The majority of miRNA targets require slicer activity of ARGONAUTE1 for repression at the mRNA level.
Abstract
MicroRNAs (miRNAs) are key posttranscriptional regulators of gene expression in animals and plants. They guide RNA-induced silencing complexes to complementary target mRNA, thereby mediating mRNA degradation or translational repression. ARGONAUTE (AGO) proteins bind directly to miRNAs and may catalyze cleavage (slicing) of target mRNAs. In animals, miRNA target degradation via slicing occurs only exceptionally, and target mRNA decay is induced via AGO-dependent recruitment of deadenylase complexes. Conversely, plant miRNAs generally direct slicing of their targets, but it is unclear whether slicer-independent mechanisms of target mRNA decay also exist, and, if so, how much they contribute to miRNA-induced mRNA decay. Here, we compare phenotypes and transcript profiles of ago1 null and slicer-deficient mutants in Arabidopsis (Arabidopsis thaliana). We also construct conditional loss-of-function mutants of AGO1 to allow transcript profiling in true leaves. Although phenotypic differences between ago1 null and slicer-deficient mutants can be discerned, the results of both transcript profiling approaches indicate that slicer activity is required for mRNA repression of the vast majority of miRNA targets. A set of genes exhibiting up-regulation specifically in ago1 null, but not in ago1 slicer-deficient mutants was also identified, leaving open the possibility that AGO1 may have functions in gene regulation independent of small RNAs.
MicroRNAs (miRNAs) are important elements of posttranscriptional gene regulation in eukaryotes. These 20- to 24-nucleotide noncoding RNAs form 1:1 stoichiometric complexes with proteins of the ARGONAUTE (AGO) family and use base pairing to guide AGOs to complementary mRNA (Hammond et al., 2001; Schirle and MacRae, 2012). In plants, AGO1, one of 10 AGO paralogs in Arabidopsis (Arabidopsis thaliana; Vaucheret, 2008), associates with nearly all miRNAs and is responsible for most miRNA-guided gene regulation (Vaucheret et al., 2004; Baumberger and Baulcombe, 2005; Qi et al., 2005; Mi et al., 2008).
AGO recruitment to an mRNA target generally leads to repression of protein production, but several different mechanisms may be employed. In both plants and animals, targets may be translationally repressed with little or no effect on mRNA levels (Wightman et al., 1993; Aukerman and Sakai, 2003; Doench et al., 2003; Chen, 2004; Gandikota et al., 2007; Brodersen et al., 2008). In animals, there is good evidence for the existence of mechanisms of repression both at initiation and postinitiation levels (Olsen and Ambros, 1999; Seggerson et al., 2002; Pillai et al., 2005; Maroney et al., 2006; Nottrott et al., 2006; Petersen et al., 2006; Fukaya et al., 2014), but the precise molecular nature of these mechanisms has not been clearly defined. In plants, mechanisms of translational repression by miRNAs remain nearly completely unknown, and although genetic screens have identified some genes required for the process (Brodersen et al., 2008; Yang et al., 2012a; Li et al., 2013; Reis et al., 2015), none of them provides clear insight into how AGO1 recruitment to an mRNA may bring about its translational repression.
mRNA degradation is another common outcome of AGO recruitment. AGO proteins may use intrinsic RNase H-like activity to cleave (“slice”) target mRNA (Liu et al., 2004). This mode requires highly complementary miRNA-target interactions, notably around the cleavage site (Hutvágner and Zamore, 2002; Martinez and Tuschl, 2004). The catalytic center of AGO proteins harbors two MgII-ions whose coordination requires an Asp-Glu-Asp-His/Asp (DEDH/D) catalytic tetrad (Nakanishi et al., 2012; Sheng et al., 2014). Slicing is commonly observed in plants, but not in animals where extended base-pairing between miRNAs and targets occurs only exceptionally (Axtell, 2013). In animals, target mRNA decay is accelerated by recruitment of the major deadenylases CCR4-NOT and PARN (Braun et al., 2011; Chekulaeva et al., 2011; Fabian et al., 2011). Recruitment of these complexes requires the adaptor protein GW182/TNRC6 that binds to CCR4-NOT and PARN and uses Trp side chains in intrinsically disordered regions to interact directly with hydrophobic pockets in AGO proteins (Schirle and MacRae, 2012; Pfaff et al., 2013). An exact counterpart of this mechanism is unlikely to exist in plants because no homolog of GW182/TNRC6 is present in plant genomes. Nonetheless, it remains a valid question whether other mechanisms exist that may ensure miRNA-guided, yet slicer-independent, mRNA degradation in plants.
A thorough study of base-pairing requirements for repression of transiently overexpressed reporters by coexpressed miRNAs showed that central base pairing was necessary for miRNA activity in this experimental setup, suggesting an essential contribution of AGO1 slicing to miRNA target repression (Liu et al., 2014). Nonetheless, the observation that central base pairing is required for efficient translational repression of a luciferase reporter in vitro, even when assayed with a catalytically deficient AGO1 (Iwakawa and Tomari, 2013), indicates that a requirement for central pairing in a miRNA:mRNA interaction does not necessarily imply that slicing is employed as the dominant mechanism to bring about mRNA repression. Furthermore, central complementarity is not always required for target regulation in plants (Dugas and Bartel, 2008), nor is it always sufficient, since determinants of miRNA-guided regulation can be found in sequences flanking the target site (Li et al., 2014).
Solid evidence that slicing of miRNA targets occurs in planta has come from PCR-mediated detection of 3′-cleavage fragments whose 5′-ends precisely match the AGO1 cleavage site predicted from miRNA:mRNA hybrids (Kasschau et al., 2003). It is not clear, however, how large a fraction of miRNA-induced mRNA degradation is accounted for by direct AGO1-catalyzed slicing, and it remains an assumption that this mechanism is the unique biochemical route for target mRNA degradation. Direct experiments linking the extent of slicing to the extent of miRNA-guided mRNA degradation have not been reported, and in principle, it is possible that slicer-independent modes of miRNA target regulation contribute substantially to repression at the mRNA level.
In this study, we analyze the implication of slicer activity in miRNA-mediated degradation of target mRNA in vivo using stable and conditional mutants defective in slicer activity of AGO1. We show that in contrast to ago1 null mutants, slicer-defective mutants tend to have normal miRNA and miRNA* levels. Our transcriptome analyses of stable and conditional mutants defective in slicer activity of AGO1 indicate that in young seedlings, slicing by AGO1 accounts for most miRNA-induced mRNA degradation, since an overwhelming majority of miRNA targets shows similar mRNA up-regulation in ago1 null and in ago1 slicer-deficient mutants. Some candidates, including a validated miR395 target, were also identified whose mRNA levels are strongly up-regulated in ago1 null mutants but remain comparable to the wild type in slicer-deficient mutants. Thus, the existence of mechanisms for miRNA-mediated mRNA decay independent of slicer activity in plants cannot be entirely excluded, but their use is not widespread under the conditions employed here.
