The negative role of AtWRKY57 in Botrytis infection results from transcriptional competition between transcription factors WRKY57 and WRKY33 and their actions on downstream target JAZ1 and JAZ5 genes.
Abstract
Although necrotrophic pathogens cause many devastating plant diseases, our understanding of the plant defense response to them is limited. Here, we found that loss of function of WRKY57 enhanced the resistance of Arabidopsis (Arabidopsis thaliana) against Botrytis cinerea infection. Further investigation suggested that the negative regulation of WRKY57 against B. cinerea depends on the jasmonic acid (JA) signaling pathway. Chromatin immunoprecipitation experiments revealed that WRKY57 directly binds to the promoters of JASMONATE ZIM-DOMAIN1 (JAZ1) and JAZ5, encoding two important repressors of the JA signaling pathway, and activates their transcription. In vivo and in vitro experiments demonstrated that WRKY57 interacts with nuclear-encoded SIGMA FACTOR BINDING PROTEIN1 (SIB1) and SIB2. Further experiments display that the same domain, the VQ motif, of SIB1 and SIB2 interact with WRKY33 and WRKY57. Moreover, transient transcriptional activity assays confirmed that WRKY57 and WRKY33 competitively regulate JAZ1 and JAZ5, SIB1 and SIB2 further enhance these competitions of WRKY57 to WRKY33. Therefore, coordinated regulation of Arabidopsis against B. cinerea by transcription activators and repressors would benefit plants by allowing fine regulation of defense.
In nature, pathogens often challenge plants; however, only a few pathogens are capable of successfully colonizing a specific host, suggesting the existence of potent recognition and defense mechanisms (Birkenbihl et al., 2012). Generally, there are two types of microbial pathogens that differ in their lifestyles: necrotrophic and biotrophic (Glazebrook, 2005). Plant resistance against necrotrophic pathogens involves sacrificing infected cells through triggering cell death; thus, the maintenance of plant cell viability is the main the defense strategy against necrotrophic pathogens. Necrotrophic pathogens make nutrients accessible for growth and for completion of their life cycles via offsetting plant cells. By contrast, biotrophic pathogens require living plant cells for growth and reproduction. They have a tendency to maintain intact colonized tissues for long-term nutritional benefits (Spoel et al., 2007).
Different phytohormone signaling pathways are used by plants to distinguish distinctive pathogens and to activate appropriate responses (Pieterse et al., 2009). Plant responses against biotrophic pathogens are generally mediated by salicylic acid (SA; Vlot et al., 2009), whereas responses to necrotrophs are regulated by ethylene (ET) and jasmonic acid (JA; Farmer et al., 2003). In Arabidopsis (Arabidopsis thaliana), the JA-insensitive coi1 mutant is impaired in resistance to the necrotrophic fungal pathogen Botrytis cinerea (Penninckx et al., 1996, 1998; Thomma et al., 1998) and enhanced resistance to necrotrophic pathogens is often correlated with the accumulation of a phytoalexin, camalexin (Thomma et al., 1999; Ferrari et al., 2003). However, normal expression levels of JA-regulated PDF1.2 and accumulation of camalexin are associated with susceptibility to necrotrophic pathogens (Mengiste et al., 2003; Ferrari et al., 2003; Veronese et al., 2004), suggesting that there might be other, as yet unknown, defense pathways. Overall, our understanding of plant defense against necrotrophic pathogens is limited.
The genetic control of the resistance of Arabidopsis against B. cinerea is complex (Rowe and Kliebenstein, 2008). Multiple pharmacological and genetic studies have revealed that the outcome of host-B. cinerea interactions are influenced by plant genes and products. These include secondary cell wall formation and cutin biosynthesis enzymes (Tang et al., 2007; Hernández-Blanco et al., 2007; Voisin et al., 2009; Raiola et al., 2011), the TIR domain-encoding protein RLM3 (Staal et al., 2008), the RING E3 ligases HUB1 and BOI (Dhawan et al., 2009; Luo et al., 2010), the receptor-like kinase BIK1 (Veronese et al., 2006), the MAPK MPK3 (Ren et al., 2008), and several autophagy genes (Lenz et al., 2011; Lai et al., 2011). Arabidopsis leaves infected by B. cinerea induce massive transcriptional reprogramming in the host, as demonstrated by global transcriptional profiling studies, suggesting that key regulators are involved in this process (Rowe et al., 2010; Ferrari et al., 2007; AbuQamar et al., 2006).
The WRKY superfamily of transcript factors regulates host defenses against various phytopathogens (Pandey and Somssich, 2009). In particular, WRKY33 mainly functions in resistance against the necrotrophs B. cinerea and Alternaria brassicicola (Zheng et al., 2006). WRKY33 is thought to positively regulate the JA signaling pathway and negatively regulate the SA signaling pathway, based on the lower expression of JA-response genes and the higher expression of PR genes in wrky33 compared with wild-type plants under B. cinerea treatment. Moreover, WRKY33 interacts with MPK4 and MKS1 within the nucleus to form a trimeric complex under natural growth conditions. However, upon elicitation by the microbe-associated molecular pattern flg22 or upon challenge with the hemibiotrophic pathogen Pseudomonas syringae, WRKY33 is released from the trimeric complex and subsequently binds to the PAD3 promoter to transduce pathogen-triggered host signals (Qiu et al., 2008; Andreasson et al., 2005). It is not clear whether the same signal transduction cascade exists during resistance against necrotrophic pathogens because WRKY33 is phosphorylated by the mitogen-activated protein kinases MPK3 and MPK6 in vivo under B. cinerea challenge (Mao et al., 2011). In addition, recent studies suggested that WRKY33 interacts with ATG18a, a critical autophagy protein, within the cell nucleus (Lai et al., 2011). Two VQ motif-containing proteins, SIB1 and SIB2, interact with WRKY33 and function as activators of WRKY33 in plant defense against necrotrophic pathogens (Lai et al., 2011). Transcriptomic analysis, biochemical experiments, and genetic studies revealed that WRKY33 regulates directly the expression of various distinct components of defense pathways, such as JAZ1, JAZ5 (encoding jasmonate ZIM-domain proteins (JAZ)), ORA59, TRX-H5, and GLIP1, which are crucial in enhancing appropriate host responses against B. cinerea (Birkenbihl et al., 2012).
In this study, we report the identification and functional analysis of WRKY57 in compromised plant defense against the necrotrophic pathogen B. cinerea. WRKY 57 belongs Group IIc of WRKY transcription factor family (Eulgem et al., 2000). Loss of function of WRKY57 enhanced resistance against B. cinerea infection, and this resistance was associated with the JA signaling pathway, especially COI1. By chromatin immunoprecipitation (ChIP), JAZ1 and JAZ5, direct targets of WRKY33, were identified as direct target genes of WRKY57 in vivo. Using the yeast two-hybrid assay, we revealed that WRKY57 interacts with two VQ motif-containing proteins, SIB1 and SIB2, which also interact with WRKY33. In addition, mapping of the interaction domain of SIB1 and SIB2 with WRKY57 confirmed that the same VQ motif of SIB1 and SIB2 interact with WRKY33 and WRKY57. Furthermore, transient transcriptional activity assays showed that WRKY57 and WRKY33 competitively regulate JAZ1 and JAZ5, and SIB1 and SIB2 further enhanced these competitions of WRKY57 to WRKY33. These results suggested that WRKY57 compromises B. cinerea resistance via competing with WRKY33 to transcriptionally regulate JAZ1 and JAZ5 in Arabidopsis. This synergistic regulation by transcription activators and repressors would benefit Arabidopsis by allowing fine regulation of the response against B. cinerea.
