Abstract
Aims
The aim of the present study was to describe the real‐life usage patterns of paracetamol.
Methods
The Echantillon Généraliste de Bénéficiaires (EGB) database, the permanent 1/97 representative sample from the French national healthcare insurance system, was searched in 2011 to identify usage patterns, concomitant chronic diseases and use of cardiovascular medication in users prescribed single‐ingredient (SP) and combination (CP) paracetamol, representing 85% of all sales.
Results
Of 526 108 subjects aged ≥15 years in the EGB, 268 725 (51%) had paracetamol dispensed on ≥1 occasion; of these, 207 707 (77%) were dispensed only SP and 61 018 (23%) received CP with or without SP. SP users were younger (48.3 years vs. 50.5 years), and 57% of SP users vs. 58% of CP users were female. Chronic comorbidities were more common in CP than SP users. SP users had, on average, 3.4 dispensings per year vs. 5.0 for CP users, for 36 defined daily doses (DDD, 3 g) of SP vs. 53 DDD per year for CP; 49% SP users bought 14 DDD or fewer; 15% bought >60 DDD. Use of paracetamol increased with age from about 16 DDD per year in 15–30‐year‐olds to over 90 DDD per year in patients above the age of 75; 53% of patients ≤60 years bought fewer than 14 DDD per year, whereas 55% of those >60 bought more than 30 DDD per year. More than half the dispensings exceeded the legal per‐box limit of 8 g.
Conclusions
Over 50% of the French adult population were dispensed paracetamol at least once over the course of a year, generally for short‐term use. Considering recent misgivings on the real efficacy and safety of paracetamol, such widespread use might have important public health consequences.
Keywords: adults, drug utilization, paracetamol, risk factors
What is Already Known about this Subject
Although paracetamol is used mostly in mild‐to‐moderate pain conditions and in chronic arthritis, and its usage pattern might be derived from these indications, there are few or no studies of actual usage patterns and drug exposure or burden.
What this Study Adds
Over 50% of all adults in the database were dispensed paracetamol at least once in 2011.
The median amount of paracetamol dispensed over 1 year was seven defined daily doses (or 21 g), and this increased with age.
Only 15% of subjects received more than 60 days' worth of paracetamol in a year.
Introduction
Paracetamol (acetaminophen in the USA) is a widely used, relatively weak analgesic with a mostly unknown mechanism of action, although it has been suggested that it may act as a weak cyclooxygenase 2 inhibitor 1. As it has been marketed for over 70 years, it has a reputation of being safe, except in overdose 2, 3. However, there have been recent reports that paracetamol might not be as safe 4, 5 or as effective 6, 7, 8, 9, 10, 11 as commonly believed.
Paracetamol is available over the counter (OTC) without prescription in most countries, and therefore its use is not captured in population databases, be they electronic health records or claims reimbursement databases. In France, even though it is also available OTC, paracetamol will be reimbursed if prescribed. It will then be found in the national claims databases. In fact, paracetamol is the most widely prescribed drug in France, with 2.4 billion defined daily doses (DDD, 3 g), equivalent to >7.5 kilotons paracetamol reimbursed over 3 years 3, 5, 12. Comparing overall sales data to reimbursed quantities, it is estimated that about 85% of all paracetamol sales can be found in the national claims databases 12. As prescribed drugs are mostly free of charge within the national healthcare system, this may explain why France has the highest per capita sales of paracetamol among the seven countries (France, UK, Italy, Netherlands, Ireland, Portugal, Greece) in the Study of Acute Liver transplantation (SALT) (51.5 g per inhabitant per year, vs., for example, 34.9 g in the UK and 24.1 g in Ireland) 3.
In spite of its widespread use, little is known of the usage patterns of paracetamol 13. Taking advantage of its presence in the French claims databases, the aim of the present study was to describe the usage pattern of single‐ingredient paracetamol (SP) and paracetamol in combination with other agents (CP) in France, much as we had studied OTC and prescription nonsteroidal anti‐inflammatory drugs (NSAIDs) 14.
