Overflow in triacylglycerol is insufficient to explain carbon storage during nutrient deprivation.
Abstract
Because of the potential importance of algae for green biotechnology, considerable effort has been invested in understanding their responses to nitrogen deprivation. The most frequently invoked reasons proposed for the accumulation of high cellular levels of triacylglycerol (TAG) and starch are variants of what may be termed the “overflow hypothesis.” According to this, growth inhibition results in the rate of photosynthetic energy and/or carbon input exceeding cellular needs; the excess input is directed into the accumulation of TAG and/or starch to prevent damage. This study was aimed at providing a quantitative dataset and analysis of the main energy and carbon flows before and during nitrogen deprivation in a model system to assess alternative explanations. Cellular growth, biomass, starch, and lipid levels as well as several measures of photosynthetic function were recorded for cells of Chlamydomonas reinhardtii cultured under nine different autotrophic, mixotrophic, and heterotrophic conditions during nutrient-replete growth and for the first 4 d of nitrogen deprivation. The results of a 13C labeling time course indicated that in mixotrophic culture, starch is predominantly made from CO2 and fatty acid synthesis is largely supplied by exogenous acetate, with considerable turnover of membrane lipids, so that total lipid rather than TAG is the appropriate measure of product accumulation. Heterotrophic cultures accumulated TAG and starch during N deprivation, showing that these are not dependent on photosynthesis. We conclude that the overflow hypothesis is insufficient and suggest that storage may be a more universally important reason for carbon compound accumulation during nutrient deprivation.
The current rate of utilization of fossil fuel products for energy and the chemical industry is unsustainable. Among the alternative renewable replacements, microalgal oil and biomass production have shown considerable promise (Ohlrogge et al., 2009; Atabani et al., 2012; Chisti, 2007; Williams and Laurens, 2010) because many algae are capable of rapid photoautotrophic growth to high cell densities and can accumulate high dry weight percentages of triacylglycerol (TAG; Hu et al., 2008; Wijffels and Barbosa, 2010; Jones and Mayfield, 2012). TAG accumulation is induced in many microalgal taxa by a number of stresses including salt and nutrient deprivation (Murphy, 2001). Beginning over 100 years ago (Beijerinck, 1904), many researchers have reported that nitrogen (N) deprivation induces high levels of accumulation of TAG and starch in a range of microalgal species at the expense of decreased cell growth and slower metabolism (Liu and Benning, 2013; Martin and Goodenough, 1975; Shifrin and Chisholm, 1981; Hu et al., 2008; Granum et al., 2002; Spoehr and Milner, 1949). This has led to interest in understanding the underlying physiological and adaptive functions of algal TAG production during N deprivation to help guide engineering of higher oil yields.
In recent years, studies of the effects of nitrogen deprivation have revealed many changes in the structure and function of networks across metabolism and other cellular functions (Miller et al., 2010; Blaby et al., 2013; Schmollinger et al., 2014; Goodenough et al., 2014; Juergens et al., 2015). Such studies and mutant screens have led to the identification of enzymes involved in TAG synthesis (Merchant et al., 2012; Li et al., 2012) and pointed to putative regulatory networks (Gargouri et al., 2015). However, the interpretation of system-wide molecular changes and the choice of which among them to target for further investigation and practical purposes is uncertain and is strongly influenced by the prevailing views of the function of TAG accumulation.
Several explanations have been proposed for the induction of TAG accumulation in algae under stress, ranging from storing reduced carbon as an energy source for survival and/or future recovery, to lipid reorganization during photosynthetic down-regulation and/or subsequent up-regulation, to photoprotection (Grossman et al., 2010; Kohlwein, 2010; Klok et al., 2014; Murphy, 2001; Akita and Kamo, 2015; Khozin-Goldberg et al., 2005). Roessler (1990) appears to have been the first to postulate that algae accumulate TAG as a sink for excess photosynthetic energy and reductant to prevent photochemical damage. In this explanation, photosynthetic carbon and energy assimilation that can no longer be directed to growth when population increase is inhibited by nutrient deficiency or other stresses results in overflow products, particularly TAG (Du and Benning, 2016). The concept of overflow metabolism has traditionally been associated with the export of metabolites by heterotrophic microbes (Neijssel and Tempest, 1975) and has more recently also been applied to photosynthetic metabolism in cyanobacteria (Hays and Ducat, 2015; Courchesne et al., 2009; Gründel et al., 2012) and higher plants (Weise et al., 2011). In microalgal work, the idea of photosynthetic overflow (excess photosynthetic energy and carbon) as the driver of oil accumulation has become a widely accepted explanation (Hu et al., 2008; Li et al., 2012; Solovchenko, 2012; Li et al., 2013; Klok et al., 2014; Du and Benning, 2016). This overflow hypothesis (OH) strongly influences research efforts and the interpretation of results in biological and engineering studies of microalgal metabolism and TAG accumulation and also has important implications for the ecophysiology of photosynthetic microbes. While frequently invoked in interpreting the results of nutrient deprivation studies, the OH has not been systematically assessed.
Such systematic assessments should include a quantitative analysis of the major carbon and energy flows during stress-induced oil accumulation and should be carried out over a range of ecophysiologically and practically meaningful conditions in relevant model organisms (Smith et al., 2010). Despite a burgeoning literature on microalgal TAG accumulation under N deprivation, such studies are lacking. The green alga Chlamydomonas reinhardtii is the most widely studied species in research on microalgal oil production. Although it produces less TAG than some species and has a low tolerance of extreme conditions, its robust growth, ease of culture, ability to grow heterotrophically, and the wealth of existing data and available genetic tools (including mutant collections, transformation tools, and a well annotated genome sequence), make it attractive for biofuel research (Rochaix, 2002; Merchant et al., 2012; Merchant et al., 2007).
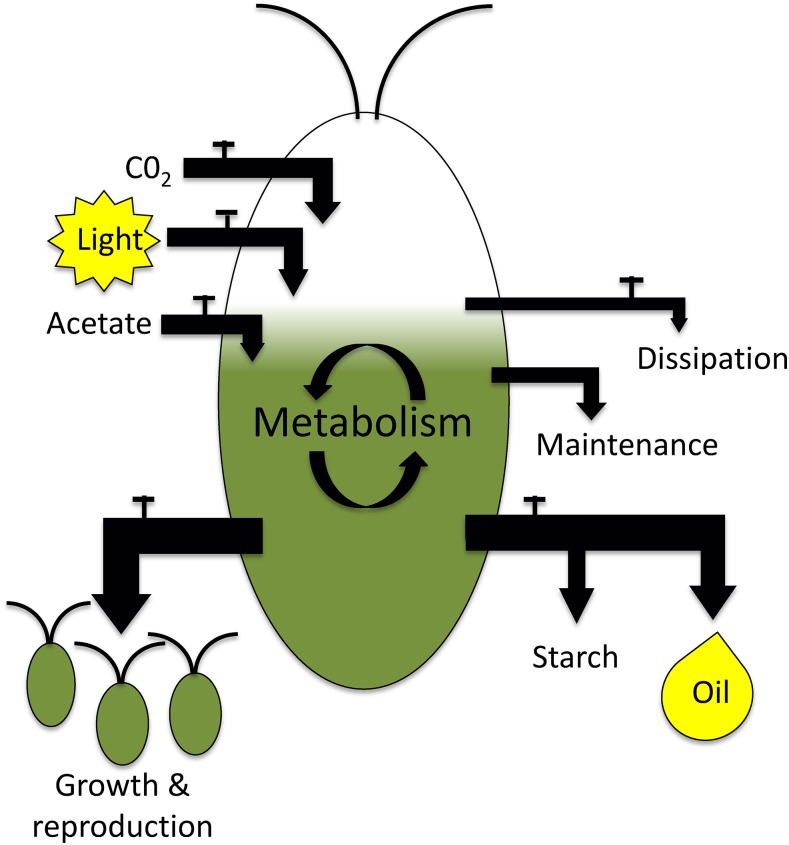
Carbon (C) and energy (E) balances are established tools in ecology and metabolic engineering and are employed to assess growth and yields during commercial applications. Widely used for monitoring and improving bacterial and yeast fermentation processes, measuring cellular C and E influxes and outflows has also been used in several cyanobacterial and algal studies (Watanabe et al., 1995; Slade and Bauen, 2013; Chapman et al., 2015; Wagner et al., 2006). Figure 1 shows the C and E entering a Chlamydomonas cell (photosynthesis and/or acetate uptake) as well as the major C and E outputs (growth, maintenance, dissipation, and TAG and starch production). Figure 1 also illustrates the overflow hypothesis, in which nutrient deprivation causes a redirection of output fluxes from reproduction to TAG and starch synthesis. Quantifying the relationships between C and E fluxes before and during stress conditions can point to processes such as dissipative metabolic or photochemical processes and can be used to make quantitative tests of predictions implied by the OH and alternative explanations for TAG accumulation.
Figure 1.
Illustration of the major carbon and energy inputs and outputs of Chlamydomonas cells. CO2 provides C input, light provides energy input, and acetate provides both. Incoming energy can be dissipated as heat, consumed for maintenance, or used in cell growth or biosynthesis of storage compounds (starch and oil). Incoming carbon is used in growth, stored in starch or oil, or respired as CO2. The taps indicate sites of regulation by the cell for which the literature provides support.
Here, we present measurements of cellular growth, biomass, starch, TAG, and total fatty acid accumulation as well as changes in photosynthetic fluxes and substrate uptake rates across a range of light levels with and without exogenous acetate during the course of 96 h of N deprivation. Results from a 13C labeling time-course experiment highlight the involvement of multiple lipid pools during N deprivation, so that (1) total lipid rather than TAG alone is the appropriate pool for C and E balances, and (2) lipids are preferentially formed from exogenous fixed carbon (when available) rather than from photosynthate, the latter contributing more to starch production. TAG and starch accumulated to significant levels in the dark after N depletion, demonstrating that TAG accumulation is not dependent upon photosynthetic activity. Using the photosynthetic, biomass, and uptake rates, C and E influxes and outflows are compared to predictions deduced from the overflow hypothesis. Several aspects of the findings do not support the OH in its straightforward form. We conclude that the overflow hypothesis is insufficient to explain carbon accumulation during nutrient deprivation. Based on these findings and a reinterpretation of the literature, we suggest that starch and oil synthesis are better explained as means for the storage of biosynthetic precursors and/or chemical energy for survival of nutrient deprivation and to aid in recovery.
