Abstract
Aims
The aim of the study was to develop a list of hospital based paediatric prescribing indicators that can be used to assess the impact of electronic prescribing or clinical decision support tools on paediatric prescribing errors.
Methods
Two rounds of an electronic consensus method (eDelphi) were carried out with 21 expert panellists from the UK. Panellists were asked to score each prescribing indicator for its likelihood of occurrence and severity of outcome should the error occur. The scores were combined to produce a risk score and a median score for each indicator calculated. The degree of consensus between panellists was defined as the proportion that gave a risk score in the same category as the median. Indicators were included if a consensus of 80% or higher was achieved and were in the high risk categories.
Results
Each of the 21 panellists completed an exploratory round and two rounds of scoring. This identified 41 paediatric prescribing indicators with a high risk rating and greater than 80% consensus. The most common error type within the indicators was wrong dose (n = 19) and the most common drug classes were antimicrobials (n = 10) and cardiovascular (n = 7).
Conclusions
A set of 41 paediatric prescribing indicators describing potential harm for the hospital setting has been identified by an expert panel. The indicators provide a standardized method of evaluation of prescribing data on both paper and electronic systems. They can also be used to assess implementation of clinical decision support systems or other quality improvement initiatives.
Keywords: clinical decision support, consensus, medication errors, paediatrics, prescribing errors, quality indicators
What is Already Known about this Subject
Prescribing errors are common in the paediatric setting.
Prescribing indicators can be used to measure or monitor the accuracy of prescribing.
There are no validated paediatric prescribing indicators for the hospital setting.
What this Study Adds
A set of 41 prescribing indicators specific for the UK hospital paediatric setting were identified.
A standardized method for assessing the impact of electronic prescribing on high risk medicines was provided.
Introduction
The use of medication to treat disease, alleviate symptoms and prevent illness is the most common intervention used in healthcare. The vast majority of medication does not cause harm. However, all medicines carry some level of risk. Medication errors are common in hospital practice 1 and evidence suggests possibly more common in children 2. Determining the harm caused by these errors is vital to be able to understand how interventions might be targeted to reduce the risk of harm. Methods for determining harm vary considerably. Some studies use a severity scale for determining harm, scored by the researcher or by obtaining consensus between a number of healthcare professionals 3, 4.
The same methodologies for identifying prescribing errors and harm in adult patients have been used in the paediatric setting. Prescription review often by hospital pharmacists yields large numbers of potential prescribing errors often with low or no harm 4, 5. This makes it difficult to determine the impact of any change or improvement.
Trigger tools look for indicators of harm rather than specific errors. For example a high international normalized ratio (INR) indicates that a potential error with warfarin may have occurred and requires checking to confirm this. Triggers for the paediatric setting have been described in the literature. Stockwell et al. 6 recently published a paediatric harm measurement tool which contained 51 triggers, including 21 medication related triggers. Trigger tools such as this provide a standard method of identifying errors but they require extensive retrospective case note review in order to identify firstly the trigger and then any subsequent medication related harm.
Prescribing indicators are a valid, standardized way of measuring or monitoring an area of prescribing where changes in prescribing or putative improvement require evaluation either prospectively or retrospectively. Adult prescribing indicators have been developed in several settings in the UK 7, 8, 9, 10. Thomas et al. 11 published a set of adult prescribing indicators for the hospital setting. By using an eDelphi methodology, consensus on a set of 81 indicators was achieved. They describe prescribing errors that have the risk of causing significant harm.
The aim of this research was to create a set of paediatric prescribing indicators for the hospital setting that can be used to assess the impact of electronic prescribing.
Method
While evidence‐based medicine is the gold standard approach to care, there remain vast swathes of medicine where evidence is lacking or incomplete. This is often due to the rare nature of a condition and the subsequent difficulty in running a randomized controlled trial. The Delphi method has been used in numerous areas of health services research including guideline development 12, outcome measures for primary health care research 13, drug related mortality 14, high acuity paediatric conditions 15 and the design of a paediatric pharmaceutical care model 16. Importantly the method has been used extensively to develop prescribing indicators for general practice 8, 9, 17, 18, 19, 20 and hospital adult in‐patients 11. Based on the validated use thus far, the Delphi technique was selected to gain consensus opinion on paediatric prescribing indicators, from a range of both paediatric physicians and pharmacists.