Methods
Data source
Data were extracted from the Echantillon Généraliste de Bénéficiaires (EGB) database, a permanent representative 1/97 sample of the nationwide Système National d'Informations Inter‐Régimes de l'Assurance Maladie (SNIIRAM) database. SNIIRAM is the population claims database of individuals who are covered by the French National Health Insurance Systems (94% of the French population in 2010) 15, 16. It contains anonymized demographic characteristics and records of healthcare reimbursements, as well as dispensing data for all prescribed and reimbursed medicines 14, 17, in addition to data on hospital admissions, deaths and chronic diseases, with full coverage of all expenses. Nonprescribed self‐medicated paracetamol or ibuprofen are not registered, but in a previous study we found that 84% of paracetamol sales and 70% of ibuprofen sales were reimbursed by the healthcare system and could be identified in this database 5, 12, 14. In France, there are no sales of medicines outside pharmacies.
Study population
The study cohort included all patients in the EGB aged ≥15 years with at least one dispensing of any oral paracetamol preparation between 1 January 2011 and 31 December 2011. Patients in the study cohort were followed for 365 days after the first dispensing in 2011.
Demographic characteristics included age at the first paracetamol dispensing, gender and registration for chronic diseases (Affections de Longue Durée, ALDs). ALDs are diagnoses that result in full coverage of all medical expenses related to the disease 17, 18, 19. Prevalent ALDs were those that were present at the time of inclusion. All‐type ALD was considered, as well as any of the five cardiovascular ALDs: stroke; chronic lower‐limb arterial disease with ischaemic events; severe heart failure, severe arrhythmias, severe heart valve disease or severe congenital heart defects; severe arterial hypertension; coronary heart disease. Use of other drugs, including NSAIDs, amoxicillin, aspirin, cardiovascular drugs antithrombotic agents and antidiabetic agents, during follow‐up was also identified by their anatomical, therapeutic and chemical (ATC) codes (http://www.whocc.no/atc_ddd_index/), which are included in the EGB database..
Exposure definition
SC and CP preparations were identified by their ATC codes. The ATC code for SP is N02BE01; CP preparations were identified by the following codes: N02BE51 (combinations of paracetamol excluding psycholeptic agents), N02BE71 (combinations of paracetamol and psycholeptic agents) and N02AA59 (combinations of paracetamol and codeine).
Paracetamol users were classified into exclusive SP users and CP users. Exclusive SP users were dispensed only SP during the study period, whereas CP users had at least one dispensing of CP, but could also receive SP.
Paracetamol exposure was described by the number of dispensings, number of DDD per dispensing and total number of DDD dispensed over the study period. The DDD for SP was 3 g, as described by the Woirld Health Organization Collaborating Centre for drug statistics methodology (http://www.whocc.no/atc_ddd_index/). If the DDD was not available, the recommended daily dose in the 2012 French national drug formulary (VIDAL® dictionary, Paris) was used.
Statistical analysis
The statistical analyses were carried out using SAS® (SAS Institute, Cary, NC, USA) 9.2, and were limited to descriptive analyses. There was no prior hypothesis to test and no formal statistical comparisons were made. Considering the number of subjects in the samples, any descriptive difference greater than 0.1% was considered statistically significant, and 95% confidence intervals (CIs) would be less than 1% of the point estimates 14.
Ethical approval
The study was conducted using a fully anonymized database that, by decree, requires no specific ethical or data protection approval. It was registered with the French research institute INSERM and the overseeing body for the use of EGB data. Data used in the present study can be made available for any validation or verification, although, by law, the data cannot be forwarded or leave the country; all validations will need to be done on site.
Results
In 2011, the EGB database included a total population of 526 108 patients aged ≥15 years. Among them, 268 725 patients (51%) had at least one dispensing of paracetamol in 2011. Of these, 207 707 (77%) were SP users and 61 018 (23%) were CP users. Table 1 shows the demographic characteristics of the two populations of paracetamol users. Gender distribution was similar between the two groups (57% of SP users were female vs. 58% for CP). Exclusive SP users were younger (mean age ± standard deviation: 48.3 ± 20.2 years) than CP users (50.5 ± 18.7 years); CP users had more ALD, including diabetes, coronary heart disease and hypertension (Table 1).