RESULTS
Growth Rates in N-Replete Conditions
To characterize the effect of light and trophic conditions on Chlamydomonas growth rates, cells were grown in defined media either with acetate as a fixed carbon source (Tris acetate phosphate [TAP] media) or without acetate (high salt [HS] media) at light levels of 0, 5, 15, 40, and 160 μmol photons m−2 s−1. No growth was observed in the dark in HS media and this condition was not further considered.
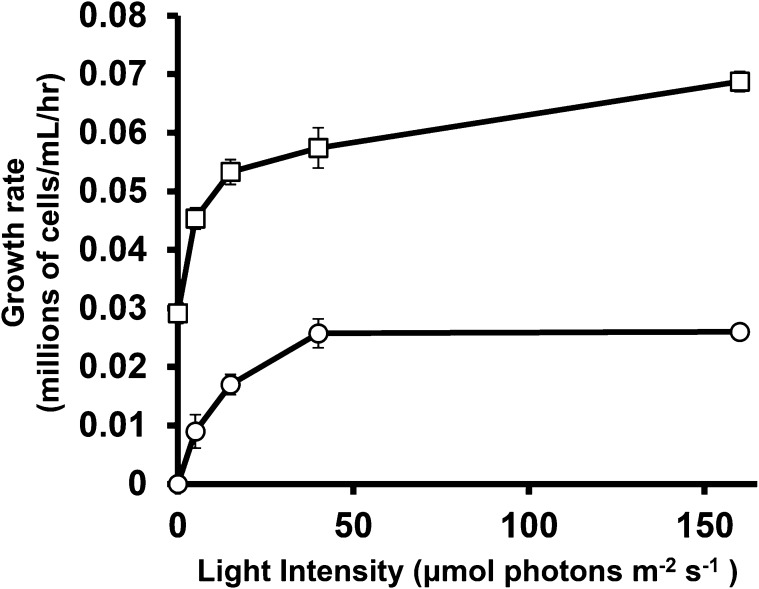
Cell growth measured during exponential growth is presented as relative or specific growth rate in Figure 2 (see “Materials and Methods”). This range of light intensity spans heterotrophic growth in the dark in TAP media, light-limited autotrophic and mixotrophic conditions, and saturating light levels. Illumination was kept below levels (at or above 200 μmol photons m−2 s−1) where cells showed chlorosis and reduced growth rates indicative of light stress and/or photoinhibition (Juergens et al., 2015; Peers et al., 2009; Bonente et al., 2012). Increasing illumination from 40 to 160 μmol photons m−2 s−1 did not increase growth rate, suggesting that these cells are carbon limited (Spalding, 1989; Goldman et al., 1974). This suggestion was supported by the observation that specific growth rates increased approximately 2-fold (from 0.025/h to 0.055/h ± 0.0006) for autotrophically grown cells at 160 μmol photons m−2 s−1 when humidified ambient air was circulated through the culture flasks to increase CO2 availability.
Figure 2.
Growth rates during exponential growth in nitrogen-replete media with acetate (TAP media; squares) and or without (HS media; circles) under continuous illumination at light levels from 0 to 160 μmol photons m−2 s−1. Rates are presented as millions of new cells produced per milliliter of culture per hour by a culture at a density of 106 per mL. These represent the specific, or relative growth rates (RGR), which are the rate constants for exponential growth according to the formula n = No.eµt, where n is the number of cells per mL at time T, No is the initial cell density, and µ is the relative growth rate. RGR is inversely related to doubling time: RGR = Ln (2)/tdoubling. Error bars indicate ±sd (n = 3 biological replicates).
Biomass
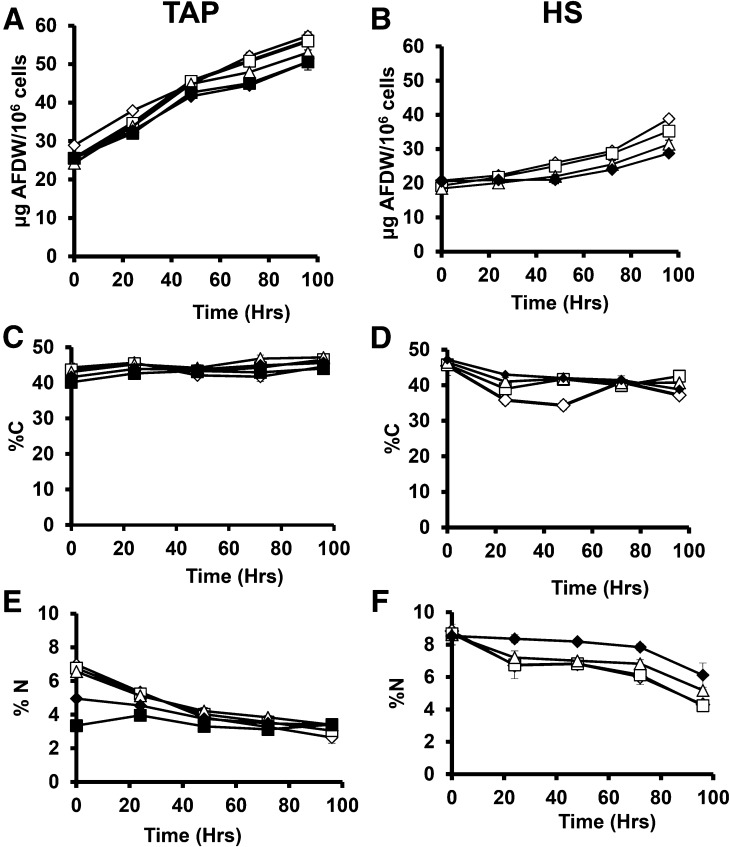
Biomass measured as ash free dry weight (AFDW) is shown in Figure 3, A and B. Cellular biomass in N replete cells is very similar across light levels, with mixotrophic and heterotrophic cultures (TAP medium) having approximately 25% more AFDW than autotrophic cultures. Cells cultured in TAP medium increased ∼2-fold in cell biomass over 96 h, whereas HS cells increased less in biomass, especially in the first 48 h of deprivation.
Figure 3.
Cellular biomass and percentage of carbon and nitrogen for N-replete and N-deprived cultures. Biomass is given as AFDW per million cells (A and B), %C (C and D), and %N (E and F) and is reported for cultures grown in TAP (A, C, and E) and HS (B, D, and F) media and deprived of N at time 0. Empty diamonds, cells cultured at 160 photons m−2 s−1 (μE); empty squares, 40 μE; empty triangles, 15 μE; filled diamonds, 5 μE; filled squares, 0 μE. Error bars indicate ±sd (n = 3).
Biomass and C and N contents of cells were measured to quantify net carbon accumulation rates and cellular nitrogen contents as a proxy for nitrogenous biomass (protein plus nucleic acids). Cellular C and N contents measured by elemental analysis are shown in Figure 3, C to F, and C:N ratio is shown in Supplemental Figure S3. Carbon accounts for ∼40 to 47% of cell dry weight during growth in N replete media (Fig. 3, C and D), levels that changed little during N deprivation in TAP media, whereas C levels decreased in cells deprived of N in HS media. Nitrogen levels as a percentage of dry weight were higher in nitrogen replete cells growing autotrophically than in TAP media and fell significantly during N deprivation (except for heterotrophic cultures). The falling cellular %N during deprivation is due to the accumulation of biomass components lacking N, so that total N contents per cell were little changed. A rising C:N ratio was previous reported during the first 24 h of N deprivation in a mixotrophic culture(Schmollinger et al., 2014).
The Accumulation of TAG and Starch
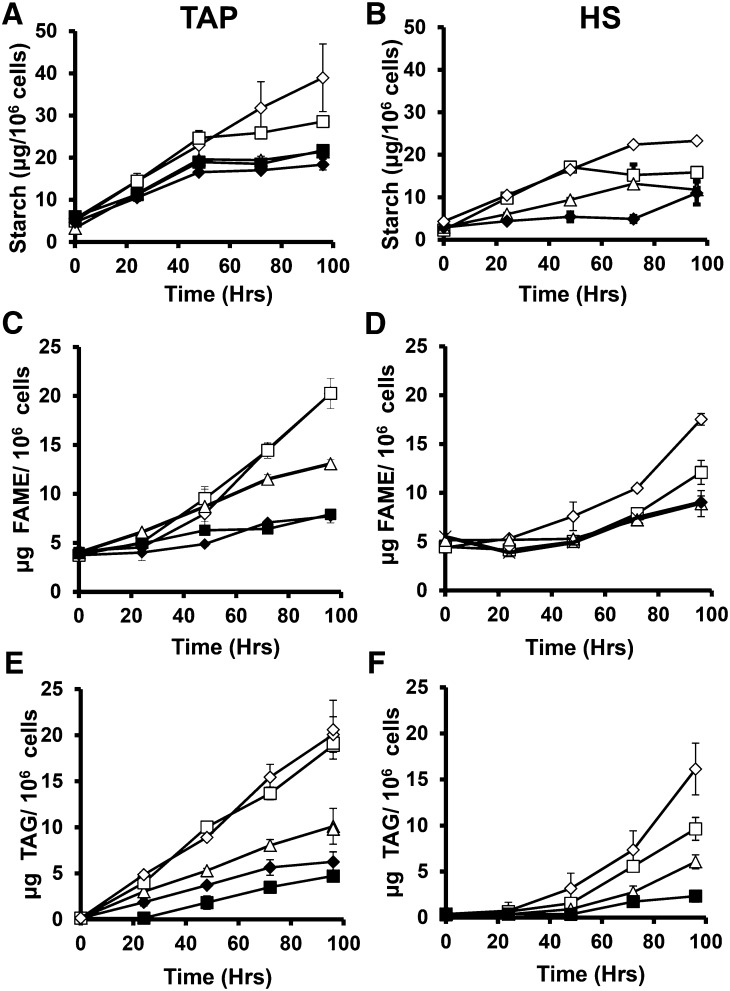
The first compound known to accumulate to high levels during N deprivation is starch (Klein, 1987), and the up-regulation of starch synthesis gene expression has been reported during N deprivation (Miller et al., 2010; Ball et al., 1990; Juergens et al., 2015). Increases in starch levels during N deprivation are shown in Figure 4; they account for the majority of the increases in cellular dry weight. Starch levels rose linearly for 48 h after N deprivation in both TAP and HS media, with higher rates at higher light levels and in TAP compared to HS media. After 48 h starch accumulation rates decreased, with cessation of starch accumulation by 96 h for all but the cells cultures at the highest light level in TAP media and those at the lowest light levels in HS media.
