Abstract
Objective
To determine the feasibility of cervical conization and sentinel lymph node (SLN) mapping as a fertility-sparing strategy to treat stage I cervical cancer and estimate the tumor margin status needed to achieve no residual carcinoma in the cervix.
Methods
We identified all patients who desired fertility-preservation and underwent SLN mapping with cervical conization for stage I cervical cancer from 9/2005–8/2012. Relevant demographic, clinical, and pathological information was collected.
Results
Ten patients were identified. Median age was 28 years (range,18–36). None of the patients had a grossly visible tumor. The initial diagnosis of invasive carcinoma was made either on a loop electrosurgical excision procedure (LEEP) or cone biopsy. All patients underwent preoperative radiologic evaluation (MRI and PET-CT). None of the patients had evidence of gross tumor or suspicion of lymph node metastasis on imaging. Stage distribution included: IA1 with lymphovascular invasion, 7(70%); and microscopic IB1, 3(30%). Histology included: squamous cell carcinoma, 8(80%); adenocarcinoma, 1(10%); and clear cell carcinoma, 1(10%). Nine patients underwent repeat cervical conization with SLN mapping, and 1 patient underwent post-conization cervical biopsies and SLN mapping. None of the patients had residual tumor identified on the final specimen. The median distance from the invasive carcinoma to the endocervical margin was 2.25mm, and the distance from the invasive carcinoma to the ectocervical margin was 1.9mm. All collected lymph nodes were negative for metastasis. After a median follow-up of 17 months (range,1–83), none of the patients were diagnosed with recurrent disease and 3 patients (30%) achieved pregnancy.
Conclusion
Cervical conization and SLN mapping appears to be an acceptable treatment strategy for selected patients with small-volume stage I cervical cancer. Tumor clearance of ≥2mm appears to correlate well with no residual on repeat conization. A larger sample size and longer follow-up is needed to establish the long-term outcomes of this procedure.
Keywords: stage I cervical cancer, conization, sentinel lymph node mapping
Introduction
The fertility-sparing standard of care for patients with International Federation of Gynecology and Obstetrics (FIGO) stage IA1 cervical cancer with lymphovascular invasion (LVI), IA2 disease, and small-volume IB1 disease is radical trachelectomy with pelvic lymph node sampling. The choice of procedure depends on several factors, including tumor characteristics, reproductive potential, patient preference, and experience of the surgeon. The radical trachelectomy has demonstrated excellent oncologic outcomes, with an overall risk of recurrence of less then 5% [1]. The postoperative complications of a radical trachelectomy are manageable, although they do occur in up to 21% of patients [2]. The reproductive outcomes of the radical trachelectomy are promising. Approximately two thirds of patients are able to conceive, and the rate of preterm deliveries is approximately 25% [3, 4].
The surgical benefit of a radical trachelectomy lies in the resection mimicking a radical hysterectomy but sparing the uterine fundus [5]. Several studies have shown that 41–68% of patients do not have residual cancer on final pathologic evaluation [1, 2]. The incidence of parametrial involvement is also low in patients with negative pelvic lymph nodes [6]. Those studies established a future direction for fertility-sparing surgery in cervical cancer patients–conization and pelvic lymph node sampling. Theoretically, these procedures should offer the same oncologic outcomes as a radical trachelectomy but with less morbidity. The largest report on the topic includes a cohort of 35 patients who underwent large loop excision of the transformation zone and pelvic lymph node sampling [7]. There were no preterm deliveries or second trimester losses, and none of the patients recurred after a median follow-up of 56 months (range, 16–132) [7].
We began including the radical trachelectomy in our fertility-sparing surgical armamentarium in 2001. As our experience broadened, we began offering conization and sentinel lymph node (SLN) mapping as a fertility-sparing procedure to a select group of patients with favorable pathologic characteristics in 2005. The objective of this study is to describe our initial experience with patients selected for an ultraconservative fertility-sparing procedure (cervical cone biopsy with SLN mapping) and to report postoperative complications, and initial reproductive and oncologic outcomes.