RESULTS
ago1 Null and Slicer-Deficient Mutants Produce Severe, But Distinguishable Morphological Phenotypes
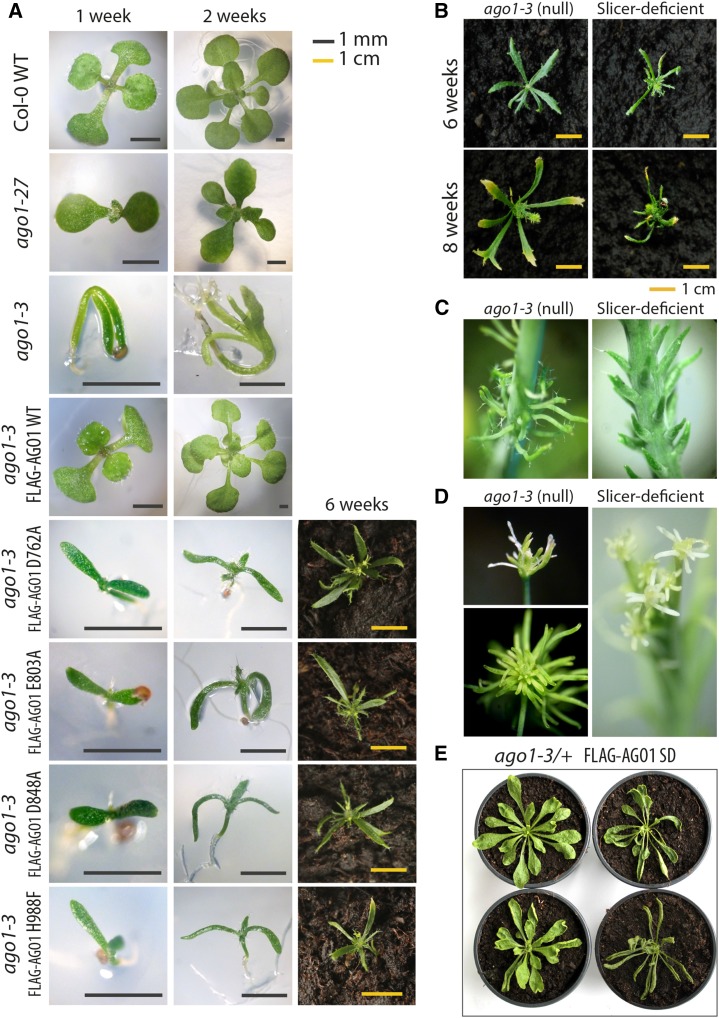
Mutant alleles of AGO1 in Arabidopsis produce very different morphological phenotypes depending on the biochemical function affected by the mutation and on the degree of reduction of function (Morel et al., 2002; Kidner and Martienssen, 2004, 2005; Poulsen et al., 2013). Our accompanying study reports on the construction of point mutants in all four metal-coordinating residues in AGO1 and shows that slicer activity is either completely abolished or strongly reduced (Arribas-Hernández et al., 2016), consistent with the two-metal ion mechanism for slicing previously proposed based on structures of bacterial Ago proteins (Sheng et al., 2014). To evaluate the in vivo effect of each of the catalytic tetrad mutants on morphological phenotype and miRNA target regulation, we constructed stable transgenic lines expressing FLAG-tagged point mutants (D762A, E803A, D848A, and H988F) in the ago1-3 background, in which a nonsense mutation (G42STOP) confers total loss of AGO1 activity (Bohmert et al., 1998). All four mutants produced severe and very similar morphological phenotypes that were distinguishable not only from hypomorphic ago1, but also from ago1 null alleles (Fig. 1A). Slicer-deficient seedlings showed filament-like cotyledons similar to ago1-3, but in contrast to ago1-3, the seedlings grew upright from the medium after germination, while the null mutants lay horizontally on the medium for 2 to 3 weeks (Fig. 1A). Despite a high percentage of seedling mortality between 10 and 20 d after germination, some null and slicer-deficient plants grew to produce small rosettes that were bigger in null than in slicer-deficient mutants (Fig. 1B). Null mutants formed a few flat, narrow leaves, while leaves of slicer-deficient mutants were radially symmetrical and needle-like. After 8 weeks, the plants formed short stems bearing filamentous cauline leaves. The size and distribution of cauline leaves on the stem was homogeneous in slicer-deficient plants, whereas null mutants produced clusters of filaments that adopted flower-like arrangements in some cases (Fig. 1C). Both kinds of plants formed sterile flowers with sepals and petals of filamentous shape and aberrant reproductive organs (Fig. 1D). Although a low level of slicer activity remained detectable in the E803A mutant (Arribas-Hernández et al., 2016), its phenotype was identical to that of mutants in the other three catalytic residues in all aspects that distinguish them from the null allele.
Figure 1.
Developmental phenotype of ago1 slicer-deficient plants. A, Seedlings of Col-0 wild type, ago1-27 (hypomorphic allele), ago1-3, the complementation line of ago1-3 with wild-type AGO1P:FLAG-AGO1, and slicer-deficient point mutants D762A, E803A, D848A, and H988F. B, Rosettes of ago1-3 null and slicer-deficient mutants. C, Cauline leaves of ago1-3 null and slicer-deficient mutants. D, Flowers and aberrant reproductive organs of ago1-3 null and slicer-deficient mutants. E, Representative T1 plants of slicer-deficient (SD) AGO1P:FLAG-AGO1 in Col-0 wild type or ago1-3/+. A wide variability of defects was found among T1 plants of the four point mutants, but not in plants transformed with the equivalent wild-type transgene.
Slicer-Deficient AGO1 Exerts a Dominant-Negative Effect on Wild-Type Plants
Expression of slicer-deficient AGO1 enhances the morphological and molecular phenotypes of the hypomorphic ago1-25 allele (Carbonell et al., 2012). These dominant-negative effects were probably due to competition of the slicer-deficient protein with the residual endogenous AGO1 activity of ago1-25, by sequestration of miRNAs or miRNA-target complexes (Carbonell et al., 2012). A dominant-negative effect on wild-type plants was also described. Similarly, we observed dominant-negative phenotypes upon transgenic expression of slicer-deficient AGO1 in ago1-3/+ or AGO1 wild-type plants. The number and severity of aberrant phenotypes found in T1 plants transformed with slicer-deficient constructs was clearly higher than in those transformed with FLAG-AGO1WT, for which a low percentage of plants had weak defects caused by cosuppression (Mallory and Vaucheret, 2009). The broad variability of defects ranged from smaller rosettes and upward-curling leaves to sterile plants with severe deformities (Fig. 1E). This variability did not correlate with AGO1 protein accumulation analyzed by western blotting (Supplemental Fig. S1), excluding the possibility that enhanced cosuppression underlies the phenotypes observed.
Dominant-Negative Effects on AGO10 May Explain Some Phenotypic Differences between ago1 Null and ago1 Slicer-Deficient Mutants
Although loss of function of the close AGO1 homolog AGO10 does not affect development in the Col-0 accession, double null ago1ago10 mutant plants are embryonically lethal (Mallory et al., 2009), suggesting that ago1 null plants are viable due to residual regulation of miRNA targets by AGO10 in the absence of AGO1. We reasoned that some of the differences observed between ago1 null and slicer-deficient plants could also be due to a dominant effect of slicer-deficient AGO1 on the activity of AGO10. To test this possibility, we crossed ago1-3/+ FLAG-AGO1D762A to ago10-1 and selected ago10-1ago1-3/+ FLAG-AGO1D762A families in the F2 population. As previously described for ago10-1ago1-3 (Mallory et al., 2009), we could not identify any ago10-1ago1-3 FLAG-AGO1D762A in the progeny of these plants. The same was true for ago10-1ago1-3 FLAG-AGO1H988F. These results suggest that ago1 slicer-deficient mutants do not possess enough regulatory activity of miRNA targets to confer viability in the absence of AGO10. Thus, the observation of some phenotypic traits more severe in ago1 slicer-deficient than in null mutants (e.g. extent of loss of leaf polarization) could be due to a dominant-negative effect on AGO10. In contrast, other molecular explanations may underlie those phenotypes for which ago1 slicer-deficient mutants were closer to the wild type than ago1 null, most notably the upright growth upon germination.
miRNA Populations in Slicer-Deficient AGO1 Plants
miRNA levels are reduced in strong AGO1 loss-of-function mutants (Vaucheret et al., 2004). To test whether this is an effect of loss of slicer activity, we performed northern analyses of miRNA and miRNA* species with total RNA isolated from seedlings of ago1-3, ago1-3 expressing the complementing FLAG-AGO1 transgene, or ago1-3 expressing one of three slicer-deficient mutants (D762A, E803A, and H988F). The seedlings were grown in liquid culture and harvested 13 d after germination (DAG). As ago1 null and slicer-deficient plants grow slowly, the developmental stage at harvest differed between mutant and wild-type plants. Thus, we included an additional control of wild-type plants harvested 8 DAG at a developmental stage (expanded cotyledons) that may be comparable to mutants at 13 DAG. Northern analyses showed that while miR159, miR160, and miR166 levels were substantially reduced in ago1-3, they were unaffected in slicer-deficient AGO1 mutants (Fig. 2A). Sequencing of total small RNA populations showed that this was a recurrent pattern for the vast majority of miRNAs (Fig. 2B; Supplemental Table S1). A few miRNAs showed higher levels in ago1 null than in ago1 slicer-deficient mutants (miR398) or showed higher levels in both ago1 null and slicer-deficient mutants compared to the wild type (miR172, miR824, and miR5026; Fig. 2, A and B). Up-regulation of miR172 could be explained by stabilization through preferential loading into AGO2 (Zhang et al., 2011), whose levels are higher in ago1 null and slicer-deficient mutants (see below), probably due to defective repression of AGO2 by miR403 (Allen et al., 2005). Two miRNA*s (miR160* and miR173*) were clearly more abundant in ago1 null, but not in slicer-deficient mutants (Fig. 2A). Although several miRNA*s are also loaded into AGO2 (Zhang et al., 2011), overaccumulation of AGO2 is not sufficient to explain the difference in miRNA* levels in null and slicer-deficient plants because AGO2 levels are similarly increased in both groups (see below). We conclude that the general trend of loss of miRNA in ago1 null mutants does not involve slicer activity. Examples of miRNAs with a strict requirement for slicer activity for biogenesis, similar to the conserved animal miRNA miR-451 (Cheloufi et al., 2010; Cifuentes et al., 2010), were also not identified.