Figure 4.
Starch, FAME, and TAG levels before and during nitrogen deprivation. Starch (A and B), FAME (C and D), and TAG (E and F) levels are reported in μg per million cells for cultures in TAP (A, C, and E) and HS (B, D, and F) media. Empty diamonds, cells cultured at 160 μmol photons m−2 s−1 (μE); empty squares, 40 μE; empty triangles, 15 μE; filled diamonds, 5 μE; filled squares, 0 μE. Error bars indicate ±sd (n = 3).
During nitrogen-replete growth cultures contained close to 5 µg of total fatty acid per million cells (Fig. 4, B and C) and no TAG was detected (Fig. 4, E and F). During N deprivation in mixotrophic cultures, TAG levels were detected at 24 h, while little or no TAG was detected in HS cultures until 48 h. Levels of starch and TAG were previously reported by Fan et al. (2012) and by Siaut et al. (2011) for several Chlamydomonas strains cultured at 50 and 150 μmol photons m−2 s−1, respectively, after 2 or 3 d of N deprivation. Our results for these time points in cells supplied with 40 and 160 μmol photons m−2 s−1 are similar to those previously reported (except for mutant strains lacking starch). The observation that TAG accumulates during nitrogen deprivation in Chlamydomonas cells cultured heterotrophically in the dark separates the induction of TAG accumulation from photosynthesis. High levels of TAG accumulation have been reported in heterotrophically grown Chlorella species (Miao and Wu, 2004; Liu et al., 2011), and TAG accumulation was also observed in heterotrophically cultured Chlamydomonas after carbon starvation (Singh et al., 2014) and during N deprivation in the presence of high levels (60 mm) of acetate (Fan et al., 2012).
The increases in total cellular fatty acid methyl ester (FAME) levels we observed were lower and slower to start than increases in TAG. This is consistent with previous reports that membrane lipid levels fall during N deprivation and that some fatty acids used in TAG synthesis come from membrane lipids (Yoon et al., 2012; Moellering and Benning, 2011; Juergens et al., 2015). However, the extent to which fatty acids newly synthesized during N deprivation enter bulk membrane lipid pools is not known. The source(s) of carbon entering TAG and the fate of carbon assimilated during nitrogen deprivation are relevant to the consideration of cellular metabolic input and output fluxes during TAG synthesis and were therefore probed in a labeling experiment.
13C Labeling Results
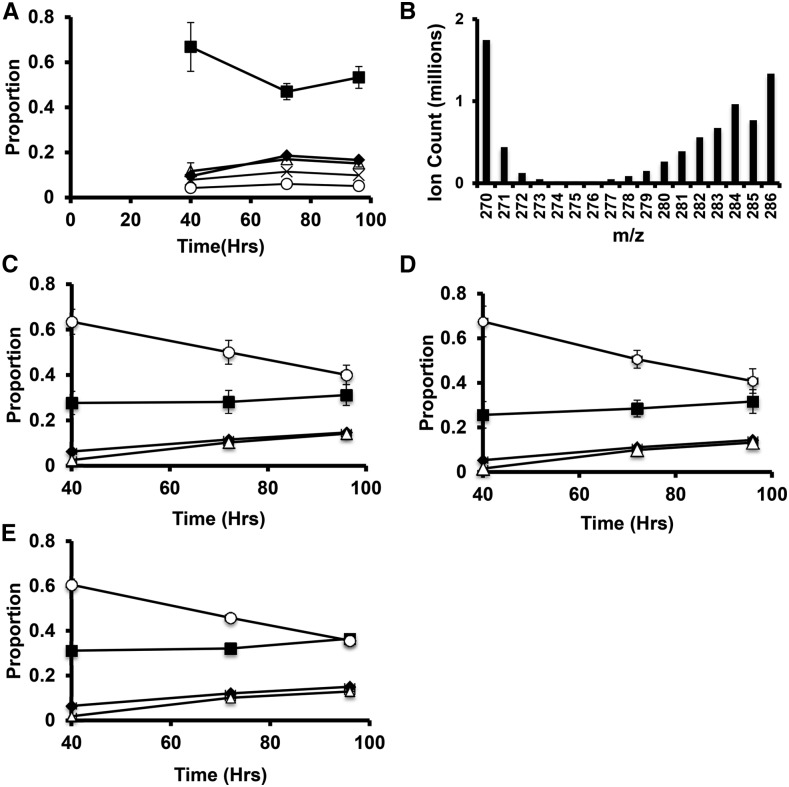
Chlamydomonas cells growing in nitrogen-replete TAP media were transferred to TAP media without N and containing 13C labeled acetate (100% 13C1,2). After 40 h, cells were transferred to TAP media containing unlabeled acetate and lacking N for the remainder of the 96h deprivation period. Cells cultured mixotrophically at 160 μmol photons m−2 s−1 were used as these conditions are similar to those used in many prior studies and showed the highest sustained starch and lipid accumulation rates. Cells were labeled for the first 40 h of N deprivation, while starch accumulation rates were highest and moved to unlabeled medium to determine the origins of starch and TAG carbon and to monitor fatty acid turnover during continued deprivation. After 40 h of N deprivation in the presence of 13C-acetate, the proportion of starch-derived ions detected that were labeled with 13C was found to be low, with over 65% of the ions containing no 13C label (Fig. 5A) and over 80% of the total carbon being 12C. Label levels increased modestly during the initial washout period between 40 and 72 h (∼28% of starch carbon at 72 h originated from the 13C acetate taken up between 0 and 40 h). Label levels in starch did not significantly change between 72 and 96 h. Thus, photosynthetic CO2 fixation and/or cellular biomass components made before deprivation is the dominant source of carbon for net starch synthesis during N deprivation under mixotrophic conditions. In such cultures, the total starch levels per cell doubled between 40 and 96 h (Fig. 4A), during which time no 13C label was present in the medium, so we had expected a dilution of the 13C label in starch. The lack of a decrease in fractional labeling level between 40 and 96 h shows that 13C assimilated during nitrogen deprivation (0–40 h) contributed significantly to starch synthesis later.
Figure 5.
13C labeling of fatty acids and starch during N deprivation in cells cultured in TAP media at 160 μmol photons m−2 s−1. Cells grown to log phase in N-replete media were moved at time 0 to TAP media lacking N with 100% uniformly labeled 13C acetate. After 40 h, the media was replaced with TAP media lacking N and containing unlabeled acetate for the remainder of the time course. The proportions of different mass isomers are plotted as a function of time. Panel A shows mass isomers of the m/z = 319 fragment ion containing carbons 2-6 of the Glc monomers of starch. Filled squares represent M+0, filled diamonds M+1, empty triangles m+2, X’s M+3, and empty circles M+4. Panel B is a histogram of the mass isomer distributions for a representative 16:0 FAME mass spectrum showing the abundance of highly labeled molecules together with unlabeled molecules (M+0 and naturally occurring M+1 masses). C to E represent mass isomer distributions for ions of intact C16:0 FAME molecules from TAG, MGDG, and polar lipid fractions, respectively. Filled squares represent unlabeled molecules (M+0); filled diamonds, M+1 (one 13C atom); empty triangles, M+2; empty circles, the sum of M+3 through M+16 (fully labeled). Error bars indicate range (n = 2).
Labeling in fatty acids from the experiment described above is also shown in Figure 5. Figure 5B shows the distribution of mass isomers from a representative mass spectrum of palmitate (C16:0, as the methyl ester) from cells collected at 40 h. Labeling results for C16:0 from TAG and two membrane lipid fractions (galactolipids, which are major components of chloroplast membranes, and a polar lipid fraction containing lipids from both chloroplast and extraplastidic membranes) are shown in Figure 5, C to E. Analogous data for other abundant fatty acids are given in the Supplementary Figures S4 and S5. After 40 h of labeling and nitrogen deprivation, approximately 70% of the fatty acid molecules from TAG and membrane lipids contained one or more 13C labeled carbons. In the majority of fatty acid molecules, over half the carbon atoms were 13C and the most abundant labeled mass isomer was the fully labeled one (13C in all positions) and approximately half the total carbon in cellular fatty acids was 13C. The preponderance of fatty acid molecules in all pools were either unlabeled or highly labeled, and since the total fatty acid content of the cultures approximately doubled during the 40-h labeling period, we infer that 75% or more of the carbon used to synthesize fatty acids during N deprivation is derived from acetate taken up during deprivation. High fractional labeling in galactolipids and polar lipids (Fig. 5, C and D) after 40 h of N deprivation shows that membrane lipids are synthesized at a significant rate during nitrogen deprivation. Since the cellular levels of membrane lipids fall by more than 50% during this time (Juergens et al., 2015), it is clear that substantial rates of simultaneous synthesis and breakdown of membrane lipids are occurring. Therefore, we consider that total cellular lipid rather than TAG is the appropriate pool to be measured when evaluating net carbon and energy fluxes into accumulated intracellular compounds during nitrogen deprivation.
After replacement of labeled with unlabeled acetate, the proportion of fatty acid molecules that were highly 13C-labeled fell, while the proportion of unlabeled molecules rose; this is consistent with dilution of highly labeled with newly synthesized unlabeled fatty acids. The similarity in C16:0 labeling patterns in different lipid pools is consistent with the continued flux of newly synthesized fatty acids into membrane lipids as well as TAG between 40 and 96 h. In addition, during this “chase” period, there is an increase in the proportion of fatty acid molecules with low levels of labeling (M+1 and M+2), which we attribute to flow of C from nonlipid compounds synthesized during the initial stages of N deprivation into lipid synthesis at later stages.