Table 1.
Demographic characteristics of paracetamol users
| Single‐ingredient paracetamol‐only users N = 207 707 | Combined paracetamol users N = 61 018 | |
|---|---|---|
| Age (years) | ||
| Mean (± SD) | 48.3 (20.2) | 50.5 (18.7) |
| Median [p25% – p75%] | 47.0 [32.0; 63.0] | 50.0 [36.0; 64.0] |
| 15–30 | 47 709 (23.0) | 9969 (16.3) |
| 31–45 | 52 194 (25.1) | 15 670 (25.7) |
| 46–60 | 47 129 (22.7) | 16 996 (27.9) |
| 61–75 | 35 355 (17.0) | 11 172 (18.3) |
| >75 | 25 320 (12.2) | 7211 (11.8) |
| Female, n (%) | 118 367 (57.0) | 35 625 (58.4) |
| Any ALD, n (%) | 44 756 (21.5) | 16 069 (26.3) |
| Stroke, n (%) | 1506 (0.7) | 451 (0.7) |
| Lower‐limb arterial disease with ischaemia, n (%) | 2067 (1.0) | 804 (1.3) |
| Severe heart failure, severe arrhythmias, severe heart valve diseases, severe congenital heart defects, n (%) | 3512 (1.7) | 1156 (1.9) |
| Diabetes type I, II, n (%) | 9355 (4.5) | 3298 (5.4) |
| Severe arterial hypertension, n (%) | 6137 (3.0) | 2127 (3.5) |
| Coronary artery diseases, n (%) | 4469 (2.2) | 1402 (2.3) |
ALD, Affections de Longue Durée, (long‐term chronic disease resulting in full healthcare coverage); SD, standard deviation.
SP users had an average of three dispensings over the year, compared with five for CP, with an average of 8.3 DDD per SP dispensing vs. 8.4 for CP. Over the course of the year, SP users bought a mean of 36 DDD, with a median of 16 DDD per year. CP bought a mean of 53 DDD, with a median of 25 DDD. Forty‐nine per cent of SP users bought 14 DDD or fewer over the year, and 25% of CP users bought more than 60 DDD over the year (Table 2).
Table 2.
Dispensing pattern of paracetamol users
| Single‐ingredient paracetamol only users N = 197 888 | Combined paracetamol users N = 59 739 | |
|---|---|---|
| Number of dispensings | ||
| Mean (SD) | 3.4 (3.1) | 5.0 (4.0) |
| Median [p25% – p75%] | 2.0 [1.0; 4.0] | 4.0 [2.0; 7.0] |
| 1 | 63 186 (30.4) | 7075 (11.6) |
| 2–3 | 79 050 (38.1) | 20 668 (33.9) |
| 4–6 | 37 760 (18.2) | 17 617 (28.9) |
| 7–12 | 22 506 (10.8) | 12 011 (19.7) |
| >12 | 5205 (2.5) | 3647 (6.0) |
| Average DDD per dispensing: | ||
| Mean (SD) | 8.3 (6.0) | 8.4 (5.8) |
| Median [p25% – p75%] | 7.0 [5.0;10.0] | 7.0 [5.0;10.0] |
| Total number of DDD dispensed in one year | ||
| Mean (SD) | 35.9 (58.8) | 53.1 (80.8) |
| Median [p25% – p75%] | 16.0 [8.0; 35.0] | 25.0 [12.0; 59.0] |
| 1–7 | 45 738 (22.0) | 8749 (14.3) |
| 8–14 | 55 372 (26.7) | 10 379 (17.0) |
| 15–21 | 32 010 (15.4) | 8516 (14.0) |
| 22–28 | 12 563 (6.0) | 5346 (8.8) |
| >28 | 62 024 (29.9) | 28 028 (45.9) |
| 1–30 | 150 060 (72.2) | 34 465 (56.5) |
| 31–60 | 26 972 (13.0) | 11 530 (18.9) |
| >60 | 30 675 (14.8) | 15 023 (24.6) |
| Dispensings of other drugs during follow‐up | ||
| NSAIDs, n (%) | 109 985 (53.0) | 43 463 (71.2) |
| Amoxicillin, n (%) | 73 874 (35.6) | 24 651 (40.4) |
| Aspirin, n (%) | 7104 (3.4) | 2211 (3.6) |
| Antithrombotic agents, n (%) | 34 920 (16.8) | 12 684 (20.8) |
| Other cardiovascular drugs, n (%) | 76 871 (37.0) | 25 903 (42.5) |
| Antidiabetic drugs, n (%) | 14 832 (7.1) | 5050 (8.3) |
DDD, defined daily doses; NSAID, nonsteroidal anti‐inflammatory drug; SD, standard deviation.
