Processes that generate metabolite diversity and reprogram hormone biosynthesis are coordinately regulated to impart stress tolerance in Arabidopsis.
Abstract
Secondary metabolites play a key role in coordinating ecology and defense strategies of plants. Diversity of these metabolites arises by conjugation of core structures with diverse chemical moieties, such as sugars in glycosylation. Active pools of phytohormones, including those involved in plant stress response, are also regulated by glycosylation. While much is known about the enzymes involved in glycosylation, we know little about their regulation or coordination with other processes. We characterized the flavonoid pathway transcription factor TRANSPARENT TESTA8 (TT8) in Arabidopsis (Arabidopsis thaliana) using an integrative omics strategy. This approach provides a systems-level understanding of the cellular machinery that is used to generate metabolite diversity by glycosylation. Metabolomics analysis of TT8 loss-of-function and inducible overexpression lines showed that TT8 coordinates glycosylation of not only flavonoids, but also nucleotides, thus implicating TT8 in regulating pools of activated nucleotide sugars. Transcriptome and promoter network analyses revealed that the TT8 regulome included sugar transporters, proteins involved in sugar binding and sequestration, and a number of carbohydrate-active enzymes. Importantly, TT8 affects stress response, along with brassinosteroid and jasmonic acid biosynthesis, by directly binding to the promoters of key genes of these processes. This combined effect on metabolite glycosylation and stress hormones by TT8 inducible overexpression led to significant increase in tolerance toward multiple abiotic and biotic stresses. Conversely, loss of TT8 leads to increased sensitivity to these stresses. Thus, the transcription factor TT8 is an integrator of secondary metabolism and stress response. These findings provide novel approaches to improve broad-spectrum stress tolerance.
Plants produce a wide variety of secondary metabolites having diverse physical and chemical properties. This enables secondary metabolites to play an important role in the growth, development, and innate immunity of plants (Wink, 2010). This huge diversity in the structure and function of secondary metabolites arises through various biochemical processes, such as conjugation, hydroxylation, and methylation, among others, which act on a relatively small number of core metabolites (Hartmann, 1996). Conjugation of metabolites is an active process that changes the basic chemical properties of acceptor metabolites, resulting in novel bioactivity, subcellular mobility, stability, and compartmentalization. Among various conjugation processes, glycosylation (addition of sugar moieties to aglycones) and deglycosylation (reversal of glycosylation) are among the most prominent (Bowles et al., 2005).
Several core aglycone molecules undergo regio- and sterio-selective glycosylation involving different sugar molecules, resulting in glycosides with diverse chemical structures and properties (Bowles et al., 2006; Vaistij et al., 2009). For example, 300 out of the 5,000 flavonoid forms arise from conjugation of a single flavonol, quercetin (Harborne and Baxter, 1999). Similarly, glycosylation of phytohormones is an important mechanism through which pools of bioactive hormones are regulated (Bajguz, 2007; Bajguz and Piotrowska, 2009; Westfall et al., 2013). Thus, glycosylation plays an indirect role toward regulating defense and stress response mechanism.
Among higher plants, nearly 4% of their respective genomes encode specialized enzymes, collectively known as carbohydrate-active enzymes (CAZy), most of which are involved in glycosylation processes (Lombard et al., 2014). As the chemical diversity of flavonoids is mainly generated through glycosylation, flavonoids serve as an excellent model to understand the coordination of multiple processes that regulate metabolite glycosylation. Previous studies have shown that the basic helix-loop-helix (bHLH) transcription factor TRANSPARENT TESTA8 (TT8; AT4G09820) regulates the expression of key enzymes such as BAN (for Banyuls) and DFR (for Dihydroflavonol 4-reductase) from the flavonoid biosynthesis pathway (Nesi et al., 2000; Baudry et al., 2004). TT8 loss-of-function lines showed altered chromatographic profiles of both aglycone (Pelletier et al., 1999) and glycosylated forms of the two flavonoids kaempferol and quercetin (Narasimhan et al., 2003). However, it is not known whether TT8 directly regulates genes involved in the glycosylation of these flavonoids or any other aglycone targets. Herein, we have used TT8 loss-of-function and induced overexpression lines to investigate: (1) glycosylation of flavonoids; (2) coordinated regulation of components of metabolite glycosylation; and (3) other biological processes directly coregulated with metabolite glycosylation. We hypothesize that TT8 affects glycosylation of flavonoids and possibly other metabolite classes by regulating a number of CAZy.
Integration of metabolomics and transcriptomics datasets have provided new insights into biochemical processes by linking lower level biological entities (such as DNA, RNA, and metabolites) with higher organizational levels (such as physiological and phenotypic response; Joyce and Palsson, 2006; Saito and Matsuda, 2010; Rai and Saito, 2016), so as to generate testable hypothesis in plants and other systems (Kresnowati et al., 2006; Hirai et al., 2007). We therefore studied whether the glycosylation process might be coordinately regulated by TT8 using systems biology approaches.
This study provides direct evidence to show that at least two strategies in plant defense, namely, generation of metabolite diversity and phytohormone-mediated reprogramming of stress response processes, are coordinately regulated by a common regulatory mechanism. Our results highlight the importance of using systems-level approaches to uncover novel relationships among cellular processes.
RESULTS
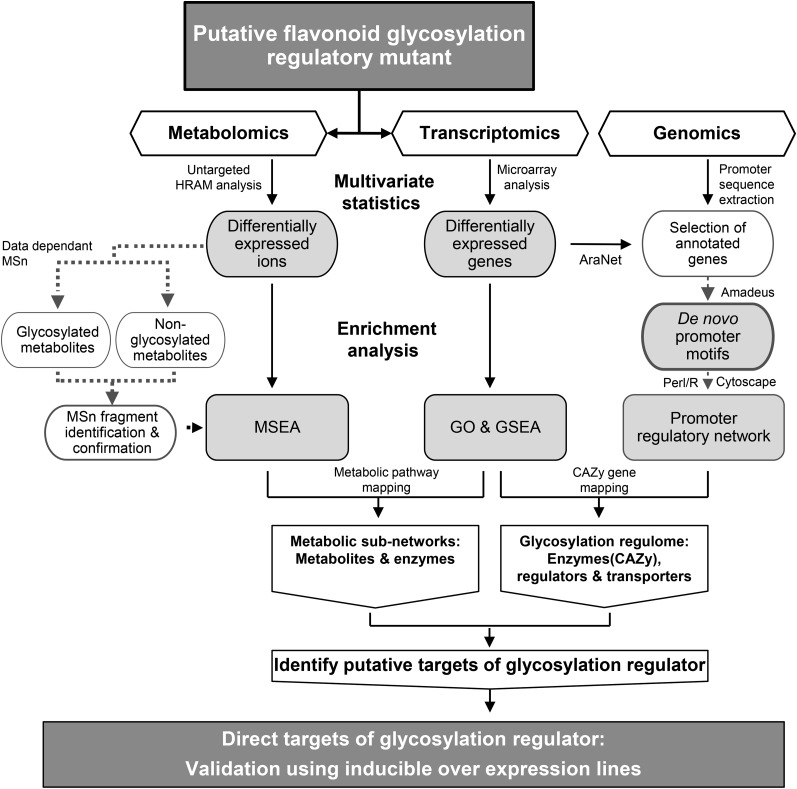
To discover components of the glycosylation machinery and its regulatory network, we developed an integrative omics strategy (Fig. 1). It consists of three tracks, namely, high-mass-accuracy metabolomics to identify targets of glycosylation, transcriptomics to identify genes involved in glycosylation, and transcriptome-dependent in silico genomics to reveal promoter relationships between CAZy and genes associated with other biological processes. These three tracks provide complementary information that is integrated at the level of metabolic networks. We then provide both computational and biochemical evidence to validate the role of TT8 in directly regulating key members of its glycosylation network.
Figure 1.
Integrative omics strategy to identify metabolic targets and regulome of a flavonoid glycosylation regulator. Differential metabolites and transcripts were identified from untargeted metabolite and expression profiling of a putative glycosylation regulatory mutant, tt8 (Ws). Glycosylated metabolites affected in tt8 (Ws) were identified by mass fragmentation. Integration workflow consists of two stages: (1) mapping enriched metabolites and gene sets onto metabolic pathways; and (2) mapping of enriched genes onto regulatory network based on promoter motif similarity between differentially expressed genes. The metabolites and enzymes of the metabolic subnetworks obtained from the first stage are further mapped onto the glycosylation regulome from the second stage. Finally, the overlapping putative targets of the regulator are validated using inducible overexpression (in Col background) and mutant lines (in Ws background) based on their reciprocal patterns of accumulation. GO, Gene ontology; GSEA, gene set enrichment analysis; MSEA, metabolite set enrichment analysis; HRAM, high-resolution accurate mass.
Loss of TT8 Affects Glycosylation of Flavonoids and Nucleotides
Untargeted metabolomics analysis of tt8 (Ws) and WT (Ws) (wild type in Wassilewskija [Ws] background) seedlings (Supplemental Fig. S1, A and B) showed changes in the levels of flavonoids and nucleotides, which were not previously reported to be associated with TT8. Principal coordinate analysis showed that the metabolite profiles of tt8 (Ws) and WT (Ws) were vastly different, with the primary component accounting for nearly 47% of the variation between these lines. Comparison of tt8 (Ws) and WT (Ws) revealed 1,259 mass features to be with 2-fold or higher difference in abundance (false discovery rate [FDR] < 0.05). Similarly, comparison of metabolite profiles of tt8 (Ler) with WT (Ler) (wild type in Landsberg erecta [Ler] background) showed that the primary component accounted for only 23% of variation between these lines, and only 611 mass features were differential with 2-fold or higher difference in abundance (FDR < 0.05; Supplemental Fig. S1C). Smaller differences in the metabolite profiles of the Ler background are consistent with the reports that tt8 (Ws) has a stronger phenotype than tt8 (Ler) (Nesi et al., 2000). Flavonols, flavanones, and anthocyanins formed the largest class of differential metabolites affected by the loss of TT8 (Supplemental Table S1). Consistent with previous reports (Pelletier et al., 1999; Nesi et al., 2000), kaempferol and quercetin aglycones were up-regulated in tt8 (Ws). Here, we report eight additional flavonoid aglycones whose glycosylated forms have not yet been shown to be affected in tt8 (Ws) (Supplemental Table S1). Overall, TT8 loss resulted in significant down-regulation of 18 out of 29 glycosylated flavonoids. Glycosylation of flavonoid aglycones to specific classes of sugars showed opposite trends. Glycoside conjugation with classes of sugars had different influences of TT8. For example, the majority of glycoside-conjugated forms of quercetin and kaempferol were down-regulated in tt8 (Ws), while rhamnoside-conjugated forms were mostly up-regulated (Supplemental Table S1).