Expert panel selection
A list of potential panellists was generated by the research team from networks via the Royal College of Paediatrics and Child Health (RCPCH) and the Neonatal and Paediatric Pharmacists Group (NPPG). Additional contacts were made through research links with a National Institute of Healthcare Research (NIHR) programme grant investigating the impact of electronic prescribing. An e‐mail invitation was sent to 39 potential panellists requesting their participation, along with a summary of the proposed research. Panellists were general paediatricians, paediatric pharmacists and paediatric clinical pharmacologists from across the UK. Panellist information was collected on the total number of years of paediatric experience and experience with electronic prescribing systems. Out of the 39 people invited, 24 agreed to participate. This achieved the target number of at least 20 panel members, comparable with the number used in a similar Delphi study 11.
Identifying potential indicators
Information was gathered from a variety of sources on paediatric prescribing errors by the lead researcher and assessed for its suitability according to the inclusion and exclusion criteria, by the research team. The sources used were:
Inclusion
The indicator describes a prescribing error relating to a specific drug.
The indicator is specific to the hospital paediatric setting.
Exclusion
The indicator describes a prescribing practice not routinely undertaken in paediatric hospital settings.
The indicator describes an error that would not be amenable to clinical decision support or electronic prescribing.
Extraction of data for the indicator from hospital records is not likely to be feasible.
The indicator describes a failure to monitor.
The indicator describes errors relating to the administration or dispensing of a drug.
The eDelphi process
Exploratory round
The 24 panellists were sent the initial list of indicators for the exploratory round. They were instructed to review each indicator for relevance and possible modification to ensure clarity. They also had the opportunity at this stage to suggest additional indicators that had not been identified by the research team. The additional indicators were collated and reviewed and, if appropriate, included in the final indicator list used for round 1 of the eDelphi process. Panellists were also made aware of the reasons for exclusion of any suggested indicators.
Round 1
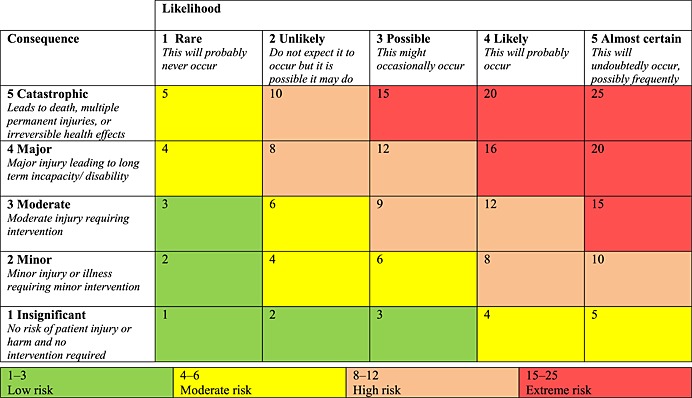
In round 1 panellists were asked to rate each indicator for its likelihood of occurrence and severity of harm should it occur. The scoring system used was based on the National Patient Safety Agency scale in common use in UK hospitals 22 (Table 1) and allowed identification of indicators with the greatest clinical risk. The panellist scores were converted into a risk score using the matrix. The median risk scores for each indicator were then calculated, allowing the indicators to be divided into groups based on their risk scores.
Table 1.
Scoring likelihood and severity of the errors occurring (from the UK National Patient Safety Risk Matrix [22])
Round 2
In round 2, each panellist was sent the indicators, the median likelihood and severity scores from the panel and the individual panellist's original scores from round 1. Panellists were then asked to review their scores in light of the median scores and were given the opportunity to either maintain their original judgement or modify their scores in line with the majority of the group. The median scores were then re‐calculated for each indicator and the level of consensus determined. Indicators with a median risk score greater than 8 (high or extreme) and at least 80% consensus were then considered to have achieved an adequate level of consensus and therefore included into the final list.
Results
Prior to the exploratory round, a total of 179 potential indicators were identified from the resources listed above. The research team reviewed each indicator against the inclusion and exclusion criteria resulting in a final list of 100 indicators, 77 indicators were identified from a single source, 23 from two or more sources.