Material and Methods
After obtaining Institutional Review Board approval, we indentified all patients with stage IA1 cervical cancer with LVI, IA2 disease, and microscopic IB1 disease who underwent cervical cone biopsy and SLN mapping followed by selective pelvic lymphadenectomy of any suspicious lymph nodes. The mapping was performed via cervical injection of blue dye, as previously described [8]. Preoperatively, relevant demographic and clinical information was abstracted from the patients’ medical records. Radiologic studies (MRI and/or PET-CT) were ordered on all patients. Postoperative complications and obstetrical outcomes were analyzed up to the last day of follow-up.
Pathologic data were collected from the final pathology report. Pathologic ultrastaging was performed on SLNs. For the purpose of this study, we also assessed the cervical cone biopsy specimens for resection margin status, including the endocervical and ectocervical (stromal) margins, distance from tumor to negative margin, and presence of residual tumor. For the cases that underwent a second cone, we also microscopically measured the distance from the prior biopsy site to the final margin. The pathologic study was done by specialty-trained gynecologic pathologists. A descriptive statistical analysis was performed.
Results
Between 9/2005 and 8/2012, we identified 10 patients with invasive cervical carcinoma who underwent cervical cone biopsy and SLN mapping. The median age of all patients was 28 years (range, 18–36), and the median body mass index (BMI) was 24 (range, 20–31). The majority of patients (9 [90%]) were nulliparous.
None of the patients had a grossly visible tumor, and all the patients were referred to our institution after the initial diagnosis of invasive cervical carcinoma was made either on a loop electrosurgical excision procedure (LEEP) or a cone biopsy. All pathology specimens were reviewed, and disease distribution was as follows: stage IA1 with LVI, 7 patients (70%), and microscopic stage IB1, 3 patients (30%). Histology included 8 (80%) squamous cell carcinomas, 1 (10%) adenocarcinoma, and 1 (10%) clear cell carcinoma. Of the patients with stage IB1 disease, none had LVI. The tumors of the 3 stage IBI patients had the following dimensions: Patient #1: stromal invasion - 2 mm and horizontal spread - 8 mm; Patient #2: stromal invasion - 4 mm and horizontal spread - 11 mm; and Patient #3: stromal invasion - 6 mm and horizontal spread - 8 mm.
All patients had a comprehensive radiologic workup preoperatively, which included an MRI and PET-CT. There was no evidence of residual tumor on MRI. Preoperative PET-CT was negative for lymph node uptake in all patients, and findings were suggestive of postoperative changes in the cervix.
All patients had pathologic reevaluation of the cervix. Repeat cervical conization was performed on 9 patients. In 1 patient, cervical conization was omitted due to a previous large cone; we performed cervical biopsies on that patient. All patients underwent SLN mapping followed by selective pelvic lymphadenectomy of any suspicious lymph nodes and side-specific lymphadenectomy if one pelvic side wall did not map. Cervical cerclage was not placed in any patient. The median duration of the procedure was 135 minutes (range, 95–168), and the median estimated blood loss was 20 ml (range, 10–50). Bilateral SLN mapping was achieved in all cases. Figure 1 illustrates a lymphatic channel draining into the external iliac lymph node, and Figure 2 shows an SLN. Paraaortic lymph node sampling was not performed in any patient. In 1 patient with stage IA1 invasive squamous cell carcinoma, a hysterectomy was performed per patient choice 3 months after the cervical cone. The patient requested the completion hysterectomy due to fear of recurrence, although she was counseled that a conservative approach was reasonable. The hysterectomy specimen for that case was negative for any residual carcinoma.