Interestingly, loss of TT8 affected nucleotides and their glycosylated forms. Five out of eight aglycone nucleotides and seven out of 20 glycosylated nucleotides were up-regulated in tt8 (Ws) (Supplemental Table S1). Specifically, a majority of the nucleotide sugars of UDP were down-regulated by more than 6-fold. The different types of sugars involved in nucleotide glycosylation (10 types) were more than the sugars involved in flavonoid glycosylation (seven types). Loss of TT8 resulted in greater diversity among the classes of conjugated sugars in nucleotides when compared with flavonoids. Taken together, we observed glycosylation of both primary and secondary metabolites being affected in tt8 (Ws) lines, with aglycones being up-regulated and their different glycosylated forms being effectively down-regulated when compared with WT (Ws).
Sugar Metabolism and Glycosylation-Associated Processes Are Affected by TT8
To identify genes affected by TT8 loss, we performed gene expression profiling for tt8 (Ws) and WT (Ws) (Supplemental Fig. S1D). Consistent with previous reports (Nesi et al., 2000; Baudry et al., 2004), known targets of TT8, namely, BAN and DFR, along with a number of biosynthesis genes from phenylpropanoid and flavonoid pathways, were down-regulated in tt8 (Ws) with respect to WT (Ws).
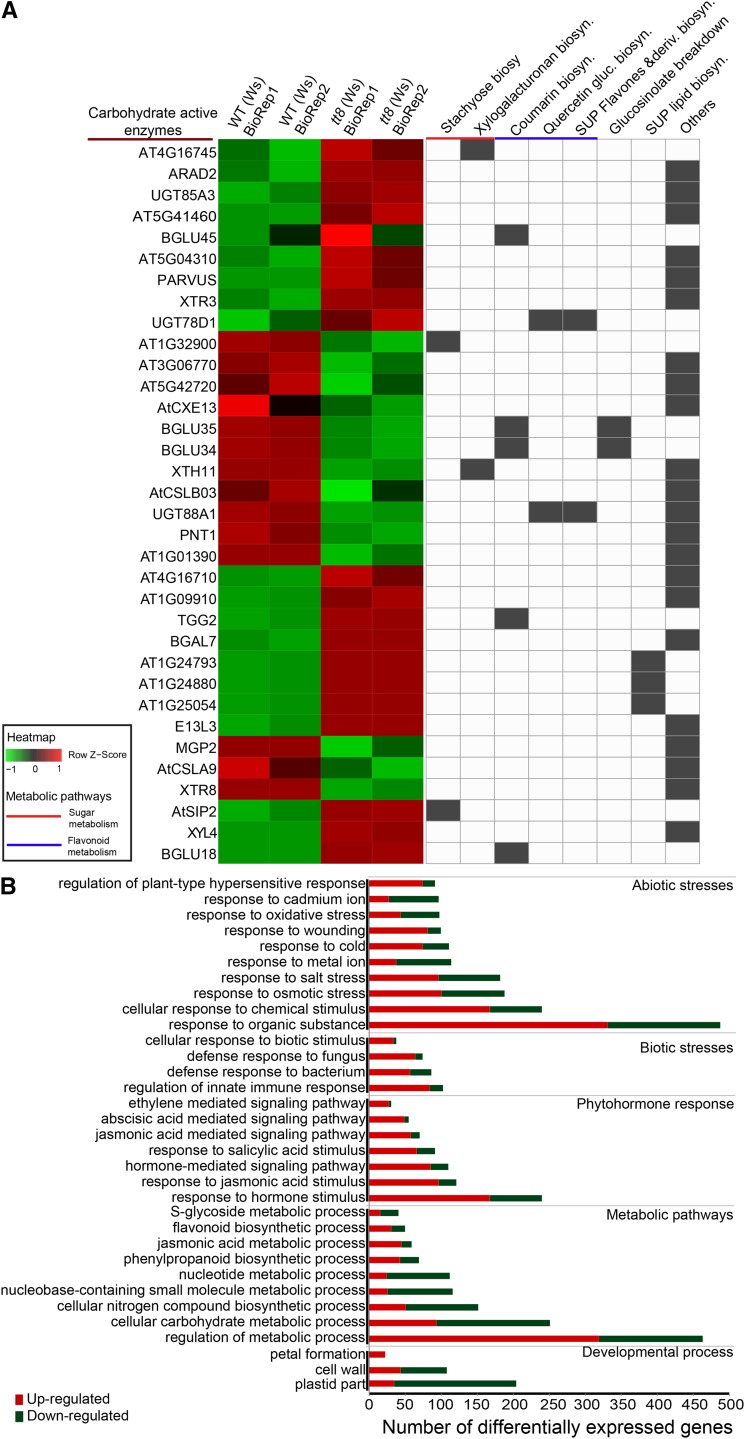
As metabolomics analysis of tt8 (Ws) lines showed significant perturbation in the levels of glycosylated metabolites, we expected changes in the gene expression levels of CAZy. Consistent with our hypothesis, loss of TT8 significantly affected CAZy gene levels, with 34 genes being differentially expressed by greater than 2-fold (Fig. 2) and nearly 10% of the total (129/1,194) CAZy genes significantly affected by more than 1.5-fold. Expression levels of several CAZy genes were consistent with the trends shown in their associated metabolite levels. For instance, UGT78D1, a glycosyltransferase that conjugates rhamnosides to kaempferol (Jones et al., 2003), was up-regulated by 6.5-fold (Fig. 2A; Supplemental Table S2), showing the same trend as that of rhamnoside-conjugated forms of kaempferol in tt8 (Ws) (Supplemental Table S1). Similarly, UGT88A1, which glucosylates quercetin (Lim et al., 2004), was down-regulated by 2.5-fold (Fig. 2A; Supplemental Table S2), while three forms of quercetin glucosides were down-regulated in tt8 (Ws) (Supplemental Table S1). Both UGT78D1 and UGT88A1 are associated with several pathways in sugar conjugation.
Figure 2.
CAZy and stress response-associated gene ontology categories enriched in tt8. A, Heatmap on the left shows relative levels of differential gene abundances classified under CAZy, with absolute fold change > 2 and FDR < 0.05, and computed as z-scores using heatmap2 function in R. Each row represents a gene and column an average of the two replicates each of tt8 (Ws) and WT (Ws) in each slide. In the matrix on the right, each column represents a pathway from AraCyc, with the presence of a gene in that pathway shown in gray. Fourteen out of 34 genes belong to sugar, flavonoid, or hormone biosynthesis. Genes not classified under these pathways were annotated as others. B, Gene set enrichment analysis was performed on 1,284 differentially expressed genes using the PlantGSEA tool. The top 33 enriched gene ontology categories (FDR < 0.05) with the number of differentially expressed genes affected in each category are shown here. Within each of the enriched gene ontology categories, the number of up- and down-regulated genes are shown in red and green, respectively.
By integrating metabolomics and transcriptomics data using subnetwork enrichment analysis, we identified 26 metabolic subnetworks to be significantly affected in tt8 (Ws) compared with WT (Ws) (Supplemental Table S3). The phenylpropanoid pathway was the most enriched pathway, mainly due to the large number of glycosylated flavonoids and their corresponding enzymes being affected in the tt8 (Ws) line. Interestingly, the inositol phosphate pathway, which is associated with signaling mechanism in defense responses, was also significantly enriched. These two pathways were followed by nucleotides and sugar metabolism (based on the number of gene and metabolite entities, and the FDR of enrichment). Sugar metabolism networks, including that of Fru, Man, and pyruvate pathways, were enriched together with glycolysis and TCA cycle (Supplemental Table S3). Branches of the TCA cycle that lead to amino acid pathways, such as Arg, Pro, and Cys and Met metabolism, were also enriched. Subnetworks associated with the pentose phosphate and amino acid pathways that contribute precursors to the components of the nucleotide network were significantly enriched, suggesting indirect influence of the enriched sugar metabolism network on nucleotide metabolism.
In addition to the sugar and nucleotide metabolic subnetworks, a number of sugar transporters (such as SUC6 and SUC7), Suc synthases (such as SUS2, SUS3, and SUS5), and sugar-binding proteins (such as STP6) were down-regulated by more than 1.5-fold in tt8 (Ws). Taken together, gene expression analysis showed disruption of sugar conjugation machinery in tt8 (Ws) with the members of sugar metabolism and glycosylation machinery such as Suc synthases, sugar-binding proteins, sugar transporters, glycosyltransferases, and hydrolases being affected, thus explaining the widespread perturbation in glycosylation of metabolites.
Abiotic and Biotic Stress Responses and Stress Phytohormone Biosynthesis Networks Are Affected by TT8
Gene set enrichment analysis revealed that 14 biological processes associated with abiotic and biotic stress responses were differentially altered (Fig. 2B). Genes associated with stress response constituted nearly 30% of the total number of differentially expressed genes (Supplemental Table S4). Abiotic and biotic stress responses, hormone response, metabolic pathways, and developmental functions were the major biological processes affected by TT8 loss (Fig. 2B). Genes associated with biotic stress response showed overall up-regulation, while abiotic stress categories showed a mixed trend, with hormone response and biosynthesis processes being effectively up-regulated in tt8 (Ws). Subnetworks belonging to phytohormone biosynthesis, namely, jasmonates in the α-linolenic acid pathway, were significantly up-regulated by the loss of TT8 (Supplemental Table S3).