The exploratory stage and rounds 1 and 2 were completed by 21 of the 24 panellists who had originally agreed to take part. Table 2 summarizes the panellists' levels of experience, profession and location type. The panel comprised of eight pharmacists with a total of 181 years of paediatric experience and 13 paediatricians with a total of 256 years experience. Panellists had a total of 91 years of experience with electronic prescribing.
Table 2.
Demographic details of expert panel members
| Position | Years of experience | Years of electronic prescribing experience | Type of hospital |
|---|---|---|---|
| Senior paediatric pharmacist | 35 | 2 | General teaching |
| Clinical pharmacy manager | 25 | 3 | Specialist childrens |
| Neonatal pharmacist | 32 | 0 | General |
| Consultant pharmacist | 26 | 21 | General teaching |
| Medication safety pharmacist | 20 | 11 | General teaching |
| Clinical pharmacist | 12 | 5 | Specialist childrens |
| Associate professor of Child Health | 18 | 1 | Specialist childrens |
| Consultant paediatrician | 19 | 1 | Specialist childrens |
| Consultant paediatrician | 24 | 1 | Specialist childrens |
| Consultant neonatologist | 19 | 0 | Specialist childrens |
| Specialist registrar | 10 | 0 | Specialist childrens |
| Consultant paediatrician | 30 | 0 | General teaching |
| Senior lecturer paediatric pharmacology | 20 | 0 | Specialist childrens |
| Consultant paediatrician | 20 | 14 | General teaching |
| Lead Informatics pharmacist | 22 | 15 | General teaching |
| Paediatric pharmacist | 9 | 3 | Specialist childrens |
| Consultant neonatologist | 20 | 0 | General |
| Consultant paediatrician | 19 | 10 | General |
| Consultant paediatrician | 17 | 4 | General |
| Consultant paediatrician | 19 | 0 | General |
| Consultant paediatrician | 14 | 0 | General |
During the exploratory round, 75 new indicators were proposed by the panel and reviewed by the research team, 34 of which were included in round 1. In addition, nine of the original indicators were removed and one reworded following the comments and suggestions of the panel. Typical reasons for exclusion were that the indicator described a cause of an error rather than an error itself, that the indicator was non‐specific and would relate to numerous drugs or that the issue would be captured by another indicator. This resulted in a final list of 125 indicators for round 1.
Following two rounds of scoring, 41 of the indicators were considered high risk by consensus. These are summarized in Table 3. None of the indicators was assessed as extreme risk by the panellists.
Table 3.
High risk indicators from the eDelphi process with >80% consensus
| Indicator | Possible outcome | Therapeutic class | Error type | Level of consensus |
|---|---|---|---|---|
| Domperidone prescribed at >1.2mg kg–1 day–1 maximum 20mg (prolongation of QT interval, sudden cardiac death) | Increased risk of arrhythmias and sudden cardiac death | Gastrointestinal | Dosing | 86% |
| Prescription of NSAIDS in suspected toxic shock syndrome (contraindicated but patients are pyrexial) | Risk of enhanced cytokine release contributing to shock, organ failure etc. | Musculoskeletal | Clinical contraindication | 81% |
| Baclofen dose not reduced in response to decreased renal function (eGFR <90 ml min–1 1.73 m–2) | Increased risk of toxic effects | Musculoskeletal | Dosing | 90% |
| Midazolam prescribed for procedural sedation at a dose inappropriate for the route of administration | Risk of supratherapeutic or subtherapeutic dose of midazolam | Anaesthesia | Dosing | 81% |
| Digoxin dose not reviewed in light of reduced renal function | Risk of supratherapeutic doses increasing risk of adverse effects | Cardiovascular | Dosing | 95% |
| Potassium‐sparing diuretic (excluding aldosterone antagonists) prescribed to a patient also receiving an ACE inhibitor or angiotensin II receptor antagonist (increased risk of severe hyperkalaemia) | Increased risk of severe hyperkalaemia | Cardiovascular | Drug–drug interaction | 90% |
| Amiodarone prescribed to a patient on digoxin without review of the digoxin dose | Risk of digoxin toxicity | Cardiovascular | Drug–drug interaction | 81% |
| β‐adrenoceptor blocking drug prescribed to a patient with asthma (increased risk of bronchospasm and acute deterioration) | β‐adrenoceptor blocking drugs are known to cause bronchoconstriction in asthmatics, and can cause acute deterioration | Cardiovascular | Clinical contraindication | 81% |