Light Absorption, Utilization Efficiency, and Dissipation
To gauge photosynthetic light capture rates, levels of chlorophyll (Chl) and the efficiency of photosystem II were measured. Chl levels are shown in Figure 6, A and B, for cells cultured in TAP and HS medium, respectively. Nitrogen-replete cells growing under mixotrophic conditions contained more Chl than autotrophically growing cells, with the exception of mixotrophic cells at the lowest light levels. During nitrogen deprivation, Chl levels fell continuously in mixotrophically cultured cells, whereas under autotrophic conditions, Chl levels fell during the first 2 d and did not change significantly thereafter.
Figure 6.
Chlorophyll, photosynthetic efficiency, and NPQ. A and B, Chl levels in Tap and HS conditions, respectively. C and D, Maximum photosynthetic efficiency from dark adapted cells for TAP and HS conditions, respectively. E and F, Photosynthetic efficiency in the light for TAP and HS conditions, respectively. G and H, Photosynthetic efficiency in TAP and HS conditions, respectively. In all cases, 160 μmol m−1 s−1 is indicated by hollow diamonds, 40 μmol m−1 s−1 by hollowed squares, 15 μmol m−1 s−1 by hollowed triangles, 5 μmol m−1 s−1 by solid diamonds, and 0 μmol m−1 s−1 by solid squares. Error bars indicate sd (n = 3).
Both theoretical quantum yield at photosystem II (Fv/Fm) and light-driven yields (ΦII) were measured by fluorescence spectroscopy as measures of the efficiency with which absorbed light drives linear electron flow. In TAP media, Fv/Fm and ΦII (Fig. 6, C and E, respectively) declined through the nitrogen deprivation period, with the exception of cells under 5 μmol photons m−2 s−1. In autotrophic cells, Fv/Fm and ΦII (Fig. 6, D and F) did not significantly change during nitrogen deprivation.
During photosynthetic stress conditions and at high light levels, cells dissipate excess absorbed light energy, by nonphotochemical quenching (NPQ) (Müller et al., 2001). We measured NPQ before and during N deprivation under mixotrophic (Fig. 6G) and autotrophic (Fig. 6H) conditions using the same light level for NPQ measurement as the level under which the cells had been cultured. NPQ levels recorded before and throughout nitrogen deprivation for all cultures were substantially lower than values reported in previous studies in which much higher light levels were used for measurement than for growth (Terauchi et al., 2010; Peers et al., 2009; Niyogi et al., 1997). Fan et al. (2012) reported increases in NPQ to modest levels in N-deprived Chlorella pyrenoidosa. The absence of significant increases in NPQ after N deprivation suggests that there is little or no light stress due to excess energy intake under these conditions.
Photosynthetic Fluxes
Photosynthetic fluxes were assessed by measuring oxygen evolution and electrochromic shift (ECS) absorbance spectroscopy as indicators of linear and cyclic electron flow respectively (Fig. 7). Oxygen evolution rates at 160 μmol photons m−2 s−1 for both autotrophic and heterotrophic cultures decreased markedly during N deprivation (Fig. 7, A and B). At 40 μmol photons m−2 s−1 and below, net oxygen fluxes were much less during nitrogen-replete growth but remained largely unchanged during deprivation. Mixotrophically cultured cells at lower light levels and autotrophic cells were net oxygen consumers. ECS decay rates, which reflect the proton fluxes across thylakoid membranes that drive photosynthetic ATP synthesis, decreased severalfold during nitrogen deprivation for cells under the highest light level for both mixotrophic and autotrophic cells (Fig. 7, C and D). At lower light levels, ECS values for N-replete cells were significantly less than at 160 μmol photons m−2 s−1 and decreased moderately or were not significantly changed during nitrogen deprivation.
Figure 7.
Oxygen evolution and ECS measurements. Net oxygen evolution (A and B) and ECS decay rates (indicating proton fluxes across the thylakoid membrane; C and D) for cells grown in TAP (A and C) and HS (B and D) media. C and D are presented as per 109 cells instead of 106 used in the rest of the manuscript. Results for cells cultured at 160 μmol m−2 s−1 are indicated by empty diamonds, 40 μmol m−2 s−1 by empty squares, 15 μmol m−2 s−1 by empty triangles, 5 μmol m−2 s−1 by filled diamonds, and 0 μmol m−2 s−1 by filled squares. Error bars indicate sd (n = 3).
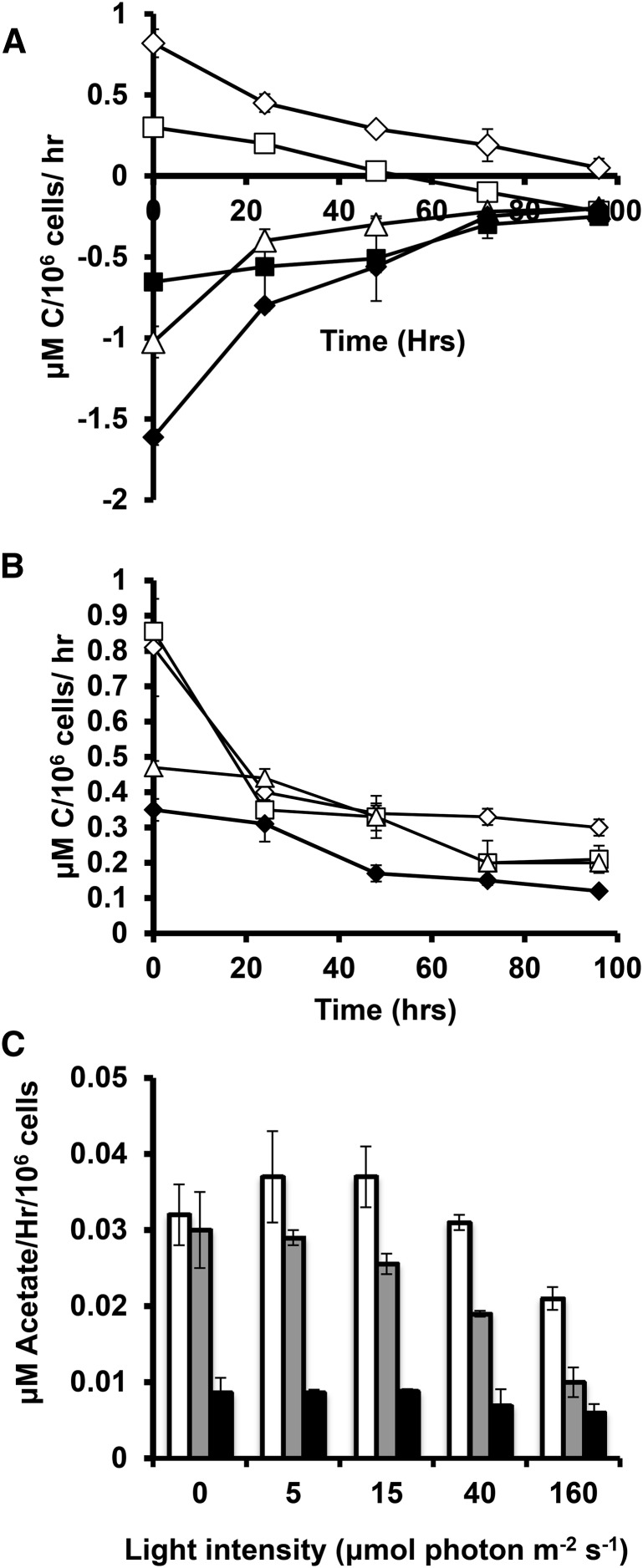
Carbon Assimilation and Release
Net CO2 uptake and efflux rates were measured before and during N deprivation (Fig. 8, A and B). In TAP media, cultures under 160 and 40 μmol photons m−2 s−1 of illumination and in HS media at all light levels showed net CO2 assimilation when provided with nitrogen, while cultures exposed to lower light levels or in the dark in TAP media were net CO2 producers. During nitrogen deprivation, net CO2 uptake and output rates decreased, with TAP-grown cells at 40 μmol photons m−2 s−1 becoming net CO2 producers after 2 d of deprivation. Acetate uptake rates for mixotrophic and heterotrophic cultures (Fig. 8C) were higher for cells at lower light intensities and in the dark and decreased during nitrogen deprivation. Thus, both photosynthetic CO2 assimilation and consumption of external fixed carbon decreased strongly during N deprivation, with decreases being more marked at higher light levels.
Figure 8.
Carbon assimilation rates. Net CO2 uptake in TAP (A) and HS (B) media. Measurements of cells cultured at 160 μmol m−2 s−1 are indicated by empty diamonds, 40 μmol m−2 s−1 by empty squares, 15 μmol m−2 s−1 by empty triangles, 5 μmol m−2 s−1 by filled diamonds, and 0 μmol m−1 s−1 by filled squares. Acetate uptake in TAP cultures in C for N-replete cells (white), 0 to 24 h of N deprivation (gray), and 24 to 96 h of deprivation (black). Error bars indicate sd (n = 3).
DISCUSSION
The first observation of microalgal TAG accumulation under nitrogen deprivation was made over a century ago (Beijerinck, 1904), and the potential to utilize this phenomenon for biofuels has been recognized for over 70 years (Harder and von Witsch, 1942a, 1942b). Among several explanations offered, the OH, now at least 25 years old (Roessler, 1990), currently holds sway over much of the interpretation of algal TAG accumulation data. The OH explains the synthesis of TAG and/or starch by algae during nutrient deprivation as a response to excess photosynthetic energy and/or carbon assimilation. The results of this study allow a thorough assessment to be made of the OH in its straightforward form for the first time by making a series of inferences from it about the relationships between photosynthetic carbon and energy uptake rates and the rates of accumulation of TAG and starch and comparing these expectations to the data.