Use of paracetamol increased with age, from about 16 DDD in patients aged 15–30 years, to over 90 DDD in patients above the age of 75 years (Figure 1). The distribution of usage in DDD per year was clearly different between those who were ≤60 years and >60 years: 53% of patients aged ≤60 years bought fewer than 14 DDD and only 9% of them bought more than 60 DDD, whereas 37% of users aged ≥60 years bought more than 60 DDD of paracetamol (Figure 2). Women bought slightly more paracetamol than men, with 35% buying more than 30 DDD vs. 28% in men. The proportion of SP and CP users were not different among age groups (around 80% and 20%, respectively, in all age groups) (Figure 3).
Figure 1.
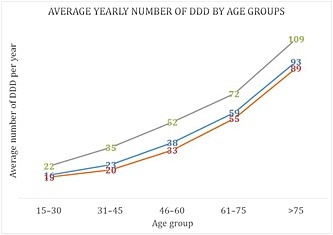
Average yearly number of defined daily doses (DDD) of single‐ingredient (SP) or combined (CP) paracetamol dispensed, by age groups. ( ) All users, (
) All users, ( ) SP users, (
) SP users, ( ) CP users
) CP users
Figure 2.
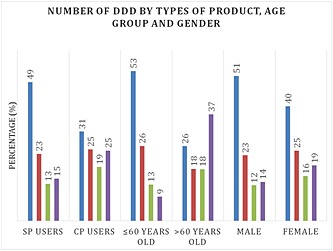
Number of DDD by types of product, age group and gender. ( ) 1‐14 DDD, (
) 1‐14 DDD, ( ) 15‐30 DDD, (
) 15‐30 DDD, ( ) 30‐60 DDD, (
) 30‐60 DDD, ( ) 30‐60 DDD
) 30‐60 DDD
Figure 3.
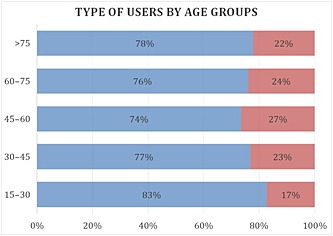
Type of paracetamol users by age group. ( ) SP users, (
) SP users, ( ) CP users
) CP users
During the 1‐year follow‐up, SP users also bought NSAIDs (53%), amoxicillin (36%), aspirin (3.4%), antithrombotic agents (including low‐dose aspirin), cardiovascular drugs (17%) and antidiabetics (7%). CP users bought more NSAIDs, amoxicillin, aspirin, antithrombotic agents, other cardiovascular drugs and antidiabetics than SP users (Table 2).
Discussion
Little is known about the usage patterns of paracetamol, either in the form of SP (available OTC) or as CP, mostly in combination with opiates such as codeine, available only by prescription.