| Low molecular weight heparin prescribed to a patient with renal impairment without dose adjustment (increased risk of bleeding) | Increased risk of bleeding with the dose of low molecular weight heparin is not adjusted for renal function | Cardiovascular | Dosing | 86% |
| Antiplatelet prescribed to a patient with a concurrent bleeding disorder (increased risk of bleeding) | High risk of bleeding when antiplatelets prescribed to patients with a past medical history of bleeding disorders | Cardiovascular | Clinical contraindication | 81% |
| Prescribing of intravenous heparin infusion for treatment of thromboembolic event using the wrong dose or infusion rate based on local protocol (risk of toxicity or therapeutic failure) | Risk of supratherapeutic or subtherapeutic dose of heparin | Cardiovascular | Dosing | 86% |
| Prescribing of intravenous salbutamol infusion using the wrong dose or infusion rate (risk of toxicity or therapeutic failure) | Risk of supratherapeutic or subtherapeutic dose of salbutamol | Respiratory | Dosing | 81% |
| Two concomitant opiate analgesics that are not in line with the WHO pain ladder (injudicious use of two opiates risk of toxicity) | Increased risk of opioid toxicity | CNS | Therapeutic Duplication | 86% |
| Dose of paracetamol prescribed inappropriate for route of administration (potential overdose due to change in route or misreading of BNF) | Risk of paracetamol overdose | CNS | Dosing | 81% |
| Prescribing of incorrect or inequivalent morphine (opiate) dose via multiple routes. (risk of toxicity) | Oral and intramuscular doses are not equivalent, risk of therapeutic failure or toxicity | CNS | Dosing | 81% |
| Phenytoin dose not reviewed in light of low albumin (potential for toxicity) | Increased risk of phenytoin toxicity | CNS | Dosing | 86% |
| Penicillin containing compound prescribed to a penicillin allergic patient without reasoning (e.g. a non‐allergy such as diarrhoea or vomiting entered as an allergy where the indication for penicillin is compelling) (risk of hypersensitivity reactions) | Contraindicated in patients with history of penicillin allergy. Risk of hypersensitivity reaction | Anti‐microbial | Allergy | 81% |
| Nitrofurantoin prescribed to a patient with renal impairment, avoid if eGFR <60ml min–1 1.73 m– 2 (risk of peripheral neuropathy and inadequate concentration in urine) | Risk of peripheral neuropathy and reduced therapeutic effect | Anti‐microbial | Dosing | 80% |
| Ceftriaxone prescribed at a total daily dose of 50mg kg–1 instead of 80mg kg–1 for severe infection/sepsis in a patient >1 month of age (risk of under dosage) | Potential subtherapeutic dose for severe infection/sepsis | Anti‐microbial | Dosing | 90% |
| Meropenem prescribed at a dose of 20mg kg–1 instead of 40mg kg–1 for meningitis or respiratory exacerbation of CF (potential under treatment) | Potential subtherapeutic dose for severe infection/sepsis | Anti‐microbial | Dosing | 86% |
| Gentamicin prescribed to a patient with at least mild renal impairment without dose frequency adjustment (increased risk of toxicity) | Increased risk of toxicity | Anti‐microbial | Dosing | 81% |
| Gentamicin dose calculated based on actual body weight rather than ideal body weight in an obese patient (risk of excessive dosing and toxicity) | Risk of excessive dosing and toxicity | Anti‐microbial | Dosing | 100% |
| Macrolide antibacterial prescribed concomitantly with warfarin without appropriate dose adjustment or increased INR monitoring (increased risk of bleeding) | Macrolide antibacterials can reduce the metabolism of warfarin, causing an increase in the INR and an increased risk of bleeding | Anti‐microbial | Drug–drug interaction | 90% |
| Co‐prescribing of macrolides with interacting drug (QT prolongation) | Risk of prolongation of QT interval and ventricular arrhythmia | Anti‐microbial | Drug–drug interaction | 86% |
| Co‐prescribing of a macrolide with ciclosporin or tacrolimus (increases plasma levels of anti‐rejection agent) | Increased plasma concentration of ciclosporin | Anti‐microbial | Drug–drug interaction | 86% |
| Vancomycin prescribed intravenously over less than 60 min (rapid infusion of vancomycin can cause severe reactions) | Increased risk of infusion reactions | Anti‐microbial | Administration | 81% |