RESULTS
Expression Pattern of WRKY57 under Biotic Stresses
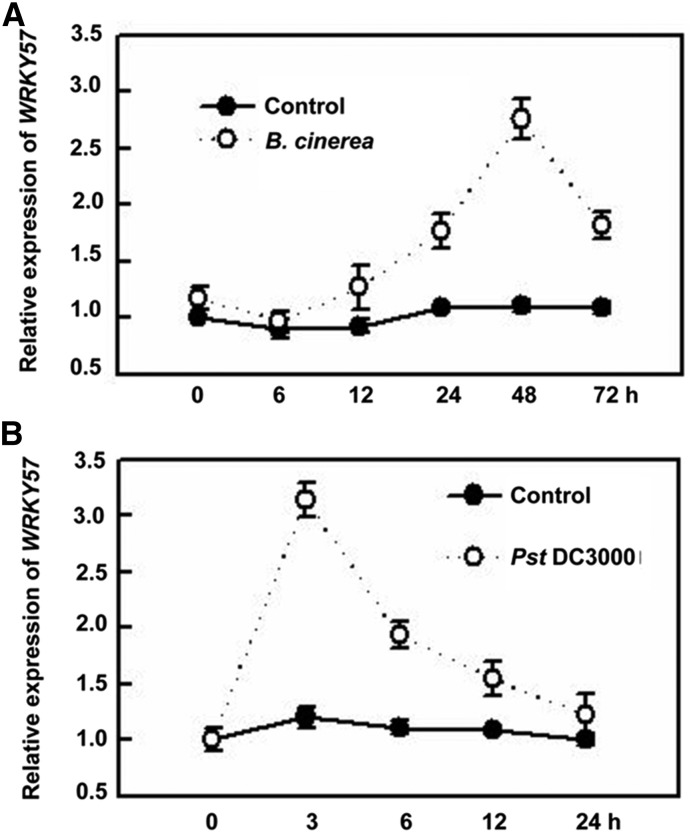
In our previous research, we confirmed that transcription factor WRKY57 is involved in JA-induced leaf senescence (Jiang et al., 2014). There is a considerable overlap between pathogen-related and senescence-related genes (Weaver et al., 1998; Quirino et al., 1999); therefore, WRKY transcription factors might function as both positive and negative regulators to fine-tune the complex signaling networks of plant defense and senescence. Besides their role in leaf senescence, jasmonates (JAs) also act as key phytohormones in the defense against necrotrophic fungi (Turner et al., 2002). Therefore, to investigate whether WRKY57 is involved in JA-related defense against necrotrophic fungi, we checked the expression levels of WRKY57 upon challenge with B. cinerea, a necrotrophic fungus that causes gray mold disease in many plant species. Expression of WRKY57 was moderately induced by B. cinerea treatment (Fig. 1A). Previous studies showed that JA induces plant susceptibility to the bacterium strain Pst DC3000 of P. syringae pv. tomato (Zheng et al., 2006). We also checked the expression levels of WRKY57 upon Pst DC3000 treatment. Unlike the expression pattern upon B. cinerea treatment, expression of WRKY57 was induced rapidly by Pst DC3000 at 3 h (Fig. 1B).
Figure 1.
Expression of WRKY57 in response to biotic stresses. A, Wild-type plants were sprayed with B. cinerea and leaf samples were taken at the indicated time points to quantify the transcript levels of WRKY57 by real-time RT-PCR. B, Wild-type plants were sprayed with Pst DC3000 and leaf samples were taken at the indicated time points to quantify the transcript levels of WRKY57 by real-time RT-PCR. Error bars represent se of five biological replicates (n = 5).
According to these results, we speculated that WRKY57 may function in the basal defense process in Arabidopsis.
Loss of WRKY57 Function Enhances Basal Defense against B. cinerea
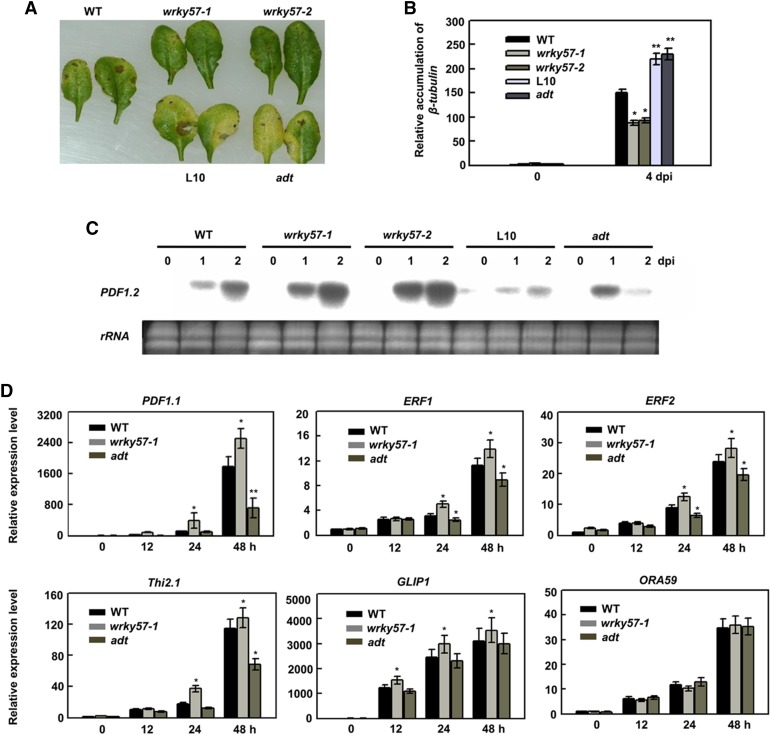
To confirm our speculation that WRKY57 functions in the basal defense process in Arabidopsis, two wrky57 mutants wrky57-1 (Salk_076716) and wrky57-2 (Salk_095225) and two overexpression lines, Line10 and adt, described in our previous research (Jiang et al., 2014), were used for further analysis. To determine the possible roles of the WRKY57 in plant defense, we examined the responses of the wild-type, two mutants, and two overexpression plants to B. cinerea. Five-week-old plants were inoculated with a B. cinerea spore suspension at a density of 5 × 104 spores/mL. At 4-d post-infection (dpi), leaves showing necrotic symptoms were evaluated for disease severity. B. cinerea infection caused necrotic symptoms; however, necrosis remained at localized sites in the wrky57-1 and wrky57-2 plants (Fig. 2A). Only 20% of the leaves of wrky57 mutant plants had disease symptoms at 4 dpi (Fig. 2A). However, necrotic symptoms rapidly increased in severity during infection of the wild-type plants and overexpression plants, and approximately 40 and 65% of the leaves were severely decayed at 4 dpi, respectively (Fig. 2A). Furthermore, lower levels of β-tubulin mRNA of B. cinerea accumulated at 4 dpi in the two mutant plants (Fig. 2B). In addition, higher expression levels of several JA- and B. cinerea-related genes (such as PDF1.2, PDF1.1, Thi2.1, ERF1, and ERF2) in the wrky57 mutants than in the wild type, and especially in overexpressing plants, further supported these phenotypes (Fig. 2, C and D). Thus, these results confirmed that disruption of WRKY57 significantly enhanced resistance to the fungal pathogen and that WRKY57 plays a negative role in B. cinerea defense.
Figure 2.
Loss of WRKY57 function enhances resistance of Arabidopsis against B. cinerea. A, Responses to B. cinerea using whole-plant inoculation. Wild-type, wrky57-1, wrky57-1, WRKY57OE-L10, and adt plants were inoculated by spraying spore suspension at a density of 5 × 104 spores/mL and kept at high humidity. Photographs of representative leaf were taken 4 d after inoculation. The experiments were repeated three times with similar results. B, Estimation of the biomass of the fungal pathogen on infected plant. Total RNA was isolated from the plants 4 d after inoculation and quantified with a B. cinerea Actin gene probe to determine the biomass of the fungal pathogen on infected plants. Values are mean + se (n = 5 experiments, *P < 0.05, **P < 0.01). C, Expression of PDF1.2 after B. cinerea infection. Wild-type wrky57-1, wrky57-2, WRKY57OE-L10, and adt plants were inoculated with B. cinerea. The inoculated leaves were collected at the indicated days after inoculation for RNA isolation. RNA gel blot analysis was performed with a 32P-labeled PDF1.2 DNA fragment. Ethidium bromide staining of rRNA is shown for the assessment of equal loading. The experiments were repeated three times with similar results. D, Relative expression levels of PDF1.1, Thi2.1, ERF1, ERF2, GLIP1, and ORA59 in the leaves of 4-week-old wild type, wrky57-1, and adt after B. cinerea treatment. Values are the mean ± se (*P < 0.05, **P < 0.01) of three independent experiments.
