Scholar]
- 59. Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010; 375: 998–1006. [DOI] [PubMed] [Google Scholar]
- 60. Santos RD, Duell PB, East C, Guyton JR, Moriarty PM, Chin W, et al. Long‐term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2‐year interim results of an open‐label extension. Eur Heart J 2015; 36: 566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Akdim F, Tribble DL, Flaim JD, Yu R, Su J, Geary RS, et al. Efficacy of apolipoprotein B synthesis inhibition in subjects with mild‐to‐moderate hyperlipidaemia. Eur Heart J 2011; 32: 2650–9. [DOI] [PubMed] [Google Scholar]
- 62. Akdim F, Visser ME, Tribble DL, Baker BF, Stroes ES, Yu R, et al. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low‐density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol 2010; 105: 1413–9. [DOI] [PubMed] [Google Scholar]
- 63. Akdim F, Stroes ES, Sijbrands EJ, Tribble DL, Trip MD, Jukema JW, et al. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol 2010; 55: 1611–8. [DOI] [PubMed] [Google Scholar]
- 64. Thomas GS, Cromwell WC, Ali S, Chin W, Flaim JD, Davidson M. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double‐blind, placebo‐controlled trial. J Am Coll Cardiol 2013; 62: 2178–84. [DOI] [PubMed] [Google Scholar]
- 65. Cromwell WC, Thomas GS, Boltje I, Chin W, Davidson M. Safety and efficacy of mipomersen administered as add‐on therapy in patients with hypercholesterolemia and high cardiovascular risk. J Clin Lipidol 2012; 6: 291–2. [Google Scholar]
- 66. Cromwell WC, Santos RD, Blom DJ, Marais DA, Lachmann RH, Gaudet D, et al. Mipomersen, a first‐in‐class apolipoprotein B synthesis inhibitor, lowers lipoprotein (a) in patients with homozygous familial hypercholesterolemia. J Clin Lipidol 2010; 4: 221. [Google Scholar]
- 67. Stein EA, Dufour R, Gagne C, Gaudet D, East C, Donovan JM, et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double‐blind, placebo‐controlled trial to assess efficacy and safety as add‐on therapy in patients with coronary artery disease. Circulation 2012; 126: 2283–92. [DOI] [PubMed] [Google Scholar]
- 68. Tardif J‐C, Ceska R, Burgess L, Soran H, Gouni‐Berthold I, Wagener G, et al. Apolipoprotein B synthesis inhibition by mipomersen reduces LDL‐C when added to maximally tolerated lipid‐lowering medication in patients with severe heterozygous hypercholesterolemia. J Clin Lipidol 2011; 5: 219–20. [Google Scholar]
- 69. Duell PB, Rose JE, Selvey S, Alam S, Mittleman R. Long term efficacy and safety of mipomersen during 4 years of treatment in a cohort of patients with heterozygous familial hypercholesterolemia and coronary artery disease (CAD). J Clin Lipidol 2013; 7: 276. [Google Scholar]
- 70. Tsimikas S, Witztum J, Catapano A. Effect of mipomersen on lipoprotein (A) in patients with hypercholesterolemia across four phase III studies. J Am Coll Cardiol 2012; 59: E1494. [Google Scholar]
- 71. Visser ME, Wagener G, Baker BF, Geary RS, Donovan JM, Beuers UHW, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low‐density lipoprotein cholesterol in high‐risk statin‐intolerant patients: a randomized, double‐blind, placebo‐controlled trial. Eur Heart J 2012; 33: 1142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Patel N, Hegele RA. Mipomersen as a potential adjunctive therapy for hypercholesterolemia. Expert Opin Pharmacother 2010; 11: 2569–72. [DOI] [PubMed] [Google Scholar]
- 73. McGowan MP, Tardif JC, Ceska R, Burgess LJ, Soran H, Gouni‐Berthold I, et al. Randomized, placebo‐controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid‐lowering therapy. PLoS One 2012; 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Goemans NM, Tulinius M, Van Den Akker JT, Burm BE, Ekhart PF, Heuvelmans N, et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. New Engl J Med 2011; 364: 1513–22. [DOI] [PubMed] [Google Scholar]
- 75. Goemans N, Voit T, McDonald C, Watson C, Kraus J, Rolfe K, et al. Drisapersen treatment for Duchenne muscular dystrophy: results of a 96‐week follow‐up of an open‐label extension study following two placebo‐controlled trials. Neurology 2014; 83: e34–40. [Google Scholar]