| Amphotericin B prescribed without additionally stating both brand name and the dose in mg kg–1 (risk of fatal overdose due to confusion between lipid based and non‐lipid | Specification of brand name to reduce risk of wrong formulation being administered and resulting toxicity | Anti‐microbial | Drug name | 90% |
| Failure to adjust dose or frequency of ganciclovir in the presence of altered renal function (risk of toxicity or treatment failure) | Risk of supratherapeutic or subtherapeutic levels of ganciclovir | Anti‐microbial | Dosing | 80% |
| Aciclovir prescribed at a dose of 250mg m–2 instead of 500mg m–2 for herpes simplex encephalitis in patients aged between 3 months and 12 years | Risk of treatment failure | Anti‐microbial | Dosing | 90% |
| Soluble insulin prescribed to a patient on a when required basis (increased risk of serious episodes of hypoglycaemia and nocturnal hypoglycaemia post dose) | Increased risk of serious episodes of hypoglycaemia and nocturnal hypoglycaemia especially if given more than one stat dose. Not managing the long‐term condition | Endocrine | Clinical contraindication | 85% |
| Failure to increase of hydrocortisone to ‘sick day doses’ from ‘maintenance’ doses in those adrenally suppressed | Reduces risk of shock | Endocrine | Dosing | 95% |
| Dose reduction of immunosuppressant not made despite low white cell count (risk of neutropenia) | Increased risk of neutropenia and subsequent infection, (list of common immunosuppressant will be included during data collection) | Immunosuppressant | Dosing | 90% |
| Failure to prescribe folinic acid rescue therapy following high dose methotrexate chemotherapy (risk of methotrexate toxicity) | Risk of methotrexate toxicity | Immunosuppressant | Drug omission | 80% |
| Methotrexate prescribed to a patient with a clinically significant drop in white cell count or platelet count (risk of bone marrow suppression) | Risk of bone marrow suppression | Immunosuppressant | Clinical contraindication | 90% |
| Oral methotrexate prescribed to a patient with an inappropriate frequency (increased risk of toxicity) | Oral methotrexate should be dosed once weekly, and the prescription clear as to which day of the week this should be | Immunosuppressant | Dosing | 100% |
| Methotrexate prescribed to a patient with abnormal liver function tests (risk of liver toxicity) | Risk of liver toxicity | Immunosuppressant | Clinical contraindication | 85% |
| Methotrexate prescribed concomitantly with trimethoprim (increased risk of haematological toxicity) | Trimethoprim suppresses activity of dihydrofolate reductase ‐ potential for additive effect to produce folate deficiency. Increased risk of haematological toxicity when methotrexate given with trimethoprim (including trimethoprim containing compound ‐ co‐trimoxazole) | Immunosuppressant | Drug–drug interaction | 85% |
| Allopurinol prescribed concomitantly with mercaptopurine (allopurinol enhances effect of mercaptopurine and increases risk of toxicity) | Increased risk of toxicity and enhanced effects of mercaptopurine when given concomitantly. The dose of mercaptopurine should be one quarter of usual dose | Immunosuppressant | Drug–drug interaction | 80% |
| Potassium chloride supplements continued for longer than is required (based on age appropriate local reference ranges approx 3.5–5.3 mmol l–1) (increased risk of hyperkalaemia) | Failure to act on potassium chloride monitoring and continuing treatment for longer than required risks hyperkalaemia | Nutrition | Dosing | 81% |
| Potassium chloride infusions exceeding 40 mmol l–1 prescribed to administered via the peripheral route (peripheral administration risks venous pooling, which can lead to sudden high concentrations of potassium chloride being delivered to the heart provoking an arrhythmia) | Intravenous administration of potassium chloride solutions exceeding 40mmol l–1 should be prescribed via the central route to avoid arrhythmias | Nutrition | Administration | 86% |
| A prescription for a drug for a patient with a known allergy to that drug (risk of anaphylaxis) | Risk of anaphylaxis | General | Allergy | 100% |
The 41 indicators include 34 different drugs or classes from the following therapeutic groups, gastrointestinal (n = 1), cardiovascular (n = 7), respiratory (n = 1), central nervous system (n = 3), antimicrobials (n = 10), endocrine (n = 2), immunosuppression (n = 6), fluids and electrolytes (n = 1), musculoskeletal (n = 2) and anaesthesia (n = 1).
