Abstract
Aim
The aim of the study was to assess ticagrelor's effects on inhibition of platelet aggregation (IPA), P2Y12 reaction units (PRU, measure of platelet P2Y12 receptor blockade), pharmacokinetic (PK) parameters and safety in Chinese patients with stable coronary artery disease (CAD).
Methods
This was an open label, single centre, randomized study. Thirty‐six patients on low dose aspirin (75–100 mg day–1) received ticagrelor 45, 60 or 90 mg (single dose, days 1 and 7; twice daily, days 3–6). IPA (final extent), PRU and ticagrelor and AR‐C124910XX plasma concentrations were determined.
Results
On day 1, peak IPA >80% occurred 2–6 h post‐dose (all doses). PRU was markedly reduced at 1 h vs. baseline (all doses). With ticagrelor 45 and 90 mg twice daily, maximum IPA (mean, SD) was 91% (13%), and 99% (3%), and maximum PRU reduction from baseline (mean, SD) was 82% (17%) and 92% (9%), respectively. Approximate dose‐proportional increases (mean [%CV]; 45 vs. 90 mg twice daily) in ticagrelor C max (616 [37] vs. 1273 [43] ng ml–1) and AUC (3882 [42] vs. 8206 [51] ng ml–1 h) and AR‐C124910XX parameters were seen. Pharmacodynamic and PK differences between 45 and 60 mg were small. No safety issues were identified.
Conclusions
In Chinese patients with CAD, ticagrelor (45, 60 and 90 mg) markedly reduced platelet aggregation. The IPA and PRU magnitude increased generally with increasing doses. However, the mean pharmacodynamic differences between 45 and 60 mg doses were small. Following single and multiple doses, the mean C max and AUC values of ticagrelor and AR‐C124910XX increased approximately dose proportionally between 45 and 90 mg doses.
Keywords: Chinese patients, coronary artery disease, ticagrelor, pharmacodynamics, pharmacokinetics
What is Already Known about this Subject
Ticagrelor (an oral, direct acting, reversibly binding P2Y12 receptor antagonist) is approved for preventing atherothrombotic events in patients with acute coronary syndromes.
Ticagrelor bioavailability is ~40% higher in Asians than Caucasians.
Pharmacodynamics, pharmacokinetics and safety of ticagrelor have not been evaluated previously in Chinese patients with stable coronary artery disease.
What this Study Adds
In Chinese patients with coronary artery disease, ticagrelor (45, 60 or 90 mg twice daily) markedly inhibited platelet aggregation.
Dose‐related changes in pharmacodynamic/pharmacokinetic parameters occurred with ticagrelor.
Ticagrelor exposure was consistent with previous Asian studies and higher than in Caucasian studies.
Introduction
Ticagrelor, an orally administered, direct acting, reversibly binding P2Y12 receptor antagonist, inhibits ADP‐induced platelet aggregation 1, 2 and inhibits cellular uptake of adenosine via equilibrative nucleoside transporter 1 inhibition 3. Ticagrelor is indicated to reduce the rate of cardiovascular death, myocardial infarction (MI) and stroke in patients with acute coronary syndromes (ACS) or a history of MI 4.
Efficacy and safety of ticagrelor (180 mg loading dose, 90 mg twice daily thereafter) plus aspirin vs. clopidogrel plus aspirin were established in the phase III Platelet Inhibition and Patient Outcomes (PLATO) trial 5. Ticagrelor significantly reduced the primary composite end point (MI/stroke/death from vascular causes) vs. clopidogrel 5. No significant increase in overall rates of major bleeding was seen with ticagrelor vs. clopidogrel, although ticagrelor was associated with a higher rate of major bleeding not related to coronary artery bypass grafting 5. The prospective Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin‐Thrombolysis in Myocardial Infarction 54 (PEGASUS‐TIMI 54) study showed that long term (1–3 years) therapy with ticagrelor (60 mg or 90 mg twice daily) and low dose aspirin (75–100 mg day–1) in patients with a prior MI (>12 months previously) significantly reduced the primary composite end point (MI/stroke/death from vascular causes), with an increase in major bleeding vs. placebo 6.
Ticagrelor is rapidly absorbed with a linear pharmacokinetic (PK) profile 7, 8. Ticagrelor does not require metabolic activation but is extensively metabolized via cytochrome P450 (CYP) 3 A4/5 to the active metabolite AR‐C124910XX 9, 10, 11. Both compounds are reversible P2Y12 receptor antagonists 1, 2. Due to direct and reversible binding, ticagrelor‐induced platelet inhibition is rapid and related to plasma concentrations 9, 12, 13.
Patient populations can differ widely in responses to certain drugs, including antiplatelet agents 14. Many factors, including ethnicity 15, can affect drug metabolism and disposition. A key factor is ethnic differences in drug metabolizing enzymes 16, 17. For example, genetic polymorphisms occur in the CYP3A family 18, with unique CYP3A polymorphisms seen in Chinese subjects 19.
Compared with Caucasians, mean exposure to ticagrelor and AR‐C124910XX is ~40% higher in Asians, for example, healthy Chinese 20 and Japanese 21 volunteers, and Japanese and Asian patients with stable coronary artery disease (CAD) 22. However, in PLATO, the efficacy and safety of ticagrelor vs. clopidogrel for the Asian (n = 1056) and Chinese (n = 383) subgroups were broadly consistent with those in the overall study population 5. Multiple ticagrelor dosing resulted in high levels of inhibition of platelet aggregation (IPA) in healthy Japanese volunteers 21 and in Japanese and Asian patients with stable CAD 22. However, pharmacodynamic (PD) data for ticagrelor in Chinese patients are limited.
The primary objective of this study was to determine the IPA profiles of single and multiple doses of ticagrelor, at three doses (45, 60 and 90 mg) in Chinese patients with stable CAD on chronic low dose aspirin. Secondary objectives included investigation of PRU, PK and safety profiles of ticagrelor in this patient population.