- 76. Flanigan KM, Voit T, Rosales XQ, Servais L, Kraus JE, Wardell C, et al. Pharmacokinetics and safety of single doses of drisapersen in non‐ambulant subjects with Duchenne muscular dystrophy: results of a double‐blind randomized clinical trial. Neuromuscul Disord 2014; 24: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goemans N, Tulinius M, Wilson R, Wardell C, Bedwell P, Campion G. G.P.113: Drisapersen (DRIS) treatment for Duchenne muscular dystrophy (DMD): results of up to 188 weeksGÇÖ follow‐up of an open‐label extension study. Neuromuscul Disord 2014; 24: 829. [Google Scholar]
- 78. Van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma‐Rus A, Bremmer‐Bout M, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. New Engl J Med 2007; 357: 2677–86. [DOI] [PubMed] [Google Scholar]
- 79. Voit T, Topaloglu H, Straub V, Muntoni F, Deconinck N, Campion G, et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory, randomised, placebo‐controlled phase 2 study. Lancet Neurol 2014; 13: 987–96. [DOI] [PubMed] [Google Scholar]
- 80. Switaj T, Lasek W. Technology evaluation: HYB‐2055, Hybridon. Curr Opin Mol Ther 2005; 7: 376–83. [PubMed] [Google Scholar]
- 81. Hwang JJ, Park A, Amin A, Martin RR, Sullivan T, Burns T, et al. A phase I study of HYB2055 in patients (pts) with advanced solid malignancies. J Clin Oncol 2004; 22: 3111. [Google Scholar]
- 82. Idera Pharmaceuticals, Inc. Idera Pharmaceuticals Presents Phase 1 Clinical Data for IMO‐3100, Lead Candidate for the Treatment of Autoimmune Diseases, and Provides Program Update, 21 October 2010.
- 83. Kim YH, Girardi M, Duvic M, Kuzel T, Link BK, Pinter‐Brown L, et al. Phase I trial of a Toll‐like receptor 9 agonist, PF‐3512676 (CPG 7909), in patients with treatment‐refractory, cutaneous T‐cell lymphoma. J Am Acad Dermatol 2010; 63: 975–83. [DOI] [PubMed] [Google Scholar]
- 84. Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1‐like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B‐class CpG oligodeoxynucleotide TLR9 agonist. J Immunother 2004; 27: 460–71. [DOI] [PubMed] [Google Scholar]
- 85. Pashenkov M, Goess G, Wagner C, Hormann M, Jandl T, Moser A, et al. Phase II trial of a Toll‐like receptor 9‐activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol 2006; 24: 5716–24. [DOI] [PubMed] [Google Scholar]
- 86. Thompson JA, Kuzel T, Drucker BJ, Urba WJ, Bukowski RM. Safety and efficacy of PF‐3512676 for the treatment of stage IV renal cell carcinoma: an open‐label, multicenter phase I/II study. Clin Genitourin Cancer 2009; 7: E58–65. [DOI] [PubMed] [Google Scholar]
- 87. Zent CS, Smith BJ, Ballas ZK, Wooldridge JE, Link BK, Call TG, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF‐03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma 2012; 53: 211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McHutchison JG, Bacon BR, Gordon SC, Afdhal NH, Jacobson IM, Muir A, et al. Human pharmacologic activity of a new TLR9 agonist antiviral, CPG 10101 (ACTILON). Hepatology 2004; 40: 697A. [Google Scholar]
- 89. McHutchison JG, Bacon BR, Gordon SC, Lawitz E, Shiffman M, Afdhal NH, et al. Phase 1B, randomized, double‐blind, dose‐escalation trial of CPG 10101 in patients with chronic hepatitis C virus. Hepatology 2007; 46: 1341–9. [DOI] [PubMed] [Google Scholar]
- 90. Vicari AP, Schmalbach T, Lekstrom‐Himes J, Morris ML, Al‐Adhami MJ, Laframboise C, et al. Safety, pharmacokinetics and immune effects in normal volunteers of CPG 10101 (ACTILON), an investigational synthetic toll‐like receptor 9 agonist. Antivir Ther 2007; 12: 741–51. [PubMed] [Google Scholar]
- 91. Balak DMW, van Doorn MBA, Rissmann R, Sullivan T, Burggraaf J, Arbeit RD. Results from a randomized, double‐blind, placebo‐controlled, monotherapy trial of IMO‐8400 demonstrate clinical proof‐of‐concept for Toll‐like receptor 7, 8 and 9 antagonism in psoriasis. AAD 2015 Poster 1805. San Francisco, CA: American Academy of Dermatology.