The most frequent error type identified as high risk was dosing (n = 19) with drug–drug interactions (n = 7) and clinical contraindications (n = 6) the next two most frequent error types.
Discussion
The eDelphi process has identified 41 high risk prescribing indicators for the paediatric hospital setting. They can potentially be used to monitor the impact of electronic prescribing or clinical decision support tools. To the authors' knowledge, this is the first set of prescribing indicators for paediatric patients in the hospital setting.
The consensus process used to derive the indicators involved a panel consisting of 21 paediatricians and paediatric pharmacists all of whom completed two rounds of scoring, limiting any bias introduced by missing responses.
Nearly half (n = 19) of the final 41 indicators related to dosing errors. This is not surprising since dose errors account for the majority of the indicators identified for rounds 1 and 2. This is likely influenced by the fact that dosing errors are the most common error type reported in paediatrics 23, 24, 25. Drugs with known risks such as gentamicin, phenytoin and methotrexate were included in the dosage indicators. However, ‘lower risk’ drugs such as meropenem, ceftriaxone and domperidone are also present. This may reflect, in the case of the antimicrobials, the relatively serious clinical indications in which these drugs are used and the need to prescribe the correct dose to avoid treatment failure as well as heightened awareness as a result of antimicrobial stewardship or, in the case of domperidone, the relatively recent publicity relating to adverse reactions 26.
Previously published work has identified high alert medicines within paediatrics. Maaskant et al. 27 published a list containing 14 specific drugs and four medication classes of high alert medications. Comparing this with our prescribing indicators shows that 10 of the individual drugs and three of the drug classes are duplicated. The four high alert drugs not identified in our prescribing indicators are all infusions commonly used in intensive care areas, such as dopamine and norepinephrine. Reference to errors involving infusions was excluded from our research because the reported incidents all related to errors occurring as a result of incorrect administration or infusion preparation rather prescribing. The high alert drug class from Masskant et al.'s 27 report that is not included in our prescribing indicators relates to parenteral nutrition. Errors reported relating to parenteral nutrition concern administration or preparation errors rather than prescribing. This possibly reflects UK practice in terms of these medications where standard prescriptions and electronic systems for parenteral nutrition have been developed to prevent errors at the prescribing stage.
Stockwell et al. 6 published a list of paediatric triggers developed using an eDelphi technique and an international panel. From their list of 21 triggers relating to medicines, 11 also appear in our paediatric prescribing indicator list. The triggers describe adverse events that could result from any incorrect use of a medicine. For example the administration of Digibind® could be triggered by an error in the prescribing, dispensing, administration or monitoring of digoxin. This is an appropriate way of identifying an adverse event after it has occurred. Our indicators, however, are specific for the prescribing process and can be used to identify errors at the prescribing stage, which may be in advance of the medicine being administered. This can tell us whether quality improvement interventions such as ePrescribing can prevent the ‘potential’ for harm occurring.
Many of the paediatric indicators for the exploratory round were derived from the adult indicators previously published 11. The final list of 41 paediatric indicators contains 28 indicators modified from the research conducted in adult medicine. Many of the remaining indicators were related to specific paediatric settings or medicines not usually classed as high risk in adults as such as meropenem, as discussed above.
Reports of the incidence of prescribing errors in the paediatric setting vary between 7 and 13% 24, 28. This is partly because there is no standard definition of what and how to collect information about errors. Studies use different data collection methods and different definitions of medication error 29. This lack of standardization makes comparison between reports difficult to assess.
Prescribing indicators can be used to assess the impact of a safety improvement intervention by standardizing both pre‐ and post‐implementation data collection. The objective nature of these data would allow comparisons and conclusions to be drawn and provide more robust evidence across healthcare settings. The standardization means that, for the first time, comparisons can be made between hospitals and different initiatives.
The indicators can also be used to optimize the capability of electronic prescribing systems, such as with the provision of complex clinical decision support to highlight and avert such errors at the point of prescribing. This also has the potential to focus alerts on high risk areas, with the advantage of reducing alert fatigue 30.