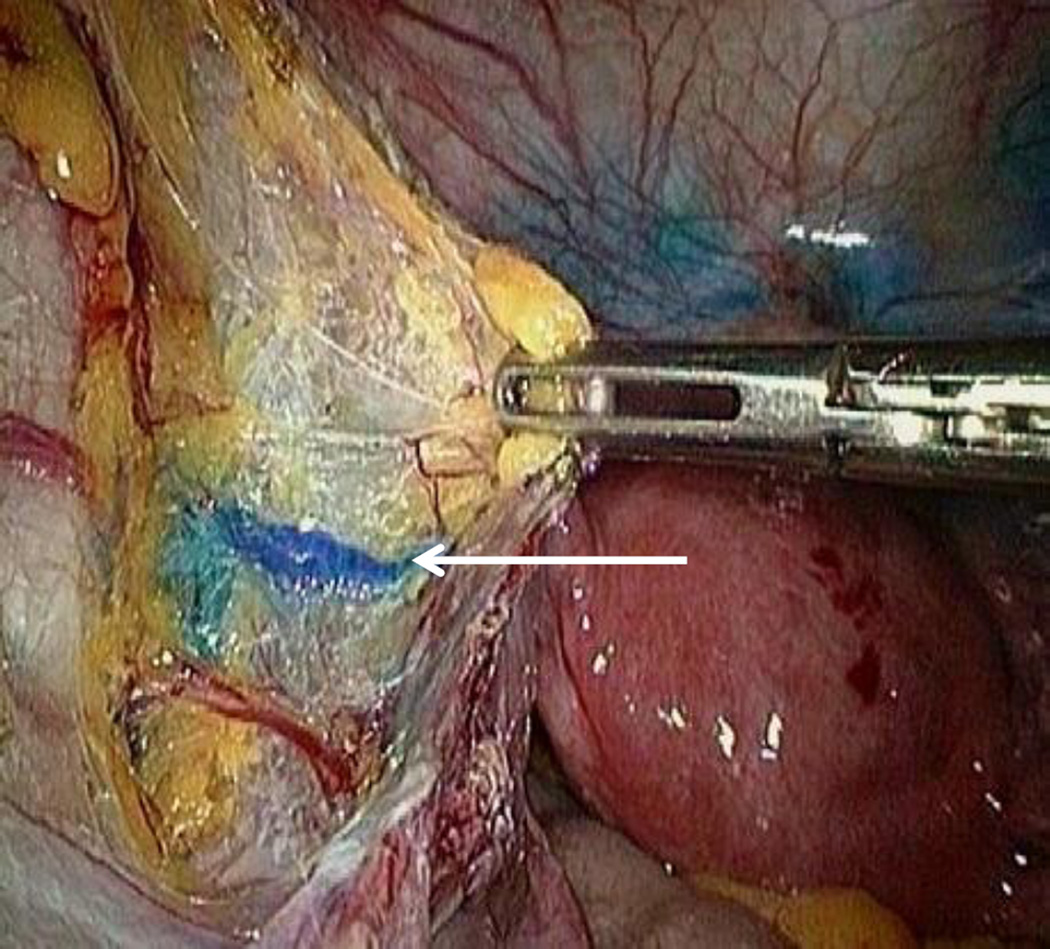
Figure 1.
A 33-year-old female with stage IA1 squamous cell carcinoma and positive lymphovascular invasion. The arrow indicates a lymphatic channel draining into the external iliac sentinel lymph node.