- 92. Graham MJ, Lee RG, Bell TA, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein c‐iii reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res 2013; 112: 1479–90. [DOI] [PubMed] [Google Scholar]
- 93. Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med 2014; 371: 2200–6. [DOI] [PubMed] [Google Scholar]
- 94. Liu Q, Bethune C, Dessouki E, Grundy J, Monia BP, Bhanot S. ISIS‐FXIRx, a novel and specific antisense inhibitor of factor XI, caused significant reduction in FXI antigen and activity and increased aPTT without causing bleeding in healthy volunteers. Blood 2011; Conference: 53rd Annual Meeting of the American Society of Hematology: 18 Nov 2011. [Google Scholar]
- 95. Buller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 2015; 372: 232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Limmroth V, Barkhof F, Desem N, Diamond MP, Tachas G. CD49d antisense drug ATL1102 reduces disease activity in patients with relapsing‐remitting MS. Neurology 2014; 83: 1780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reesink HW, Janssen HLA, Zeuzem S, Lawitz E, Rodriguez‐Torres M, Patel K, et al. Final results‐randomized, double‐blind, placebo‐controlled safety, anti‐viral proof‐of‐concept study of miravirsen, an oligonucleotide targeting MIR‐122, in treatment‐naive patients with genotype 1 chronic HCV infection. J Hepatol 2012; 56: S26. [Google Scholar]
- 98. Janssen HLA, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez‐Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. New Engl J Med 2013; 368: 1685–94. [DOI] [PubMed] [Google Scholar]
- 99. Viney N, Hughes SG, Singleton W, Crooke RM, Graham MJ, Su J, et al. ISIS APO (a) Rx, an antisense inhibitor to apolipoprotein (a), reduces plasma levels of Lp (a) and oxidized phospholipids/apoB‐100 in healthy volunteers. Eur Heart J 2014; Conference: European Society of Cardiology: 01 Sep 2014. [Google Scholar]
- 100. Geary RS, Bradley JD, Watanabe T, Kwon Y, Wedel M, Van Lier JJ, et al. Lack of pharmacokinetic interaction for ISIS 113715, a 2'‐0‐methoxyethyl modified antisense oligonucleotide targeting protein tyrosine phosphatase 1B messenger RNA, with oral antidiabetic compounds metformin, glipizide or rosiglitazone. Clin Pharmacokinet 2006; 45: 789–801. [DOI] [PubMed] [Google Scholar]
- 101. Brandt TA, Crooke ST, Ackermann EJ, Xia S, Morgan ES, Liu Q, et al. Isis 113715, a novel PTP‐1B antisense inhibitor, improves glycemic control and dyslipidemia and increases adiponectin levels in T2DM subjects uncontrolled on stable sulfonylurea therapy. Diabetes 2010; ; Conference: 70th Scientific Sessions of the American Diabetes Association Orlando: 2010. [Google Scholar]
- 102. PRNewswire . ATL1103 Phase II Trial – Successful Efficacy Results, 2 September 2014.
- 103. Isis Pharmaceuticals, Inc. Isis Pharmaceuticals Reports Final Phase 2 Data on ISIS‐GCGR RX, Press release, 16 June 2014.