To understand whether wrky57 mutants are susceptibility to a virulent strain of P. syringae, plants were inoculated with the bacteria. The wrky57 mutants showed no differences compared with the wild-type and overexpressing plants (Supplemental Fig. S1A), and bacterial growth displayed only insignificant differences among wild-type, wrky57 mutant, and overexpressing plants (Supplemental Fig. S1B). Moreover, the expression levels of marker gene, PR1, checked by northern blotting, was also slightly changed in wrky57 mutants compared with the wild-type and overexpressing plants (Supplemental Fig. S1B). Thus, disruption of WRKY57 did not add to its susceptibility to the bacterial pathogen.
WRKY57’s Negative Regulation of Defense Is Related to the JA Signaling Pathway
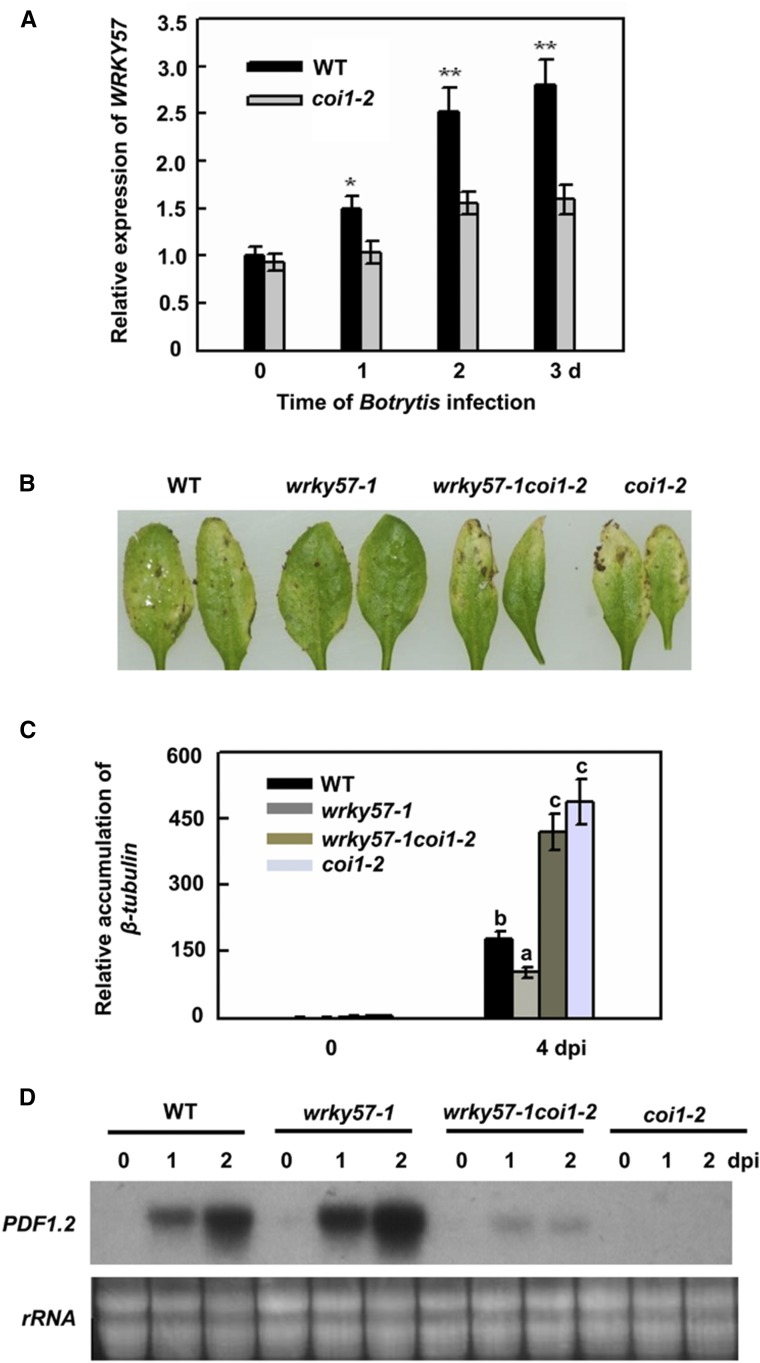
To investigate the relationship between WRKY57 and the JA signaling pathway, we checked the expression levels of WRKY57 in wild-type and coi1-2 plants upon B. cinerea treatment. As shown in Figure 3A, the expression levels of WRKY57 were lower in coi1-2 than in wild-type plants. These results suggested that the expression of WRKY57 is partly dependent on COI1 under B. cinerea treatment. To further study the potential role of WRKY57 in fungal pathogen defense, we sprayed the B. cinerea spore suspension onto the wild-type, wrky57-1, and wrky57-1coi-1-2 double-mutant plants. Only 20% of the leaves of wrky57-1 mutant plants had disease symptoms at 4 dpi (Fig. 3B). However, necrotic symptoms rapidly increased in severity during infection in the wild-type, wrky57-1coi1-2, and coi1-2 plants, and approximately 40%, 75%, and 85% of the leaves were severely decayed, respectively (Fig. 3B). Consistent with this phenotype, the β-tubulin mRNA of B. cinerea accumulated higher levels in the coi1-2wrky57 and coi1-2 plants at 4 dpi (Fig. 3C). Moreover, weak expression levels of PDF1.2 were observed in coi1-2wrky57 (Fig. 3D). These results suggested that WRKY57 might be involved in the JA signaling pathway to negatively modulate basal defense against B. cinerea.
Figure 3.
The negative regulation of WRKY57 against B. cinerea relates to the JA signaling pathway. A, Wild-type and coi1-2 plants were spray-inoculated with B. cinerea and leaf samples were taken at the indicated time points to quantify the transcript levels of WRKY57 by real-time RT-PCR. Error bars represent se of five biological replicates (n = 5, *P < 0.05, **P < 0.01). B, Responses to B. cinerea using wild-type, coi1-2, and wrky571-coi1-2 whole-plant inoculation. All plants were inoculated by spraying spore suspension at a density of 5 × 104 spores/mL, then kept at high humidity. Photographs of representative leaf were taken 4 dpi. The experiments were repeated three times with similar results. C, Estimation of the biomass of the fungal pathogen on an infected plant. Total RNA was isolated from the plants 4 d after inoculation and quantified with a B. cinerea Actin gene probe to determine the biomass of the fungal pathogen on infected plants. Values are mean + se (n = 5 experiments), and different letters above columns indicate significant differences based on Tukey’s test (P < 0.05). D, Expression of PDF1.2 in wild-type, coi1-2, and wrky571-coi1-2 plants after B. cinerea infection. Wild-type, coi1-2, and wrky571-coi1-2 plants were inoculated with B. cinerea. The inoculated leaves were collected at the indicated days after inoculation for RNA isolation. RNA gel blot analysis was performed with a 32P-labeled PDF1.2 DNA fragment. Ethidium bromide staining of rRNA is shown for the assessment of equal loading. The experiments were repeated three times with similar results.