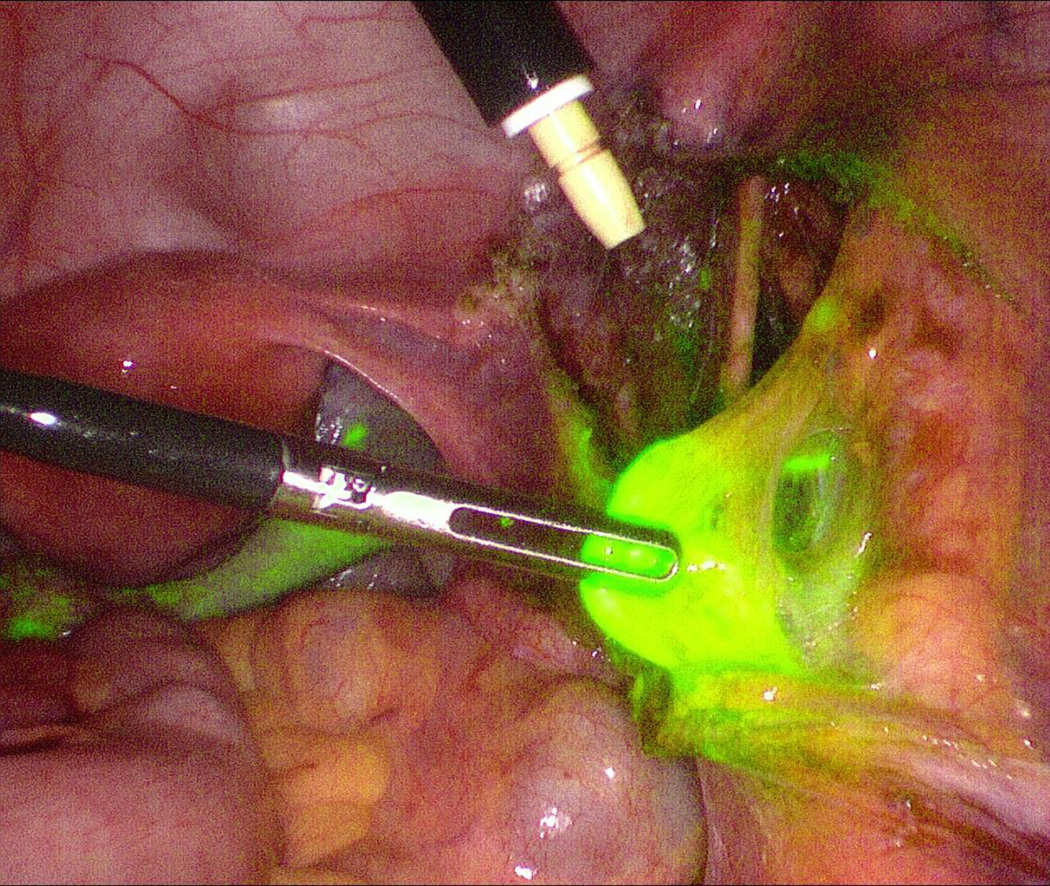
Figure 2.
A right external iliac sentinel lymph node in a patient with stage IB1 cervical adenocarcinoma. The sentinel lymph node mapping procedure was performed using indocyanine green via a cervical injection.
The pathologic characteristics of the cone specimens are presented in Table 1. Pathology slides were available for review in 9 of 10 patients. Margins in first cone or LEEP specimens were positive for invasive carcinoma in 3 patients (33%). For the remaining 6 patients, we performed additional pathologic evaluation of the initial cone or LEEP to measure the distance from the invasive carcinoma to the endocervical margin and to the ectocervical (stromal) margin. The median distance from the invasive carcinoma to the endocervical margin was 2.25 mm (range, 0.5–3.5), and the distance from the invasive carcinoma to the ectocervical margin was 1.9 mm (range, 1–4). Nine patients underwent a repeat cervical cone biopsy, and the specimens from those cases were evaluated in a similar fashion. Of note, none of the patients had residual invasive cervical carcinoma in the second cone specimen. The median distance from the prior biopsy site to the new endocervical margin in the repeat cone was 5 mm (range, 1.2–13), and the median distance from the prior biopsy site to the new ectocervical margin was 4 mm (range, 0.5–9). The median number of SLNs examined was 4 (range, 2–8). The median number of non-SLNs examined was 2 (range, 0–9). All SLNs and non-SLNs were negative for metastasis. Ultrastaging was also performed on the SLNs, and they remained negative.
Table 1.