Figure 2.
miRNA populations in slicer-deficient AGO1 plants. Analysis of miRNAs and miRNA*s in total RNA extracted from 8- or 13-d-old seedlings of Arabidopsis stable transgenic lines expressing the wild type or slicer-deficient FLAG-AGO1 in the ago1-3 null background. A, Northern-blot analyses. The same membranes were rehybridized with different miRNA probes. U6 was used as loading control. B, Normalized reads/million of miRNAs, averaged from two biological replicates. The SD (slicer-deficient) column corresponds to the average between the normalized reads obtained for the three AGO1 slicer-deficient mutants analyzed (D762A, E803A, and H988F). Data from miRNA families showing a similar distribution among genotypes were pooled. Only miRNA families with more than 20 reads in total are shown (the complete data set can be found in Supplemental Table S1). Green-yellow color codes indicate relative expression levels of the same miRNA across the different genotypes. Orange-white color codes in the right-most column indicate abundance of the different miRNAs. The membranes 1 to 4 used to hybridize miRNA probes in A are the same as the ones used for siRNA analysis in Figure 3A of Arribas-Hernández et al. (2016). Accordingly, the U6 loading controls are the same in the corresponding panels.
Slicer-Deficient ago1 Alleles May Maintain a Low Level of Translational Repression of Some Targets
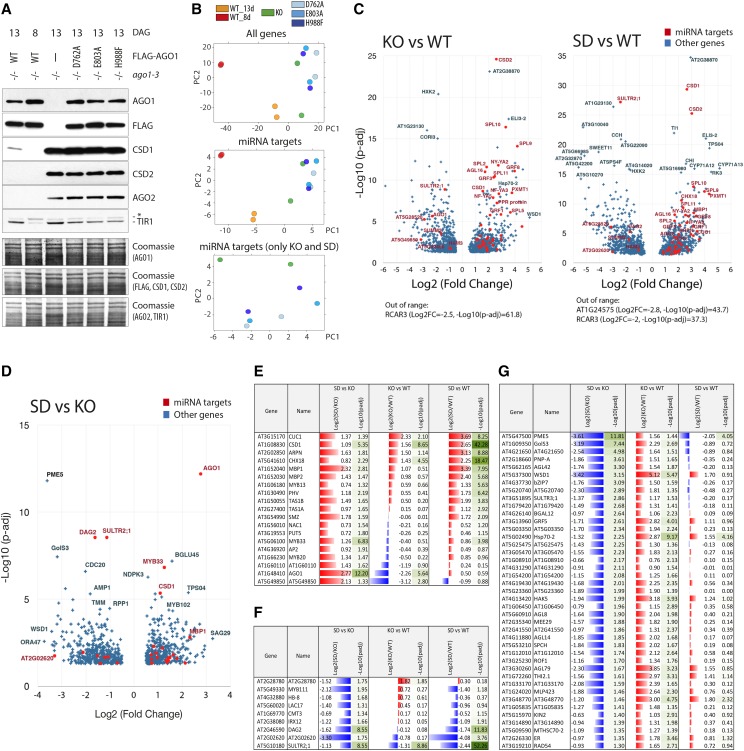
To test the effect of slicer-deficient AGO1 on miRNA target regulation, we used the same set of young seedlings harvested at 13 and 8 d after germination as described above. We initially tested the abundance of a few miRNA targets at the protein level and found similar up-regulation in ago1 null and slicer-deficient mutants compared to the wild type for CSD1, CSD2 (miR398), and AGO2 (miR403; Fig. 3A). Nevertheless, the levels of the miR393 target TIR1 were higher in ago1-3 null seedlings, suggesting that miRNA-mediated regulation of this target might be maintained to some extent in ago1 slicer-deficient seedlings.
Figure 3.
AGO1 slicer-deficient alleles do not provide evidence for slicer-independent miRNA target regulation. A, Western-blot analysis of protein extracts from 8- or 13-d old whole seedlings of ago1-3 and stable transgenic lines expressing the wild type or slicer-deficient FLAG-AGO1 (D762A, E803A, and H988F) in the ago1-3 null background. Three identical membranes were cut horizontally according to the expected protein sizes and were used for incubation with antibodies detecting the miRNA targets CSD1 (miR398), CSD2 (miR398), AGO2 (miR403), and TIR1 (miR393). AGO1 and FLAG antibodies were used to monitor expression of FLAG-AGO1 in the transgenic lines. Coomassie staining serves as loading control. B to G, Analysis of RNA-seq data from poly(A+) RNA isolated from seedlings described in A. Two biological replicates of each genetic background were analyzed. B, PCA. KO, ago1-3 null (knockout); SD, ago1-3 expressing slicer-deficient AGO1 mutants. C and D, Volcano plot showing differential expression between 13-d-old seedlings of ago1-3 (KO) compared to ago1-3 FLAG-AGO1 (WT) and averaged data from slicer-deficient samples (SD) compared to the wild type (C) or SD compared to KO (D). Only significantly regulated genes are plotted (|log2(fold change)| > 0.5, adjusted P value < 0.05). Validated or predicted miRNA targets are highlighted in red. E, miRNA targets upregulated in SD compared to KO. F, miRNA targets downregulated in SD compared to KO. G, Genes significantly downregulated in SD compared to KO and significantly upregulated in KO compared to wild-type plants. In E to G, only genes with |log2(fold change)| > 0.5, adjusted P value < 0.05 are included. Adjusted P values were calculated using Wald test with Benjamini-Hochberg adjustment in all cases.
Global Expression Patterns between ago1 Null and Slicer-Deficient Mutants Are Similar
Next, we performed transcriptome-wide analysis by RNA-seq (Supplemental Table S2) to test comprehensively whether slicer-deficient AGO1 can promote mRNA decay by cleavage-independent mechanisms. Principal component analysis (PCA) showed that ago1 null and slicer-deficient seedlings clustered together and apart from the wild type (Fig. 3B, top), as may be expected from the phenotype of the plants (Fig. 1A). Distinct clustering of the null and slicer-deficient samples was only observed by principal component 2 (PC2), when we left out wild-type samples and limited the analysis to miRNA targets (Fig. 3B). Thus, although some gene expression differences exist between ago1 null and slicer-deficient plants, the PCA indicates that the gene expression patterns between the two groups are related.
Most miRNA Target mRNAs Are Similarly Up-Regulated in ago1 Null and Slicer-Deficient Seedlings
To complete a more detailed analysis of gene expression differences of miRNA targets, we first studied the differential expression of miRNA targets between the two wild-type controls (8 and 13 DAG) and ago1 mutant plants. The results revealed that gene expression patterns of the two sets of AGO1 wild-type samples differed substantially and that differences in accumulation of target transcripts were more consistent with the expected pattern of up-regulation in ago1 null mutants when the 13 DAG control was used as a reference (Supplemental Fig. S2). Thus, we used only plants harvested at the same age (13 DAG) for further analysis.