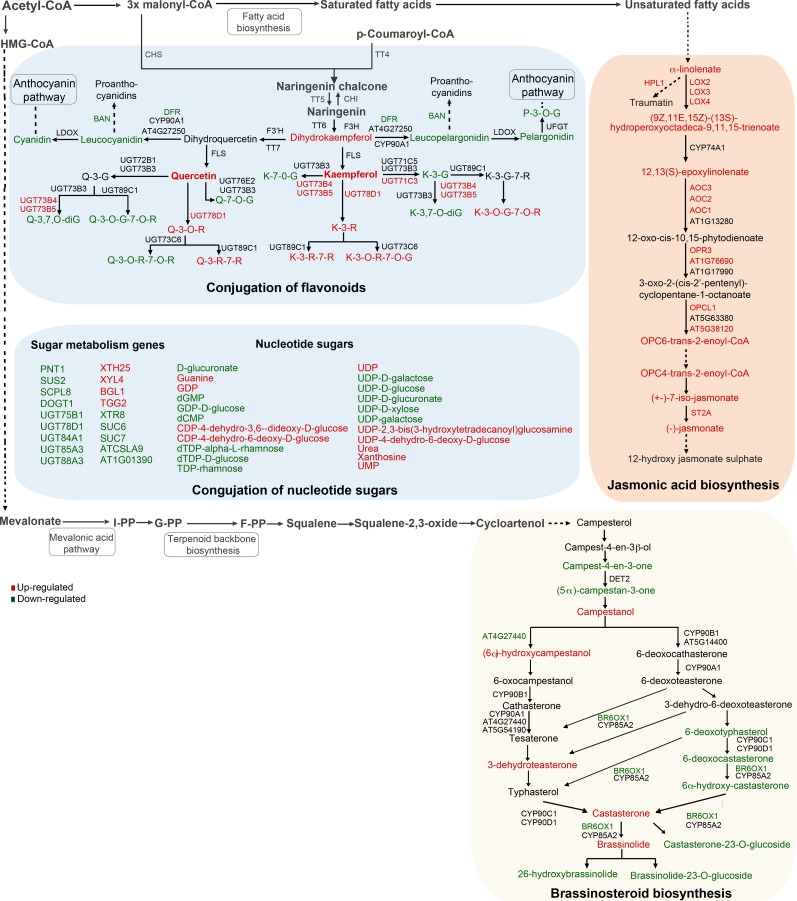
From the jasmonic acid biosynthesis pathway, nine out of 12 differential metabolites that belong to both initial and late steps of this pathway were up-regulated between 2- to 12-fold in the tt8 (Ws) line (Fig. 3; Supplemental Table S1). Interestingly, both glycosylated and amino-conjugated forms of jasmonate were not affected in tt8 (Ws). Similar to the levels of metabolite intermediates, expression levels of nine genes from the jasmonic acid biosynthesis pathway, 14 jasmonic acid response genes (including JAZ1–JAZ12 with the exception of JAZ3), and jasmonate signaling gene MYC2 were all up-regulated in tt8 (Ws) (Supplemental Table S2). The effect of TT8 loss extended upstream of the jasmonic acid biosynthesis pathway to its fatty acid precursor, α-linolenic acid. This corroborates the previous study that showed the inhibitory effect of TT8 on fatty acid biosynthesis based on biochemical evidence (Chen et al., 2014).
Figure 3.
TT8 loss affects flavonoid glycosylation, jasmonic acid, and brassinosteroid biosynthesis pathways. Differentially expressed metabolites and genes, with FDR < 0.05 and absolute fold change > 2, were mapped onto the top 3 enriched pathways, namely, biosynthesis of flavonoids and their conjugation, jasmonic acid, and brassinosteroids. In the conjugation of flavonoids section, the abbreviations are as follows: K, kaempferol; Q, quercetin; G, glucoside; and R, rhamnoside. Up- and down-regulated genes and metabolites are shown in red and green, respectively. Loss of TT8 leads to coordinated increase in transcript and metabolic levels of jasmonic acid pathways, whereas the other two pathways are down-regulated. TT8 loss also affected nucleotide sugar levels along with sugar metabolism enzymes, transporters, and sugar-binding proteins. Selected pathways shown here are based on AraCyc 8.0 and cross-validated with KEGG Arabidopsis metabolic maps.
Biosynthesis of brassinolide from campesterol occurs through two alternative routes, one through (6α)-hydroxycampestanol and second through 6-deoxycathasterone in Arabidopsis (Arabidopsis thaliana). Although both branches for brassinolide biosynthesis are active in the wild type (Noguchi et al., 2000), in the tt8 (Ws) line the (6α)-hydroxycampestanol branch was specifically up-regulated while the 6-deoxycathasterone branch was down-regulated, thus suggesting a preferred route for brassinolide biosynthesis in tt8 (Ws) (Fig. 3). The key genes, BR6OX1 and AT4G27440, were down-regulated by more than 2-fold, while components of the brassinosteroid signaling cascade, such as SERK4 (AT2G13790) and SERK5 (AT2G13800), were up-regulated by more than 2-fold. Unlike jasmonate signaling, expression levels of several key brassinosteroid signaling and response genes, such as DWF4, BRI1, BAX1, BZR1, BIN1, BIN2, and BES1, were not affected.
TT8 Regulatory Network Includes Genes Associated with CAZy, Phytohormone Biosynthesis, and Stress Response
To determine whether genes that are differentially expressed in tt8 (Ws) share transcription factor binding sites (TFBS) and thus possibly are regulated under a common mechanism, we used a network-guided guilt-by-association approach (Lee et al., 2010). TFBS-based relationships were uncovered at a regulome level (encompassing a set of regulatory components such as genes, enzymes, proteins, and metabolites), and enriched 8-mer and 10-mer de novo promoter motifs (Linhart et al., 2008) were analyzed to obtain shared TFBS between differentially expressed genes (Supplemental Table S5).
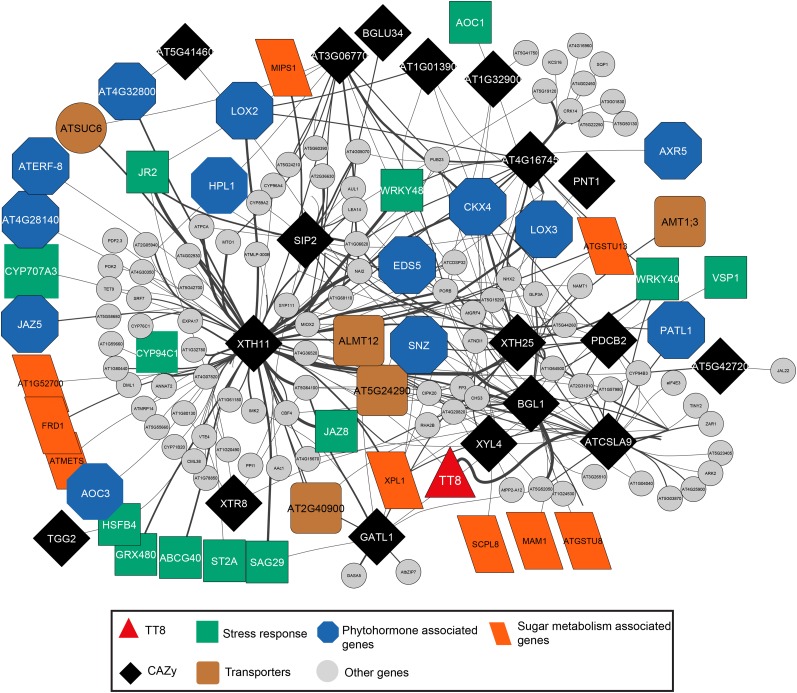
We identified members of the TT8 glycosylation regulome by constraining the promoter network to CAZy genes. This rationale was based on our results that showed metabolite levels of glycosylated flavonoids and nucleotides and expression levels of CAZy genes involved in glycosylation processes being affected in tt8 (Ws). Using this criterion, genes that shared promoter motifs with the CAZy genes were used to form an undirected network consisting of nodes representing genes and the edges representing shared motifs (Fig. 4; Supplemental Table S6). Several genes involved in carbohydrate and sugar conjugation processes, such as glucosidases, Glc transporters, and glucosyltransferases, shared a high number of motifs with each other. EmBP-1, Squamosa, and MYB, along with other bZIP-related binding domains, were overrepresented in the glycosylation regulome (Supplemental Table S7). Interestingly, genes associated with stress response, jasmonic acid biosynthesis, and cytochrome P450 genes involved in the initial steps of brassinosteroid biosynthesis also shared a high number of promoter motifs with CAZy genes (Supplemental Table S6). Of the 10 gene-metabolite pairs enriched in the jasmonate subnetwork (Fig. 3), eight genes form a part of the TT8 regulome (Fig. 4).
Figure 4.
CAZy coding genes share motif similarity with stress response and phytohormone-associated genes. Differentially expressed genes form the nodes, while the edges are based on sharing of promoter motifs. To identify relationships between sugar metabolism genes and those of other pathways in the regulome, a constraint was applied to connect nodes of sugar metabolism genes that share a minimum of 14 motifs (top 25 percentile) with other genes. This constrained regulatory network is shown here. The width of the edges corresponds to the number of shared motifs between the two nodes, with high similarity resulting in thicker edges. This glycosylation regulome consists of 18 enzymes (CAZy) connected mostly with stress response genes (13 genes) and phytohormone-associated genes (13 genes), followed by transporters (five genes) and and transferases, hydrolases, and oxidases (nine genes). Other genes in the glycosylation network but not directly annotated to any of the above functional processes are shown as gray circles.