In this representative sample of the French population, 51% of the population aged ≥15 years were prescribed and dispensed paracetamol at least once in a year. This was mostly SP, with 23% being prescribed CP at least once. The usage patterns in SP and CP users are likely to correspond to different indications, the former for acute pain episodes, with short‐term intermittent use 20, and the latter for more chronic indications such as osteoarthritis and rheumatoid arthritis, in the same way as for NSAIDs 21, 22, 23. The average SP user was around 48years of age, which is similar to the findings in other studies of common pain 20. Users of CP received an average of 53 DDD over the year, which is similar to the findings for prescription‐only NSAIDs 14. Forty‐four per cent of CP users received more than 30 DDD (compared with 40% for prescription‐only NSAIDs). SP users bought an average of 36 DDD over a year but 49% received fewer than 14 DDD per year, compared with 78% for OTC‐strength NSAIDs 14.
Forty percent were also dispensed antibiotics, predominantly amoxicillin, which suggests the use of paracetamol for acute infectious episodes 24. This certainly could contribute to the high rate of prescribed paracetamol; patients seeing their physician for painful febrile episodes would be prescribed paracetamol along with antibiotics and other medicines. The added risk of this combination on hepatotoxicity is unknown.
The present study did not include actual OTC (non‐prescription) usage of paracetamol bought directly from pharmacies. However, as stated above, 84% of paracetamol is prescribed in France 12. The remaining 16% that is bought directly from pharmacies might be bought by patients for whom paracetamol was not prescribed, or by those who also obtain it by prescription. Paracetamol is likely to be bought OTC for acute and unexpected painful episodes in young patients who do not routinely see their physician, or in combination with antihistamines or vasoconstrictors for flu or the common cold. Chronic paracetamol users or patients utilizing medical resources (e.g. related to concomitant diseases or to the indication itself, if, for example, an antibiotic is prescribed) would generally obtain a prescription for paracetamol, which results in not having to pay for the drug; even though it is not expensive, repeated usage would result in unnecessary expense. As with OTC‐dose NSAIDs 14, the nonreimbursed fraction of SP probably has little effect on the utilization pattern described here. It would possibly increase the number of short‐term users, or minimally increase the overall annual burden of paracetamol use in the users we identified.
To reduce the risk of overdose‐related hepatotoxicity, the total amount of paracetamol per box is limited in France to 8 g, or 2.7 DDD. In 2011, the average amount dispensed per episode was 8.3 DDD (25 g), with a median of 7.0 DDD (21 g), showing that the limitation to 8 g per box is essentially not reflected in actual dispensed amount. How that affects the risk of accidental or voluntary overdose is not certain. France has one of the highest per‐capita use of paracetamol (51.5 g per inhabitant per year, compared with 24.1 g per inhabitant per year in Ireland), with a rate of liver transplantation for acute paracetamol poisoning one‐tenth of that in Ireland (0.16 per million inhabitants per year compared with 1.16 per million) 3, even though the quantities dispensed on each occasion in France are easily within the toxic range.
In the aspirin and ibuprofen new tolerability (PAIN) study 20, the average use of analgesics for common pain episodes was 3.3 DDD (10 g paracetamol) over 5 days. The limited quantity allowed per box (8 g or 2.7 DDD) would not be enough to cover one painful episode, forcing patients to buy more than one box, as shown by the average dispensing observed here (8.3 DDD or 25 g), which is more than needed for one episode, and could in fact be enough for two separate painful episodes. If only 3.3 DDD are used from the average dispensing, this means that at least one box remains unused. This might justify increasing the unit size of boxes to 10 g or 12 g paracetamol, to treat one average painful episode and avoid the potential accumulation of unused drug. This would probably have little effect on the risk of overdose hepatotoxicity 3, but could reduce the probability of self‐medication and inadvertent overdosing.
Paracetamol usage per patient increased with age. This is consistent with the use of paracetamol for chronic age‐related diseases such as osteoarthritis, and the reluctance to use NSAIDs in older patients because of the risk of renal or cardiovascular adverse events with prescription‐dose NSAIDs. However, paracetamol seems to have little real effectiveness in chronic pain conditions such as osteoarthritis or low‐back pain 6, 7, 8, 9, 25. In elderly patients, the reduced metabolism and detoxification processes might also be of concern. There is a real need for further studies to re‐evaluate the risks and merits of paracetamol and other routine painkillers.