We next compared transcripts whose differential expression between either ago1 null and wild type or ago1 slicer-deficient and wild type was significant (|log2(fold change)| > 0.5, adjusted P value < 0.05; Supplemental Table S3). The data indicated that miRNA targets tend to be upregulated in both null and slicer-deficient ago1 mutants when compared to the wild type (Fig. 3C). Indeed, volcano plots showing differential expression between ago1 slicer-deficient and ago1 null mutants showed few differences (Fig. 3D), in particular for annotated miRNA targets of which 27 were found to have differential expression between the two groups (Fig. 3, D–F; Supplemental Table S4). Contrary to expectation, approximately two-thirds of them showed up-regulation in slicer-deficient plants compared to ago1-3 (Fig. 3E). Thus, for many miRNA targets, loss of repression is even more pronounced in slicer-deficient mutants than in null mutants, perhaps as a consequence of residual repression by AGO10 that is counteracted by the presence of slicer-deficient AGO1. These analyses show that for the majority of miRNA targets, mutants defective in slicer activity of AGO1 do not reveal a contribution to miRNA-mediated mRNA decay independent of slicing.
Known miRNA Targets Repressed by Slicer-Deficient AGO1
We next focused on the nine annotated miRNA targets that showed lower transcript accumulation in slicer-deficient ago1 compared to KO plants (Fig. 3F). Among them, only the miR395 target At2g28780 (Sunkar and Zhu, 2004) behaved like a bona fide miRNA target with significantly higher mRNA levels in ago1-3 null mutants compared to the wild type (Fig. 3F). For the rest of these candidates, up-regulation in ago1-3 compared to the wild type was not significant, suggesting that miRNA-mediated regulation may not directly underlie differential regulation between ago1-3 and slicer-deficient mutants. Thus, catalytic activity of AGO1 is generally required to bring about degradation of known miRNA targets in Arabidopsis.
Identification of Transcripts Repressed by Slicer-Deficient AGO1
We hypothesized that novel, nonsliceable targets with mismatches at the 10th to 11th nucleotide position might be found in our data if they are regulated by slicing-independent mRNA decay. Such targets would be missed not only by prediction algorithms that consider central pairing a requirement for plant miRNA:target interactions, but also by analyses of degradome sequencing data that require alignments of the 5′-ends of many reads to a predicted slicer site to identify a functional miRNA:target interaction. We therefore searched our RNA-seq data for mRNAs with significantly lower expression in ago1 slicer-deficient than in ago1 null (log2(fold change) < −0.5, adjusted P value < 0.05) and significantly higher expression in ago1 null compared to the wild type (log2(fold change) > 0.5, adjusted P value < 0.05). This produced a short list of 41 candidates (Fig. 3G). We used psRNA target (Dai and Zhao, 2011) with relaxed parameters to search this mRNA set for potential miRNA target sites. psRNA target uses a Smith-Waterman algorithm to find miRNA-mRNA alignments and introduces extra penalty for mismatches only in the seed region of nucleotides 2 to 7 of the miRNA, but not for central mismatches (Dai and Zhao, 2011). Most of the candidates produced low-confidence predictions, often with nonconserved miRNAs of low sequence complexity, suggesting that they do not represent important regulatory interactions (Supplemental Table S5). Six candidates produced credible target predictions, including the transcription factors AGL14 and GRF5, and the sulfate transporter SULTR3 that all belong to gene families known to be controlled by miRNAs (Meng et al., 2011). Since the accompanying manuscript demonstrates that slicer activity of AGO1 is not required for miRNA-induced production of secondary siRNAs (Arribas-Hernández et al., 2016), we considered the possibility that intact repression in ago1 slicer-deficient mutants might be a consequence of production of secondary siRNAs that could bring about slicing in complex with AGO proteins other than AGO1. However, inspection of small RNA sequencing data did not yield clear examples of genes with small RNA peaks in the wild type and ago1 slicer-deficient mutants, but not in ago1-3. Thus, although our analysis of differential gene expression between ago1 null and slicer-deficient mutants may identify a few bona fide miRNA targets, we conclude overall that the results obtained with young seedlings expressing catalytically defective AGO1 mutants do not provide solid evidence for miRNA-mediated mRNA decay independent of slicing in plants as it occurs in animals.
A Cre-Lox Allele of AGO1 Confers Conditional Loss of AGO1 Function
It is a possible pitfall of our analysis of stable slicer-deficient ago1 mutants that it is limited to strongly developmentally affected embryonic tissues because most ago1 null or slicer-deficient seedlings die at the cotyledon stage. To extend our analysis of the effect of AGO1 slicer deficiency to true leaves, we constructed a transgenic system for conditional loss of AGO1 cleavage activity. The Cre-Lox vector pX6 (Zuo et al., 2001) was engineered to contain wild-type 3xHA-tagged AGO1 flanked by LoxP sequences, with the endogenous AGO1 promoter driving its expression (Fig. 4A). In this system, estradiol induces the phage recombinase Cre, which in turns excises the DNA located between LoxP sequences from the genome. Successful excision is monitored by expression of GFP (Fig. 4, A and B). We introduced this construct into Col-0 wild type, ago1-3, or ago1-3 expressing wild type or slicer-deficient FLAG-AGO1. While the Cre-Lox HA-AGO1 construct fully complemented ago1-3 (Fig. 4B, right panel), seedlings germinated on media containing 10 μm estradiol expanded normal cotyledons but showed clear ago1 mutant phenotypes from the emergence of the first true leaves (Fig. 4B, left panel). These phenotypic defects were accompanied by GFP fluorescence (Fig. 4B, bottom panel), consistent with excision of pAGO1:3xHA-AGO1. We verified by western analysis that 3xHA-AGO1 protein levels were reduced in seedlings germinated on estradiol (Fig. 4C). Interestingly, the reduction was nearly complete in a Col-0 wild-type background or ago1-3 lines expressing FLAG-AGO1WT, but only partial in ago1-3 containing no additional transgene or slicer-deficient FLAG-AGO1 (Fig. 4C, left panel). The AGO1-miR168 autoregulatory loop that maintains AGO1 homeostasis may at least partially underlie this effect (Vaucheret et al., 2004, 2006). We could not find conditions for estradiol treatment that led to full reduction of HA-AGO1 levels in pX6 3xHA-AGO1/ago1-3 seedlings. Although only partial reduction of AGO1 abundance was observed upon germination on estradiol-containing media, clear up-regulation of the miR398 target CSD2 in these conditions indicated that substantial loss of AGO1 function was achieved in response to estradiol (Fig. 4C, right panel), in agreement with the morphological defects observed in these plants (Fig. 4B). Thus, the Cre-Lox system confers conditional loss of AGO1 function despite the modest effect on AGO1 protein levels.
Figure 4.
A Cre-Lox allele of AGO1 to study the effect of slicing on miRNA regulation. A, Schematic representation of the T-DNA containing AGO1P:3xHA-AGO1-AGO1T in Cre-LoxP DNA-excision system used to construct conditional loss of AGO1 function in the ago1-3 background (adapted from Zuo et al., 2001). Expression of the XVE chimeric transcription factor is controlled by the constitutive G10-90 promoter. XVE binding to β-estradiol leads to transcriptional activation of CRE recombinase from the OLexA-46 promoter. CRE excises the DNA segment placed between the Lox P sites from the host genome, leading to loss of 3xHA-AGO1 and to fusion of the G10-90 promoter with the downstream GFP. B, Phenotype of 20-d-old ago1Cre-Lox seedlings germinated on MS-agar media with or without 10 μm β-estradiol. GFP fluorescence in root tips of induced plants is shown below. C, Western-blot analyses of protein extracted from the aerial part of seedlings germinated on ±10 μm estradiol and harvested 15 d after germination. All lines analyzed contain the pX6 3xHA-AGO1 transgene in the different backgrounds indicated. Coomassie staining is used as loading control. Note efficient disappearance of 3xHA-AGO1 protein upon CRE induction only in lines with an additional wild-type copy of AGO1.