The pathologic characteristics of specimens
| Patient | Margin status after first cone or LEEP |
Distance from invasive carcinoma to endocervical margin in the first cone or LEEP (mm) |
Distance from invasive carcinoma to ectocervical margin in the first cone or LEEP (mm) |
Residual invasive carcinoma in the second cone specimen |
Distance from prior biopsy site to endocervical margin in the second cone |
Distance from prior biopsy site to ectocervical margin in the second cone |
|---|---|---|---|---|---|---|
| 1 | Positive | - | - | No | 13 | 4 |
| 2 | Negative | 3 | 1.8 | No | 3 | 1 |
| 3 | Negative | 1.5 | 1.5 | No | 12 | 9 |
| 4 | Positive | - | - | No | 1.2 | 0.5 |
| 5 | Negative | 3 | 2.2 | No | 4 | 3 |
| 6 | Negative | 1 | 1 | No | 9 | 5 |
| 7 | Positive | - | - | No | 5 | 5 |
| 8 | Negative | 3.5 | 2 | No | 7.5 | 2.2 |
| 9 | Negative | 0.5 | 4 | No | 7 | 7.5 |
| 10 | * | * | * | ** | ** | ** |
LEEP, loop electrosurgical excision procedure
the specimen was not available for the pathologic review
cervical conization was not done. We performed random cervical biopsies for this patient.
All patients were discharged on the same day. Major complications were not documented in our patients. One patient had an episode of mild vaginal bleeding that resolved spontaneously and did not require intervention. None of the patients received adjuvant postoperative therapy. With a median follow-up of 17 months (range, 1–83), no recurrences were observed and 3 patients (30%) achieved spontaneous pregnancy.
Discussion
Our study demonstrates that cervical cone biopsy and laparoscopic SLN mapping followed by selective pelvic lymphadenectomy is a feasible surgical approach in patients with small-volume invasive cervical carcinoma. This conclusion is based on the fact that none of the patients required additional therapy postoperatively and none of the patients required a conversion to hysterectomy due to the presence of high-risk features. We previously reported that approximately one third of patients selected for radical trachelectomy will require additional treatment–post-trachelectomy chemoradiation or completion hysterectomy, mainly due to positive pelvic lymph nodes [2]. In our series, none of the patients had positive pelvic lymph nodes, underlying the importance of patient selection to achieve favorable outcomes.
We reviewed the pathology of all initial cone or LEEP specimens performed at other institutions to exclude high-risk histologies and determine margin status. Moreover, we broadly utilized preoperative imaging of different modalities (MRI, PET-CT) to delineate the extent of disease and evaluate for the presence of metastatic disease. In our series of 10 patients, there was no evidence of residual disease in the cervix on MRI; and PET-CT was negative for lymphatic spread. None of the patients had a residual invasive carcinoma in the final cone specimen, and all collected lymph nodes were negative for metastasis. Therefore, we achieved 100% concordance between pelvic MRI assessing residual disease in the cervix, PET-CT evaluating for lymphatic spread, and final pathologic evaluation.
The presence of LVI in the patients with stage IA1 disease should not be considered a contraindication for cervical conization and pelvic lymph node sampling. In our study, 7 patients with stage IA1 (70%) had LVI. However, all collected lymph nodes were negative for metastasis. In our opinion, the presence of LVI is a risk factor for nodal spread that requires pelvic lymph node sampling since lymph nodes may potentially harbor cancer cells and positive pelvic lymph nodes are a contraindication for fertility-sparing procedures. Several other authors have reported the importance of pelvic lymph node evaluation prior to fertility-sparing procedures in recent years, and our findings are in concordance with them [9, 10].
In our study, we performed additional histologic evaluation to determine the distance from the tumor to the resection margins. As shown in Table 1the margins vary significantly (1–13 mm). At this time, we are not able to make a definitive recommendation in regards to minimal margin status required for adequate local and distant control due to the small number of patients, but tumor clearance of ≥2 mm on conization appears to correlate well with no residual tumor on repeat conization. To the best of our knowledge, this is the first report describing the detailed margin status in this population. Follow-up prospective observational study is required to determine the optimal resection margin distance.