TT8 Actively Reprograms Hormone Biosynthesis and Sugar Conjugations
To test whether TT8 is directly associated with the regulation of metabolite glycosylation, jasmonic acid and brassinosteroid biosynthesis, and key genes of stress response processes, dexamethasone (DEX)-inducible 2x35S:TT8:GR overexpression lines in Columbia (Col) background were generated. Three lines of evidence show that DEX-induced TT8:GR (Col) overexpression lines are functional. First, consistent with previous reports (Baudry et al., 2004, 2006), these lines, upon DEX treatment, show increased expression of direct targets of TT8, namely, BAN and DFR (Fig. 5B, “Controls” BAN and DFR; Supplemental Fig. S2E). Promoter fragments of BAN were also induced to nearly 5.5-fold in DEX-induced TT8:GR (Col) overexpression lines with respect to mock-treated TT8:GR (Col) lines in chromatin immunoprecipitation (ChIP)-PCR assay (Fig. 6B; Supplemental Fig. S3; Supplemental Tables S8 and S9). This corroborates earlier reports (Debeaujon et al., 2003), which show that the enrichment of the same promoter fragment is sufficient to activate BAN. Thus, the significant induction of BAN serves as a positive control for DEX-induced TT8:GR (Col) overexpression lines. Second, TT8 has been reported to play an important role in trichome formation (Maes et al., 2008). In this study, DEX-induced TT8:GR (Col) overexpression lines show a significantly higher number of trichomes compared with mock-treated TT8:GR (Col) and WT (Col) lines (Supplemental Fig. S2, C and D). Last, TT8 has been shown to regulate seed coat pigmentation (Nesi et al., 2000; Zhang et al., 2003); thus, consistent with these reports, seeds of DEX-induced TT8:GR (Col) overexpression lines are darker than mock-treated TT8:GR (Col) (Supplemental Fig. S2B). Untargeted metabolite profiling also revealed levels of epicatechin, a type of proanthocyanidin (Debeaujon et al., 2003), to be 2.3-fold higher in DEX-induced TT8:GR (Col) overexpression compared with mock-treated TT8:GR (Col) lines (Supplemental Table S1).
Figure 5.
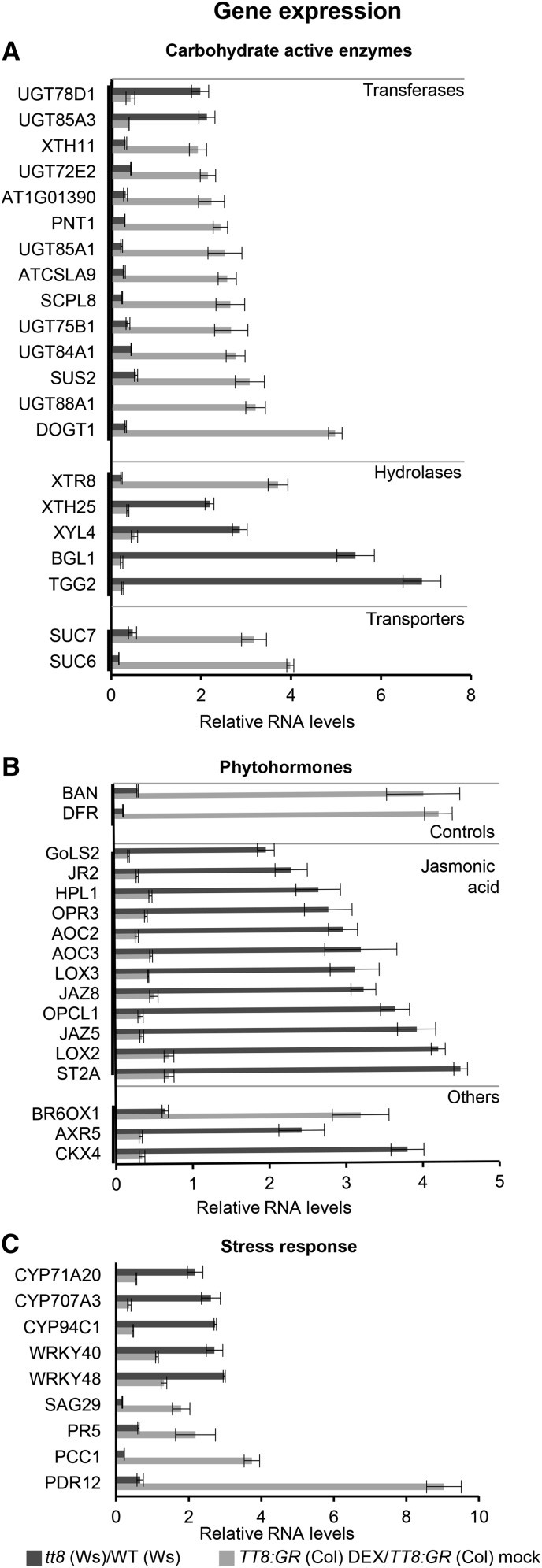
Validation of TT8 glycosylation regulome members using mutant and inducible overexpression lines. From the glycosylation regulatory network, 18 CAZy with top 3 enriched processes, 13 phytohormone-associated genes, and nine stress response-associated genes showed reciprocal expression levels in tt8 (Ws) and its DEX-induced TT8:GR (Col) overexpression lines. Relative transcript levels of genes were quantified using real-time PCR normalized against tubulin2 as control. Shown are genes associated with CAZy (A), phytohormone biosynthesis (B), and stress response (C). Light and dark gray bars represent relative transcript levels in TT8:GR (Col) DEX/TT8:GR (Col) mock and tt8 (Ws)/WT (Ws), respectively. Results are shown as mean ± se based on three replications (P < 0.05).
Figure 6.
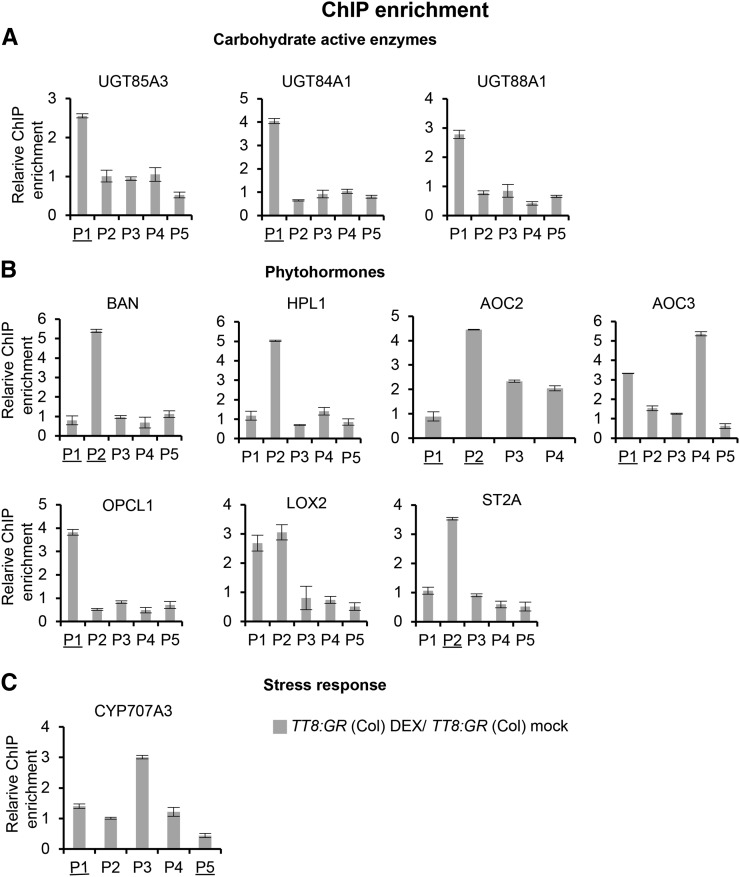
TT8 directly binds to promoters of key genes. Using ChIP-PCR, enrichment of promoter fragments of target genes was quantified using real-time PCR normalized against actin2. Relative ChIP enrichment was calculated as TT8:GR (Col) DEX/TT8:GR (Col) mock. TT8:GR (Col) lines were treated with 30 µm DEX dissolved in ethanol, while mock was treated with equivalent volume of ethanol. Fold changes were calculated based on ΔΔCt values. Shown are genes associated with CAZy (A), phytohormone biosynthesis (B), and stress response (C). Results are shown as mean ± se based on three replications (P < 0.05). Direct binding of TT8 to promoter regions of these genes results in enriched expression of promoter fragments (P1–P5). The underlined enriched fragments indicate the presence of a known bHLH binding site in that region.
Both DEX and mock-treated WT (Col) lines have similar phenotypes and germination rates under both stress-free conditions and stressed conditions of both biotic (Supplemental Fig. S4) and abiotic nature (Supplemental Fig. S6). Furthermore, both the phenotypes and trichome numbers of DEX-treated WT (Col) are similar to mock-treated WT (Col) (Supplemental Fig. S2B). These results show that DEX treatment only leads to induced overexpression in TT8:GR (Col) lines and does not induce TT8 or affect the TT8-related phenotypes nonspecifically in WT (Col) lines.
We hypothesized that members of the TT8 glycosylation regulome will show inverse relationships in their expression levels in mutant and induced overexpression lines. We selected representative genes for each functional category from the promoter network and quantified their expression levels relative to their respective controls [tt8 (Ws) versus WT (Ws) and DEX-induced TT8:GR (Col) overexpression versus mock-treated TT8:GR (Col); Supplemental Table S10]. Relative quantification of CAZy transcripts, such as transferases, sugar transporters, and hydrolases, indeed showed reciprocal expression trends, thus revealing their regulation by TT8 (Fig. 5A). For stress phytohormones, 12 jasmonic acid-associated genes (Fig. 5B) and BR6OX1 from the brassinosteroid biosynthesis pathway showed reciprocal expression trends in both loss-of-function and inducible overexpression lines. Stress response genes also showed inverse relationships in these two sets of lines (Fig. 5C). These inverse gene expression trends were also corroborated at the respective metabolite levels. Seven out of 12 up-regulated metabolite intermediates from jasmonic acid pathways in tt8 (Ws) were down-regulated in DEX-induced TT8:GR (Col) overexpression lines (Supplemental Table S1). In addition, 14 out of 29 glycosylated flavonoids that were down-regulated in tt8 (Ws) were up-regulated in the DEX-induced TT8:GR (Col) overexpression lines. These results show that TT8 expression results in active reprogramming of stress-related hormone biosynthesis, stress-associated genes, and sugar conjugation processes in Arabidopsis.