While the paediatric indicators described here are focused on the secondary care setting, many could be applicable to general practice. There are currently no primary care related exclusive paediatric trigger tools published in the literature.
Limitations
The initial list of indicators was derived from an extensive literature search and, therefore, unpublished cases of medication errors would not have been included. However, we aimed to reduce this effect by including the exploratory round so panellists had the opportunity to propose indicators they see in practice.
The work was entirely UK based and as such may not have applicability in other global settings. Lastly, as new evidence emerges and new drugs begin to be used, other potential indicators may become relevant. The adult indicators previously cited are currently under review and if the paediatric indicators described here become extensively utilized a programme of periodic review will be necessary.
Conclusions
In conclusion, paediatric prescribing errors with the potential to cause harm have been identified by an expert panel. The indicators provide an objective tool that can be used to collect routine prescribing data in either electronic or paper‐based environments. Standardization of what is collected will allow a better understanding of what errors are occurring in paediatrics. Without this knowledge, it is difficult to target quality improvement projects and also inform under‐ and post‐graduate education of paediatric prescribing.
They could also be used to refine alerting systems used in electronic prescribing to target warnings and alleviate alert fatigue.
The use of these paediatric indicators in combination with previously described adult indicators for the hospital setting provides a comprehensive tool that can be used to evaluate changes across a wider age range.
Funding information
The work has been funded entirely by University Hospitals Southampton.
Competing interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
The authors wish to thank the members of the expert panel for their contributions to this study.
Fox, A. , Pontefract, S. , Brown, D. , Portlock, J. , and Coleman, J. (2016) Developing consensus on hospital prescribing indicators of potential harm for infants and children. Br J Clin Pharmacol, 82: 451–460. doi: 10.1111/bcp.12954.
References
- 1. Franklin BD, Reynolds M, Shebl NA, Burnett S, Jacklin A. Prescribing errors in hospital inpatients: a three‐centre study of their prevalence, types and causes. Postgrad Med J 2011; 87: 739–45. [DOI] [PubMed] [Google Scholar]
- 2. Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al Medication errors and adverse drug events in pediatric inpatients. JAMA 2001; 285: 2114–20. [DOI] [PubMed] [Google Scholar]
- 3. Dean B, Barber N. A validated, reliable method of scoring the severity of medication errors. Am J Health Syst Pharm 1999; 56: 57–62. [DOI] [PubMed] [Google Scholar]
- 4. Dornan T, Ashcroft D, Heathfield H, Lewis P, Miles J, Taylor D. An in depth investigation into causes of prescribing errors by foundation trainees in relation to their medical education EQUIP study. London: General Medical Council; 2009; Available from: http://www.gmc‐uk.org/FINAL_Report_prevalence_and_causes_of_prescribing_errors.pdf_28935150.pdf (last accessed 3 March 2014).
- 5. Ghaleb M. The incidence and nature of prescribing and administration errors in paediatric inpatients. Thesis. University of London 2006. [DOI] [PubMed]
- 6. Stockwell DC, Bisarya H, Classen DC, Kirkendall ES, Lachman PI, Matlow AG, et al Development of an electronic pediatric all‐cause harm measurement tool using a modified Delphi method. J Patient Saf 2014. (ePub) Aug 26. DOI: 10.1097/PTS.0000000000000139. Available from: http://journals.lww.com/journalpatientsafety/Abstract/publishahead/Development_of_an_Electronic_Pediatric_All_Cause.99719.aspx [DOI] [PubMed] [Google Scholar]
- 7. Health and Social Care Information Centre . Prescribing indicators and comparators. Available from: http://www.hscic.gov.uk/prescribing/measures (last accessed 3 March 2015).