Methods
Patients
Patients provided written, informed consent. Eligible patients were Chinese (as per Chinese Regulatory Guidelines) men or women (non‐pregnant, surgically sterile or post‐menopausal) aged ≥18 years with documented stable CAD (stable angina pectoris 23, angina severity grade I or II 24) and taking aspirin 75–100 mg day–1. Key exclusion criteria included oral anticoagulant or dual antiplatelet use, use of strong CYP3A inhibitors/inducers or substrates with a narrow therapeutic index (within 14 days of study start), platelet count <100 000 mm3 or haemoglobin <10 g dl–1, history of/current alcohol or drug abuse, current smokers (≥5 cigarettes/week), renal dialysis, moderate or severe hepatic impairment and ACS or stent placement within 12 months of screening.
Study design and treatments
This open label, single centre, randomized study (NCT02064985; AstraZeneca study code: D5130C00086) was conducted in China from February to November 2014. The study protocol was approved by the Peking University Third Hospital Medical Science Research Ethics Committee (Beijing, China) and the National Regulatory Authority. The study was conducted according to the ethical principles established in the Declaration of Helsinki consistent with the International Conference on Harmonization/Good Clinical Practice guidelines, AstraZeneca policy on bioethics and applicable regulatory guidelines.
Eligible patients were admitted to the Clinical Pharmacology Unit on day −2 and discharged on day 8. A follow‐up visit occurred 2–5 days after the last dose of study medication. Patients were randomized to one of three groups (n = 12 patients/group), ticagrelor 45, 60 or 90 mg. For each group, a single ticagrelor dose was administered in the morning on day 1. Patients received ticagrelor twice daily on days 3–6 and a single dose on day 7. All patients maintained their daily aspirin doses at 75–100 mg day–1.
Patients refrained from consuming alcohol (from 72 h prior to enrolment until follow‐up), all grapefruit and Seville orange containing foods (from 7 days prior to study treatment until the end of treatment), any new medication (including traditional Chinese medication, from 14 days prior to study treatment until the end of treatment) and drugs of abuse (from time of consent to follow‐up).
Assessments
Pharmacodynamics
The primary PD end point was final extent IPA (observed at 6 min after ADP addition), measured by light transmission aggregometry of platelet‐rich plasma stimulated with 20 μm ADP 8. The secondary PD end point was inhibition of the P2Y12 receptor, assessed in whole blood samples within 4 h of sample collection by the VerifyNow® assay P2Y12 platelet function assay (Accumetrics, Inc., San Diego, CA, USA) according to the manufacturer's instructions and reported as PRU.
Blood samples for PD analyses were collected at 0 (pre‐dose), 0.5, 1, 2, 3, 6 and 12 h on days 1 and 7, and at 24, 36 and 48 h after dosing on day 1. Venous blood samples were collected (4.5 ml for IPA, 2 ml for PRU) into tubes containing 3.13% w/v trisodium citrate (IPA) or 3.2% w/v sodium citrate (PRU).
Pharmacokinetics
Blood samples for assessing ticagrelor and AR‐C124910XX plasma concentrations were collected at the times listed above for PD sampling. Ticagrelor and AR‐C124910XX were analyzed by Covance Pharmaceutical R&D (Beijing) Co. Ltd, Shanghai, China using a fully validated liquid chromatography tandem mass spectrometry method 25. Ranges of the calibration curves were 5–5000 ng ml–1 (ticagrelor) and 2.5–2500 ng ml–1 (AR‐C124910XX). Intra‐batch accuracy and precision were 91.9–100.9% and 4.0–8.4%, respectively (ticagrelor) and 86.8–109.2% and 5.2–16.9%, respectively, (AR‐C124910XX) 25.
Safety and tolerability
Adverse events (AEs) were monitored continuously up to the follow‐up visit. Clinical laboratory parameters (haematology, clinical chemistry, urinalysis), physical examination and vital signs were evaluated at screening, on day −1 and at follow‐up.
Data analyses
Final extent IPA from pre‐dose baseline was calculated using IPA (%) = 100*(PABL – PAt)/PABL (PABL = response at pre‐dose baseline on day 1; PAt = response at any post‐treatment time t). Area under the effect curve (AUEC; final extent) for IPA was calculated over the dosing interval from IPA‐time curves using the linear trapezoid rule.
Ticagrelor and AR‐C124910XX PK parameters were calculated using non‐compartmental methods (WinNonlin® Professional, Pharsight Corporation, Mountain View, CA, USA). Plasma concentration–time profiles were used to calculate maximum plasma concentration (C max), time to C max (t max), half‐life (t ½), area under the plasma concentration–time curve (AUC) and AUC from time 0 to 12 h (AUC0−12h). t ½ was calculated as 0.693/λz (λz = terminal rate constant calculated by least squares regression analysis of the plasma concentration–time curve obtained over the terminal log‐linear phase). AUC parameters were calculated using the linear trapezoidal rule. Accumulation ratios (Rac) were the ratio of drug concentrations at steady‐state divided by those after a single dose. An exploratory analysis using a non‐linear sigmoid maximum observed plateau effect (Emax) model investigated the PK/PD relationship for ticagrelor.
Results
Patients
Sixty‐one enrolled patients provided informed consent and 36 (n = 12/group) were randomized to the ticagrelor dose groups (45, 60 and 90 mg). All 36 patients completed the study.
Patient demographics and baseline clinical characteristics were generally well balanced across groups. Most patients were male (28/36, 78%. The range across groups was 75–83%). In the 45, 60 and 90 mg ticagrelor groups, mean ± standard deviation (SD) age was 59.3 ± 9.8, 61.3 ± 7.0 and 57.9 ± 12.6 years and mean ± SD body mass index was 28.3 ± 3.6, 25.9 ± 2.9 and 26.5 ± 2.8 kg m– 2, respectively. All patients were taking permitted medications, which were similar across groups. The most common concomitant medications were β‐hydroxy‐β‐methylglutaryl‐coenzyme A reductase inhibitors (statins) and selective β‐adrenoceptor blockers, taken by 35 (97%) and 25 (69%) patients, respectively.