Transcriptome Analysis of AGO1 Conditional Loss-of-Function Lines
We next performed RNA-seq of poly(A+) RNA isolated from ago1-3 seedlings complemented with Cre-Lox 3xHA-AGO1 and containing either FLAG-AGO1WT, slicer-deficient FLAG-AGO1 mutants, or no additional transgene, all germinated on estradiol-containing medium (Supplemental Table S6). PCA revealed clear differences in gene expression between conditional KO or slicer-deficient AGO1 plants compared to those carrying FLAG-AGO1WT (Fig. 5A). However, the conditional KO samples clustered in the middle of the three conditional slicer-deficient mutants analyzed, suggesting that the gene expression pattern did not differ substantially between these two classes. The same tendency was observed when known miRNA targets were analyzed separately, as most of them showed similar up-regulation in conditional KO or slicer-deficient AGO1 compared to plants carrying FLAG-AGO1WT (Fig. 5B; Supplemental Table S7). Direct comparison of the abundance of miRNA targets between conditional ago1 KO and slicer-deficient plants showed that the majority of target transcripts had either similar levels or higher levels in the slicer-deficient mutants (Fig. 5, B and C). This result is similar to what we observed with the stable ago1 null and slicer-deficient mutants.
Figure 5.
RNA-seq analysis upon conditional loss of AGO1 function. A to C, Analysis of RNA-seq data from poly(A+) RNA isolated from the aerial part of seedlings germinated on 10 μm estradiol and harvested 15 d after germination. Two biological replicates of each genetic background were analyzed. All lines contain the same pX6 3xHA-AGO1 transgene in different backgrounds: Col-0 wild type, ago1-3 FLAG-AGO1WT, ago1-3 (‘c-KO’ or Cond. KO), or three different ago1-3 FLAG-AGO1 slicer-deficient mutants (Cond. SD): ‘c-D762A’, ‘c-E803A’, and ‘c-H988F’. Two biological replicates of each genotype were analyzed. A, PCA. B, Volcano plots showing differential expression of validated and predicted miRNA targets. “Cond. SD” refers to the average between ‘c-D762A’, ‘c-E803A’, and ‘c-H988F’ values, and ‘WT’ refers to average values between Col-0 wild type and ago1-3 FLAG-AGO1WT. C, miRNA targets with significantly different expression (|log2(fold change)| > 0.5, adjusted P value < 0.05) between Cond. KO and Cond. SD, as described above. Adjusted P values were calculated using the Wald test with Benjamini-Hochberg adjustment.
DISCUSSION
Slicer Activity in Degradation of miRNA Target Transcripts
The transcriptome analyses performed on either stable or conditional mutants defective in AGO1 slicer activity in this study do not provide proof for the existence of slicer-independent mRNA decay of miRNA targets. Thus, miRNA-mediated deadenylation and mRNA decay is not likely to occur in plants as in animals (Huntzinger and Izaurralde, 2011). This observation is in agreement with a recent study performed in lysates of tobacco protoplasts that recapitulate miRNA-mediated repression of an in vitro-translated luciferase reporter (Iwakawa and Tomari, 2013). The authors could monitor efficient translational repression by either wild-type or slicer-deficient AGO1, but that was not accompanied by any kind of reporter-mRNA degradation other than slicing. In the green alga Chlamydomonas reinhardtii, small interfering RNA-mediated translational inhibition in vivo without deadenylation has also been reported (Ma et al., 2013).
Possible Pitfalls of Use of Slicer-Deficient Mutants
It is a legitimate question whether the AGO1 catalytic point mutants used here affect only catalytic activity or whether they may disrupt the overall structure to the extent that the protein is not functional. We exclude this latter possibility for the following reasons: (1) accumulation of miRNAs in slicer-deficient ago1 mutants is normal, contrary to ago1 null mutants; (2) loading of miRNAs in slicer-deficient AGO1 is very similar to AGO1WT (Arribas-Hernández et al., 2016); (3) slicer-deficient AGO1 is able to target TAS transcripts for production of tasiRNAs (Arribas-Hernández et al., 2016), (4) the three different point mutants tested behaved in the same way, despite the fact that they reside in distinct parts of the AGO structure (Poulsen et al., 2013); and (5) Arabidopsis AGO1D762A is able to repress target translation in vitro (Iwakawa and Tomari, 2013), and residual TIR1 repression was observed in this study. Nevertheless, the distinctive characteristics displayed by the mutant protein in vivo, in particular defective dissociation from target mRNAs (Carbonell et al., 2012; Arribas-Hernández et al., 2016) might change the properties of the protein. For example, there is good evidence that AGO proteins exist in two different states depending on whether the 3′-end of its guide strand is fully base-paired to a target strand because base-pairing to the 3′part is accompanied by release from the PAZ domain (Zander et al., 2014). Thus, in vivo, slicer-deficient AGO1 mutants may be locked in a single conformational state, potentially not competent to perform all functions of the wild-type protein. In this regard, we note that the miR398 targets CSD1 and CSD2 displayed similar accumulation at the protein level in ago1-3 and in slicer-deficient seedlings, despite the fact that several studies have shown these targets to be regulated in part by translational repression (Brodersen et al., 2008; Dugas and Bartel, 2008; Li et al., 2013). This does not necessarily imply total loss of function of the slicer-deficient point mutants, however. First, CSD1 and CSD2 mRNAs are among the miRNA targets most highly upregulated in slicer-deficient seedlings (Fig. 3C). It is possible that the capacity for translational repression by miR398 is saturated in these mutants such that a substantial part, indeed the majority, of CSD1 and CSD2 mRNA pools evades translational repression. Second, one may speculate, perhaps too daringly, that distinct AGO1 pools involved in slicing and translational repression exist in a dynamic equilibrium and that the translational repression pool is shifted toward the slicing pool as an indirect effect of the complete loss of slicer activity in catalytic mutants. Rigorous tests of this latter possibility must await establishment of experimental procedures to separate AGO1 pools with distinct biochemical properties in vivo.
Slicer Activity in miRNA Biogenesis and Accumulation
In animals, the biogenesis of the conserved miRNA miR-451 proceeds via direct pre-miRNA loading onto Ago2 (Cheloufi et al., 2010; Cifuentes et al., 2010), followed by slicing to generate the mature 5′-end and PARN-mediated trimming to generate the 3′-end (Yoda et al., 2013). The possible existence of similar slicer-dependent miRNA biogenesis mechanisms in plants has not been addressed previously. Our small RNA profiles of slicer-deficient AGO1 mutants expressed in a null background did not reveal examples of such miRNAs specifically dependent on AGO1 slicer activity. Furthermore, the analyses showed that the well-known reduction of steady-state miRNA levels in ago1 null mutants (Vaucheret et al., 2004) is not due to loss of slicer activity but is likely to be a consequence of lack of miRNA stabilization by the AGO1 protein, as previously suggested (Vaucheret et al., 2004). Finally, our small RNA analyses showed that a few miRNA* species distinctly overaccumulate in ago1 null, but not in AGO1 slicer-deficient mutants, probably because in the absence of AGO1 protein, the miRNA/miRNA* duplex is rerouted to alternative AGO proteins that may preferentially load miRNA* strands. If so, biological activity of these miRNA*s may contribute to the distinct phenotypes of ago1 null and ago1 slicer-deficient mutants.
Small RNA-Independent Functions of AGO1?
Our study revealed several morphological differences between ago1 slicer-deficient and knockout plants. Nonetheless, the misregulation of miRNA targets was similar between the two groups, and in many cases exacerbated in slicer-deficient mutants. It is possible that a dominant-negative effect of slicer-deficient AGO1 on the residual activity of AGO10 in miRNA target repression explains some of these differences. Alternatively, phenotypic differences could be related to functions of AGO1 other than miRNA target repression. Indeed, the implication of AGO proteins in several pathways such as nonsense-mediated mRNA decay (Choe et al., 2010), alternative splicing (Ameyar-Zazoua et al., 2012; Yang et al., 2012b; Taliaferro et al., 2013; Alló et al., 2014), DNA double-strand break repair (Michalik et al., 2012; Wei et al., 2012), nucleosome occupancy at transcription start sites (Carissimi et al., 2015), and quality control of mRNAs encoding proteins entering the secretory pathway (Karamyshev et al., 2014) has been proposed during the last years. If that is the case, the conditional slicer-deficient alleles might be a valuable tool to investigate novel functions of plant AGO1 by decoupling these from target regulation. In this respect, our identification of transcripts up-regulated in ago1 null, but not in slicer-deficient, mutants is a useful starting point, particularly since only a few of these transcripts are likely to be miRNA targets.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All the lines are in the Arabidopsis (Arabidopsis thaliana) Col-0 ecotype. The following mutants and transgenic lines were described previously: ago1-3 (Bohmert et al., 1998), ago1-27 (Morel et al., 2002), ago10-1 (SALK_000457; Vaucheret, 2008), 35S-TIR1, and 35S-mTIR1 (Chen et al., 2015). T-DNA insertion lines were obtained from Nottingham Arabidopsis Stock Center. Construction of ago1-3 FLAG-AGO1 wild type and slicer-deficient mutants was described by Arribas-Hernández et al. (2016).