TT8 Physically Binds to the Promoters of Jasmonic Acid Biosynthesis and Sugar Conjugation Genes
Direct binding of TT8 to the promoters of selected jasmonate biosynthesis and CAZy genes were tested using ChIP-PCR. Consistent with previous reports (Debeaujon et al., 2003), promoter fragments of BAN, a direct target of TT8, were enriched in DEX-induced TT8:GR (Col) overexpression lines by 5.5-fold with respect to mock-treated TT8:GR (Col) lines (Fig. 6B). Promoter fragments of six jasmonic acid biosynthesis genes were similarly enriched in DEX-induced TT8:GR (Col) overexpression lines (Fig. 6B). Interestingly, JAZ5, which showed inverse gene expression trends relative to TT8, did not show any promoter enrichment, thus suggesting an indirect influence of TT8 on the expression of JAZ5. Among the CAZy genes, UGT84A1, UGT85A3, and UGT88A1 (Fig. 6A) and stress response gene CYP707A3 (Fig. 6C) showed more than 2.5-fold promoter enrichment in DEX-induced TT8:GR (Col) overexpression lines. The predicted bHLH binding sites in the promoter region of these genes were determined using AtcisDB (TAIR9) from the AGRIS server (Yilmaz et al., 2011), AthaMap (June 2014 version; Hehl and Bülow, 2014), and AtCOECiS (accessed June 2014; Vandepoele et al., 2009) databases. Interestingly, such regions were also enriched in the ChIP-PCR (Fig. 6), thus suggesting that TT8, being a bHLH transcription factor, likely binds to these regions. In addition, a number of de novo motifs that were earlier identified (Supplemental Tables S5 and S8) were also detected in these regions. There is a small but significant positive correlation (Pearson’s correlation coefficient r = 0.438, P = 0.002) between the number of de novo motifs and enrichment levels of the promoter fragments, with fragments that have a high number of de novo motifs showing greater enrichment. Collectively, these results suggest TT8 to be a potential master regulator that coregulates conjugation and stress response processes by directly binding to the promoters of CAZy and stress-associated biosynthesis genes.
TT8 Overexpression Improves Resistance of Arabidopsis to Virulent Pseudomonas syringae
We next tested the biological implication of coregulation of metabolite glycosylation and stress response processes by TT8. As increased jasmonic acid biosynthesis results in enhanced susceptibility to bacterial infection (Zeng et al., 2011; Gimenez-Ibanez et al., 2014), we tested susceptibility of TT8 mutant and induced overexpression lines against the pathogenic strain Pseudomonas syringae pv tomato (Pst) strain DC3000. Compared with WT (Ws), tt8 (Ws) lines were highly susceptible to Pst DC3000 (Fig. 7A). Bacterial growth assay was also consistent with this result, showing significant increase of bacterial growth in the tt8 (Ws) lines compared with WT (Ws) (Fig. 7C). On the contrary, DEX-induced TT8:GR (Col) overexpression lines showed marked reduction of bacterial infection, and significantly reduced bacterial load at 3 d postinfection compared with the mock-treated TT8:GR (Col) lines (Fig. 7, B and C). WT (Col) treated with or without DEX showed no difference in bacterial infection, and responded similarly to mock-treated TT8:GR (Col) lines (Supplemental Fig. S4). This role of TT8 in enhancing resistance against the virulent Pst DC3000 strain could be attributed to its ability to regulate jasmonic acid biosynthesis, along with the expression of several biotic stress response factors. These results highlight the direct role of TT8 in mediating biotic stress response in Arabidopsis. The ability of TT8 to directly regulate gene expression and, therefore, key metabolite levels in phytohormone pathways and stress response processes may explain this increased resistance phenotype.
Figure 7.
tt8 (Ws) leads to enhanced susceptibility, while DEX-induced TT8:GR (Col) overexpression increases resistance against Pst DC3000 infection. A, Disease symptoms in WT (Ws) and tt8 (Ws); B, DEX-induced overexpression of TT8:GR (Col) and mock-treated TT8:GR (Col) lines. Five-week-old Arabidopsis plants were spray-inoculated with a suspension of Pst DC3000 (108 cfu) or mock-treated. The images of plants showing disease symptom were taken 3 d after inoculation. C, Bacterial growth in leaves of WT (Ws), tt8 (Ws), DEX-induced TT8:GR (Col) overexpression, and mock-treated TT8:GR (Col) lines, 3 d after spray inoculation of Pst DC3000 suspension or mock treatment. Results are shown as mean ± se based on three replications (P < 0.05).
TT8 Overexpression Enhances Stress Tolerance
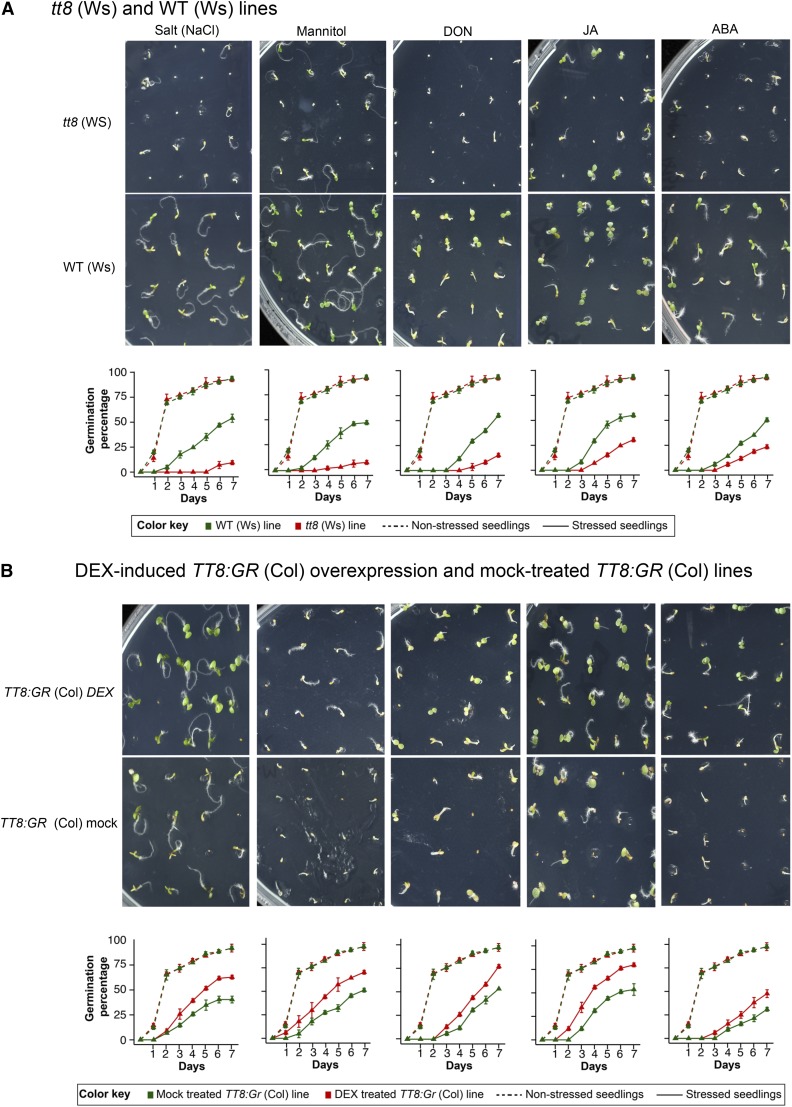
Different abiotic and biotic stresses inhibit or delay seed germination (Daszkowska-Golec, 2011). As our results show that TT8 directly regulates expression of several genes associated with stress response, stress-associated hormone biosynthesis pathways along with CAZy genes, we asked whether germination rates are influenced by TT8 during stress conditions. Hence, we recorded germination rates of TT8 loss-of-function or inducible overexpression lines under selected abiotic and biotic stress conditions over a period of 1 week. Salt (150 mm NaCl), mannitol (250 mm), and abscisic acid (ABA; 1 µm) treatments were selected to represent abiotic stress conditions, while methyl jasmonate (100 µm MeJA) and deoxynivalenol (10 mg kg−1 DON, a fungal toxin) were selected to represent biotic stresses.
The tt8 (Ws) line had significant reduction in the germination rates for all five treatments compared with WT (Ws) (Fig. 8A), while the germination rates in the tt8 (Ws) and WT (Ws) lines were similar in the nonstressed controls. Effects of the five treatments on the germination rates started appearing between day 1 for salt and day 3 for DON. These effects were most pronounced in salt, mannitol, and DON treatments with the tt8 (Ws) lines showing nearly 50% lower germination rates at the end of 1 week.
Figure 8.
Under multiple stress conditions, germination of Arabidopsis improves in a TT8-dependent manner. WT (Ws) and tt8 (Ws) were sown directly on 1× MS (2% Suc and 0.6% phytoagar) agar plates. The conditions were as follows: 150 mm NaCl, 250 mm mannitol, 10 mg kg−1 DON, 1 µm ABA, and 100 µm MeJA. TT8:GR (Col) inducible lines were sown similarly for the same treatment conditions except with addition of 30 µm DEX or equivalent volume of ethanol as mock treatment. Number of seeds germinating (three biological replicates with 28 seeds sown for each replicate) each day was calculated under more severe stress conditions of 200 mm NaCl, 500 mm mannitol, 20 mg kg−1 DON, 200 µm MeJA, and 10 µm ABA. For induced overexpression the conditions were similar, except for 15 mg kg−1 DON. Images of 6-d-old seedlings were captured for both tt8 (Ws) and WT (Ws) (A), and DEX-induced TT8:GR (Col) overexpression and mock-treated TT8:GR (Col) lines (B). Germination rates are shown as mean ± sd.