- 8. Campbell SM, Cantrill JA, Roberts D. Prescribing indicators for UK general practice: Delphi consultation study. BMJ 2000. Aug 12; 321: 425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Avery AJ, Dex GM, Mulvaney C, Serumaga B, Spencer R, Lester HE et al Development of prescribing‐safety indicators for GPs using the RAND appropriateness method. Br J Gen Pract 2011. Aug; 61: e526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avery AJ, Rodgers S, Cantrill JA, Armstrong S, Cresswell K, Eden M, et al A pharmacist‐led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost‐effectiveness analysis. Lancet 2012; 379: 1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas SK, McDowell SE, Hodson J, Nwulu U, Howard RL, Avery AJ, et al Developing consensus on hospital prescribing indicators of potential harms amenable to decision support. Br J Clin Pharmacol 2013; 76: 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998; 2: i‐iv, 1–88. [PubMed] [Google Scholar]
- 13. Hutchinson A, Fowler P. Outcome measures for primary health care: what are the research priorities? Br J Gen Pract 1992; 42: 227–31. [PMC free article] [PubMed] [Google Scholar]
- 14. Morris CJ, Cantrill JA, Hepler CD, Noyce PR. Preventing drug‐related morbidity—determining valid indicators. International J Qual Health Care 2002; 14: 183–98. [DOI] [PubMed] [Google Scholar]
- 15. Stang AS, Straus SE, Crotts J, Johnson DW, Guttmann A. Quality indicators for high acuity pediatric conditions. Pediatrics 2013; 132: 752–62. [DOI] [PubMed] [Google Scholar]
- 16. Fernandez‐Llamazares CM, Hernandez‐Gago Y, Pozas M, Cabanas MJ, Feal B, Villaronga M, et al Two‐round Delphi technique for the consensual design of a paediatric pharmaceutical care model. Pharmacol Res 2013; 68: 31–7. [DOI] [PubMed] [Google Scholar]
- 17. Campbell SM, Braspenning J, Hutchinson A, Marshall MN. Research methods used in developing and applying quality indicators in primary care. BMJ 326: 816–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cantrill JA, Sibbald B, Buetow S. Indicators of the appropriateness of long‐term prescribing in general practice in the United Kingdom: consensus development, face and content validity, feasibility, and reliability. Qual Health Care 1998; 7: 130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Avery AJ, Savelyich BS, Sheikh A, Cantrill J, Morris CJ, Fernando B, et al Identifying and establishing consensus on the most important safety features of GP computer systems: e‐Delphi study. Inform Prim Care 2005; 13: 3–12. [DOI] [PubMed] [Google Scholar]
- 20. Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008. Feb; 46: 72–83. [DOI] [PubMed] [Google Scholar]
- 21. Rosario C. Medication Safety in Children and Young people. NHS England; 2013.
- 22. National Patient Safety Agency . A risk matrix for risk managers. 2008.
- 23. National Patient Safety Agency . Safety in doses ‐ Improving the use of medicines in the NHS. 2009.
- 24. Ghaleb MA, Barber N, Franklin BD, Wong ICK. The incidence and nature of prescribing and medication administration errors in paediatric inpatients. Arch Dis Child 2010; 95: 113–8. [DOI] [PubMed] [Google Scholar]
- 25. Wong ICK, Ghaleb MA, Franklin BD, Barber N. Incidence and nature of dosing errors in paediatric medications: a systematic review. Drug Saf 2004; 27: 661–70. [DOI] [PubMed] [Google Scholar]
- 26. Medicines and Healthcare Products Regulatory Authority . Domperidone: risks of cardiac side effects. Drug Safety Update 2014; 7: A1. [Google Scholar]
- 27. Maaskant JM, Eskes A, van Rijn‐Bikker P, Bosman D, van Aalderen W, Vermeulen H. High‐alert medications for pediatric patients: an international modified Delphi study. Expert Opin Drug Saf 2013; 12: 805–14. [DOI] [PubMed] [Google Scholar]
- 28. Lewis PJ, Dornan T, Taylor D, Tully MP, Wass V, Ashcroft DM. Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf 2009; 32: 379–89. [DOI] [PubMed] [Google Scholar]
- 29. Franklin BD, Birch S, Savage I, Wong I, Woloshynowych M, Jacklin A, et al Methodological variability in detecting prescribing errors and consequences for the evaluation of interventions. Pharmacoepidemiol Drug Saf 2009; 18: 992–9. [DOI] [PubMed] [Google Scholar]
- 30. Phansalkar S, Zachariah M, Seidling HM, Mendes C, Volk L, Bates DW. Evaluation of medication alerts in electronic health records for compliance with human factors principles. J Am Med Inform Assoc 2014. Oct; 21: e332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