Seeds were surface-sterilized and stratified as described by Arribas-Hernández et al. (2016). For propagation or morphological studies, the plants were grown on soil and maintained in standard greenhouse conditions with a light cycle of 16 h light/8 h darkness or Percival growth chambers at defined day/night cycles (16 h light/21°C day, 8 h darkness/16°C night), respectively. For molecular analysis of pX6 3xHA-AGO1 lines, seeds were germinated on Murashige and Skoog (MS)-agar media (4.4 g/L MS salt mixture, 10 g/L Suc, and 8 g/L agar, pH 5.7) to grow in sterile conditions. MS medium was supplemented with 1 mL/L of 10 mm β-estradiol (Sigma-Aldrich) in DMSO when estradiol treatment was required. After 2 weeks, the plates were flash-frozen on liquid nitrogen, and the aerial parts of the seedlings were collected for analysis. For molecular analyses of stable lines of ago1 null or slicer-deficient mutants, seedlings were grown in liquid cultures as described by Arribas-Hernández et al. (2016).
Genetic Crosses and Genotyping
Floral buds of ago10-1 plants were emasculated and pollinated with stamens from ago1-3/+ FLAG-AGO1 D762A and H988F plants. F1 plants were propagated, and mutants with ago1-10 ago1-3/+ FLAG-AGO1 genotype were selected among the F2 population by genotyping and BASTA selection. DNA extraction and genotyping of ago1-3 was described by Arribas-Hernández et al. (2016). Sequences of primers for genotyping ago10-1 (LA319-320 and LBb1.3) can be found in Supplemental Table S8.
Construction of pX6U-GFP
We adapted the estrogen-receptor-based chemical-inducible system pX6-GFP (Zuo et al., 2001) for our purposes to introduce AGO1 constructs into ago1-3 plants. The system was originally designed for inducible Cre-Lox mediated DNA excision of transgenes that provide resistance to antibiotics in transgenic crops. In the pX6-GFP vector, the nptII gene for kanamycin selection is excised upon β-estradiol induction, while maintaining the gene of interest previously cloned into the plasmid inside the plant genome (Zuo et al., 2006). We modified the pX6-GFP vector for conditional knockout studies by insertion of a USER cassette within the region flanked by the two Lox P sites. In the resulting vector, the USER-cloned transgene is also excised from the plant genome by β-estradiol treatment. To introduce the cassette, a unique AvrII restriction site was created between the NPTII transcription unit and the LexA operator sequence following the QuikChange site-directed mutagenesis protocol (Stratagene). Phusion High-Fidelity DNA Polymerase (NEB) was used for PCR, and NEB 5-α Competent Escherichia coli (High Efficiency) for chemical transformation. Sequences of the primers used for PCR (LA32-LA33) are listed in Supplemental Table S8. It was not possible to obtain clones with the full-size plasmid bearing the AvrII restriction site, but NcoI/AvrII digestions showed that several clones contained a truncated plasmid with the correct mutation. Truncation may have resulted from ligation of DpnI-digested fragments of the parental plasmid to incomplete PCR products bearing the mutation. We therefore exchanged an ∼1,000-bp fragment from one of the small plasmids containing the AvrII site to the full-length parental vector by conventional restriction cloning, using unique ApaI and NruI sites. After exhaustive check of the integrity of the obtained plasmid by restriction analysis and sequencing, a single PacI-USER cassette with AvrII overhangs (Supplemental Table S8) was introduced in the reconstituted vector to generate pX6U-GFP.
Cloning of the AGO1 Construct
For construction of AGO1P:3xHA-AGO1-AGO1T, we amplified AGO1P and AGO1-AGO1T genomic DNA sequences with primers bearing USER-compatible overhangs (LA34-35 and LA36-37) matching the USER cassette on one side and the border sequences of a 3xHA epitope tag on the other. We used AtAGO1 (At1g48410) genomic DNA introduced in pGEM-T Easy (Promega) by Arribas-Hernández et al. (2016) as templates for PCR. The short 3xHA sequence was amplified from pSLF173 (Forsburg and Sherman, 1997) with the USER-compatible primers LA2-3. All PCR amplifications were performed using the uracil read-through Pfu Turbo Cx Hotstart DNA polymerase (Agilent Technologies). The three purified fragments were fused into pX6U-GFP by USER cloning (Bitinaite and Nichols, 2009). All primers used are listed in Supplemental Table S8.
Plant Transformation and Selection of Transgenic Lines
Transgenic lines were generated by floral dip transformation (Clough and Bent, 1998) with Agrobacterium tumefaciens GV3101 bearing pX6U-GFP AGO1P:3xHA-AGO1-AGO1T of ago1-3/+ plants or pCAMBIA3300U AGO1P:FLAG-AGO1-AGO1T (Arribas-Hernández et al., 2016) of the third transgenic generation (T3) of ago1-3 pX6-3xHA-AGO1 plants.
MS-agar plates were supplemented with kanamycin (35 mg/L) for selection of pX6U-GFP AGO1P:3xHA-AGO1-AGO1T lines. Media for selection of T1 seeds was additionally supplemented with ampicillin (100 mg/L) to avoid growth of A. tumefaciens. Among 110 primary transformants, two lines were identified whose T2 progeny showed clear ago1 mutant phenotypes specifically in response to β-estradiol.
Selection of primary transformants containing pCAMBIA3300U vectors was done on soil by spraying seedlings with 0.2 g/L BASTA 10 d after germination. Segregation studies of T2 and T3 populations were performed on MS-agar plates supplemented with glufosinate ammonium (Fluka; 7.5 mg/L). Single-insertion transgenic lines were selected in T2, and descendants bearing the T-DNA in homozygosity were identified in T3.
RNA-Seq
Libraries were constructed using 1 μg of TRI Reagent-extracted total RNA from 100 mg of seedling tissue as described by Arribas-Hernández et al. (2016). For preparation of mRNA libraries, we isolated mRNAs using the NEB Next Poly(A) mRNA magnetic isolation module. The libraries were prepared with the NEBNext UltraTM RNA Library Prep Kit for Illumina, following the instructions provided by the manufacturer. Agencourt AMPure XP Beads were used for the cleaning steps. We used 13 cycles of PCR for cDNA amplification, and the quality and size distribution of the libraries were controlled using an Agilent 2100 Bioanalyzer. Construction of libraries for small RNA sequencing was described by Arribas-Hernández et al. (2016). An overview of all RNA-seq and small RNA-seq libraries constructed and sequenced in this study is shown in Supplemental Table S9.
Bioinformatic Analysis of mRNAs
FASTQ files were trimmed from contaminating adapters and low-quality stretches with the cutadapt version 1.5 with options “-a AGATCGGAAGAGCACACGTCTGAACTCCAGTCAC -m30-q20” (Martin, 2011). Trimmed reads were mapped to TAIR10 genome assembly with TopHat version 2.0.12 (Kim et al., 2013) guided by the transcriptome model containing all TAIR10 genes and with multihits prefiltering. Mapped reads were selected for further analysis with “samtools view” (Li et al., 2009) using option “-q50.” PCA plots with r-log normalized counts data and differential expression analysis (Wald test, multiple testing adjustment of P values with Benjamini-Hochberg procedure) were performed in the Bioconductor environment (Gentleman et al., 2004) using DESeq2 version 1.4.5 (Love et al., 2014) workflow. The list of validated and predicted miRNA and tasiRNAs targets used for data analyses (Supplemental Table S10) was updated from Reis et al. (2015).
Small RNA Northern Blotting
Northern blots showing miRNAs were obtained following the same procedure as described by Arribas-Hernández et al. (2016). Sequences of the probes can be found in Supplemental Table S8.
Western Blotting
Western blots were performed following the same procedure as described by Arribas-Hernández et al. (2016). Information regarding antibodies is detailed in Supplemental Table S11.