Pharmacodynamics
Inhibition of platelet aggregation
With all doses, IPA occurred within 30 min on day 1. Mean (SD) final extent IPA was 27% (26), 26% (27), and 33% (31) for the 45, 60, and 90 mg doses, respectively. The greatest mean IPA was seen at 3 h with ticagrelor 45 mg (88 ± 12%) and 90 mg (96 ± 6%) and at 6 h with ticagrelor 60 mg (94 ± 6%). IPA over 48 h following a single ticagrelor dose on day 1 was dose dependent (Figure 1A). On day 1, mean AUEC was 2474, 2819 and 3301 with ticagrelor 45, 60 and 90 mg, respectively. After a single dose, mean AUEC values were 14% and 33% higher with ticagrelor 60 mg and 90 mg, respectively, vs. ticagrelor 45 mg.
Figure 1.
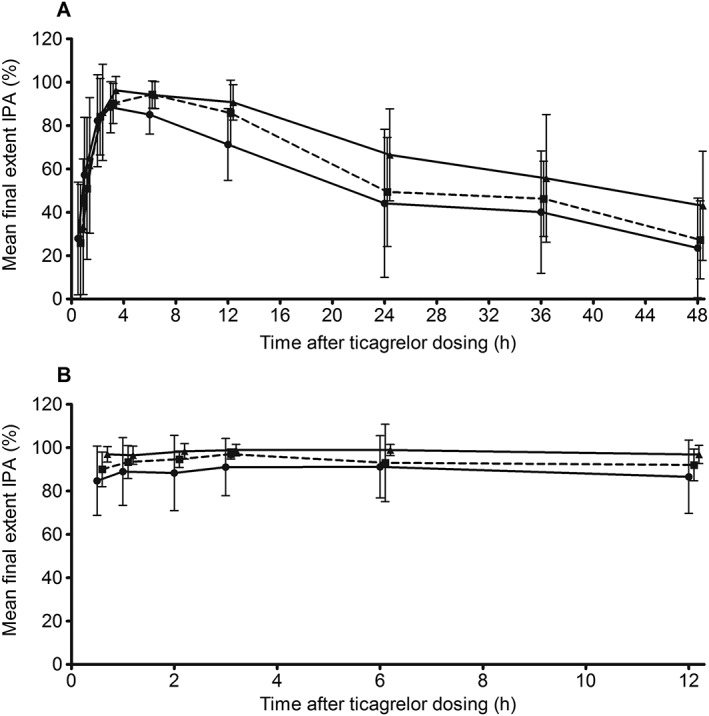
Mean (± SD) final extent IPA on (A) day 1 ( ticagrelor 45 mg (n = 12),
ticagrelor 45 mg (n = 12),  ticagrelor 60 mg (n = 12) and
ticagrelor 60 mg (n = 12) and  ticagrelor 90 mg (n = 12)) and (B) day 7 (
ticagrelor 90 mg (n = 12)) and (B) day 7 ( ticagrelor 45 mg twice daily (n = 12),
ticagrelor 45 mg twice daily (n = 12),  ticagrelor 60 mg twice daily (n = 12) and
ticagrelor 60 mg twice daily (n = 12) and  ticagrelor 90 mg twice daily (n = 12)) in Chinese patients with stable coronary artery disease treated with low dose aspirin plus ticagrelor 45 mg, 60 mg or 90 mg (single dose on days 1 and 7 and twice daily dosing on days 3–6). Note: data were collected at the time points listed in the Methods section, but data points are offset to avoid overlap. IPA inhibition of platelet aggregation
ticagrelor 90 mg twice daily (n = 12)) in Chinese patients with stable coronary artery disease treated with low dose aspirin plus ticagrelor 45 mg, 60 mg or 90 mg (single dose on days 1 and 7 and twice daily dosing on days 3–6). Note: data were collected at the time points listed in the Methods section, but data points are offset to avoid overlap. IPA inhibition of platelet aggregation
On day 7, following twice daily dosing, mean final extent IPA following ticagrelor 45, 60 and 90 mg over the dosing interval were >85%, >90%, and >95%, respectively (Figure 1B). The greatest mean (SD) IPA was seen at 3 h with ticagrelor 45 mg (91 ± 13%) and 60 mg (97 ± 3%) and at 6 h with ticagrelor 90 mg (99 ± 3%). IPA generally increased with increasing ticagrelor dose. Differences in mean IPA between doses at each time point were small. Mean AUEC values on day 7 were 1070 (45 mg), 1120 (60 mg) and 1177 (90 mg), respectively. With multiple dosing, mean AUEC values were 5% (60 mg) and 10% (90 mg) higher vs. ticagrelor 45 mg.
Inhibition of the P2Y12 receptor
On day 1, mean PRU at 1 h post‐dosing was markedly reduced vs. pre‐dose levels with all ticagrelor doses (Figure 2A). Mean PRU reduction was larger with ticagrelor 90 mg vs. 45 mg and only small differences occurred between the 45 mg and 60 mg doses.
Figure 2.

Mean (± SD) PRU on (A) day 1 ( ticagrelor 45 mg (n = 12),
ticagrelor 45 mg (n = 12),  ticagrelor 60 mg (n = 12) and
ticagrelor 60 mg (n = 12) and  ticagrelor 90 mg (n = 12)) and (B) day 7 (
ticagrelor 90 mg (n = 12)) and (B) day 7 ( ticagrelor 45 mg twice daily (n = 12),
ticagrelor 45 mg twice daily (n = 12),  ticagrelor 60 mg twice daily (n = 12) and
ticagrelor 60 mg twice daily (n = 12) and  ticagrelor 90 mg twice daily (n = 12)) in Chinese patients with stable coronary artery disease treated with low dose aspirin plus ticagrelor 45 mg, 60 mg or 90 mg (single dose on days 1 and 7 and twice daily dosing on days 3–6). Note: data were collected at the time points listed in the Methods section but data points are offset to avoid overlap. PRU P2Y12 reaction units
ticagrelor 90 mg twice daily (n = 12)) in Chinese patients with stable coronary artery disease treated with low dose aspirin plus ticagrelor 45 mg, 60 mg or 90 mg (single dose on days 1 and 7 and twice daily dosing on days 3–6). Note: data were collected at the time points listed in the Methods section but data points are offset to avoid overlap. PRU P2Y12 reaction units
On day 7, ranges of mean PRU values over the dosing interval were 48–98, 36–81 and 17–32 with ticagrelor 45, 60 and 90 mg, respectively (Figure 2B). Mean reductions in PRU from baseline over the dosing interval were 64–82% (45 mg dose), 69–87% (60 mg dose), and 89–94% (90 mg dose). The maximum reduction in mean (SD) PRU from baseline was seen at 2 h with ticagrelor 45 mg (82 ± 17%) and at 3 h with 60 mg (87 ± 13%) and with 90 mg ticagrelor (92 ± 9%). Reductions in mean PRU values and % reductions in PRU from baseline were dose dependent.