First, if accumulation is driven by surplus energy or carbon from photosynthesis, no accumulation is expected to occur in the dark. Starch accumulation rates in the dark were equal to or greater than rates for autotrophic cultures at all light levels and were exceeded only by mixotrophic cultures at the higher two light levels. We conclude either that starch accumulation during nitrogen deprivation is primarily driven by factors unrelated to photosynthetic overflow or that there is a separate additional explanation for its accumulation under heterotrophic conditions. TAG accumulation in the dark was also significant, although net fatty acid accumulation was modest compared to cultures in the light. Indeed, heterotrophic cells from taxa as diverse as fungi bacteria and mammals have been shown to accumulate TAG under nutrient deprivation (Alvarez and Steinbüchel, 2002; Frenk et al., 1958; Packter and Olukoshi, 1995; Morin et al., 2011; Murphy, 2001).
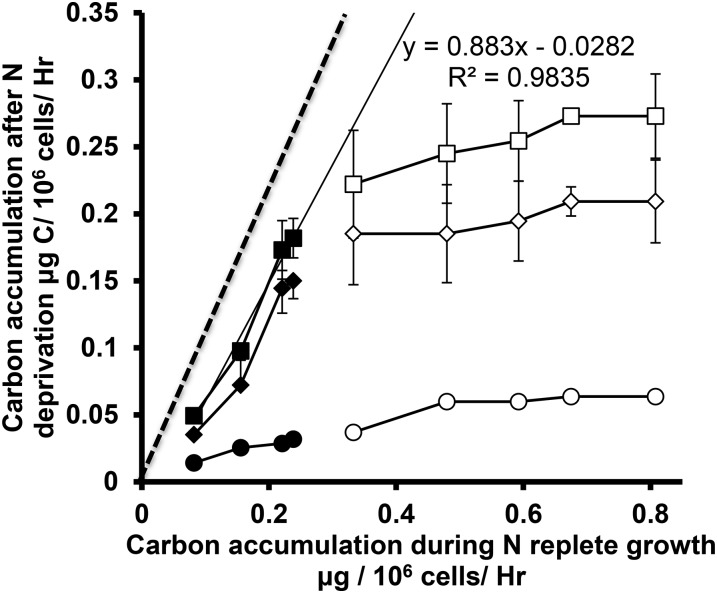
Second, the OH links growth before nutrient deprivation to TAG production afterward; initially, we examine carbon balances. Figure 9 shows a comparison of carbon accumulation rates before and during the first 24 h after N deprivation. The first 24 h after deprivation were selected for comparison with N-replete growth since at this time photosynthetic function is most similar to predeprivation rates. In this work, as elsewhere (Fan et al., 2012; Siaut et al., 2011), starch was found to be the dominant carbon sink, with total FAME accounting for <15% of net carbon accumulation during the first 24 h. Total FAME accumulation rates only match and begin to exceed those for starch after 48 h, by which time photosynthetic fluxes are much lower. For autotrophic cells across all light levels the total carbon accumulation rates after deprivation are close to 90% of those during N replete growth, which is consistent with the OH as applied to total C. For cells cultured under mixotrophic or heterotrophic conditions, total carbon accumulation rates after deprivation are not well explained by the rates during N-replete growth. Although there is a trend with increasing light levels in TAP media toward greater total C accumulation rates before and after deprivation, the slope corresponds to only 11% and the trend does not extrapolate meaningfully to low light and lower growth rates. Concerning the origins of carbon from which TAG and starch are synthesized, 13C acetate labeled both these pools in mixotrophic cultures that were grown at the highest light level to maximize photosynthetic inputs. Photosynthesis is not the dominant metabolic source of acetyl-CoA used in TAG synthesis in mixotrophy. Fan et al. (2012) have suggested that TAG accumulation is dependent upon total carbon precursor availability rather than only CO2 from photosynthesis. Supplemental Figures S6 and S7 show an alternative version of this data in the form of carbon per light energy absorbed. Here, similar conclusions are derived in that high light intensities have lower efficiencies of light use.
Figure 9.
Cellular carbon accumulation rates before and during the first 24 h after nitrogen deprivation. Accumulation during nitrogen-replete growth was derived from growth rates and total cellular carbon contents; carbon accumulation after deprivation is due to starch and lipid production. Filled symbols represent autotrophic conditions (HS media), while empty ones represent mixotrophic and heterotrophic conditions (TAP media). Squares indicate Y values for total carbon accumulation (starch plus total FAME), while diamonds and circles represent starch and total FAME, respectively. Within each series, increasing light levels correspond to symbols from left to right. Error bars depict ±sd (n = 3). The dashed line corresponds to a slope of 1, and the solid line is the least-squares best fit to the autotrophic total carbon accumulation results.
Third, regarding redox energy balance, the OH posits that the function of TAG synthesis during nutrient deprivation is to consume excess photosynthetically produced reductant and thereby prevent photodamage due to over reduction of electron transport chain components. We would expect from this that cellular fatty acid levels should rise most rapidly when net oxygen production is highest (early on in nutrient deprivation), which is not the case (compare Fig. 4, C and D, with Fig. 7, A and B). Since cells at the highest light level were CO2 limited during N-replete growth, these might be expected to show either high rates of NPQ or elevated fatty acid accumulation rates compared to starch early during deprivation, neither of which was observed.
Related to the potential role of TAG synthesis in mitigating overreduction in the electron transport chain is a fourth prediction, one that has received apparent support from past studies. In this version of the OH, following N deprivation cells experience stress from excess light energy uptake leading to the activation of energy dissipative mechanisms such as NPQ and/or the Mehler reaction in addition to TAG synthesis. Increases in NPQ under nutrient deprivation have been previously observed, reaching levels that account for a substantial proportion of light energy reaching the photosystems (White et al., 2011; Wilson et al., 2007; Allen et al., 2008; Antal et al., 2006). However, those measurements of NPQ were made under much higher light levels than those under which cultures were maintained. Measurements of NPQ under light levels under which cells had been cultured (Fig. 6) show stable or decreasing NPQ in TAP media, while in HS media, cells exhibit decreasing NPQ following N deprivation. Slow decreases in photosynthetic efficiency, Chl levels, and gas exchange rates in parallel with low and decreasing levels of NPQ indicate that a coordinated down-regulation of photosynthetic structure and function that is used to match energy supply and demand under a wide range of energy input rates, making the idea of overflow from a mismatch of supply and demand less appealing.
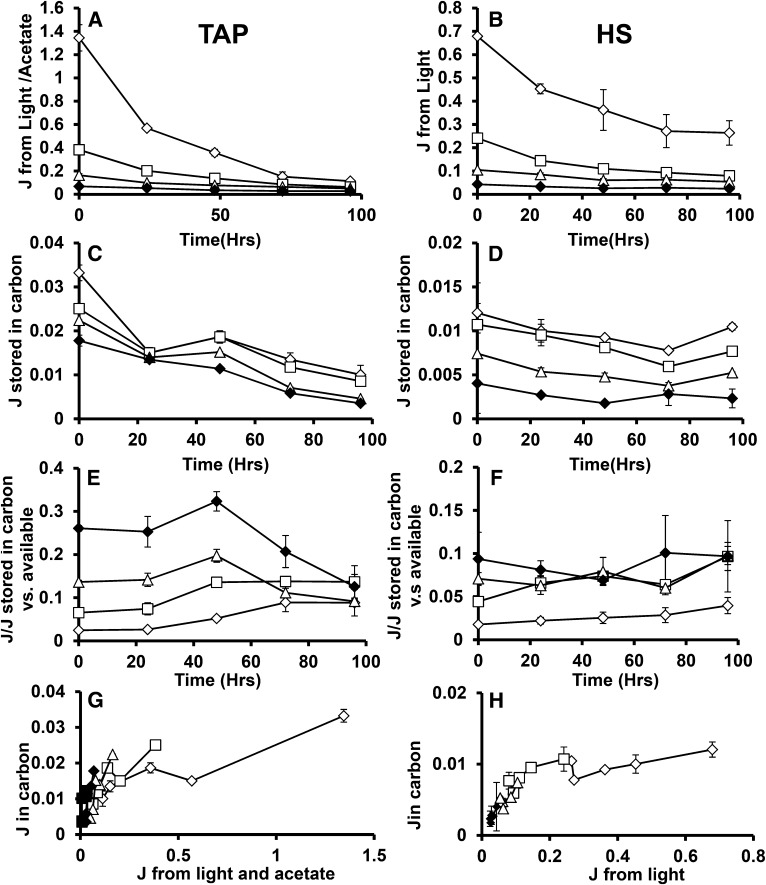
Using the results of this study, it is possible to assess the relationship between cellular energy inputs and the accumulation of TAG and starch. Figure 10, A and B, shows the rates of cellular energy intake in joules that are calculated from the results of Figure 6 as the product of illumination level, chlorophyll absorbance (based on cellular chlorophyll levels and cellular absorbance spectra), ΦII, and the energy of photons at 700 nm plus the heat of combustion of acetate taken up by mixotrophic and heterotrophic cells. This estimate takes account of energy losses before entering photosynthetic metabolism at the photosystem level and therefore offers direct insight into metabolic energy use efficiency. The photosynthetic energy capture rates span a range of approximately 50-fold under nitrogen-replete conditions, a range that narrows during N deprivation since the capture rates fall more strongly in cells cultured under lower light levels. The biomass energy accumulation rate is shown in Figure 10, C and D, which is derived from the heat of combustion of cellular biomass estimated from cellular composition and the heats of combustion of major cellular components. During N deprivation, the biomass accumulation rates are almost entirely accounted for by starch and total lipid accumulation. Cellular energy deposition rates (as well as uptake rates) are lower for autotrophic than for mixotrophic cultures at equivalent light levels (Fig. 10, A–D). The proportion of energy taken in by cells that is stored in biomass is shown in Figure 10, E and F. This varies from ∼2% for cells at higher light levels, up to 75% for heterotrophic cells after 48 h of nitrogen deprivation. Figure 10, G and H, presents energy stored in biomass versus energy available to the cells. Further, higher energy availability does not give rise to proportional increase in energy stored in biomass. The OH would lead one to expect higher or at least comparable proportions of light energy to be directed to carbon accumulation at higher light levels. Alternatively or additionally, a threshold of light capture rates might be expected to be needed to result in photosynthetic energy overflow that triggers C accumulation. Neither prediction is consistent with observation. Supplemental Figures S6 and S7 present this data on a per carbon basis instead of per energy.