Conclusion
Over 50% of the French population buy prescribed paracetamol at least once in a year, mostly in quantities consistent with short‐term use. The increased use with increasing patient age is consistent with concomitant chronic disease and a reluctance to use NSAIDs in this population, in spite of evidence of poor efficacy of paracetamol. Considering the increasing concerns about the potential risks of paracetamol, even in non‐overdose quantities 5, the recommendations on the use of paracetamol should be revisited.
Competing Interests
The authors declare no conflict of interest, except NM, who, over the last 25 years, has received consulting fees from manufacturers of ibuprofen (Boots Healthcare, Reckitt‐Benciser, Pfizer), diclofenac (Novartis), ketoprofen (Sanofi) and paracetamol (Sanofi, Pfizer), although not necessarily related to these drugs. Bordeaux PharmacoEpi receives funding from various pharmaceutical companies and governmental bodies to conduct research on marketed drugs at the request of regulatory authorities, or as a consequence of research grant applications. None of these concern the subject at hand.
This study was financed internally by Bordeaux PharmacoEpi. MD received a doctoral grant from the French Embassy in Hanoi, which we gratefully acknowledge. All other authors worked on their own time or were seconded to the study by the department as part of their training/research activities.
Contributors
MD, SEG, FS, CD, PB and NM designed and discussed the study background and logistics, AA and RS provided technical and statistical support for data extractions and analysis. MD and AA conducted the data analysis and produced the results. All authors reviewed and discussed the results and contributed to their interpretation, and reviewed the interim and approved the final version of the paper.
Duong, M. , Gulmez, S. E. , Salvo, F. , Abouelfath, A. , Lassalle, R. , Droz, C. , Blin, P. , and Moore, N. (2016) Usage patterns of paracetamol in France. Br J Clin Pharmacol, 82: 498–503. doi: 10.1111/bcp.12957.
References
- 1. Green K, Drvota V, Vesterqvist O. Pronounced reduction of in vivo prostacyclin synthesis in humans by acetaminophen (paracetamol). Prostaglandins 1989; 37: 311–5. [DOI] [PubMed] [Google Scholar]
- 2. Hamlyn AN, Douglas AP, James O. The spectrum of paracetamol (acetaminophen) overdose: clinical and epidemiological studies. Postgrad Med J 1978; 54: 400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gulmez SE, Larrey D, Pageaux GP, Bernuau J, Bissoli F, Horsmans Y, et al Liver transplant associated with paracetamol overdose: results from the seven‐country SALT study. Br J Clin Pharmacol 2015; 80: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts E, Delgado Nunes V, Buckner S, Latchem S, Constanti M, Miller P, et al Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis 2015; 75: 552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, Jove J, et al Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case‐population SALT study. Drug Saf 2013; 36: 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore A. Up to 4000 mg of paracetamol a day is ineffective for acute low back pain. Evid Based Med 2015; 20: 100. [DOI] [PubMed] [Google Scholar]
- 7. da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, et al Effectiveness of non‐steroidal anti‐inflammatory drugs for the treatment of osteoarthritis pain: a network meta‐analysis. Lancet 2016. doi: 10.1016/S0140‐6736(16)30002‐2. [DOI] [PubMed] [Google Scholar]
- 8. Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CW, Day RO, et al Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta‐analysis of randomised placebo controlled trials. BMJ 2015; 350: h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moore RA, Derry S, Wiffen PJ, Straube S, Aldington DJ. Overview review: comparative efficacy of oral ibuprofen and paracetamol (acetaminophen) across acute and chronic pain conditions. Eur J Pain 2015; 19: 1213–23. [DOI] [PubMed] [Google Scholar]