Pharmacokinetics of ticagrelor and AR‐C124910XX (Table 1)
Table 1.
Pharmacokinetic parameters for ticagrelor and AR‐C124910XX on day 1 and day 7 in Chinese patients with stable coronary artery disease treated with low dose aspirin plus ticagrelor 45 mg twice daily, 60 mg twice daily or 90 mg twice daily
| Parameter * | Ticagrelor 45 mg twice daily (n = 12) | Ticagrelor 60 mg twice daily (n = 12) | Ticagrelor 90 mg twice daily (n = 12) | |||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | |
| Ticagrelor | ||||||
| C max (ng ml –1 ) | 464 (38) | 616 (37) | 414 (34) | 689 (34) | 822 (37) | 1273 (43) |
| t max (h) † | 2.00 (1.00–2.00) | 2.00 (1.00–3.03) | 3.00 (1.00–6.00) | 2.00 (1.00–3.00) | 2.00 (1.00–3.03) | 2.00 (1.00–3.00) |
| t ½ (h) | 10.72 (16.18) | – | 9.42 (13.53) | – | 10.14 (17.54) | – |
| AUC (ng ml –1 h) | 3220 (51) | – | 3633 (32) | – | 6234 (54) | – |
| AUC0−12h (ng ml–1 h) | 2114 (42) | 3882 (42) | 2313 (30) | 4351 (37) | 3983 (42) | 8206 (51) |
| R ac | – | 1.84 (23.6) | – | 1.88 (20.7) | – | 2.06 (22.8) |
| AR‐C124910XX | ||||||
| C max (ng ml –1 ) | 88.3 (24.6) | 144 (26) | 77.1 (54) | 180 (50) | 139 (38) | 301 (32) |
| t max (h) † | 2.00 (2.00–3.00) | 2.00 (1.00–6.00) | 3.00 (2.00–6.00) | 2.00 (2.00–3.00) | 3.00 (2.00–3.03) | 2.54 (2.00–3.10) |
| t ½ (h) | 12.65 (22.94)‡ | – | 11.38 (24.27)‡ | – | 11.62 (24.64) | – |
| AUC (ng ml−1 h) | 922 (29)‡ | – | 1108 (35)‡ | – | 1644 (31) | – |
| AUC0−12h (ng ml–1 h) | 464 (19) | 1069 (25) | 504 (55) | 1314 (41) | 836 (30) | 2254 (37) |
| R ac | – | 2.30 (24.7) | – | 2.61 (28.3) | – | 2.70 (27.4) |
Values are geometric mean (percentage coefficient of variation) unless otherwise indicated;
Median (range);
n = 11.
AUC area under the plasma concentration–time curve; AUC0−12h area under the plasma concentration–time curve from zero to 12 h; C max maximum plasma concentration; Rac accumulation ratio; t ½ half‐life; t max time to maximum plasma concentration.
Plasma concentration vs. time profiles for ticagrelor and AR‐C124910XX (after multiple ticagrelor dosing) are shown in Figure 3. Following single and multiple doses, ticagrelor AUC0−12h and C max increased approximately dose proportionally between 45 mg and 90 mg doses. Differences in ticagrelor AUC0−12h and C max values between the 45 mg and 60 mg doses were small.
Figure 3.
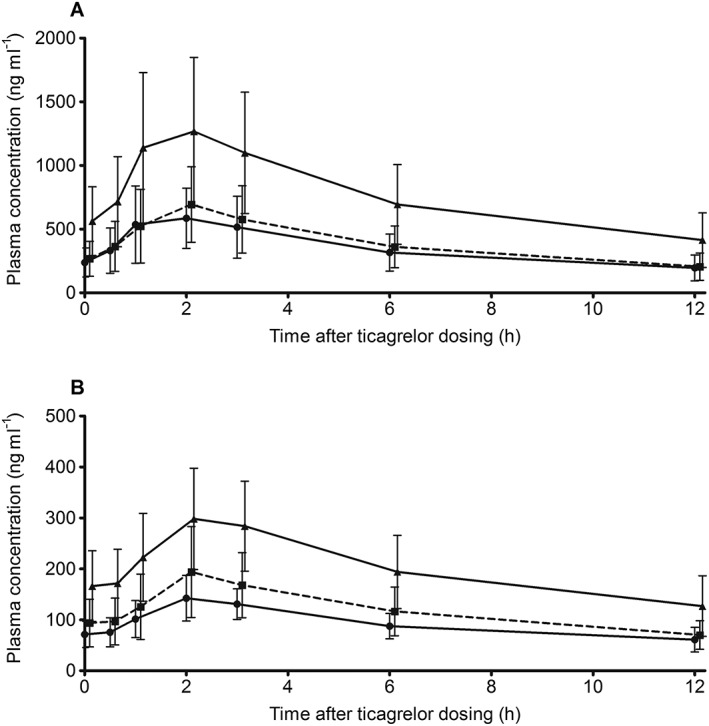
Mean (± SD) plasma concentrations on day 7 of (A) ticagrelor ( ticagrelor 45 mg (n = 12),
ticagrelor 45 mg (n = 12),  ticagrelor 60 mg (n = 12),
ticagrelor 60 mg (n = 12),  ticagrelor 90 mg (n = 12)) and (B) AR‐C124910XX (
ticagrelor 90 mg (n = 12)) and (B) AR‐C124910XX ( ticagrelor 45 mg (n = 12),
ticagrelor 45 mg (n = 12),  ticagrelor 60 mg (n = 12) and
ticagrelor 60 mg (n = 12) and  ticagrelor 90 mg (n = 12)) in Chinese patients with stable coronary artery disease treated with low dose aspirin plus ticagrelor 45 mg, 60 mg or 90 mg (single dose on days 1 and 7 and twice daily dosing on days 3–6). Note: data were collected at the time points listed in the Methods section but data points are offset to avoid overlap
ticagrelor 90 mg (n = 12)) in Chinese patients with stable coronary artery disease treated with low dose aspirin plus ticagrelor 45 mg, 60 mg or 90 mg (single dose on days 1 and 7 and twice daily dosing on days 3–6). Note: data were collected at the time points listed in the Methods section but data points are offset to avoid overlap
After single and multiple ticagrelor doses, AUC0−12h and C max of AR‐C124910XX increased dose proportionally from ticagrelor 45 mg to 90 mg doses. Very small differences in these parameters occurred between ticagrelor 45 mg and 60 mg.