WRKY57 Induces the Expression of JAZ1 and JAZ5 Directly upon B. cinerea Treatment
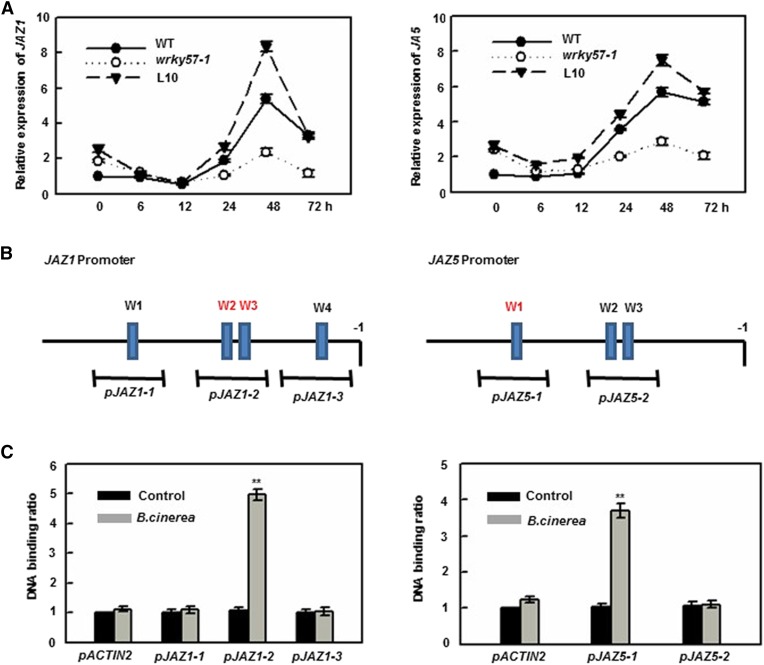
WRKY57’s negative regulation of defense against B. cinerea was related to JA signaling; therefore, we focused our attention on the JAZ proteins. There are 12 JAZs in Arabidopsis and these proteins serve as negative regulators of the JA-signaling pathway (Chini et al., 2007). To understand their expression patterns, we determined their relative expression levels in the wild type and wrky57-1 before and after B. cinerea treatment. As shown in Figure 4A and Supplemental Figure S2, six JAZs (JAZ1, JAZ5, JAZ6, JAZ7, JAZ8, and JAZ10) were induced by B. cinerea, and their induced expressions were compromised in wrky57-1. To further investigate whether these genes are direct targets of WRKY57, ChIP experiments were conducted, which showed that WRKY57 binds to the promoters of the JAZ1 and JAZ5 genes via a W-box sequence (Fig. 4, B and C). These results demonstrated that WRKY57 regulated JAZ1 and JAZ5 expression directly and positively upon B. cinerea treatment, which then suppressed resistance to fungal infection.
Figure 4.
WRKY57 directly induces the expression of JAZ1 and JAZ5. A, Relative transcript levels of JAZ1 and JAZ5. Wild-type, wrky57-1, and WRKY57OE-L10 plants were inoculated with B. cinerea. The inoculated leaves were collected at the indicated h after inoculation for RNA isolation and quantified the transcript levels of JAZ1 and JAZ5 by real-time RT-PCR. Error bars represent se of five biological replicates (n = 5). B, Promoter structure of the JAZ1 and JAZ5 genes and fragments used in the ChIP assay. W1, W2, etc. denote each W-box, numbered from left to right with sequence sites relative to the start code. Lines indicate the sequences detected by ChIP assays. C, Real-time RT-PCR data from ChIP assay with antibody against Myc with the ACTIN2 promoter (pACTIN2) as a negative control. Values are mean + se (n = 5 experiments, **P < 0.01).
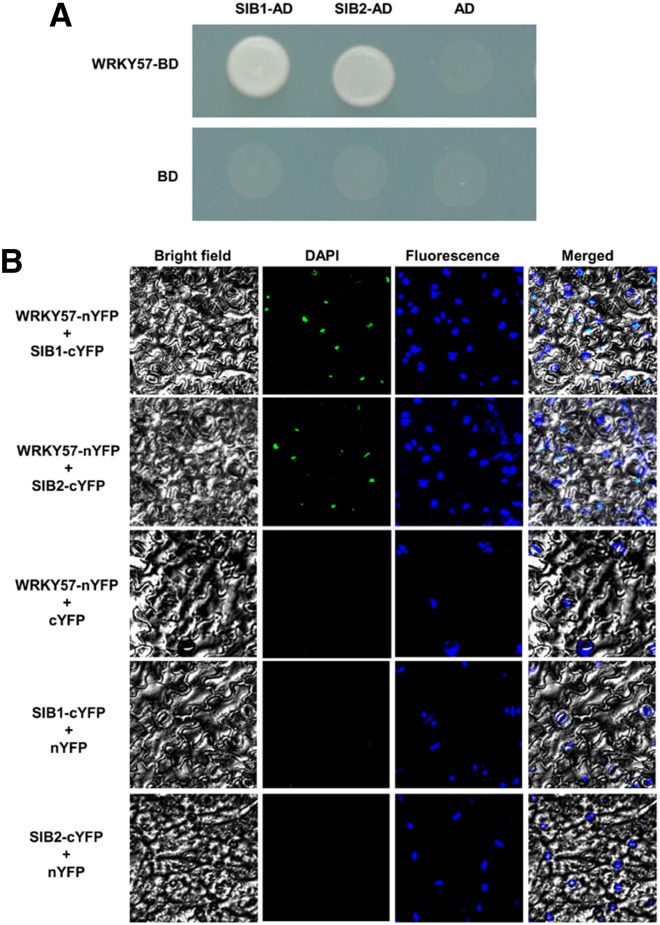
WRKY57 Physically Interacts with SIB1 and SIB2
Increasing evidence suggested that WRKY proteins function by forming protein complexes with other interactors (Xu et al., 2006; Miao and Zentfraf, 2007; Shang et al., 2010; Hu et al., 2013). To search for potential partners of the WRKY57 protein, we employed the yeast two-hybrid system, as described in our recent study (Jiang et al., 2014). Interestingly, among the candidate interactors, SIB1 and SIB2 (which had been reported to interact with WRKY33) were frequently represented. To confirm their interaction in yeast, their open-reading frame sequences were fused with the activation domain (AD) of the pGAD-T7 vector and used for further interaction experiments with WRKY57. As shown in Figure 5A, WRKY57 interacted strongly with SIB1 and SIB2. To determine whether these interactions also occur in plant cells, we employed the bimolecular fluorescence complementation (BiFC) system. Full-length WRKY57, SIB1, and SIB2 cDNAs were fused to the N-terminal region of the yellow fluorescent protein (YFP). Agrobacterial cells harboring the corresponding interaction pairs were infiltrated into Nicotiana benthamiana leaves. In parallel, empty vectors in combination with each fusion construct were coinfiltrated into tobacco leaves. After 2 d of incubation, YFP signals were observed by fluorescence microscopy. The samples coinfiltrated with an interaction pair showed YFP fluorescence in the cell nuclei, whereas all control samples failed to give any YFP signal (Fig. 5B). These results indicated that WRKY57 and its partners colocalize and interact in plant cell nuclei.
Figure 5.
WRKY57 physically interacts with SIB1 and SIB2. A, Yeast-two-hybrid assays. Interaction was indicated by the ability of cells to grow on synthetic dropout medium lacking Leu/Trp/His/Ade and containing 5 mm 3-aminotriazole. The Gal4 DNA binding domain was fused with WRKY57 (shown as BD-WRKY57) and the Gal4 activation domain was fused with SIB1 or SIB2 (shown as AD-SIB1 and AD-SIB2). The Gal4 DNA binding domain expressed by pGBKT7 was used as a negative control. B, BiFC assays. Fluorescence was observed in the nuclear compartments of N. benthamiana leaf epidermal cells that resulted from complementation of the C-terminal part of YFP fused with WRKY57 (WRKY57-cYFP) and the N-terminal part of YFP fused with SIB1 or SIB2 (SIB1-nYFP and SIB2-nYFP). No signals were observed from the negative controls. BD, binding domain.