Accession Numbers
All high-throughput sequencing data have been deposited in the European Nucleotide Archive under accession number E-MTAB-4529.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Dominant-negative effect of slicer-deficient AGO1 on wild-type plants.
Supplemental Figure S2. miRNA target regulation in ago1 mutants versus different wild-type controls.
Supplemental Figure S3. Test of TIR1 antibody.
Supplemental Table S1. Normalized miRNA reads per million in seedlings of ago1-3, ago1-3 FLAG-AGO1WT, and ago1-3 FLAG-AGO1 slicer-deficients.
Supplemental Table S2. mRNAseq total reads in seedlings of ago1-3, ago1-3 FLAG-AGO1WT, and ago1-3 FLAG-AGO1 slicer-deficients (stable lines).
Supplemental Table S3. Gene expression fold changes between ago1-3, ago1-3 FLAG-AGO1WT, and ago1-3 FLAG-AGO1 slicer-deficients (stable lines).
Supplemental Table S4. Comparative expression of genes differentially regulated in ago1-3 FLAG-AGO1 slicer-deficient relative to ago1-3 (null) seedlings (stable lines).
Supplemental Table S5. Target predictions for decay candidates.
Supplemental Table S6. mRNA-seq total reads in ago1Cre-Lox alleles (conditional KO or slicer-deficient ago1).
Supplemental Table S7. Gene expression fold changes between conditional KO or slicer-deficient ago1 and wild-type controls (Cre-Lox alleles).
Supplemental Table S8. Primers and probes.
Supplemental Table S9. RNA-seq and small RNA-seq libraries.
Supplemental Table S10. miRNA and tasiRNA targets in Arabidopsis thaliana.
Supplemental Table S11. Antibodies.
Supplementary Material
Acknowledgments
We thank Theo Bølsterli and his team for plant care, Mathias Henning Hansen for invaluable help with plant and seed handling, Miranda van Wonterghem for help with line selection, and Lena Bjørn Johansen for help with northern-blot hybridizations. We acknowledge the Nottingham Arabidopsis Stock Centre for providing seeds of T-DNA insertion lines, Ning Han for seeds of 35S-TIR1 and 35S-mTIR1 lines, Nam-Hai Chua for pX6-GFP, Dan Kliebenstein for the CSD1 antibody, Olivier Voinnet for the AGO2 antibody, and Lionel Navarro for the TIR1 antibody. We thank Andrea Barghetti for testing the TIR1 antibody with 35S-TIR1 lines. We also thank the EMBL GeneCore facility, Vladimir Benes, and Bettina Haase for assistance with library preparation for mRNAseq analyses and Antonin Marchais for help extracting information on miRNA read counts.
Glossary
- miRNA
microRNA
- DAG
days after germination
- PCA
principal component analysis
Footnotes
Articles can be viewed without a subscription.
This work was supported by the Novo Nordisk Foundation (Hallas Møller Fellowship), the European Research Council (Micromecca 282460 to P.B.), and Augustinus Fonden.
References
- Allen E, Xie Z, Gustafson AM, Carrington JC (2005) MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207–221 [DOI] [PubMed] [Google Scholar]
- Alló M, Agirre E, Bessonov S, Bertucci P, Gómez Acuña L, Buggiano V, Bellora N, Singh B, Petrillo E, Blaustein M, et al. (2014) Argonaute-1 binds transcriptional enhancers and controls constitutive and alternative splicing in human cells. Proc Natl Acad Sci USA 111: 15622–15629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameyar-Zazoua M, Rachez C, Souidi M, Robin P, Fritsch L, Young R, Morozova N, Fenouil R, Descostes N, Andrau JC, et al. (2012) Argonaute proteins couple chromatin silencing to alternative splicing. Nat Struct Mol Biol 19: 998–1004 [DOI] [PubMed] [Google Scholar]
- Arribas-Hernández L, Marchais A, Poulsen C, Haase B, Hauptmann J, Benes V, Meister G, Brodersen P (2016) The slicer activity of ARGONAUTE1 is required specifically for phasing, not production, of trans-acting siRNAs in Arabidopsis. Plant Cell 28: 1563–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ. (2013) Classification and comparison of small RNAs from plants. Annu Rev Plant Biol 64: 137–159 [DOI] [PubMed] [Google Scholar]
- Baumberger N, Baulcombe DC (2005) Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci USA 102: 11928–11933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitinaite J, Nichols NM (2009) DNA cloning and engineering by uracil excision. Curr Protoc Mol Biol 3: 21 [DOI] [PubMed] [Google Scholar]
- Bohmert K, Camus I, Bellini C, Bouchez D, Caboche M, Benning C (1998) AGO1 defines a novel locus of Arabidopsis controlling leaf development. EMBO J 17: 170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JE, Huntzinger E, Fauser M, Izaurralde E (2011) GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol Cell 44: 120–133 [DOI] [PubMed] [Google Scholar]
- Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science 320: 1185–1190 [DOI] [PubMed] [Google Scholar]
- Carbonell A, Fahlgren N, Garcia-Ruiz H, Gilbert KB, Montgomery TA, Nguyen T, Cuperus JT, Carrington JC (2012) Functional analysis of three Arabidopsis ARGONAUTES using slicer-defective mutants. Plant Cell 24: 3613–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carissimi C, Laudadio I, Cipolletta E, Gioiosa S, Mihailovich M, Bonaldi T, Macino G, Fulci V (2015) ARGONAUTE2 cooperates with SWI/SNF complex to determine nucleosome occupancy at human transcription start sites. Nucleic Acids Res 43: 1498–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekulaeva M, Mathys H, Zipprich JT, Attig J, Colic M, Parker R, Filipowicz W (2011) miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat Struct Mol Biol 18: 1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ (2010) A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465: 584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303: 2022–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Hu L, Han N, Hu J, Yang Y, Xiang T, Zhang X, Wang L (2015) Overexpression of a miR393-resistant form of transport inhibitor response protein 1 (mTIR1) enhances salt tolerance by increased osmoregulation and Na+ exclusion in Arabidopsis thaliana. Plant Cell Physiol 56: 73–83 [DOI] [PubMed] [Google Scholar]
- Choe J, Cho H, Lee HC, Kim YK (2010) MicroRNA/Argonaute 2 regulates nonsense-mediated messenger RNA decay. EMBO Rep 11: 380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes D, Xue H, Taylor DW, Patnode H, Mishima Y, Cheloufi S, Ma E, Mane S, Hannon GJ, Lawson ND, Wolfe SA, Giraldez AJ (2010) A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 328: 1694–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dai X, Zhao PX (2011) psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res 39: W155–W159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench JG, Petersen CP, Sharp PA (2003) siRNAs can function as miRNAs. Genes Dev 17: 438–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas DV, Bartel B (2008) Sucrose induction of Arabidopsis miR398 represses two Cu/Zn superoxide dismutases. Plant Mol Biol 67: 403–417 [DOI] [PubMed] [Google Scholar]
- Fabian MR, Cieplak MK, Frank F, Morita M, Green J, Srikumar T, Nagar B, Yamamoto T, Raught B, Duchaine TF, Sonenberg N (2011) miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat Struct Mol Biol 18: 1211–1217 [DOI] [PubMed] [Google Scholar]
- Forsburg SL, Sherman DA (1997) General purpose tagging vectors for fission yeast. Gene 191: 191–195 [DOI] [PubMed] [Google Scholar]
- Fukaya T, Iwakawa HO, Tomari Y (2014) MicroRNAs block assembly of eIF4F translation initiation complex in Drosophila. Mol Cell 56: 67–78 [DOI] [PubMed] [Google Scholar]
- Gandikota M, Birkenbihl RP, Höhmann S, Cardon GH, Saedler H, Huijser P (2007) The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J 49: 683–693 [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ (2001) Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293: 1146–1150 [DOI] [PubMed] [Google Scholar]
- Huntzinger E, Izaurralde E (2011) Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 12: 99–110 [DOI] [PubMed] [Google Scholar]
- Hutvágner G, Zamore PD (2002) A microRNA in a multiple-turnover RNAi enzyme complex. Science 297: 2056–2060 [DOI] [PubMed] [Google Scholar]
- Iwakawa HO, Tomari Y (2013) Molecular insights into microRNA-mediated translational repression in plants. Mol Cell 52: 591–601 [DOI] [PubMed] [Google Scholar]