- 10. Bailey E, Worthington HV, van Wijk A, Yates JM, Coulthard P, Afzal Z. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth. Cochrane Database Syst Rev 2013; 12: CD004624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Derry S, Moore RA. Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev 2013; 4: CD008040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore N, Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, et al Choice of the denominator in case population studies: event rates for registration for liver transplantation after exposure to NSAIDs in the SALT study in France. Pharmacoepidemiol Drug Saf 2013; 22: 160–7. [DOI] [PubMed] [Google Scholar]
- 13. Curhan GC, Bullock AJ, Hankinson SE, Willett WC, Speizer FE, Stampfer MJ. Frequency of use of acetaminophen, nonsteroidal anti‐inflammatory drugs, and aspirin in US women. Pharmacoepidemiol Drug Saf 2002; 11: 687–93. [DOI] [PubMed] [Google Scholar]
- 14. Duong M, Salvo F, Pariente A, Abouelfath A, Lassalle R, Droz C, et al Usage patterns of 'over‐the‐counter' vs. prescription‐strength nonsteroidal anti‐inflammatory drugs in France. Br J Clin Pharmacol 2014; 77: 887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merliere Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique 2010; 58: 286–90. [DOI] [PubMed] [Google Scholar]
- 16. Moulis G, Lapeyre‐Mestre M, Palmaro A, Pugnet G, Montastruc JL, Sailler L. French health insurance databases: what interest for medical research? Rev Med Interne 2015; 36: 411–7. [DOI] [PubMed] [Google Scholar]
- 17. Bezin J, Pariente A, Lassalle R, Dureau‐Pournin C, Abouelfath A, Robinson P, et al Use of the recommended drug combination for secondary prevention after a first occurrence of acute coronary syndrome in France. Eur J Clin Pharmacol 2014; 70: 429–36. [DOI] [PubMed] [Google Scholar]
- 18. Blin P, Dureau‐Pournin C, Foubert‐Samier A, Grolleau A, Corbillon E, Jove J, et al Parkinson's disease incidence and prevalence assessment in France using the national healthcare insurance database. Eur J Neurol 2015; 22: 464–71. [DOI] [PubMed] [Google Scholar]
- 19. Blin P, Dureau‐Pournin C, Lassalle R, Abouelfath A, Droz‐Perroteau C, Moore N. A population database study of outcomes associated with vitamin K antagonists in atrial fibrillation. Br J Clin Pharmacol 2016; 81: 569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore N, Van Ganse E, Le Parc J, Wall R, Schneid H, Farhan M, et al The PAIN study: paracetamol, aspirin and ibuprofen new tolerability study. A large scale, randomized clinical trial comparing the tolerability of aspirin, ibuprofen and paracetamol for short‐term analgesia. Clin Drug Investig 1999; 18: 89–98. [Google Scholar]
- 21. Moore N. Place of OTC analgesics and NSAIDs in osteoarthritis. Inflammopharmacology 2003; 11: 355–62. [DOI] [PubMed] [Google Scholar]
- 22. Depont F, Fourrier A, Merliere Y, Droz C, Amouretti M, Begaud B, et al Channelling of COX‐2 inhibitors to patients at higher gastrointestinal risk but not at lower cardiovascular risk: the COX2 inhibitors and tNSAIDs description of users (CADEUS) study. Pharmacoepidemiol Drug Saf 2007; 16: 891–900. [DOI] [PubMed] [Google Scholar]
- 23. Moore N, Verschuren X, Montout C, Callens J, Kong SX, Begaud B. Excess costs related to non‐steroidal anti‐inflammatory drug utilization in general practice. Therapie 2000; 55: 133–6. [PubMed] [Google Scholar]
- 24. Blin P, Blazejewski S, Lignot S, Lassalle R, Bernard MA, Jayles D, et al Effectiveness of antibiotics for acute sinusitis in real‐life medical practice. Br J Clin Pharmacol 2010; 70: 418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davies RA, Maher CG, Hancock MJ. A systematic review of paracetamol for non‐specific low back pain. Eur Spine J 2008; 17: 1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