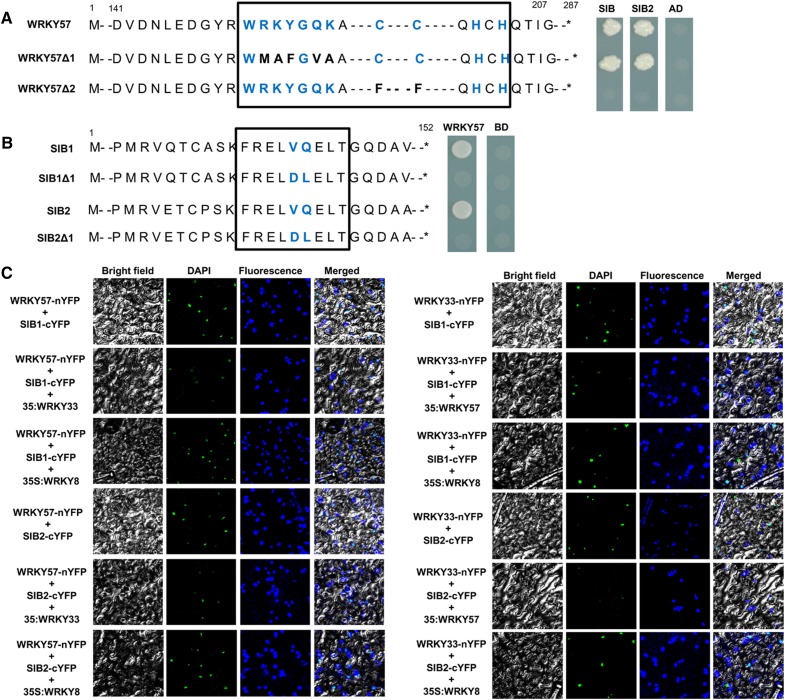
The Zinc-Finger-Motif and VQ-Motif Are Important for the Interaction of WRKY57 and SIB1 and SIB2
Given that WRKY57 and WRKY33 physically interact with SIB1 and SIB2, and that the function of the WRKY57 is opposite to that of WRKY33 in defense against B. cinerea, we questioned whether there is a competitive relationship between WRKY33 and WRKY57 for SIB1 and SIB2. To investigate whether the same region of SIB1 and SIB2 that interacts with WRKY33, the VQ domain, is required for interaction with WRKY57, we overlapped the VQ domain of SIB1 and SIB2 and then introduced it into the AD of the pGADT7 vector. The interaction between these derivatives and the WRKY57 protein revealed that the VQ domain was specifically responsible for the interaction (Fig. 6A). Therefore, we speculated that the WRKY57 protein may interfere with interactions among the SIB1, SIB2, and WRKY33 proteins.
Figure 6.
WRKY57 and WRKY33 competitively interact with SIB1 and SIB2. A, The conserved WRKYGQK of WRKY57 is not essential for interaction with SIB1 and SIB2. Left: Diagram of full-length and mutated WRKY57 constructs. Right: Interactions were indicated by the ability of yeast cells to grow on synthetic dropout medium lacking Leu, Trp, His, and Ade. The empty pGADT7 prey vector was used as a negative control. B, The VQ domains of SIB1 or SIB2 are necessary for interaction with WRKY57. Left: Diagram of full-length and mutated SIB1 or SIB2 constructs. Right: Interactions were indicated by the ability of yeast cells to grow on synthetic dropout medium lacking Leu, Trp, His, and Ade. The empty pGBKT7 prey vector was used as a negative control. In A and B, AD and BD refer to the pGADT7 and pGBKT7 vector, respectively. C, Competition between WRKY57 and WRKY33 for interaction with SIB1 and SIB2. WRKY8 is a negative control.
To test this possibility, we performed BiFC analysis. As in control, when fused WRKY57-nYFP was coexpressed with fused SIB1-cYFP or SIB2-cYFP in N. benthamiana leaves (Fig. 6C), the YFP signal was very strong in the nucleus. However, when a third protein interacting with SIB1 and SIB2, WRKY33, was coexpressed with the fused WRKY57-nYFP and SIB1-cYFP or SIB2-cYFP, the YFP signal became weaker than in the control (Fig. 6C). When a different protein, WRKY8, which cannot interact with SIB1 or SIB2, was coexpressed with WRKY57-nYFP and SIB1-cYFP or SIB2-cYFP, the YFP signal was the same strength as in the control. Similarly, when WRKY57 was coexpressed with fused WRKY33-nYFP and SIB1-cYFP or SIB2-cYFP in N. benthamiana leaves, the YFP signal also became weaker than when WRKY33-nYFP and SIB1-cYFP or SIB2-cYFP were coexpressed alone (Fig. 6C). Taken together, these results indicated that WRKY57 and WRKY33 competitively interact with SIB1 and SIB2.
To further examine which domain of WRKY57 proteins is critical for the interactions with SIB1 and SIB2, pGBKT7 vectors containing a WRKY domain mutant or a zinc-finger domain mutant were used. The results showed that the zinc-finger domain of WRKY57 was necessary for interaction with both the SIB1 and SIB2 proteins (Fig. 6B).
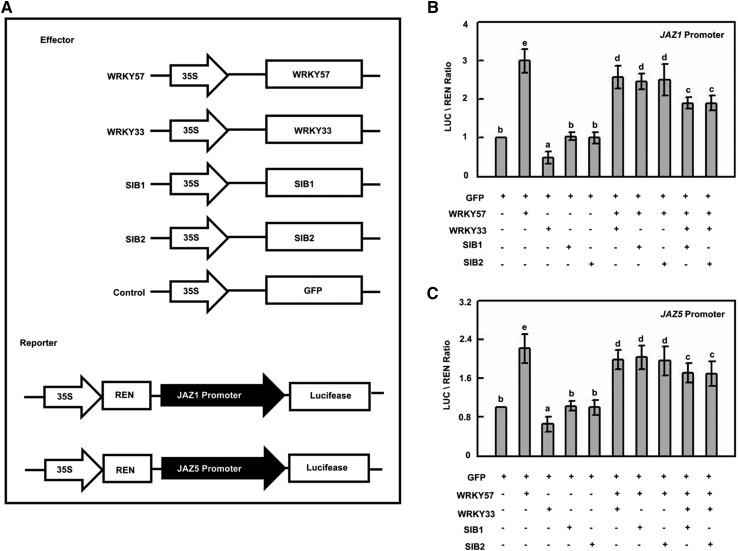
WRKY57 Competes with WRKY33 to Regulate JAZ1 and JAZ5
Considering the competing interaction between WRKY33 and WRKY57 with SIB1 and SIB2, and their opposite functions in Arabidopsis against B. cinerea, we speculated that the WRKY57 protein might interfere with the interactions among the SIB1, SIB2, and WRKY33 proteins and further affect the downstream target genes regulated by WRKY33. To confirm our hypothesis, we next generated reporter constructs PJAZ1-LUC and PJAZ5-LUC in which the luciferase (LUC) reporter was driven by the promoters of JAZ1 and JAZ5, respectively (Fig. 7A), the direct targets of WRKY33 (Birkenbihl et al., 2012) and WRKY57 (Fig. 4). As shown in Figure 7, B and C, the expression of PJAZ1-LUC and PJAZ5-LUC was not affected by SIB1 and SIB2. However, WRKY57 significantly induced the expression of PJAZ1-LUC and PJAZ5-LUC in the transient transcriptional activity assays, and WRKY33 displayed the opposite effect (Fig. 7, B and C). We also found that WRKY57 attenuated the repressed expression of PJAZ1-LUC and PJAZ5-LUC by WRKY33, and that SIB1 and SIB2 further enhanced this attenuation (Fig. 7, B and C). Collectively, these results suggested that WRKY57 competes with WRKY33 to regulate the expression of JAZ1 and JAZ5. Moreover, SIB1 and SIB2 further significantly enhanced this competitive regulatory relationship of WRKY57 to WRKY33 (Fig. 7).
Figure 7.
WRKY57 competes with WRKY33 to regulate its downstream target genes. A, The schematic diagram shows the constructs used in the transient transcriptional activity assays of B and C. B, Transient transcriptional activity assays show that activation of JAZ1 promoter by WRKY57 is repressed by WRKY33 and SIB1/SIB2. The JAZ1-LUC reporter was cotransformed with the indicated constructs (10 mg for total constructs). Values are mean + se (n = 5 experiments), and different letters above columns indicate significant differences based on Tukey’s test (P < 0.05). C, Transient transcriptional activity assays show that activation of the JAZ5 promoter by WRKY57 is repressed by WRKY33 and SIB1/SIB2. The JAZ5-LUC reporter was cotransformed with the indicated constructs (10 mg for total constructs). Values are mean + se (n = 5 experiments), and different letters above columns indicate significant differences based on Tukey’s test (P < 0.05).