Mean t ½ and median t max estimates for ticagrelor and AR‐C124910XX were independent of ticagrelor dose. Mean accumulation ratios following 45, 60 and 90 mg twice daily dosing were 1.8 to 2.1 (ticagrelor) and 2.3 to 2.7 (AR‐C124910XX).
Pharmacokinetic/pharmacodynamic relationship
PK/PD analyses demonstrated that final extent IPA increased with increasing ticagrelor plasma concentrations (Figure 4). Plasma concentration–IPA profiles and Emax model parameters were comparable among the three ticagrelor doses (data not shown). From pooled data, Emax and half maximal effective concentration (EC 50) estimates were 99.7% and 28.8 ng ml–1, respectively.
Figure 4.
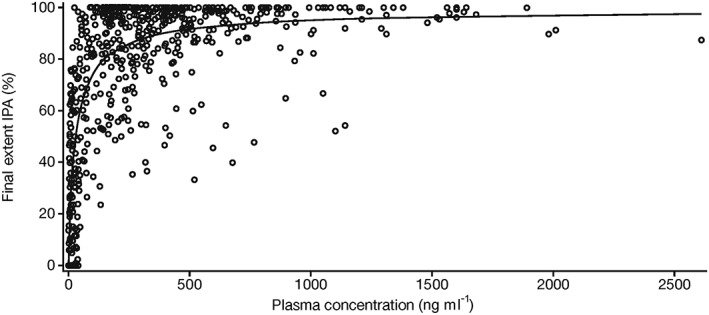
Scatter plot of ticagrelor plasma concentration vs. final extent IPA in Chinese patients with stable coronary artery disease. The prediction curve shown in the figure is based on a sigmoid Emax model. Emax maximum observed plateau effect; IPA inhibition of platelet aggregation. ( ) Ticagrelor, (
) Ticagrelor, ( ) Predicted ticagretor
) Predicted ticagretor
Safety and tolerability
All ticagrelor doses were generally well tolerated. No patients discontinued due to AEs and no deaths or serious AEs occurred. The proportion of patients with at least one AE was similar across groups (Table 2). Overall, 13 patients had an AE. Five of these had an AE considered to be causally related to ticagrelor. One had mild diarrhoea and moderate occult blood (45 mg), two had mild dyspnoea (60 mg) and one had mild dyspnoea and one had mild haemoptysis (90 mg). Overall, all AEs, except diarrhoea, dyspnoea, cardiac discomfort and hypoglycaemia, were reported only in one patient. The majority of AEs were mild. No clinically relevant changes in laboratory parameters, physical examinations or vital signs occurred.
Table 2.
Number of Chinese patients with stable coronary artery disease with AEs following treatment with low dose aspirin plus ticagrelor 45 mg twice daily, 60 mg twice daily or 90 mg twice daily
| Ticagrelor 45 mg twice daily (n = 12) | Ticagrelor 60 mg twice daily (n = 12) | Ticagrelor 90 mg twice daily (n = 12) | |
|---|---|---|---|
| Patients with any AE, n (%) | 4 (33) | 4 (33) | 5 (42) |
| Diarrhoea | 1 (8) | 1 (8) | 2 (17) |
| Dyspnoea | 0 | 2 (17) | 1 (8) |
| Hypoglycaemia | 1 (8) | 1 (8) | 0 |
| Cardiac discomfort | 0 | 1 (8) | 1 (8) |
| Blurred vision | 0 | 1 (8) | 0 |
| Ventricular extrasystoles | 0 | 0 | 1 (8) |
| Toothache | 0 | 0 | 1 (8) |
| Tinea pedis | 1 (8) | 0 | 0 |
| Palpitations | 0 | 1 (8) | 0 |
| Pain in extremity | 0 | 0 | 1 (8) |
| Positive occult blood | 1 (8) | 0 | 0 |
| Non‐cardiac chest pain | 1 (8) | 0 | 0 |
| Hyperhidrosis | 0 | 1 (8) | 0 |
| Haemoptysis | 0 | 0 | 1 (8) |
| Gingival bleeding | 0 | 1 (8) | 0 |
| Frequent bowel movements | 0 | 0 | 1 (8) |
| Epistaxis | 0 | 1 (8) | 0 |
| Electrocardiogram ST‐T change | 0 | 1 (8) | 0 |
| Constipation | 0 | 0 | 1 (8) |
AE, adverse event.
Discussion
A single ticagrelor dose (45, 60 or 90 mg) resulted in a rapid onset of IPA (within 30 min) and high IPA levels (>85%) with multiple doses in Chinese patients with stable CAD. A rapid reduction in PRU was seen with a single dose and large reductions in PRU vs. baseline occurred with multiple ticagrelor dosing. Ticagrelor was rapidly absorbed and exposure to ticagrelor and AR‐C124910XX increased approximately dose proportionally between 45 and 90 mg. For IPA, PRU and exposure to ticagrelor and AR‐C124910XX, a dose response was observed between ticagrelor 45 and 90 mg, although small differences occurred between ticagrelor 45 mg and 60 mg. The AE profile with ticagrelor in Chinese patients with stable CAD was consistent with that seen in Asian and non‐Asian populations.