Figure 10.
Joules of energy available and stored during N deprivation. Calculated joules of energy available to cells in TAP (A) and HS (B) media. Joules stored in carbon during N+ and N deprivation for TAP and HS are presented in C and D, respectively. The ratios of joules stored in carbon versus that available to the cell is presented for TAP and HS media in E and F. G and H present joules of energy in biomass versus that available from inputs, demonstrating their relationship across light intensities. In all cases, 160 μmol photons m−2 s−1 (μE) is indicated by hollow diamonds, 40 μE by hollowed squares, 15 μE by hollowed triangles, 5 μE by solid diamonds, and 0 μE by solid squares. Error bars indicate sd (n = 3).
The results of this study can also be used to test the predictions of alternative hypotheses for TAG and starch accumulation during nutrient deprivation. One potential function for TAG accumulation is that it serves to preserve carbon precursors derived from the reduction of cellular membrane pools. This would predict that total FAME levels should not fall during the period of active membrane lipid decrease; Figure 4, C and D, shows that cellular FAME levels are stable or rise modestly during the 24 to 48 h when chloroplast membrane lipid levels fall dramatically (Juergens et al., 2015). Also consistent with this hypothesis is the observation that the acetyl-CoA used for TAG synthesis in mixotrophic cells is mostly not derived from photosynthetically fixed carbon. It has been reported in a study of a nutrient deprived snow alga that intact arachidonic acid molecules in membranes are transferred into TAG (Khozin-Goldberg et al., 2000, 2005).
Another potential fitness benefit of the accumulation of TAG and starch is for survival of extended periods of deprivation. Using the measured respiration rates of cells after deprivation, we can estimate the length of time for which the quantities of carbon stored as TAG and starch that such cells contain can sustain cellular maintenance. The amounts of additional starch and total FAME per cell accumulated during 96 h of N deprivation are sufficient to sustain the respiration rates observed at 96 h for an average of 3.6 d with a range of 2 to 6 d across conditions. This is a minimum or “worst case” estimate in three ways: (1) light and/or exogenous fixed carbon uptake is assumed to cease completely after 96 h; (2) respiration rates are assumed to fall no further after 96 h; (3) no more starch or FAME is utilized after a return to predeprivation levels. Thus, the quantities of storage compounds are sufficient for survival of prolonged nutrient deprivation. Further experiments are underway to assess these alternative explanations.
CONCLUSION
This study was aimed at providing a quantitative description of metabolic and photosynthetic functional rates across a full range of autotrophic, mixotrophic, and heterotrophic conditions before and during nutrient deprivation. The results allow an unbiased assessment of the overflow hypothesis of algal TAG accumulation, which currently looms large in the pure and applied literature. Some of the results, such as the trend toward higher rates of TAG accumulation at higher light levels, and the correlation between total net carbon assimilation before and after nutrient deprivation in autotrophic cells are consistent with the OH, although they are also compatible with alternative, storage-based explanations. Other observations, including starch and TAG accumulation in heterotrophic cultures, the timing of TAG accumulation relative to maximal photosynthetic rates, and the apparent absence of correlation between light stress or excess reductant and TAG or starch accumulation, show that the OH is insufficient as an explanation for the C accumulating response of algae during nutrient deprivation.
We suggest that alternative explanations for starch and TAG accumulation, in particular the provision of metabolic precursors and chemical potential energy for survival of N deprivation and recovery, are more likely and should be explored. The correlation between nutrient deprivation and the accumulation of TAG in photosynthetic algae has been known for over a century; its significance for green biotechnology development as well as for the ecophysiology of nutrient cycles makes understanding the adaptive causal links involved important.
MATERIALS AND METHODS
Culturing
Chlamydomonas reinhardtii strain cc400 cw-15 ++mt was obtained from the Chlamydomonas Research Center and grown at 23°C in 200 mL liquid TAP media (Gorman and Levine, 1965) and Sueoka HS media (Sueoka, 1960) in 1-liter flasks shaken at 125 rpm under continuous illumination at 160, 40, 15, 5, and 0 μmol photons m−2 s−1 and ambient CO2 concentrations. The acetate concentration in TAP media is 17.5 mm and the nitrogen from NH4Cl is 9.5 mm in HS compared to 7.0 mm in TAP. Cell growth was determined by optical density measurements at 750 nm using a DU 800 spectrophotometer (Beckman Coulter). TAP and HS media were used to allow comparisons to the literature, in which these media are very commonly used for autotrophic and mixotrophic growth. Growth rate and photosynthetic fluorescence parameters during nitrogen deprivation were compared for cells in TAP media without acetate (TP) and cells in HS media (Supplemental Figs. S1 and S2). These were not significantly different, showing that the presence of acetate is the predominant cause of differences between cells cultured in HS and TAP media.
Cultures were grown to cell densities of between 0.15 (2.5 million cells/mL) and 0.3 OD750 (5 million cells/mL) before N deprivation to minimize self-shading, which becomes significant in denser cultures. Cells were counted during time-course experiments using a Z series Coulter Counter cell and particle counter (Beckman Coulter) and confirmed with hemocytometry. Growth rates were estimated during exponential phase growth according to the formula n = N0exp(µt), where N0 is the initial cells number of cells per milliliter, N is the density at time t hours, and µ is the specific or relative growth rate. The specific growth rate is inversely related to the doubling time as µ = ln(2)/tdoubling. For N deprivation, cells were centrifuged and resuspended in TAP media lacking ammonium chloride (nitrogen source). Supernatant media was carefully removed from the pellet with a pipette. Separate samples were taken for each measured parameter, using multiple flasks started at the same cell density, especially for parameters requiring large amounts of cells such as ash free dry weights.
Chlorophyll Concentration
Cells were collected by centrifugation of 1 mL culture, and Chl was extracted in 1 mL of 80% acetone for 20 min from pelleted samples after the supernatant culture medium was removed. After extraction, samples were pelleted by centrifugation and the supernatant was used for analysis. Chl was quantified spectroscopically as described (Ritchie, 2006) using a DU 800 spectrophotometer (Beckman Coulter).
Ash Free Dry Weight
Fifty milliliters of culture volume was harvested at each time point, and cells were filtered onto Millipore Glass Fiber Prefilters (Millipore) under reduced pressure. Filtered cells were dried in an oven at 100°C for 4 h and weighed on a Sartorius CP225D analytical balance. Weighed samples were then heated 540°C to incinerate the cell biomass and the filters were reweighed (Zhu and Lee, 1997). AFDW was taken as the difference between sample weights before and after incineration.
Carbon and Nitrogen Contents
Aliquots of 50 mL of culture were harvested by centrifugation at each time point. Cells were then pelleted by centrifugation and frozen at −78°C then lyophilized for 12 h. Dried samples were weighed on a Sartorius CP225D analytical balance in tin capsules, which were then submitted to Duke Environmental Stable Isotope Laboratory (Duke University) for elemental analysis.
Lipid Analyses
Cells harvested by centrifugation at 0°C and total lipids were extracted from samples containing the equivalent of ∼10 mg of dry cell mass by the method of Folch et al. (1957). Briefly, cells were extracted with 1 mL of CHCl3:MeOH (1:2, v/v), vortexing samples for 2 min. The sample was then centrifuged and the supernatant was collected; the extraction was repeated two more times and the supernatant extracts were pooled. Extracts were dried under flowing N2 gas at room temperature and stored at −20°C until analysis.
Total TAG was quantified using thin-layer chromatography separation and densitometry. Briefly, total lipid extracts were loaded onto Analtech uniplate silica gel HL plates (Analtech) and separated with hexane:diethyl ether:acetic acid (70:30:1 v/v). After iodine staining, samples were scanned with a Cannon image class mf4690 scanner. ImageJ software (National Institutes of Health) was used to quantitate TAG levels from the images. Conversion of integrated density into weight of TAG was performed by comparison with a biological standard sample (96 h N-deprived Chlamydomonas TAG extract quantified by gas chromatography flame ionization detection [GCFID] of its FAMEs).
FAMEs were quantified by GCFID after derivatization of lipid samples. C15 TAG in toluene was first added to total cell lipid extracts as internal standard. Total lipid extracts were treated with 150 μL 2 m methanolic KOH in 1 mL hexane and vortexed for 5 min at room temperature completion. Then, 6 n HCl was added to neutralize the pH, samples were vortexed for 1 min, and the hexane phase was transferred to another glass tube and dried under flowing N2 gas at room temperature and stored at −20°C. Remaining free fatty acids were derivatized with silylation agent N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide (Sigma-Aldrich) in pyridine. Samples were dried, resuspended in 1 mL hexane, and quantified using an Agilent GCFID with a DB-23 column (Agilent) as previously described(Pollard et al., 2015).
Oxygen Production and Consumption Rates
Changes in dissolved oxygen in cell suspensions were measured with a NEOFOX analyzer FOXY-R probe with a FOXY-AF-MG coating (Ocean Optics) as previously described (Juergens et al., 2015).The O2 sensor was immersed in 2 mL of culture in a capped 3-mL cuvette with stirring. Net oxygen evolution was measured for 5 min at the same illumination level as the culture had been grown in and O2 consumption was measured for 1 min in the dark immediately after the light period.
In Vivo Fluorescence Spectroscopy
All spectroscopic measurements were performed with biological triplicates at each time point as previously described (Juergens et al., 2015). Light-induced absorbance and chlorophyll fluorescence yield were measured using a kinetic spectrophotometer/fluorometer (Sacksteder et al., 2001; Livingston et al., 2010; Hall et al., 2013) modified for liquid samples by replacing the leaf holder with a temperature-controlled, stirring-enabled cuvette holder (standard 1-cm path length). Cells were maintained under far-red light LED (730 nm) for 20 min to oxidize the plastoquinone pool for accurate F0 measurements. After dark/far-red adaptation, the first saturating pulse for Chl fluorescence measurements was applied with a pulsed measuring beam (505-nm peak emission LED) filtered through a BG18 (Edmund Optics) glass filter. The sample was then illuminated with the respective photosynthetic photon flux density using a pair of LEDs (Luxeon III LXHL-PD09; Philips) with maximal emission at 620 nm, directed toward opposite sides of the cuvette, perpendicular to the measuring beam. Fluorescence yields from saturating pulses were measured under actinic light and averaged over six measurements, separated by 120-s intervals. Both absorption and fluorescence measuring pulses were 20 to 35 µs in duration and attenuated to produce less than 0.1% increase in chlorophyll fluorescence yield in dark-adapted samples. The first dark interval relaxation kinetics trace measuring the ECS kinetics (one trace per biological replicate) was recorded after 3 min of actinic illumination, followed by 1 min of dark. Actinic LEDs were calibrated using a Licor LI190 PAR quantum sensor.