With the advancement of cervical cancer screening, we are now able to detect the disease at an earlier stage, and with delayed childbearing, fertility-sparing procedures are in high demand for the treatment of small-volume invasive cervical cancer. Sonoda et al reported that up to 48% of patients with early-stage cervical cancer are potentially eligible for fertility-sparing procedures [11]. Therefore, cervical cone biopsy and laparoscopic pelvic lymph node evaluation has the potential to become a widely accepted surgical approach in the treatment of a select group of patients with early-stage, small-volume invasive cervical cancer. This approach is supported by the elimination of complex vaginal or abdominal surgery (radical trachelectomy), the absence of significant morbidity, and favorable oncologic and reproductive outcomes.
Conclusion
Cervical cone biopsy and laparoscopic pelvic lymph node sampling is a feasible surgical approach in select patients with small-volume invasive cervical carcinoma. Our study demonstrates favorable oncologic and reproductive outcomes, with the absence of major postoperative morbidity. Tumor clearance of ≥2 mm appears to correlate well with no residual tumor in the remaining cervix on repeat conization. However, our results need to be interpreted with caution due to the small sample size and relatively short follow-up.
References
- 1.Marchiole P, Benchaib M, Buenerd A, et al. Oncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent's operation): a comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH) Gynecol Oncol. 2007;106:132–141. doi: 10.1016/j.ygyno.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Rustum NR, Neubauer N, Sonoda Y, et al. Surgical and pathologic outcomes of fertility-sparing radical abdominal trachelectomy for FIGO stage IB1 cervical cancer. Gynecol Oncol. 2008;111:261–264. doi: 10.1016/j.ygyno.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim CH, Abu-Rustum NR, Chi DS, et al. Reproductive outcomes of patients undergoing radical trachelectomy for early-stage cervical cancer. Gynecol Oncol. 2012;125:585–588. doi: 10.1016/j.ygyno.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Jolley JA, Battista L, Wing DA. Management of pregnancy after radical trachelectomy: case reports and systematic review of the literature. Am J Perinatol. 2007;24:531–539. doi: 10.1055/s-2007-986680. [DOI] [PubMed] [Google Scholar]
- 5.Lanowska M, Morawietz L, Sikora A, et al. Prevalence of lymph nodes in the parametrium of radical vaginal trachelectomy (RVT) specimen. Gynecol Oncol. 2011;121:298–302. doi: 10.1016/j.ygyno.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Schmeler KM, Frumovitz M, Ramirez PT. Conservative management of early stage cervical cancer: is there a role for less radical surgery? Gynecol Oncol. 2011;120:321–325. doi: 10.1016/j.ygyno.2010.12.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biliatis I, Kucukmetin A, Patel A, et al. Small volume stage 1B1 cervical cancer: Is radical surgery still necessary? Gynecol Oncol. 2012;126:73–77. doi: 10.1016/j.ygyno.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Rustum NR, Sonoda Y, Gemignani ML. Sentinel lymph node identification for early-stage cervical cancer. Gynecol Oncol. 2007;104(2 Suppl 1):2–4. doi: 10.1016/j.ygyno.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Vercellino GF, Piek JM, Schneider A, et al. Laparoscopic lymph node dissection should be performed before fertility preserving treatment of patients with cervical cancer. Gynecol Oncol. 2012;126:325–329. doi: 10.1016/j.ygyno.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Palaia I, Musella A, Bellati F, et al. Simple extrafascial trachelectomy and pelvic bilateral lymphadenectomy in early stage cervical cancer. Gynecol Oncol. 2012;126:78–81. doi: 10.1016/j.ygyno.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Sonoda Y, Abu-Rustum NR, Gemignani ML, et al. A fertility-sparing alternative to radical hysterectomy: how many patients may be eligible? Gynecol Oncol. 2004;95:534–538. doi: 10.1016/j.ygyno.2004.07.060. [DOI] [PubMed] [Google Scholar]