Dose selection and limitations
The highest ticagrelor dose (90 mg twice daily) used in our study is the recommended clinical dose, after a single 180 mg loading dose, for preventing atherothrombotic events in patients with ACS 4. Previous ethnic‐bridging studies showed that exposure to ticagrelor and AR‐C124910XX with multiple dosing is ~40% higher in healthy Chinese 20 and Japanese volunteers 21 and in Japanese patients with stable CAD 22 vs. Caucasian subjects. Thus, based on this observation, ticagrelor 45 mg was selected as the lowest dose in our study. The mid‐dose of ticagrelor (60 mg) was included because this dose was investigated in PEGASUS‐TIMI 54, a long term placebo‐controlled study with ticagrelor in patients with a prior MI 6.
Our study has several limitations. The small sample size (12 patients/dose group) limits the ability to compare the results among ticagrelor doses. Additionally, the current findings may not reflect those that may occur in the wider patient population due to this small sample size. The variability of the PD and PK parameters in our study was large, thereby impacting on data interpretation. However, this variability is in keeping with previous ticagrelor studies in healthy Caucasian 7, 8, Chinese 20 and Japanese 21 volunteers and Japanese patients with CAD 22. The IPA and PRU tests used in this study are widely used ‘standard’ tests of platelet function 26. However, the simple test conditions may not reflect the in vivo effects of antiplatelet agents due to the complexity of platelet aggregation 27. Certain sample and assay factors may also affect the results 28. Moreover, data on the association of ex vivo platelet function tests with clinical outcomes in cardiovascular disease are limited 29, 30.
Pharmacodynamics
The ticagrelor 90 mg dose in our study was bioequivalent to the 100 mg dose of an earlier formulation used in previous studies. In Chinese patients with CAD, the ticagrelor 90 mg dose resulted in a rapid IPA onset after a single dose. Moreover, the greatest IPA effect with ticagrelor 90 mg observed in this population was consistent with that in earlier studies. For example, in Caucasians, the greatest mean IPA occurred at 2 h (88%) in healthy volunteers 7 and at 4 h (84%) in patients with atherosclerosis 10 after a single ticagrelor 100 mg dose. In healthy Japanese volunteers (ticagrelor 100 mg) and Japanese patients with CAD (ticagrelor 90 mg), following a single dose of ticagrelor, the greatest mean IPA was seen at 4 h (i.e. 95% 21 and 70% 22, respectively).
With multiple ticagrelor dosing, mean final extent IPA was high in Chinese patients with CAD at all doses, with only small differences between dose levels. This observation was also in keeping with previous studies. For example, in Caucasian subjects, ticagrelor 50–300 mg twice daily resulted in 80–100% IPA in healthy volunteers 8 and 60–90% IPA in patients with atherosclerosis 10. In healthy Japanese and Caucasian volunteers given ticagrelor 100 mg twice daily, the mean final extent IPA over the dosing interval was 99% and 85%, respectively 21. The mean IPA at the end of the dosing interval was 57% with 45 mg twice daily and 67% with 90 mg twice daily in Japanese patients with stable CAD 22. Collectively, these data demonstrate that ticagrelor twice daily dosing (Caucasian subjects 50–300 mg twice daily; Japanese subjects 45, 90, and 100 mg twice daily, Chinese subjects 45, 60 and 90 mg twice daily) results in high final extent IPA in healthy volunteers and patients of different ethnicities.
The current study is one of the first studies to report PRU with ticagrelor in Chinese patients. The majority of previous clinical studies reporting the effect of ticagrelor at 90 mg twice daily on PRU have been in patient populations mainly composed of Caucasians. For example, in patients with stable CAD, mean PRU was approximately 20 (94% reduction from baseline, 2–4 h after ticagrelor 180 mg) to 70 (78% reduction from baseline, 8 h after ticagrelor 90 mg twice daily) in the RESPOND trial 31, and ~50 (~83% reduction, 2 h after ticagrelor 90 mg twice daily) in the ONSET‐OFFSET trial 12. In patients with ACS in the PLATO platelet substudy, mean PRU was approximately 10–70 (78–97% reduction from baseline, 4 h after ticagrelor 180 mg) 30. The present results in Chinese patients with CAD are consistent with these previous results, as a marked reduction in PRU was seen. Furthermore, a dose response in PRU reduction was seen between ticagrelor 45 and 90 mg twice daily.
Pharmacokinetics
Ticagrelor and AR‐C124910XX PK parameters in Chinese patients with stable CAD were consistent with previously reported findings. In these patients, ticagrelor was rapidly absorbed and metabolized, and ticagrelor and AR‐C124910XX t max and t ½ values were similar to those reported in healthy Caucasian volunteers. For example, for ticagrelor, median t max was ~1.3–2 h 7, 9 and mean t ½ was 7.7–13.1 h 7. Moreover, comparable results were also reported in a healthy Chinese volunteer study with single (90 and 180 mg) and multiple (90 and 180 mg twice daily) ticagrelor doses, for example, median t max 2 h and mean t ½ range 10.9–14.9 h for ticagrelor 20. As expected, these parameters were independent of ticagrelor dose in our study.
With ticagrelor 90 mg twice daily, exposures to ticagrelor and AR‐C124910XX in Chinese patients with CAD were consistent with those reported in healthy Chinese volunteers. For example, mean (coefficient of variation) ticagrelor AUC was 7168 (35) ng ml–1 h and C max was 915 (32) ng ml–1 20. The present exposure results were also generally consistent with those for Japanese patients with CAD, e.g. ticagrelor, AUC 6080 (41) ng ml–1 h 22. Although a direct comparison of ticagrelor PK parameters between Chinese and Caucasian subjects was not possible because the two ethnic groups have not been included in the same study, a general cross‐study comparison was previously reported 20. This comparison demonstrated that exposure to ticagrelor and AR‐C124910XX was ~40% higher in healthy Chinese volunteers vs. various groups of Caucasian subjects, i.e. healthy volunteers: ticagrelor AUC 4108 (43) ng ml–1 h 8, patients with stable atherosclerosis: ticagrelor AUC 5530 (48) ng ml–1 h 10 and patients with ACS, ticagrelor AUC 4762 ± 2443 (SD) ng ml–1 h 32. The present ticagrelor and AR‐C124910XX exposure data in Chinese patients with stable CAD also confirm the conclusion of higher exposure in Chinese subjects. Higher exposures to both the parent drug and active metabolite have also been reported in healthy Japanese vs. Caucasian volunteers 21.