CO2 Production and Consumption Rates
CO2 exchange measurements were carried out with a LICOR XT 6400 (LICOR) infrared gas analyzer. Air was continuously circulated through 250-mL flasks containing 50 mL culture, which was maintained under culture incubation conditions, and CO2 levels were recorded for the air entering and leaving the flask. Input CO2 levels were adjusted so that returning air contained 400 ppm.
Starch Analysis
Total Glc contained in starch was measured after amyloglucosidase and amylase digestion with the Megazyme total starch analysis kit, similar to Work et al. (2010). Briefly, pellets remaining after extraction of lipids from cells with 2:1 methanol:chloroform were autoclaved for 1 h in 0.1 m acetate buffer, pH 4.8, then treated with α-amylase and amyloglucosidase for 1 h at 55°C. Free Glc was quantitated with a colorimetric assay at 510 nm using a starch assay kit (Megazyme) according to the manufacturer’s instructions.
Acetate Analysis
Total acetate in culture media was measured with an acetate analysis Kit (K-ACETRM; Megazyme). TAP media samples were diluted 5-fold to be within the linear response range, and 1.5-mL subsamples were used for NADH consumption measurement by absorbance changes at 340 nm. Briefly, acetic acid and ATP are converted to acetyl phosphate and ADP by acetate kinase. The forward reaction is maintained by phosphotransacetlyase reaction of acetyl phosphate and coenzyme A to form acetyl-CoA and inorganic phosphate. Added phosphoenolpyruvate and ADP are converted to pyruvate and ATP by pyruvate kinase to maintain ATP levels and to produce pyruvate in proportion to the original levels of acetate. d-Lactate dehydrogenase then converts the pyruvate and NADH into d-lactic acid and NAD+.
Calculation of Carbon and Energy Balances
Acetate and oxygen uptake rates were converted into μg C/106 cells/h. Net oxygen fluxes were assumed to represent net CO2 fluxes at a 1:1 ratio with 12 g C/mol O2. Acetate consumption was multiplied by 2 mol C/mole acetate. Carbon accumulation rates in biomass were obtained from the percentage of carbon of the biomass and the biomass accumulated. FAME and starch mass accumulation rates were converted to μg C/106 cells/h by multiplying by the mass fraction of carbon for those molecules (72 g C per 162 g polymerized starch and 204 g C/267 g FAME).
13C Labeling
Algal cells were fed 100% uniformly 13C labeled acetate in TAP media without N for the first 40 h after N deprivation. Cells were then centrifuged and resuspended in unlabeled TAP media without N for a further 56 h (96 h of deprivation total). Samples were collected at 40, 72, and 96h following N deprivation. Starch and lipids were extracted and treated as above, and Glc from starch samples was derivatized with methoxyamine and TMS as described (Roessner et al., 2001). Labeling in FAME samples was analyzed by GC-MS as described previously (Allen et al., 2007), while Glc labeling was analyzed on a GC-MS using the same chromatography and MS parameters previously used for amino acids by Chen et al. (2011).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. N+ growth rates of Chlamydomonas in HS, TAP, and TAP-acetate.
Supplemental Figure S2. N deprivation chlorophyll fluorescence measurements in TP and HS media from cells in 160 μE.
Supplemental Figure S3. Carbon-to-nitrogen (C:N) ratios during N deprivation.
Supplemental Figure S4. 13C labeling of C16:3 fatty acids during N deprivation in cells cultured in TAP media at 160 μmol m−1 s−1.
Supplemental Figure S5. 13C labeling of C18 fatty acids during N deprivation in cells cultured in TAP media at 160 μmol m−1 s−1.
Supplemental Figure S6. A metric of potential usable light by photosystem II.
Supplemental Figure S7. Starch and FAME per absorbed light.
Supplementary Material
Acknowledgments
We thank Drs. David Kramer and Thomas Sharkey for generously providing equipment for making photosynthesis-related measurements and Dr. Sean Weise for advice on CO2 gas exchange measurements.
Glossary
- TAG
triacylglycerol
- OH
overflow hypothesis
- HS
high salt
- TAP
Tris acetate phosphate
- AFDW
ash free dry weight
- Chl
chlorophyll
- NPQ
nonphotochemical quenching
- ECS
electrochromic shift
- GCFID
gas chromatography flame ionization detection
- FAME
fatty acid methyl ester
References
- Akita T, Kamo M (2015) Theoretical lessons for increasing algal biofuel: Evolution of oil accumulation to avert carbon starvation in microalgae. J Theor Biol 380: 183–191 [DOI] [PubMed] [Google Scholar]
- Allen AE, Laroche J, Maheswari U, Lommer M, Schauer N, Lopez PJ, Finazzi G, Fernie AR, Bowler C (2008) Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc Natl Acad Sci USA 105: 10438–10443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DK, Shachar-Hill Y, Ohlrogge JB (2007) Compartment-specific labeling information in 13C metabolic flux analysis of plants. Phytochemistry 68: 2197–2210 [DOI] [PubMed] [Google Scholar]
- Alvarez HM, Steinbüchel A (2002) Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol 60: 367–376 [DOI] [PubMed] [Google Scholar]
- Antal TK, Volgusheva AA, Kukarskikh GP, Krendeleva TE, Tusov VB, Rubin AB (2006) [Examination of chlorophyll fluorescence in sulfur-deprived cells of Chlamydomonas reinhardtii]. Biofizika 51: 292–298 [PubMed] [Google Scholar]
- Atabani AE, Silitonga AS, Badruddin IA, Mahlia TMI, Masjuki HH, Mekhilef S (2012) A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev 16: 2070–2093 [Google Scholar]
- Ball SG, Dirick L, Decq A, Martiat JC, Matagne RF (1990) Physiology of starch storage in the monocellular alga Chlamydomonas reinhardtii. Plant Sci 66: 1–9 [Google Scholar]
- Beijerinck M. (1904) Das Assimilationsprodukt der Kohlensäure in den Chromatophoren der Diatomeen. Recl Trav Bot Neerl 1: 28–32 [Google Scholar]
- Blaby IK, Glaesener AG, Mettler T, Fitz-Gibbon ST, Gallaher SD, Liu B, Boyle NR, Kropat J, Stitt M, Johnson S, et al. (2013) Systems-level analysis of nitrogen starvation-induced modifications of carbon metabolism in a Chlamydomonas reinhardtii starchless mutant. Plant Cell 25: 4305–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M (2012) Acclimation of Chlamydomonas reinhardtii to different growth irradiances. J Biol Chem 287: 5833–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SP, Paget CM, Johnson GN, Schwartz JM (2015) Flux balance analysis reveals acetate metabolism modulates cyclic electron flow and alternative glycolytic pathways in Chlamydomonas reinhardtii. Front Plant Sci 6: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Alonso AP, Allen DK, Reed JL, Shachar-Hill Y (2011) Synergy between C-13-metabolic flux analysis and flux balance analysis for understanding metabolic adaption to anaerobiosis in E. coli. Metab Eng 13: 38–48 [DOI] [PubMed] [Google Scholar]
- Chisti Y. (2007) Biodiesel from microalgae. Biotechnol Adv 25: 294–306 [DOI] [PubMed] [Google Scholar]
- Courchesne NM, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141: 31–41 [DOI] [PubMed] [Google Scholar]
- Du Z-Y, Benning C (2016) Triacylglycerol accumulation in photosynthetic cells in plants and algae. In Y Nakamura, Y Li-Beisson, eds, Lipids in Plant and Algae Development. Springer International Publishing, Cham, Switzerland, p 91 [Google Scholar]
- Fan J, Yan C, Andre C, Shanklin J, Schwender J, Xu C (2012) Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant Cell Physiol 53: 1380–1390 [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509 [PubMed] [Google Scholar]
- Frenk S, Gomez F, Ramos-Galvan R, Cravioto J (1958) Fatty liver in children; kwashiorkor. Am J Clin Nutr 6: 298–309 [DOI] [PubMed] [Google Scholar]
- Gargouri M, Park JJ, Holguin FO, Kim MJ, Wang H, Deshpande RR, Shachar-Hill Y, Hicks LM, Gang DR (2015) Identification of regulatory network hubs that control lipid metabolism in Chlamydomonas reinhardtii. J Exp Bot 66: 4551–4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JC, Oswald WJ, Jenkins D (1974) Kinetics of inorganic carbon limited algal growth. J Water Pollut Control Fed 46: 554–574 [Google Scholar]
- Goodenough U, Blaby I, Casero D, Gallaher SD, Goodson C, Johnson S, Lee JH, Merchant SS, Pellegrini M, Roth R, et al. (2014) The path to triacylglyceride obesity in the sta6 strain of Chlamydomonas reinhardtii. Eukaryot Cell 13: 591–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci USA 54: 1665–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum E, Kirkvold S, Myklestad SM (2002) Cellular and extracellular production of carbohydrates and amino acids by the marine diatom Skeletonema costatum: diel variations and effects of N depletion. Mar Ecol Prog Ser 242: 83–94 [Google Scholar]
- Grossman AR, Gonzalez-Ballester D, Shibagaki N, Pootakham W, Moseley J (2010) Responses to macronutrient deprivation. In A Pareek, SK Sopory, HJ Bohnert, Govindjee, eds, Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation. Springer, Dordrecht, The Netherlands, pp 307–348
- Gründel M, Scheunemann R, Lockau W, Zilliges Y (2012) Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology 158: 3032–3043 [DOI] [PubMed] [Google Scholar]
- Hall CC, Cruz J, Wood M, Zegarac R, De Mars D, Carpenter D, Kanazawa A, Kramer D (2013) Photosynthetic measurements with the idea spec: an integrated diode emitter array spectrophotometer/fluorometer. In T Kuang, C Lu, L Zhang, eds, Photosynthesis Research for Food, Fuel and Future—15th International Conference on Photosynthesis. Springer-Verlag, Berlin, pp 184–188
- Harder R, von Witsch H (1942a) Bericht über Versuche zur Fettsynthese mittels autotropher Microorganismen. Forschungsdienst Sonderheft 16:270–275 [Google Scholar]
- Harder R, von Witsch H (1942b) Die Massenkultur von Diatomeen. Ber Deutsch Bot Ges 60: 146–152 [Google Scholar]