DISCUSSION
Although numerous advances have been made in understanding the mechanisms of WRKY transcription factors in defense against B. cinerea, the specific mechanisms by which different WRKY transcription factors cooperate or oppose each other are not well understood. Here, we revealed that WRKY57 compromised the resistance of Arabidopsis against B. cinerea depending on the JA signaling pathway and the competition with WRKY33 to regulate its downstream target genes, JAZ1 and JAZ5.
Our investigation demonstrated that the relative expression of WRKY57 was induced by infection with B. cinerea (Fig. 1A). More importantly, resistance of Arabidopsis to B. cinerea was significantly improved in the wrky57-1 and wrky57-2 mutants and compromised in the WRKY57 overexpression lines (Fig. 2A), which were further characterized by relative accumulation of β-tubulin (Fig. 2B). Consequently, the expression level of PDF1.2 was significantly enhanced in wrky57-1 and wrky57-2 mutants upon B. cinerea treatment and, by contrast, impaired in WRKY57 overexpression lines (Fig. 2C). These results confirmed that WRKY57 negatively regulated resistance against B. cinerea in Arabidopsis.
The JA signaling pathway plays a key role in resistance to B. cinerea (Turner et al., 2002) and our previous studies revealed that WRKY57 is involved in JA-induced leaf senescence (Jiang et al., 2014). Thus, we suspected the compromised resistance against B. cinerea induced by WRKY57 is related to the JA pathway. Several results supported our speculations. First, the B. cinerea-induced expression level of WRKY57 was decreased in coi1-2, suggesting that expression of WRKY57 is partly depended on COI1 (Fig. 3A). Furthermore, the impaired resistance of coi1-2 against B. cinerea was partly compromised by wrky57-1 (Fig. 3B). Correspondingly, the compromised expression of PDF1.2 in coi1-2 was also slightly enhanced in wrky57-1coi1-2 double mutants (Fig. 3D). Therefore, the negatively regulation of resistance against B. cinerea by WRKY57 relates to the JA signal pathway.
ChIP studies have shown that the W-box sequences in the promoters of defense genes are constitutively occupied by WRKY proteins, especially in the presence of pathogen infection or elicitor treatment (Turck et al., 2004). Our ChIP assay indicated that WRKY57 could bind to the promoters of JAZ1 and JAZ5, suggesting that they are direct targets of WRKY57 (Fig. 4). Their down-regulated expression in wrky57-1 and their up-regulation in overexpression line10 suggested that WRKY57 is a positive regulator of JAZ1 and JAZ5 (Fig. 4). The positive regulation of WRKY57 was consistent with our previous study in which WRKY57 was shown to function as a positive regulator of RD29A and NCED3 (Jiang et al., 2012); however, this was opposite to our previous study in which WRKY57 functioned as a negative regulator of SEN4 and SAG12 in JA-induced leaf senescence (Jiang et al., 2014). These results suggested that WRKY57 was a multifunctional protein involved in different signaling pathways and regulating various target genes. In this case, the negative regulation of WRKY57 against B. cinerea resulted from directly enhancing the expressions of JAZ1 and JAZ5.
Expression analysis showed that B. cinerea induced expression of WRKY57, and this induction is dependent on COI1 (Fig. 3A), demonstrating a negative feed-back loop to regulate defense response. As widely known, JA signaling plays an important role in plant defense against B. cinerea, and this necrotrophic pathogen can activate JA signaling in plants. B. cinerea and JA up-regulate the expression of WRKY57 may establish a proper balance between defense and growth, resulting in appropriate defense response against B. cinerea parasitism while minimizing detrimental effects on plant growth and development. COI1 functions as the receptor of JA, and disruption of COI1 conferred JA insensitivity on plants (Yan et al., 2009). It is very possible that B. cinerea induction of WRKY57 is dependent on JA signaling, and this induction is thus reduced in JA-insensitive coi1-2 mutants. Interestingly, similar COI1 dependency was also observed for other JA-related genes. Song et al. (2013) found that bHLH13 and bHLH17 act as negative regulators of JA signaling to modulate JA-mediated root elongation and defense responses. Expression analysis in their study showed that bHLH13 and bHLH17 genes are induced by JA and their induction also depends on COI1.
JAZ proteins are ubiquitinated via SCFCOI1 in response to JA (Thines et al., 2007). The JAZ family proteins function as repressors of the JA signaling pathway, and a recent structural and pharmacological study showed that COI1 and JAZ form a coreceptor complex (Sheard et al., 2010). In our previous study (Jiang et al., 2014), we showed that the JA-induced leaf senescence process can be antagonized by auxin via WRKY57 competitive interacting with JAZ4/8 and IAA29. In this study, we further found that WRKY57 directly target promoter sequences of JAZ1 and JAZ5 to enhance their expression and decrease B. cinerea resistance (Fig. 4). As JAZs and WRKY57 function as repressors of the JA signaling pathway (Thines et al., 2007; Jiang et al., 2014; Fig. 4), up-regulation of JAZ1, JAZ5, and JAZ8 by WRKY57 (Fig. 4; Supplemental Fig. S2) may demonstrate a self-negative feedback for WRKY57. As expected, transcriptional activation of JAZ1, JAZ5, and JAZ8 results in accumulation of JAZ proteins, which then inhibit WRKY57 through physical interaction with JAZ8. The effect of self-inhibition on WRKY57 during B. cinerea defense may set up a fine-tuning regulation between WRKY57 and the JA signaling pathway.
Arabidopsis WRKY33 is the most important transcription factor of the WRKY family for plant resistance to necrotrophic pathogens (Zheng et al., 2006; Lippok et al., 2007; Lai et al., 2011; Cheng et al., 2012; Birkenbihl et al., 2012). A recent study showed that the VQ motif of SIB1 and SIB2 recognize the C-terminal WRKY domain of WRKY33 to enhance the DNA-binding activity of WRKY33 in plant defense (Lai et al., 2011) and the highly conserved core WRKYGQK motif is dispensable for interacting with SIB1 and SIB2 (Supplemental Fig. S4). Unlike WRKY33, the WRKYGQK motif of WRKY57 is not dispensable for interacting with the VQ motifs of SIB1 and SIB2 (Figs. 5 and 6). It is unexpected that mutant of the highly conserved core WRKYGQK of WRKY57 also interacted with SIB1 and SIB2, raising the question of whether this is common to other Group I and Group IIc WRKY proteins or just unique to WRKY57. To clear this question, three Group I proteins, WRKY3, WRKY4, and WRKY33, and two Group IIc proteins, WRKY24 and WRKY75, were chosen to further confirm this. As shown in Supplemental Figure S4, the WRKYGQK sequences of WRKY33 and WRKY75 are necessary to interact with SIB1 and SIB2, whereas for WRKY3, WRKY4, and WRKY24 the WRKYGQK sequences are not essential (Supplemental Fig.S4). Thus, it is safely speculated that the highly conserved core WRKYGQK sequences of some WRKY proteins are dispensable for interacting with SIB1 and SIB2, such as WRKY57, WRKY3, WRKY4, and WRKY24. For WRKY57, different motifs play different roles under various stresses. In this case, the Zinc-finger motif of WRKY57 interacting with the VQ motif of SIB1 and SIB2 plays a negative role in Botrytis defense, whereas the WRKYGQK motif of WRKY57 interacting with the ZIM motif of the JAZ4 and II domain of IAA29 is involved in the JA-induced leaf senescence process (Fig. 6A; Jiang et al., 2014).