In the current study, for both ticagrelor and AR‐C124910XX AUC and C max, dose proportionality was seen between the 45 mg and 90 mg doses (single and multiple ticagrelor dosing), and only small differences occurred between ticagrelor 45 mg and 60 mg. However, due to the small sample size and the large data variability, the differences between the PK (and PD) parameters for the 45 mg and 60 mg doses are difficult to interpret. A dose response has also been reported with a wide range of ticagrelor doses in other populations, for example, in healthy volunteers (Caucasian 30–400 mg single dose 7, 50–200 mg twice daily 8, Chinese 90 and 180 mg single dose and twice daily 20 and Japanese 50–600 mg single dose, 100 and 300 mg twice daily 21), Japanese patients with CAD (45 and 90 mg 22) and in patients with atherosclerosis (50–200 mg twice daily 10). Collectively, these data, including those from our study, demonstrate the linear and predictable PK of ticagrelor in a diverse group of subjects 33.
Pharmacodynamic/pharmacokinetic relationship
In Chinese patients with CAD in the current study, the IPA magnitude was associated with plasma ticagrelor concentrations. Both the plasma concentration–IPA profiles and the Emax model parameters were comparable among the three ticagrelor doses used in this study. These data suggest that the ticagrelor plasma concentration achieved with the twice daily dosing at the doses studied was sufficient to achieve high levels of IPA. This relationship has also been reported in healthy Caucasian volunteers 7, 8 and in Japanese patients with CAD 22. This finding is as expected, since ticagrelor 2 and AR‐C124910XX inhibit platelet activity by directly binding to the P2Y12 receptor. Thus, following ticagrelor administration, IPA (prior to the plateau of effect) reflects the plasma concentrations of the parent drug and the active metabolite 34.
Safety
Ticagrelor was generally well tolerated in Chinese patients with stable CAD at all doses evaluated. Furthermore, the safety profile of ticagrelor in the current study was consistent with those previously observed in mainly Caucasian populations such as healthy volunteers 7, 8 and patients with atherosclerosis 10 or ACS 5. Similar findings were also seen in healthy Chinese 20 and Japanese 21 volunteers and Asian patients with stable CAD 22. Thus, no new safety concerns were identified in Chinese patients with stable CAD, albeit with a short duration of ticagrelor dosing.
Clinical implications
As discussed above, our results in Chinese patients with stable CAD demonstrated that the PK of ticagrelor are linear and predictable in this population, and are consistent with similar studies in Asian subjects 20, 21, 22. In addition, high levels of platelet inhibition are rapidly achieved and maintained in this patient population, consistent with studies in various patient groups 10, 12, 22, 30, 31. Moreover, the phase III trial, PLATO, showed that the efficacy and safety of 90 mg ticagrelor vs. clopidogrel in Asian and Chinese patients was broadly similar to those in the overall study population 5. Collectively, these results suggest that ticagrelor may have potential clinical benefits in Chinese patients with CAD.
Our data showing that the difference in mean IPA for the three ticagrelor doses was small supports the PEGASUS‐TIMI 54 trial results. In PEGASUS‐TIMI 54, ticagrelor significantly reduced the rate of the primary end point (composite of cardiovascular death, MI or stroke) vs. placebo and the magnitude of this effect was similar between the 60 and 90 mg twice daily ticagrelor doses 6. However, the rates of bleeding and dyspnoea were numerically lower with 60 mg vs. 90 mg ticagrelor, resulting in a lower rate of discontinuation of the study drug and a better safety profile with the 60 mg dose 6. Thus, in general, the 60 mg dose of ticagrelor may offer a better benefit–risk profile vs. the 90 mg dose.
Conclusions
In conclusion, Chinese patients with stable CAD, ticagrelor (45, 60 and 90 mg) markedly reduced platelet aggregation, and the magnitude of IPA and PRU changes generally increased with increasing doses. The differences in PD parameters between ticagrelor 45 and 60 mg were small. Following single and multiple doses, the mean C max and AUC values of ticagrelor and AR‐C124910XX increased approximately dose‐proportionally between 45 and 90 mg doses. There were no significant safety concerns with ticagrelor doses up to and including 90 mg in Chinese patients with stable CAD.
Competing interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that Haiyan Li (first author) has acted as a consultant and speaker for AstraZeneca in the previous 3 years, Jingchuan Guo has no conflicts of interest and Glenn Carlson and Renli Teng (corresponding author) are current employees of AstraZeneca, LP.
The authors thank the staff of the Phase I Unit at the Peking University Third Hospital, Beijing, China, who conducted the study. The authors thank Mohammad Niazi (AstraZeneca employee) for his assistance with the PK data analyses. We also thank Jackie Phillipson, PhD, from Zoetic Science (UK), who provided medical writing support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing). This support was funded by AstraZeneca.
Principal Investigator
Haiyan Li, Peking University Third Hospital, Haidian District, Beijing, China.
Contributors
HL contributed to the conception and design of the study, acquisition of data and data interpretation. JG contributed to the conception and design of the study, acquisition of data and data interpretation. GC contributed to data interpretation. RT contributed to the conception and design of the study, acquisition of data, data analyses and data interpretation.
Funding
This study was funded by AstraZeneca.
Li, H. , Guo, J. , Carlson, G. F. , and Teng, R. (2016) Pharmacodynamics, pharmacokinetics, and safety of ticagrelor in Chinese patients with stable coronary artery disease. Br J Clin Pharmacol, 82: 352–361. doi: 10.1111/bcp.12950.