- Hays SG, Ducat DC (2015) Engineering cyanobacteria as photosynthetic feedstock factories. Photosynth Res 123: 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54: 621–639 [DOI] [PubMed] [Google Scholar]
- Jones CS, Mayfield SP (2012) Algae biofuels: versatility for the future of bioenergy. Curr Opin Biotechnol 23: 346–351 [DOI] [PubMed] [Google Scholar]
- Juergens MT, Deshpande RR, Lucker BF, Park JJ, Wang H, Gargouri M, Holguin FO, Disbrow B, Schaub T, Skepper JN, et al. (2015) The regulation of photosynthetic structure and function during nitrogen deprivation in Chlamydomonas reinhardtii. Plant Physiol 167: 558–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khozin-Goldberg I, Shrestha P, Cohen Z (2005) Mobilization of arachidonyl moieties from triacylglycerols into chloroplastic lipids following recovery from nitrogen starvation of the microalga Parietochloris incisa. Biochim Biophys Acta 1738: 63–71 [DOI] [PubMed] [Google Scholar]
- Khozin-Goldberg I, Yu HZ, Adlerstein D, Didi-Cohen S, Heimer YM, Cohen Z (2000) Triacylglycerols of the red microalga Porphyridium cruentum can contribute to the biosynthesis of eukaryotic galactolipids. Lipids 35: 881–889 [DOI] [PubMed] [Google Scholar]
- Klein U. (1987) Intracellular carbon partitioning in Chlamydomonas reinhardtii. Plant Physiol 85: 892–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok AJ, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2014) Edible oils from microalgae: insights in TAG accumulation. Trends Biotechnol 32: 521–528 [DOI] [PubMed] [Google Scholar]
- Kohlwein SD. (2010) Triacylglycerol homeostasis: insights from yeast. J Biol Chem 285: 15663–15667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Moellering ER, Liu B, Johnny C, Fedewa M, Sears BB, Kuo MH, Benning C (2012) A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii. Plant Cell 24: 4670–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Han D, Yoon K, Zhu S, Sommerfeld M, Hu Q (2013) Molecular and cellular mechanisms for lipid synthesis and accumulation in microalgae: biotechnological implications. In A Richmond, Q Hu, eds, Handbook of Microalgal Culture: Applied Phycology and Biotechnology, Ed 2. Wiley-Blackwell, Oxford, pp 545–565 [Google Scholar]
- Liu B, Benning C (2013) Lipid metabolism in microalgae distinguishes itself. Curr Opin Biotechnol 24: 300–309 [DOI] [PubMed] [Google Scholar]
- Liu J, Huang J, Sun Z, Zhong Y, Jiang Y, Chen F (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresour Technol 102: 106–110 [DOI] [PubMed] [Google Scholar]
- Livingston AK, Cruz JA, Kohzuma K, Dhingra A, Kramer DM (2010) An Arabidopsis mutant with high cyclic electron flow around photosystem I (hcef) involving the NADPH dehydrogenase complex. Plant Cell 22: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NC, Goodenough UW (1975) Gametic differentiation in Chlamydomonas reinhardtii. I. Production of gametes and their fine structure. J Cell Biol 67: 587–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SS, Kropat J, Liu B, Shaw J, Warakanont J (2012) TAG, you’re it! Chlamydomonas as a reference organism for understanding algal triacylglycerol accumulation. Curr Opin Biotechnol 23: 352–363 [DOI] [PubMed] [Google Scholar]
- Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Marechal-Drouard L, et al. (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao X, Wu Q (2004) High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J Biotechnol 110: 85–93 [DOI] [PubMed] [Google Scholar]
- Miller R, Wu G, Deshpande RR, Vieler A, Gärtner K, Li X, Moellering ER, Zäuner S, Cornish AJ, Liu B, et al. (2010) Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol 154: 1737–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering ER, Benning C (2011) Galactoglycerolipid metabolism under stress: a time for remodeling. Trends Plant Sci 16: 98–107 [DOI] [PubMed] [Google Scholar]
- Morin N, Cescut J, Beopoulos A, Lelandais G, Le Berre V, Uribelarrea JL, Molina-Jouve C, Nicaud JM (2011) Transcriptomic analyses during the transition from biomass production to lipid accumulation in the oleaginous yeast Yarrowia lipolytica. PLoS One 6: e27966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ. (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40: 325–438 [DOI] [PubMed] [Google Scholar]
- Neijssel OM, Tempest DW (1975) The regulation of carbohydrate metabolism in Klebsiella aerogenes NCTC 418 organisms, growing in chemostat culture. Arch Microbiol 106: 251–258 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Bjorkman O, Grossman AR (1997) Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell 9: 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J, Allen D, Berguson B, Dellapenna D, Shachar-Hill Y, Stymne S (2009) Energy. Driving on biomass. Science 324: 1019–1020 [DOI] [PubMed] [Google Scholar]
- Packter NM, Olukoshi ER (1995) Ultrastructural studies of neutral lipid localisation in Streptomyces. Arch Microbiol 164: 420–427 [DOI] [PubMed] [Google Scholar]
- Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462: 518–521 [DOI] [PubMed] [Google Scholar]
- Pollard M, Martin TM, Shachar-Hill Y (2015) Lipid analysis of developing Camelina sativa seeds and cultured embryos. Phytochemistry 118: 23–32 [DOI] [PubMed] [Google Scholar]
- Ritchie RJ. (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89: 27–41 [DOI] [PubMed] [Google Scholar]
- Rochaix JD. (2002) Chlamydomonas, a model system for studying the assembly and dynamics of photosynthetic complexes. FEBS Lett 529: 34–38 [DOI] [PubMed] [Google Scholar]
- Roessler PG. (1990) Environmental control of glycerolipid metabolism in microalgae: Commercial implications and future research directions. J Phycol 26: 393–399 [Google Scholar]
- Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie A (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13: 11–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacksteder CA, Jacoby ME, Kramer DM (2001) A portable, non-focusing optics spectrophotometer (NoFOSpec) for measurements of steady-state absorbance changes in intact plants. Photosynth Res 70: 231–240 [DOI] [PubMed] [Google Scholar]
- Schmollinger S, Mühlhaus T, Boyle NR, Blaby IK, Casero D, Mettler T, Moseley JL, Kropat J, Sommer F, Strenkert D, et al. (2014) Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. Plant Cell 26: 1410–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin NS, Chisholm SW (1981) Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. J Phycol 17: 374–384 [Google Scholar]
- Siaut M, Cuiné S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès C, Li-Beisson Y, Peltier G (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Shukla MR, Chary KV, Rao BJ (2014) Acetate and bicarbonate assimilation and metabolite formation in Chlamydomonas reinhardtii: a 13C-NMR study. PLoS One 9: e106457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53: 29–38 [Google Scholar]
- Smith VH, Sturm BS, Denoyelles FJ, Billings SA (2010) The ecology of algal biodiesel production. Trends Ecol Evol 25: 301–309 [DOI] [PubMed] [Google Scholar]
- Solovchenko AE. (2012) Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ J Plant Physiol 59: 167–176 [Google Scholar]
- Spalding MH. (1989) Photosynthesis and photorespiration in fresh-water green algae. Aquat Bot 34: 181–209 [Google Scholar]
- Spoehr HA, Milner HW (1949) The chemical composition of Chlorella: Effect of environmental conditions. Plant Physiol 24: 120–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardi. Proc Natl Acad Sci USA 46: 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi AM, Peers G, Kobayashi MC, Niyogi KK, Merchant SS (2010) Trophic status of Chlamydomonas reinhardtii influences the impact of iron deficiency on photosynthesis. Photosynth Res 105: 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H, Jakob T, Wilhelm C (2006) Balancing the energy flow from captured light to biomass under fluctuating light conditions. New Phytol 169: 95–108 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, de la Noüe J, Hall DO (1995) Photosynthetic performance of a helical tubular photobioreactor incorporating the cyanobacterium Spirulina platensis. Biotechnol Bioeng 47: 261–269 [DOI] [PubMed] [Google Scholar]
- Weise SE, van Wijk KJ, Sharkey TD (2011) The role of transitory starch in C(3), CAM, and C(4) metabolism and opportunities for engineering leaf starch accumulation. J Exp Bot 62: 3109–3118 [DOI] [PubMed] [Google Scholar]
- White S, Anandraj A, Bux F (2011) PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresour Technol 102: 1675–1682 [DOI] [PubMed] [Google Scholar]
- Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329: 796–799 [DOI] [PubMed] [Google Scholar]
- Williams PJL, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ Sci 3: 554–590 [Google Scholar]
- Wilson A, Boulay C, Wilde A, Kerfeld CA, Kirilovsky D (2007) Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins. Plant Cell 19: 656–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work VH, Radakovits R, Jinkerson RE, Meuser JE, Elliott LG, Vinyard DJ, Laurens LM, Dismukes GC, Posewitz MC (2010) Increased lipid accumulation in the Chlamydomonas reinhardtii sta7-10 starchless isoamylase mutant and increased carbohydrate synthesis in complemented strains. Eukaryot Cell 9: 1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K, Han D, Li Y, Sommerfeld M, Hu Q (2012) Phospholipid:diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. Plant Cell 24: 3708–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CJ, Lee YK (1997) Determination of biomass dry weight of marine microalgae. J Appl Phycol 9: 189–194 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.