A previous study showed that WRKY33 acts as a transcription repressor that binds to and activates promoter sequences of its respective target genes, such as JAZ1 and JAZ5 (Birkenbihl et al., 2012). In the protoplast transient transcriptional activity assay, we showed that WRKY57 acts as transcriptional activator (Fig. 7) that binds to the promoter sequences of JAZ1 and JAZ5 (Fig. 4, B and D) to compete with the transcription regulation function of WRKY33 (Fig. 7) to JAZ1 and JAZ5. Interestingly, WRKY57 and WRKY33 bind to the same W-boxes of JAZ1 and JAZ5 promoter sequences (Fig. 4), and there is also a directly competing relationship between WRKY57 and WRKY33 binding to the same W-box (Fig. 7, B and C). However, SIB1 and SIB2 proteins significantly enhanced the competition of WRKY57 to WRKY33 (Fig. 7, B and C). The protoplast transient transcriptional activity assay is a well-established method to define transcriptional relationships. WRKY33 acts as a transcriptional repressor of JAZ1 and JAZ5 in this system (Fig. 7, B and C), which is consistent with previous observations (Birkenbihl et al., 2012). Taking our results together, we concluded that WRKY57 negatively regulates resistance of Arabidopsis against B. cinerea via competitively regulating JAZ1 and JAZ5 with WRKY33 and then weakening the transcriptional function of WRKY33 to regulate its downstream target genes, JAZ1 and JAZ5.
MATERIALS AND METHODS
Materials and Plant Growth Conditions
The screening of wrky57-1 (Salk_076716), wrky57-2 (Salk_095225) mutants and a gain-of-function mutant adt (Salk_117358), and construction of WRKY57 overexpression transgenic plants L10, have been described in our previous studies (Jiang et al., 2012, 2014).
Arabidopsis (Arabidopsis thaliana ecotype Columbia) seeds were surface-sterilized (20% [v/v] bleach for 15 min) before being sown on half-strength Murashige and Skoog (1/2 MS) medium and kept at 4°C for 3 d. One-week-old plants were transferred to soil. The plants were then kept in a growth cabinet at 22°C under long-d conditions (16-h light [100 μE m−2 s−1]/8-h dark cycle).
Expression Analysis
Five-week-old normal leaves were infected with PstDC3000 and Botrytis cinerea and then harvested for total RNA extraction. Real-time RT-PCR analysis was performed as described in our previous study (Jiang et al., 2014). Five biological replicates were conducted. The primers used for real-time RT-PCR are listed in Supplemental Table S1.
For northern-blotting analyses, the total RNA was extracted using the TRIzol reagent (Invitrogen). Approximately 20 μg of RNA was separated on an agaroseformaldehyde gel and then blotted onto nylon membranes following standard procedures. The membranes were hybridized with (α-32P)-dATP-labeled DNA probes. Hybridization was performed in PerfectHyb Plus hybridization buffer (Sigma-Aldrich) for 16 h at 68°C. The membranes were washed once for 10 min with 2× SSC (1× SSC is 0.15 m NaCl plus 0.015 m sodium citrate) and 0.5% SDS, twice for 20 min with 0.5× SSC and 0.1% SDS, once for 20 min with 0.1× SSC and 0.1% SDS at 68°C, and then exposed to x-ray films at −80°C. DNA probes for PR1 and PDF1.2 were obtained from PCR amplifications using the following gene-specific primers: PR1, 5′-TCTTCCCTCGAAAGCTCAAG-3′ and 5′-ACACCTCACTTTGGCACATC-3′; and PDF1.2, 5′-ACGGGAAGATGATGTCTGTTT-3′ and 5′-TTCAGTGGTCCTGTTGTAGACA-3′.
Pathogen Infection
B.cinerea was grown on 2 × V8 agar as described previously by Mengiste et al. (2003). To infect plants, conidia were collected from a 10-d-old culture and the spore density was adjusted in Sabouraud Maltose Broth (http://www.bd.com/ds/productCenter/242910.asp) and sprayed using a Preval sprayer (https://preval.com). Inoculated plants were maintained at high humidity with a transparent cover in a growth chamber, and symptom development was observed from 3 to 5 dpi. Biomass of the fungal pathogen was quantified by qRT-PCR of total RNA isolated from inoculated plants.
For each treatment, leaves of six to eight plants were inoculated by infiltration with the Pseudomonas syringae pv. tomato DC3000 strain containing the pVSP61 kanamycin-resistant empty plasmid vector (OD600 = 0.0001 in 10 mm MgCl2).
Chromatin Immunoprecipitation Assays
The chromatin immunoprecipitation assay was performed essentially according to previously described protocols in Mukhopadhyay et al. (2008), Shang et al. (2010), and Jiang et al. (2014). Four-week-old ProWRKY57: Myc-AtWRKY57/wrky57-1 seedlings were used. To quantitatively determine the AtWRKY57–DNA (target promoters) binding, real-time RT-PCR analysis was performed according to the procedure described previously in Mukhopadhyay et al. (2008) with the ACTIN2 3ʹ-untranslated region sequence as an endogenous control. The relative quantity value is presented as the DNA binding ratio (differential site occupancy). The primers used for PCR amplification of different promoters are listed in Supplemental Table S5. A fragment of the ACTIN2 promoter was used as a negative control. W-box information for jasmonate ZIM-domain protein (JAZ) JAZ1, JAZ5, JAZ6, JAZ7, JAZ8, and JAZ10 genes is displayed in Supplemental Figure S2.
Yeast Two-Hybrid Screening and Confirmation
The full-length AtWRKY57 cDNA was cloned into the bait vector pGBKT7, and then transformed into the yeast strain Y2H Gold (Clontech, Tokyo, Japan). Two-hybrid screening was performed via the mating protocol described in the Matchmaker Gold Yeast Two-Hybrid user manual (Clontech). To confirm protein–protein interactions, the full-length SIB1 and SIB2 CDS were cloned into the prey vector pGADT7. The primers used for yeast two-hybrid are listed in Supplemental Table S3.
Bimolecular Fluorescence Complementation Assay
The cDNA sequences of enhanced YFP fragments, 173 amino acids located in the N terminus (nYFP), and 64 amino acids located in the C terminus (cYFP), were PCR-amplified and cloned into pFGC5941 to generate pFGC-nYFP and pFGC-cYFP, respectively (Hu et al., 2013). The full-length AtWRKY57 CDS sequence was inserted into pFGC-cYFP to generate a C-terminal in-frame fusion with cYFP, while SIB1 and SIB2 CDSs were introduced into pFGC-nYFP to form N-terminal in-frame fusions with nYFP. The resulting plasmids were introduced into Agrobacterium tumefaciens (strain EHA105), and infiltration of Nicotiana benthamiana was performed as described previously in Hu et al. (2013). Infected tissues were analyzed 48 h after infiltration. YFP and DAPI (4ʹ, 6-diamidino-2-phenylindole) fluorescence was observed under a confocal laser scanning microscope (Olympus, Tokyo, Japan). The primers used for bimolecular fluorescence complementation are listed in Supplemental Table S4.
Protoplast Transfection Assays
For transient transcriptional activity assay using the firefly luciferase (LUC) reporter, the CDS sequences of WRKY57, WRKY33, SIB1, and SIB2 were cloned into pGreenII 62-SK vectors under control of 35S promoter, respectively. The 2,500-bp promoter sequences of JAZ1 and JAZ5 were amplified from genomic DNA and respectively cloned into pGreenII 0800-LUC. All primers used for making these constructs are listed in Supplemental Table S3. After protoplast preparation and subsequent transfection, LUC and renillia luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega) following the manufacturer’s instructions. Relative LUC activity was calculated by normalizing against the renillia luciferase activity. All the experiments were repeated five biological times with similar results. The primers used for transient transcriptional activity assay are listed in Supplemental Table S5.
Statistical Analysis
Statistically significant differences (*P < 0.05 or **P < 0.001) are based on the Student’s t-test computed by SigmaPlot 10.0 (http://sigmaplot.software.informer.com/10.0/). Data are the means ± se of five independent experimental replicates.
Accession Numbers
Sequence data of the loci investigated in this study can be found in The Arabidopsis Information Resource under the following accession numbers: At1g69310 (WRKY57), At2g38470 (WRKY33), At3g56710 (SIB1), and At2g41180 (SIB2).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Loss of function of WRKY57 does not create sensitivity to Pst DC3000.
Supplemental Figure S2. Relative transcript levels of JAZs.