- Karamyshev AL, Patrick AE, Karamysheva ZN, Griesemer DS, Hudson H, Tjon-Kon-Sang S, Nilsson I, Otto H, Liu Q, Rospert S, et al. (2014) Inefficient SRP interaction with a nascent chain triggers a mRNA quality control pathway. Cell 156: 146–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasschau KD, Xie Z, Allen E, Llave C, Chapman EJ, Krizan KA, Carrington JC (2003) P1/HC-Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA unction. Dev Cell 4: 205–217 [DOI] [PubMed] [Google Scholar]
- Kidner CA, Martienssen RA (2004) Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature 428: 81–84 [DOI] [PubMed] [Google Scholar]
- Kidner CA, Martienssen RA (2005) The role of ARGONAUTE1 (AGO1) in meristem formation and identity. Dev Biol 280: 504–517 [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Reichel M, Millar AA (2014) Determinants beyond both complementarity and cleavage govern microR159 efficacy in Arabidopsis. PLoS Genet 10: e1004232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu L, Zhuang X, Yu Y, Liu X, Cui X, Ji L, Pan Z, Cao X, Mo B, et al. (2013) MicroRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell 153: 562–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ (2004) Argonaute2 is the catalytic engine of mammalian RNAi. Science 305: 1437–1441 [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang F, Axtell MJ (2014) Analysis of complementarity requirements for plant microRNA targeting using a Nicotiana benthamiana quantitative transient assay. Plant Cell 26: 741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Kim EJ, Kook I, Ma F, Voshall A, Moriyama E, Cerutti H (2013) Small interfering RNA-mediated translation repression alters ribosome sensitivity to inhibition by cycloheximide in Chlamydomonas reinhardtii. Plant Cell 25: 985–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory AC, Hinze A, Tucker MR, Bouché N, Gasciolli V, Elmayan T, Lauressergues D, Jauvion V, Vaucheret H, Laux T (2009) Redundant and specific roles of the ARGONAUTE proteins AGO1 and ZLL in development and small RNA-directed gene silencing. PLoS Genet 5: e1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory AC, Vaucheret H (2009) ARGONAUTE 1 homeostasis invokes the coordinate action of the microRNA and siRNA pathways. EMBO Rep 10: 521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroney PA, Yu Y, Fisher J, Nilsen TW (2006) Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat Struct Mol Biol 13: 1102–1107 [DOI] [PubMed] [Google Scholar]
- Martin M. (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17: 1 [Google Scholar]
- Martinez J, Tuschl T (2004) RISC is a 5′ phosphomonoester-producing RNA endonuclease. Genes Dev 18: 975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Gou L, Chen D, Mao C, Jin Y, Wu P, Chen M (2011) PmiRKB: a plant microRNA knowledge base. Nucleic Acids Res 39: D181–D187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Cai T, Hu Y, Chen Y, Hodges E, Ni F, Wu L, Li S, Zhou H, Long C, et al. (2008) Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133: 116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik KM, Böttcher R, Förstemann K (2012) A small RNA response at DNA ends in Drosophila. Nucleic Acids Res 40: 9596–9603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel J-B, Godon C, Mourrain P, Béclin C, Boutet S, Feuerbach F, Proux F, Vaucheret H (2002) Fertile hypomorphic ARGONAUTE (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. Plant Cell 14: 629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Weinberg DE, Bartel DP, Patel DJ (2012) Structure of yeast Argonaute with guide RNA. Nature 486: 368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottrott S, Simard MJ, Richter JD (2006) Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat Struct Mol Biol 13: 1108–1114 [DOI] [PubMed] [Google Scholar]
- Olsen PH, Ambros V (1999) The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol 216: 671–680 [DOI] [PubMed] [Google Scholar]
- Petersen CP, Bordeleau ME, Pelletier J, Sharp PA (2006) Short RNAs repress translation after initiation in mammalian cells. Mol Cell 21: 533–542 [DOI] [PubMed] [Google Scholar]
- Pfaff J, Hennig J, Herzog F, Aebersold R, Sattler M, Niessing D, Meister G (2013) Structural features of Argonaute-GW182 protein interactions. Proc Natl Acad Sci USA 110: E3770–E3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, Bertrand E, Filipowicz W (2005) Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science 309: 1573–1576 [DOI] [PubMed] [Google Scholar]
- Poulsen C, Vaucheret H, Brodersen P (2013) Lessons on RNA silencing mechanisms in plants from eukaryotic argonaute structures. Plant Cell 25: 22–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Denli AM, Hannon GJ (2005) Biochemical specialization within Arabidopsis RNA silencing pathways. Mol Cell 19: 421–428 [DOI] [PubMed] [Google Scholar]
- Reis RS, Hart-Smith G, Eamens AL, Wilkins MR, Waterhouse PM (2015) Gene regulation by translational inhibition is determined by Dicer partnering proteins. Nat Plants 1: 14027. [DOI] [PubMed] [Google Scholar]
- Schirle NT, MacRae IJ (2012) The crystal structure of human Argonaute2. Science 336: 1037–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggerson K, Tang L, Moss EG (2002) Two genetic circuits repress the Caenorhabditis elegans heterochronic gene lin-28 after translation initiation. Dev Biol 243: 215–225 [DOI] [PubMed] [Google Scholar]
- Sheng G, Zhao H, Wang J, Rao Y, Tian W, Swarts DC, van der Oost J, Patel DJ, Wang Y (2014) Structure-based cleavage mechanism of Thermus thermophilus Argonaute DNA guide strand-mediated DNA target cleavage. Proc Natl Acad Sci USA 111: 652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16: 2001–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaferro JM, Aspden JL, Bradley T, Marwha D, Blanchette M, Rio DC (2013) Two new and distinct roles for Drosophila Argonaute-2 in the nucleus: alternative pre-mRNA splicing and transcriptional repression. Genes Dev 27: 378–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret H. (2008) Plant ARGONAUTES. Trends Plant Sci 13: 350–358 [DOI] [PubMed] [Google Scholar]
- Vaucheret H, Mallory AC, Bartel DP (2006) AGO1 homeostasis entails coexpression of MIR168 and AGO1 and preferential stabilization of miR168 by AGO1. Mol Cell 22: 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret H, Vazquez F, Crété P, Bartel DP (2004) The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev 18: 1187–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Ba Z, Gao M, Wu Y, Ma Y, Amiard S, White CI, Rendtlew Danielsen JM, Yang YG, Qi Y (2012) A role for small RNAs in DNA double-strand break repair. Cell 149: 101–112 [DOI] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G (1993) Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75: 855–862 [DOI] [PubMed] [Google Scholar]
- Yang L, Wu G, Poethig RS (2012a) Mutations in the GW-repeat protein SUO reveal a developmental function for microRNA-mediated translational repression in Arabidopsis. Proc Natl Acad Sci USA 109: 315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang H, Li L (2012b) Alternative mRNA processing increases the complexity of microRNA-based gene regulation in Arabidopsis. Plant J 70: 421–431 [DOI] [PubMed] [Google Scholar]
- Yoda M, Cifuentes D, Izumi N, Sakaguchi Y, Suzuki T, Giraldez AJ, Tomari Y (2013) Poly(A)-specific ribonuclease mediates 3′-end trimming of Argonaute2-cleaved precursor microRNAs. Cell Reports 5: 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander A, Holzmeister P, Klose D, Tinnefeld P, Grohmann D (2014) Single-molecule FRET supports the two-state model of Argonaute action. RNA Biol 11: 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao H, Gao S, Wang WC, Katiyar-Agarwal S, Huang HD, Raikhel N, Jin H (2011) Arabidopsis Argonaute 2 regulates innate immunity via miRNA393(∗)-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol Cell 42: 356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Hare P, Chua N-H (2006) Applications of chemical-inducible expression systems in functional genomics and biotechnology. In Salinas J, Sanchez-Serrano J, eds, Arabidopsis Protocols, Vol 323. Humana Press, New York, pp 329–342 [DOI] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Møller SG, Chua NH (2001) Chemical-regulated, site-specific DNA excision in transgenic plants. Nat Biotechnol 19: 157–161 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.