Trends of germination rates were reversed in the case of DEX-induced TT8:GR (Col) overexpression lines when compared with tt8 (Ws). DEX-induced TT8:GR (Col) overexpression led to nearly 20% to 30% improvement in the germination rates for all five treatments (Fig. 8B). One mechanism of resistance to DON is conversion of DON to DON-3-O-glucoside (Shin et al., 2012). Therefore, we quantified levels of DON and glycosylated DON in the tt8 (Ws) and DEX-induced TT8:GR (Col) overexpression lines (Supplemental Fig. S5). The DEX-induced TT8:GR (Col) overexpression line showed up-regulation of glycosylated DON by 6.89-fold compared with mock-treated TT8:GR (Col) lines, while no change was observed in tt8 (Ws) lines when compared with their control, WT (Ws). This showed that the improved germination rates were a result of increased glycosylation of DON in DEX-induced TT8:GR (Col) overexpression lines. Furthermore, as control, DEX-treated WT (Col) showed similar germination patterns when compared with mock-treated WT (Col) under both normal conditions and multiple stress conditions (Supplemental Fig. S6). Thus, the phenotypes observed were independent of DEX and were dependent on TT8 function. These results establish that TT8 plays a direct role in tolerance toward multiple abiotic and biotic stresses.
DISCUSSION
The diversity of secondary metabolites is critical for their role in plant stress response (Wink, 2010). In this study on the mechanism of glycosylation, which generates diverse secondary metabolites, we have discovered direct links between processes that regulate glycosylation and stress phytohormone-related pathways in Arabidopsis. By integrating systems-level datasets, we report here that (1) metabolic subnetworks of sugar conjugation, hormone biosynthesis, and stress response are affected in a TT8-dependent manner, and (2) strong promoter relationships exist between genes of CAZy and those of stress-related hormone biosynthesis and stress response processes.
Previous studies have elucidated the role of transcription factors in the regulation of flavonoid pathways and identified their regulatory partners in the process. These studies, for instance, explained that regulation of key enzymes of flavonoid biosynthesis occurs via the transcription factor complex (MYB-bHLH-WD40) that includes TT8 (Falcone Ferreyra et al., 2012). Targeted metabolic profiling of 11 transparent testa lines (mutants from the flavonoid biosynthesis pathways), including tt8, showed that the loss of these genes has a significant effect on flavonoid accumulation patterns and on the root and shoot architecture (Buer et al., 2013). While these reports have improved our understanding of the role of flavonoid regulators, their scope was limited to flavonoid pathways. This study establishes a common regulatory mechanism for glycosylation of selected primary (nucleotides) and secondary (flavonoids and brassinosteroids) metabolites. The cellular needs for metabolite glycosylation are wide ranging, and the process is highly coordinated as evident from our results, which show the involvement of CAZy, transporters, sugar-binding proteins, and sugar synthases (Fig. 3). TT8 regulation also affects central metabolism, involving both carbohydrate metabolism and amino acid biosynthesis pathways including activated nucleotide sugars (Supplemental Table S3), thus establishing TT8’s important role in multiple metabolic processes. Activated sugars are involved in the metabolism and homeostasis of signaling molecules, such as phytohormones, gibberellins, and ABA, among others (Gibson, 2004), where glycosylation regulates pools of their activated forms (Bajguz, 2007; Bajguz and Piotrowska, 2009; Westfall et al., 2013). It will be interesting to explore whether any of those functions are mediated, directly or indirectly, by TT8. As activated sugars are also involved in protein glycosylation, does TT8 therefore represent the metabolic crossroad between glycosylation of metabolites and proteins?
While glycosylation of stress hormones is well-established, here we have shown that regulation of phytohormone biosynthesis, particularly jasmonic acid and brassinosteroid biosynthesis, together with expression of CAZy genes, is mediated by a common transcription factor, TT8. Our results suggest two roles of TT8 in regulating phytohormone levels: first, directly, by binding to the promoters of their biosynthesis genes and regulating their expression levels; and second, indirectly, by regulating expression of CAZy genes, which in turn regulate active pools of phytohormones. TT8 negatively regulates jasmonate and positively regulates brassinosteroid biosynthesis, which is also consistent with previously reported antagonistic relationships between jasmonic acid and brassinosteroid pathways (Ren et al., 2009). This negative regulation of the jasmonate pathway is specific to the biosynthesis genes, but not to those of downstream effector genes JAZ5 and JAZ8. Thus, TT8 might have a functional role similar to other bHLH transcription factors such as JAM1, JAM2, and JAM3 that are negative regulators of jasmonic acid biosynthesis (Sasaki-Sekimoto et al., 2013). Negative regulation of jasmonic acid biosynthesis explains the effects on its downstream targets, namely, COL1 and MYC2 (Supplemental Table S2). Interestingly, the TT8 regulatory network also revealed TFBS that are known to be enriched in the presence of regulators such as JAZ and a number of CAZy genes associated with plant stress response and sugar-related pathways (Kang et al., 2010; Qi et al., 2011; Kazan and Manners, 2012).
Three lines of evidence from our results suggest a direct role for TT8 in stress response. First, two phytohormone biosynthesis pathways of jasmonic acid and brassinosteroids, which are implicated in stress response (Bari and Jones, 2009), are directly regulated by TT8. Second, at least eight stress response proteins, which are implicated in the tolerance against salt and drought stress (Seo et al., 2008; Liu et al., 2013; Mir et al., 2013), are directly regulated in a TT8-dependent manner (Fig. 5C), thus implicating TT8 in reprogramming defense response. Last, TT8 has a direct role in increasing the diversity of core metabolites, particularly by regulating glycosylation of brassinosteroids and flavonoids. Latter have been implicated in radical scavenging activity that improves tolerance toward oxidative and drought stress (Nakabayashi et al., 2014). The predicted outcomes of the coordinated changes in stress phytohormones and flavonoid glycosylation are corroborated by tolerance of plants toward multiple stresses in a TT8-dependent manner. Loss of TT8 renders the plants to be more sensitive to fungal toxin (DON), jasmonic acid, ABA, drought, and salt stresses, and TT8-induced overexpression improves tolerance against the same (Fig. 8). TT8 also imparts tolerance to biotic stress (Fig. 7). Taking these together, we propose a model (Fig. 9) based on the role of TT8 in stress response, by directly regulating glycosylation of metabolites, and stress-associated hormone biosynthesis and stress response processes.
Figure 9.
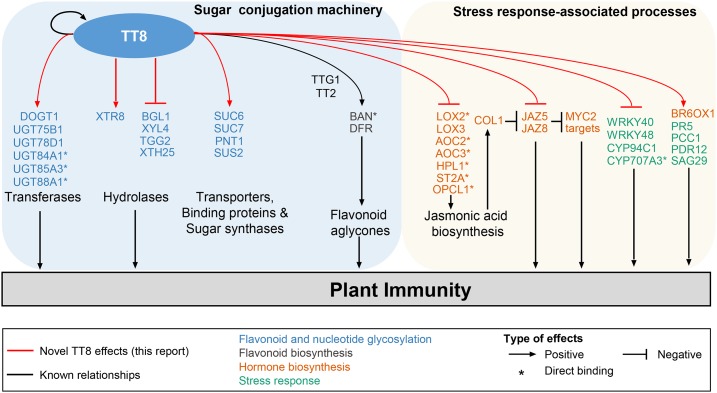
Model showing mechanism through which TT8 regulates response to biotic and abiotic stress conditions in Arabidopsis. We show that the metabolite sugar conjugation machinery is coregulated with stress response. The model involves TT8 as a key transcription factor that directly binds to the promoters of genes involved in these two processes. TT8 positively regulates flavonoids and nucleotide glycosylation, and negatively regulates jasmonic acid biosynthesis. Apart from biosynthesis genes, TT8 also regulates expression of several genes associated with stress response. In addition, TT8 regulates the gene expression levels of members of sugar conjugation machinery, such as transferases, hydrolases, transporters, binding proteins, and sugar synthases, which results in increased metabolite diversity. This model shows that TT8 is an integrator of secondary metabolism and stress response.
As biochemical processes that lead to increased diversity of secondary metabolites are coordinated with stress response in innate immunity in Arabidopsis, it will be interesting to study the presence of such direct integration in other dicot and monocot plants. These findings could, therefore, have applications in generating chemical diversity using synthetic biology approaches and in enhancing tolerance toward multiple stresses in plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants of ecotype Ws-2 and tt8-3 (deb122) (Nesi et al., 2000) were obtained from INRA, while tt8-2 and Ler-0 were obtained from the Arabidopsis Biological Resource Center. For TT8 loss of function, line tt8 (Ws) (Supplemental Fig. S2) was used, as this allele showed the strongest phenotype. tt8 (Col) being a leaky mutant is a weak allele; thus, it has no distinguishable phenotype with or without stress (Supplemental Fig. S7, “control,” “Salt (NaCl),” and “JA” stress response panels). Phenotypes of tt8 (Ler) were intermediate to those in Ws and Col background (Supplemental Fig. S8). Therefore, the mutant lines used in this study are (1) tt8-3 (Ws) for metabolomics, phenotype, and microarray analyses; and (2) tt8-2 (Ler) for additional metabolomics analyses. DEX-inducible TT8:GR overexpression lines were created by transforming 2x35S:TT8:GR in the wild-type Col-0 background by Agrobacterium-mediated floral dipping approach. TT8:GR overexpression lines were created in Col background (as it has the highest transformation efficiency among the ecotypes used here, unlike Ws background, which is highly recalcitrant for this purpose; Ghedira et al., 2013). Seeds were surface-sterilized using 30% bleach (Clorox) with 7-min incubation, followed by washing six times with autoclaved water and transfer to Murashige and Skoog (MS; Duchefa Biochemie) agar plate (1× MS media, 20 g/L Suc, and 6 g/L phytoagar, pH 5.7) at 4°C for 4 d before placing them in growth chamber at 22°C for 16-h-light/8-h-dark cycle. For inducible lines, seeds were sown directly on MS agar plates with 30 µm DEX (stock solution prepared in ethanol) or equivalent amount of ethanol for mock treatment. Photon flux density was set at 50 µmol m–2 s–1. Seedlings were harvested after 6 d for metabolites and RNA extraction. For the stress tolerance assays, Arabidopsis seeds were sown onto MS agar plate with or without salt, MeJA, mannitol, DON, and ABA. All primers used in this study are listed in Supplemental Table S9.