Supplemental Figure S3. W-box information of JAZs genes promoter sequences in detail.
Supplemental Figure S4. The highly conserved WRKYGQK is essential for WRKY33 and WRKY75, whereas it is not necessary for WRKY3, WRKY4, and WRKY23 to interact with SIB1 and SIB2.
Supplemental Table S1. List of real-time RT-PCR primer sequences.
Supplemental Table S2. List of real-time RT-PCR primer sequences for ChIP.
Supplemental Table S3. List of yeast two-hybrid primer sequences.
Supplemental Table S4. List of BiFC primer sequences.
Supplemental Table S5. List of transient transcriptional activity assay primer sequences.
Supplementary Material
Acknowledgments
We thank Yanru Hu for his careful revision of the manuscript.
References
- AbuQamar S, Chen X, Dhawan R, Bluhm B, Salmeron J, Lam S, Dietrich RA, Mengiste T (2006) Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J 48: 28–44 [DOI] [PubMed] [Google Scholar]
- Andreasson E, Jenkins T, Brodersen P, Thorgrimsen S, Petersen NHT, Zhu S, Qiu J-L, Micheelsen P, Rocher A, Petersen M, et al. (2005) The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J 24: 2579–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbihl RP, Diezel C, Somssich IE (2012) Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol 159: 266–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhou Y, Yang Y, Chi YJ, Zhou J, Chen JY, Wang F, Fan B, Shi K, Zhou YH, Yu JQ, Chen Z (2012) Structural and functional analysis of VQ motif-containing proteins in Arabidopsis as interacting proteins of WRKY transcription factors. Plant Physiol 159: 810–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Dhawan R, Luo H, Foerster AM, Abuqamar S, Du H-N, Briggs SD, Mittelsten Scheid O, Mengiste T (2009) HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis. Plant Cell 21: 1000–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5: 199–206 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Alméras E, Krishnamurthy V (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6: 372–378 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J (2007) Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol 144: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM (2003) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35: 193–205 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Hernández-Blanco C, Feng DX, Hu J, Sánchez-Vallet A, Deslandes L, Llorente F, Berrocal-Lobo M, Keller H, Barlet X, Sánchez-Rodríguez C, et al. (2007) Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 19: 890–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Jiang L, Wang F, Yu D (2013) Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25: 2907–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liang G, Yang S, Yu D (2014) Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid- and auxin-mediated signaling in jasmonic acid-induced leaf senescence. Plant Cell 26: 230–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liang G, Yu D (2012) Activated expression of WRKY57 confers drought tolerance in Arabidopsis. Mol Plant 5: 1375–1388 [DOI] [PubMed] [Google Scholar]
- Lai Z, Li Y, Wang F, Cheng Y, Fan B, Yu JQ, Chen Z (2011) Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell 23: 3824–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz HD, Haller E, Melzer E, Kober K, Wurster K, Stahl M, Bassham DC, Vierstra RD, Parker JE, Bautor J, et al. (2011) Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J 66: 818–830 [DOI] [PubMed] [Google Scholar]
- Lippok B, Birkenbihl RP, Rivory G, Brümmer J, Schmelzer E, Logemann E, Somssich IE (2007) Expression of AtWRKY33 encoding a pathogen- or PAMP-responsive WRKY transcription factor is regulated by a composite DNA motif containing W box elements. Mol Plant Microbe Interact 20: 420–429 [DOI] [PubMed] [Google Scholar]
- Luo H, Laluk K, Lai Z, Veronese P, Song F, Mengiste T (2010) The Arabidopsis Botrytis Susceptible1 Interactor defines a subclass of RING E3 ligases that regulate pathogen and stress responses. Plant Physiol 154: 1766–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S (2011) Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23: 1639–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R (2003) The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15: 2551–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Zentgraf U (2007) The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 19: 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Deplancke B, Walhout AJM, Tissenbaum HA (2008) Chromatin immunoprecipitation (ChIP) coupled to detection by quantitative real-time PCR to study transcription factor binding to DNA in Caenorhabditis elegans. Nat Protoc 3: 698–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SP, Somssich IE (2009) The role of WRKY transcription factors in plant immunity. Plant Physiol 150: 1648–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IA, Eggermont K, Terras FR, Thomma BP, De Samblanx GW, Buchala A, Métraux JP, Manners JM, Broekaert WF (1996) Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8: 2309–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Métraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, van der Ent S, Van Wees SCM (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5: 308–316 [DOI] [PubMed] [Google Scholar]
- Qiu JL, Fiil BK, Petersen K, Nielsen HB, Botanga CJ, Thorgrimsen S, Palma K, Suarez-Rodriguez MC, Sandbech-Clausen S, Lichota J, et al. (2008) Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J 27: 2214–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirino BF, Normanly J, Amasino RM (1999) Diverse range of gene activity during Arabidopsis thaliana leaf senescence includes pathogen-independent induction of defense-related genes. Plant Mol 29: 1049–1060 [DOI] [PubMed] [Google Scholar]
- Raiola A, Lionetti V, Elmaghraby I, Immerzeel P, Mellerowicz EJ, Salvi G, Cervone F, Bellincampi D (2011) Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol Plant Microbe Interact 24: 432–440 [DOI] [PubMed] [Google Scholar]
- Ren D, Liu Y, Yang K-Y, Han L, Mao G, Glazebrook J, Zhang S (2008) A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 105: 5638–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe HC, Kliebenstein DJ (2008) Complex genetics control natural variation in Arabidopsis thaliana resistance to Botrytis cinerea. Genetics 180: 2237–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe HC, Walley JW, Corwin J, Chan EKF, Dehesh K, Kliebenstein DJ (2010) Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog 6: e1000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Yan L, Liu ZQ, Cao Z, Mei C, Xin Q, Wu FQ, Wang XF, Du SY, Jiang T, et al. (2010) The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 22: 1909–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, et al. (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Qi T, Fan M, Zhang X, Gao H, Huang H, Wu D, Guo H, Xie D (2013) The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet 9: e1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Johnson JS, Dong X (2007) Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc Natl Acad Sci USA 104: 18842–18847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal J, Kaliff M, Dewaele E, Persson M, Dixelius C (2008) RLM3, a TIR domain encoding gene involved in broad-range immunity of Arabidopsis to necrotrophic fungal pathogens. Plant J 55: 188–200 [DOI] [PubMed] [Google Scholar]
- Tang D, Simonich MT, Innes RW (2007) Mutations in LACS2, a long-chain acyl-coenzyme A synthetase, enhance susceptibility to avirulent Pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiol 144: 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Nelissen I, Eggermont K, Broekaert WF (1999) Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J 19: 163–171 [DOI] [PubMed] [Google Scholar]
- Turck F, Zhou A, Somssich IE (2004) Stimulus-dependent, promoter-specific binding of transcription factor WRKY1 to Its native promoter and the defense-related gene PcPR1-1 in Parsley. Plant Cell 16: 2573–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell (Suppl) 14: S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese P, Chen X, Bluhm B, Salmeron J, Dietrich R, Mengiste T (2004) The BOS loci of Arabidopsis are required for resistance to Botrytis cinerea infection. Plant J 40: 558–574 [DOI] [PubMed] [Google Scholar]
- Veronese P, Nakagami H, Bluhm B, Abuqamar S, Chen X, Salmeron J, Dietrich RA, Hirt H, Mengiste T (2006) The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell 18: 257–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Voisin D, Nawrath C, Kurdyukov S, Franke RB, Reina-Pinto JJ, Efremova N, Will I, Schreiber L, Yephremov A (2009) Dissection of the complex phenotype in cuticular mutants of Arabidopsis reveals a role of SERRATE as a mediator. PLoS Genet 5: e1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Gan S, Quirino B, Amasino RM (1998) A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol Biol 37: 455–469 [DOI] [PubMed] [Google Scholar]
- Xu X, Chen C, Fan B, Chen Z (2006) Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18: 1310–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, Wang Z, Xie D (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21: 2220–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Qamar SA, Chen Z, Mengiste T (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48: 592–605 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.