References
- 1. Husted S, van Giezen JJJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther 2009; 27: 259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Giezen JJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, et al. Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP‐induced receptor signaling and PA. J Thromb Haemost 2009; 7: 1556–65. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther 2014; 19: 209–19. [DOI] [PubMed] [Google Scholar]
- 4. AstraZeneca . Brilinta® prescribing information, 2015. Available at: http://www.azpicentral.com/brilinta/brilinta.pdf, last accessed 1 December 2015.
- 5. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–57. [DOI] [PubMed] [Google Scholar]
- 6. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. PEGASUS‐TIMI 54 Steering Committee and Investigators. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–800. [DOI] [PubMed] [Google Scholar]
- 7. Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y(12) receptor antagonist, in healthy subjects. Eur J Clin Pharmacol 2010; 66: 487–96. [DOI] [PubMed] [Google Scholar]
- 8. Butler K, Teng R. Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol 2010; 70: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos 2010; 38: 1514–21. [DOI] [PubMed] [Google Scholar]
- 10. Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double‐blind comparison to clopidogrel with aspirin. Eur Heart J 2006; 27: 1038–47. [DOI] [PubMed] [Google Scholar]
- 11. Zhou D, Andersson TB, Grimm SW. In vitro evaluation of potential drug–drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction and differential kinetics. Drug Metab Dispos 2011; 39: 703–10. [DOI] [PubMed] [Google Scholar]
- 12. Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double‐blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009; 120: 2577–85. [DOI] [PubMed] [Google Scholar]
- 13. Husted S. Evaluating the risk–benefit profile of the direct‐acting P2Y(12) inhibitor ticagrelor in acute coronary syndromes. Postgrad Med 2011; 123: 79–90. [DOI] [PubMed] [Google Scholar]
- 14. Beitelshees AL, Voora D, Lewis JP. Personalized antiplatelet and anticoagulation therapy: applications and significance of pharmacogenomics. Pharmgenomics Pers Med 2015; 8: 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther 2015; 97: 263–73. [DOI] [PubMed] [Google Scholar]
- 16. McGraw J, Waller D. Cytochrome P450 variations in different ethnic populations. Expert Opin Drug Metab Toxicol 2012; 8: 371–82. [DOI] [PubMed] [Google Scholar]
- 17. Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 2009; 41: 89–295. [DOI] [PubMed] [Google Scholar]
- 18. Chen X, Wang H, Zhou G, Zhang X, Dong X, Zhi L, et al. Molecular population genetics of human CYP3A locus: signatures of positive selection and implications for evolutionary environmental medicine. Environ Health Perspect 2009; 117: 1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du J, Xing Q, Xu L, Xu M, Shu A, Shi Y, et al. Systematic screening for polymorphism in the CYP3A4 gene in the Chinese population. Pharmacogenomics 2006; 7: 831–41. [DOI] [PubMed] [Google Scholar]
- 20. Li H, Butler K, Yang L, Yang Z, Teng R. Pharmacokinetics and tolerability of single‐ and multiple‐doses of ticagrelor in healthy Chinese volunteers. Clin Drug Investig 2012; 32: 87–97. [DOI] [PubMed] [Google Scholar]
- 21. Teng R, Butler K. Comparison of the pharmacokinetics, pharmacodynamics and tolerability of single and multiple doses of ticagrelor in healthy Japanese and Caucasian volunteers. Int J Clin Pharmacol Ther 2014; 54: 478–91. [DOI] [PubMed] [Google Scholar]
- 22. Hiasa Y, Teng R, Emanuelsson H. Pharmacodynamics, pharmacokinetics and safety of ticagrelor in Asian patients with stable coronary artery disease. Cardiovasc Interv Ther 2014; 29: 324–33. [DOI] [PubMed] [Google Scholar]
- 23. Chinese Society of Cardiology . Guideline for diagnosis and treatment of patients with chronic stable angina. Zhonghua Xin Xue Guan Bing Za Zhi 2007; 35: 195–206. [PubMed] [Google Scholar]
- 24. Canadian Cardiovascular Society . Grading of angina pectoris. 1976. Available at: http://www.ccs.ca/images/Guidelines/Guidelines_POS_Library/Ang_Gui_1976.pdf last accessed 1 December 2015.
- 25. Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 2299–306. [DOI] [PubMed] [Google Scholar]
- 26. Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manage 2015; 11: 133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson SP. The growing complexity of platelet aggregation. Blood 2007; 109: 5087–95. [DOI] [PubMed] [Google Scholar]
- 28. Voisin S, Bongard V, Tidjane MA, Lhermusier T, Carrié D, Sié P. Are P2Y12 reaction unit (PRU) and % inhibition index equivalent for the expression of P2Y12 inhibition by the VerifyNow® assay? Role of haematocrit and haemoglobin levels. Thromb Haemost 2011; 106: 227–9. [DOI] [PubMed] [Google Scholar]
- 29. Aradi D, Collet J‐P, Mair J, Plebani M, Merkely B, Jaffe AS, et al. Platelet function testing in acute cardiac care ‐ is there a role for prediction or prevention of stent thrombosis and bleeding? Thromb Haemost 2015; 113: 221–30. [DOI] [PubMed] [Google Scholar]
- 30. Storey RF, Angiolillo DJ, Patil SB, Desai B, Ecob R, Husted S, et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes. J Am Coll Cardiol 2010; 56: 1456–62. [DOI] [PubMed] [Google Scholar]
- 31. Gurbel PA, Bliden KP, Butler K, Antonino MJ, Wei C, Teng R, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation 2010; 121: 1188–99. [DOI] [PubMed] [Google Scholar]
- 32. Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, et al. DISPERSE‐2 Investigators. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non‐ST‐segment elevation acute coronary syndrome: primary results of the DISPERSE‐2 trial. J Am Coll Cardiol 2007; 50: 1844–51. [DOI] [PubMed] [Google Scholar]
- 33. Teng R. Ticagrelor: pharmacokinetic, pharmacodynamic, and pharmacogenetic profile – an update. Clin Pharmacokinet 2015; 54: 1125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Butler K, Teng R. Pharmacokinetics, pharmacodynamics, and safety of ticagrelor in volunteers with mild hepatic impairment. J Clin Pharmacol 2011; 51: 978–87. [DOI] [PubMed] [Google Scholar]