Metabolite Profiling and Analysis
Untargeted metabolic profiling was performed as described previously (Rai et al., 2013) with modifications (described in “Supplemental Data”) using two experimental platforms, namely, Agilent quadrupole time-of-flight mass spectrometry (Q-TOF 6540) and LTQ-Orbitrap Velos (Thermo Fisher Scientific). Metabolite extracts for four biological replicates of tt8 (Ws) and WT (Ws) lines, with each replicate injected three times as technical replicates, were used in quadrupole time-of-flight mass spectrometry, while Orbitrap had three biological replicates of tt8 (Ler) and WT (Ler) lines, with three technical replicates. Raw data were analyzed using XCMS package (version 1.38; Smith et al., 2006) in R (R Core Team, 2014). Log-transformed and quantile-normalized data subjected to Mann-Whitney U test with multiple testing correction using Benjamini-Hochberg FDR (Benjamini and Hochberg, 1995) yielded 1,259 and 611 differential features between tt8 and the wild type, with FDR < 0.05 and fold change > 2 in the Ws and Ler backgrounds, respectively. A total of 101 differential features were then recursively mapped (with mass tolerance < 5 and 10 mg kg−1) onto the KEGG Arabidopsis database (2014 version; Kanehisa and Goto, 2000; Kanehisa et al., 2014) using PCDL manager (Agilent). Of these, 46 metabolites then identified at MSI Level 2 (Sumner et al., 2007) using data-dependent tandem mass spectrometry and by matching the fragmentation patterns using database search. Raw data has been deposited to the MetaboLights (Haug et al., 2013) database.
DNA Microarray Analysis
Microarray-based expression profiling using Agilent Arabidopsis 4×44k Array were conducted for WT (Ws) and tt8 (Ws) lines (described in “Supplemental Data”). The experiments were performed for four biological replicates each for WT (Ws) and tt8 (Ws) lines, with two bio-replicates of each line being hybridized on a single slide. Genespring 12.0 software (Agilent) was used to perform normalization using percentile shift to 75%. The probes were then baseline-corrected to the median, and filtered by only selecting signal values between 20th and 100th percentile with the coefficient of variance < 20%. Filtered data were then used for statistical analysis using unpaired t test with asymptotic FDR computation and FDR correction to identify 1,284 differentially expressed genes (FDR < 0.05 and fold change > 2). These were then used for gene set enrichment analysis in PlantGSEA (Yi et al., 2013) and Subpathway enrichment using iSubpathwayMiner package (Li et al., 2013). Raw data have been deposited to the National Center for Biotechnology Information Gene Expression Omnibus database (Edgar et al., 2002).
ChIP-PCR
Promoters of target genes were scanned for putative motifs and five sets of primers were designed to cover entire the promoter, with motif position taken into account and named 1 to 5 (Supplemental Fig. S3). Promoter fragment 1 starts from the ATG start codon for each gene. Quantitative real-time PCR was performed in triplicates using the CFX384 real-time PCR system (Bio-Rad) with Maxima SYBR Green qPCR mix (Thermo Fisher Scientific) on total RNA extracted from 0.1 g of 6-d-old seedlings.
Promoter Analysis and Gene Regulatory Networks
Differentially expressed genes were analyzed using the network-guided guilt-by-association approach in Aranet (Lee et al., 2010) to identify target gene group. Among the 1,284 differentially expressed genes, genes having a minimal set of information in The Arabidopsis Information Resource were selected first. This approach removed 306 genes that did not contain any annotation or those where functional/sequence similarity to other organisms could not be identified. We scanned for enriched de novo motifs using Amadeus platform (Linhart et al., 2008) in the 968 differentially expressed genes with six different combinations, defined as: Promoter length: 1500, 1000, and 500 bp upstream of transcription start site; and Motif length: 8 and 10. An adjacency matrix (Supplemental Table S5), which contained the number of shared motifs between any two genes (Supplemental Table S6), revealed the maximum motif similarity between the 23 CAZy genes and other differentially expressed genes to be 19 motifs. Using an approach similar to Vandepoele et al. (2009), we tested different constraints, such as genes sharing at least 95%, 90%, 75%, and 50% of the maximum motif similarity; these gene clusters contained 15 (sharing 18 motifs), 27 (sharing 17 motifs), 170 (sharing 14 motifs), and 596 (sharing 10 motifs) genes, respectively. To limit false positives but still have the potential to uncover new interactions, genes were selected based on a highly stringent condition, i.e. those sharing a minimum of 14 motifs (at least 75% motif similarity; Supplemental Table S6). The promoter regulatory network was constructed using this list in Cytoscape (Shannon et al., 2003). Vertices in Cytoscape-represented genes and edge width are based on the number of shared motifs, with higher number of shared motifs leading to thicker lines. PScan (Zambelli et al., 2009) was then used to identify enriched plant promoter motifs (Supplemental Table S7) in 170 genes from the glycosylation regulatory network.
Detailed materials and methods are available in the supplemental data.
Accession Numbers
Metabolomics data deposition: Accession MTBLS129. Microarray data deposition: The data discussed in this publication have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (Edgar et al., 2002) and are accessible through Gene Expression Omnibus Series accession number GSE59048.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Exploratory data analysis.
Supplemental Figure S2. Phenotypes of mutant in Ws background and inducible overexpression lines in Col background.
Supplemental Figure S3. Location of the promoter fragments of genes tested using ChIP-PCR in DEX-induced TT8:GR (Col) overexpression lines and mock-treated TT8:GR (Col) lines.
Supplemental Figure S4. Pst DC3000 infection on WT (Col) lines with DEX and mock treatment.
Supplemental Figure S5. Tandem mass spectrometry-based quantification of glycosylation of DON in tt8 (Ws)/WT (Ws) and TT8:GR (Col) DEX/TT8:GR (Col) mock.
Supplemental Figure S6. Under multiple stress conditions, germination of Arabidopsis DEX and mock WT (Col) remains similar.
Supplemental Figure S7. Under multiple stress conditions, tt8 (Col) does not have any distinguishable phenotype compared with WT (Col).
Supplemental Figure S8. Compared with WT (Ler), tt8 (Ler) is sensitive to multiple stresses.
Supplemental Table S1. Differentially expressed metabolites in tt8 (Ws) and TT8:GR (Col) lines.
Supplemental Table S2. List of differentially expressed genes in tt8 (Ws)/WT (Ws).
Supplemental Table S3. List of enriched subnetworks derived using differentially expressed genes and metabolites in tt8 (Ws)/WT (Ws).
Supplemental Table S4. Gene set enrichment analysis of differentially expressed genes in tt8 (Ws)/WT (Ws).
Supplemental Table S5. Promoter motif similarity between differentially expressed genes in tt8 (Ws)/WT (Ws).
Supplemental Table S6. Network of genes involved in the TT8 glycosylation regulome.
Supplemental Table S7. Enriched plant transcription factors of the TT8 glycosylation regulome.
Supplemental Table S8. List of genes and promoter regions enriched in ChIP-PCR.
Supplemental Table S9. List of primers used in this study.
Supplemental Table S10. Raw quantitative real-time PCR values of genes belonging to tt8 (Ws), WT (Ws), DEX-induced TT8:GR (Col) overexpression, mock-treated TT8:GR (Col), DEX-treated WT (Col), and mock-treated WT (Col) lines.
Supplementary Material
Acknowledgments
We thank Professor Prakash Kumar (National University of Singapore) and Professor Yu Hao (National University of Singapore) for their comments on the manuscript. We thank Agilent Technologies (Singapore) for their support in providing metabolomics and genomics instrumentation, software, and advice.
Glossary
- CAZy
carbohydrate-active enzymes
- FDR
false discovery rate
- TFBS
transcription factor binding sites
- DEX
dexamethasone
- ChIP
chromatin immunoprecipitation
- bHLH
basic helix-loop-helix
- ABA
abscisic acid
- MeJA
methyl jasmonate
- DON
deoxynivalenol
Footnotes
This work was supported in part by Singapore-Peking-Oxford Research Enterprise (COY-15-EWI-RCFSA/N197-1), and NUS ODPRT Grant R-154-000-569-133, NUS Environmental Research Institute and Department of Biological Sciences, National University of Singapore.
Articles can be viewed without a subscription.
References
- Bajguz A. (2007) Metabolism of brassinosteroids in plants. Plant Physiol Biochem 45: 95–107 [DOI] [PubMed] [Google Scholar]
- Bajguz A, Piotrowska A (2009) Conjugates of auxin and cytokinin. Phytochemistry 70: 957–969 [DOI] [PubMed] [Google Scholar]
- Bari R, Jones JD (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69: 473–488 [DOI] [PubMed] [Google Scholar]
- Baudry A, Caboche M, Lepiniec L (2006) TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J 46: 768–779 [DOI] [PubMed] [Google Scholar]
- Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L (2004) TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 39: 366–380 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300 [Google Scholar]
- Bowles D, Isayenkova J, Lim EK, Poppenberger B (2005) Glycosyltransferases: managers of small molecules. Curr Opin Plant Biol 8: 254–263 [DOI] [PubMed] [Google Scholar]
- Bowles D, Lim EK, Poppenberger B, Vaistij FE (2006) Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol 57: 567–597 [DOI] [PubMed] [Google Scholar]
- Buer CS, Kordbacheh F, Truong TT, Hocart CH, Djordjevic MA (2013) Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta 238: 171–189 [DOI] [PubMed] [Google Scholar]
- Chen M, Xuan L, Wang Z, Zhou L, Li Z, Du X, Ali E, Zhang G, Jiang L (2014) TRANSPARENT TESTA8 inhibits seed fatty acid accumulation by targeting several seed development regulators in Arabidopsis. Plant Physiol 165: 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszkowska-Golec A. (2011) Arabidopsis seed germination under abiotic stress as a concert of action of phytohormones. OMICS 15: 763–774 [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Nesi N, Perez P, Devic M, Grandjean O, Caboche M, Lepiniec L (2003) Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development. Plant Cell 15: 2514–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone Ferreyra ML, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghedira R, De Buck S, Nolf J, Depicker A (2013) The efficiency of Arabidopsis thaliana floral dip transformation is determined not only by the Agrobacterium strain used but also by the physiology and the ecotype of the dipped plant. Mol Plant Microbe Interact 26: 823–832 [DOI] [PubMed] [Google Scholar]
- Gibson SI. (2004) Sugar and phytohormone response pathways: navigating a signalling network. J Exp Bot 55: 253–264 [DOI] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Boter M, Fernández-Barbero G, Chini A, Rathjen JP, Solano R (2014) The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol 12: e1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB, Baxter H, editors (1999) The Handbook of Natural Flavonoids, Vol 2. Wiley-VCH Verlag, Weinheim, Germany [Google Scholar]
- Hartmann T. (1996) Diversity and variability of plant secondary metabolism: a mechanistic view. Entomol Exp Appl 80: 177–188 [Google Scholar]
- Haug K, Salek RM, Conesa P, Hastings J, de Matos P, Rijnbeek M, Mahendraker T, Williams M, Neumann S, Rocca-Serra P, et al. (2013) MetaboLights—an open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res 41: D781–D786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehl R, Bülow L (2014) AthaMap web tools for the analysis of transcriptional and posttranscriptional regulation of gene expression in Arabidopsis thaliana. Methods Mol Biol 1158: 139–156 [DOI] [PubMed] [Google Scholar]
- Hirai MY, Sugiyama K, Sawada Y, Tohge T, Obayashi T, Suzuki A, Araki R, Sakurai N, Suzuki H, Aoki K, et al. (2007) Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc Natl Acad Sci USA 104: 6478–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Messner B, Nakajima J, Schäffner AR, Saito K (2003) UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. J Biol Chem 278: 43910–43918 [DOI] [PubMed] [Google Scholar]
- Joyce AR, Palsson BO (2006) The model organism as a system: integrating ‘omics’ data sets. Nat Rev Mol Cell Biol 7: 198–210 [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M (2014) Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 42: D199–D205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SG, Price J, Lin PC, Hong JC, Jang JC (2010) The Arabidopsis bZIP1 transcription factor is involved in sugar signaling, protein networking, and DNA binding. Mol Plant 3: 361–373 [DOI] [PubMed] [Google Scholar]
- Kazan K, Manners JM (2012) JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci 17: 22–31 [DOI] [PubMed] [Google Scholar]
- Kresnowati MT, van Winden WA, Almering MJ, ten Pierick A, Ras C, Knijnenburg TA, Daran-Lapujade P, Pronk JT, Heijnen JJ, Daran JM (2006) When transcriptome meets metabolome: fast cellular responses of yeast to sudden relief of glucose limitation. Mol Syst Biol 2: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Ambaru B, Thakkar P, Marcotte EM, Rhee SY (2010) Rational association of genes with traits using a genome-scale gene network for Arabidopsis thaliana. Nat Biotechnol 28: 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Han J, Yao Q, Zou C, Xu Y, Zhang C, Shang D, Zhou L, Zou C, Sun Z, et al. (2013) Subpathway-GM: identification of metabolic subpathways via joint power of interesting genes and metabolites and their topologies within pathways. Nucleic Acids Res 41: e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EK, Ashford DA, Hou B, Jackson RG, Bowles DJ (2004) Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotechnol Bioeng 87: 623–631 [DOI] [PubMed] [Google Scholar]
- Linhart C, Halperin Y, Shamir R (2008) Transcription factor and microRNA motif discovery: the Amadeus platform and a compendium of metazoan target sets. Genome Res 18: 1180–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WX, Zhang FC, Zhang WZ, Song LF, Wu WH, Chen YF (2013) Arabidopsis Di19 functions as a transcription factor and modulates PR1, PR2, and PR5 expression in response to drought stress. Mol Plant 6: 1487–1502 [DOI] [PubMed] [Google Scholar]
- Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42: D490–D495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes L, Inze D, Goossens A (2008) Functional specialization of the TRANSPARENT TESTA GLABRA1 network allows differential hormonal control of laminal and marginal trichome initiation in Arabidopsis rosette leaves. Plant Physiol 148: 1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir R, Hernández ML, Abou-Mansour E, Martínez-Rivas JM, Mauch F, Métraux JP, León J (2013) Pathogen and Circadian Controlled 1 (PCC1) regulates polar lipid content, ABA-related responses, and pathogen defence in Arabidopsis thaliana. J Exp Bot 64: 3385–3395 [DOI] [PubMed] [Google Scholar]
- Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, et al. (2014) Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan K, Basheer C, Bajic VB, Swarup S (2003) Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol 132: 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, Lepiniec L (2000) The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 12: 1863–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Tax FE, Yoshida S, Feldmann KA (2000) Biosynthetic pathways of brassinolide in Arabidopsis. Plant Physiol 124: 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier MK, Burbulis IE, Winkel-Shirley B (1999) Disruption of specific flavonoid genes enhances the accumulation of flavonoid enzymes and end-products in Arabidopsis seedlings. Plant Mol Biol 40: 45–54 [DOI] [PubMed] [Google Scholar]
- Qi T, Song S, Ren Q, Wu D, Huang H, Chen Y, Fan M, Peng W, Ren C, Xie D (2011) The Jasmonate-ZIM-domain proteins interact with the WD-repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23: 1795–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- Rai A, Saito K (2016) Omics data input for metabolic modeling. Curr Opin Biotechnol 37: 127–134 [DOI] [PubMed] [Google Scholar]
- Rai A, Umashankar S, Swarup S (2013) Plant metabolomics: from experimental design to knowledge extraction. In Rose RJ, ed, Legume Genomics, Vol 1069 Humana Press, Totowa, NJ, pp 279–312 [DOI] [PubMed] [Google Scholar]
- Ren C, Han C, Peng W, Huang Y, Peng Z, Xiong X, Zhu Q, Gao B, Xie D (2009) A leaky mutation in DWARF4 reveals an antagonistic role of brassinosteroid in the inhibition of root growth by jasmonate in Arabidopsis. Plant Physiol 151: 1412–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Matsuda F (2010) Metabolomics for functional genomics, systems biology, and biotechnology. Annu Rev Plant Biol 61: 463–489 [DOI] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y, Jikumaru Y, Obayashi T, Saito H, Masuda S, Kamiya Y, Ohta H, Shirasu K (2013) Basic helix-loop-helix transcription factors JASMONATE-ASSOCIATED MYC2-LIKE1 (JAM1), JAM2, and JAM3 are negative regulators of jasmonate responses in Arabidopsis. Plant Physiol 163: 291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo PJ, Lee AK, Xiang F, Park CM (2008) Molecular and functional profiling of Arabidopsis pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant Cell Physiol 49: 334–344 [DOI] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Torres-Acosta JA, Heinen SJ, McCormick S, Lemmens M, Paris MP, Berthiller F, Adam G, Muehlbauer GJ (2012) Transgenic Arabidopsis thaliana expressing a barley UDP-glucosyltransferase exhibit resistance to the mycotoxin deoxynivalenol. J Exp Bot 63: 4731–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G (2006) XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem 78: 779–787 [DOI] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW, Fiehn O, Goodacre R, Griffin JL, et al. (2007) Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3: 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaistij F, Lim E-K, Edwards R, Bowles D (2009) Glycosylation of secondary Metabolites and xenobiotics. In Osbourn AE, Lanzotti V, eds, Plant-Derived Natural Products. Springer, New York, pp 209–228 [Google Scholar]
- Vandepoele K, Quimbaya M, Casneuf T, De Veylder L, Van de Peer Y (2009) Unraveling transcriptional control in Arabidopsis using cis-regulatory elements and coexpression networks. Plant Physiol 150: 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall CS, Muehler AM, Jez JM (2013) Enzyme action in the regulation of plant hormone responses. J Biol Chem 288: 19304–19311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M. (2010) Introduction: biochemistry, physiology and ecological functions of secondary metabolites. In M Wink, ed, Biochemistry of Plant Secondary Metabolism, Annual Plant Reviews, Ed 2, Vol 40. Wiley-Blackwell, Oxford, UK, pp 1–19 [Google Scholar]
- Yi X, Du Z, Su Z (2013) PlantGSEA: a gene set enrichment analysis toolkit for plant community. Nucleic Acids Res 41: W98–W103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Mejia-Guerra MK, Kurz K, Liang X, Welch L, Grotewold E (2011) AGRIS: the Arabidopsis Gene Regulatory Information Server, an update. Nucleic Acids Res 39: D1118–D1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambelli F, Pesole G, Pavesi G (2009) Pscan: finding over-represented transcription factor binding site motifs in sequences from co-regulated or co-expressed genes. Nucleic Acids Res 37: W247–W252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Brutus A, Kremer JM, Withers JC, Gao X, Jones AD, He SY (2011) A genetic screen reveals Arabidopsis stomatal and/or apoplastic defenses against Pseudomonas syringae pv. tomato DC3000. PLoS Pathog 7: e1002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130: 4859–4869 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.