Abstract
Our aim was to test the direction of causation between self-report parental monitoring (PM) and the liability to illicit drug initiation (DI) as indicated by cannabis, cocaine, and stimulants. We fitted a multiple indicator model to test causal and non-causal models based on a large, genetically informative cross-sectional sample of male twins. The sample comprises 1,778 males aged 24–62 years from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders. Data came from self-report measures of lifetime cannabis, stimulants, and cocaine initiation, and retrospective assessment of PM between ages 8–17. Multivariate modeling showed that familial aggregation in parental monitoring and drug initiation were both explained by a combination of additive genetic and shared environmental effects. Moreover, the significant association between PM and DI was best explained by a correlated liability model versus causal models. PM has typically been assumed to be an environmental, causal risk factor for drug use and has been shown to be amongst the more salient environmental risk factors for illicit drug initiation. Our data were not consistent with this causal hypothesis. Instead, a correlated liability model in which PM and risk of DI share common genetic and environmental risks provided a better fit to the data.
Drug use disorders (DUDs) are complex traits influenced by a variety of environmental factors and genetic risks. Depending on the substance, genetic risks account for 40–70% of the liability to drug initiation (DI) and DUDs (Kendler & Prescott, 2006) with increasing heritability as individuals progress to DUDs (Kendler et al., 2012). The remaining variance can be predicted by a variety of social-environmental risks often identifiable in early to mid-childhood (Caspi et al., 1996) (Casswell et al., 2002; Chassin et al., 2002; Colder et al., 2002; Dubow et al., 2008; Ellickson et al., 2004; Englund et al., 2008; Jackson & Sher, 2006; Li et al., 2001; Maggs et al., 2008; Manzardo et al., 2005; Pitkanen et al., 2008; Wiesner et al., 2007; Windle et al., 2005), which includes personality (McGue et al., 1999), externalizing behaviors (Boyle et al., 1992; Helzer et al., 1992) (Fergusson & Lynskey, 1998; Lynskey & Fergusson, 1995; Szobot & Bukstein, 2008; Young et al., 1995), and environmental factors such as parental monitoring (Dishion & Loeber, 1985; Gillespie et al., 2012b), childhood sexual or physical abuse (Glaser et al., 1977; Kendler et al., 2000a), parental attitudes towards drug use (Mcdermott, 1984) (Gillespie et al., 2012b), household drug use (Gfroerer, 1987), deviant peer group affiliation (Jones et al., 1976), drug availability (Freisthler et al., 2005a; Freisthler et al., 2005b; Gillespie et al., 2009), and participation in pro-social activities (Kendler & Myers, 2009). Among these risks, parental monitoring (PM) has been shown to be one of the most salient (Gillespie et al., 2012b; Latendresse et al., 2008, 2009).
PM is typically measured in terms of parental knowledge of children’s whereabouts and degree of parental supervision. Although PM is a significant predictor of both cannabis initiation and average cannabis consumption over time (Gillespie et al., 2012b) the genetic and environmental association between PM and the risk of DI remains unclear. In addition to the association being driven by correlated genetic and environmental risks, DI may be phenotypically contingent upon (reduced) parental monitoring or vice-a-versa. In lieu of genetically informative longitudinal data, alternative and innovative statistical methods can be applied to provide some capacity to infer causation. These include propensity analyses (Kendler & Gardner, 2010), cross-panel designs or modeling direction of causation based on pairs of genetically informative relatives measured on a single occasion (Heath et al., 1993).
Direction of causation modeling on cross-sectional and genetically informative data has been applied to a number of complex behavioral phenotypes (Duffy & Martin, 1994; Gillespie et al., 2012a; Gillespie et al., 2003; Neale et al., 1994b). Provided several assumptions are satisfied, differences in the patterns of cross-twin cross-trait correlation can allow researchers to falsify hypothesis about the direction of causation between two variables measured on a single occasion (Heath et al., 1993). Therefore using a genetically informative sample of male twins, our aim was to determine the direction of causation between PM and illicit DI. We fitted a model that predicted that the association between PM and the liability to DI was explained by shared genetic and environmental risk factor, which we then compared to models that predicted a reciprocal causation between PM and DI, and unidirectional causation (PM-to-DI and DI-to-PM).
Material & Methods
Subjects
Described in detail elsewhere (Kendler & Prescott, 2006) this report is based on data collected from a 2nd and 3rd wave of interviews between 1994 and 2004 as part of an ongoing study of adult male twins from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPUD). Briefly, twins were eligible for participation in this study if one or both twins were successfully matched to birth records, were a member of a multiple birth with at least one male, were Caucasian, and were born between 1940 and 1974. Of 9,417 eligible individuals for the 1st wave (1993–1996), 6,814 (72.4%) completed the initial interviews. At least one year later, we contacted those who had completed the initial interview to schedule the 2nd interview. The 2nd interview was completed by 83% of those eligible or 4,203 males comprising 1,189 complete twin pairs and 1,825 singletons. Twin pairs (no singletons) who completed the 2nd interview were eligible to participate in a 3rd interview (2000–2004). The VATSPUD was designed to study the nature and pattern of risk and protective factors for drug use and drug use disorders across adolescence and young adulthood. This 3rd interview was completed by 75% of those eligible or 1,778 males, aged 24–62 years (μ= 40.3, SD = 9.0) from 745 complete twin pairs and 288 singletons.
In both interviews, most subjects (~90%) were interviewed by telephone. A small number were interviewed in person because of subject preference, residence in an institutional setting (usually jail), or not having a telephone. Subjects were informed about the goals of the study and provided informed consent before interviews. Interviewers had a Master’s degree in a mental health-related field or a Bachelor’s degree in this area plus two years of clinical experience. The two members of a twin pair were each interviewed by different interviewers. The VATSPUD was approved by the Virginia Commonwealth University institutional review board.
Zygosity & Interview Protocol
Zygosity was diagnosed using a combination of self-report measures, photographs and DNA analysis.
Measures of Drug Initiation (DI) & Parental Monitoring (PM)
The 2nd interview assessed lifetime drug use for six substances: cannabis (marijuana and hashish); sedatives (quaalude, Seconal and Valium); stimulants (speed, ecstasy and Ritalin); cocaine (intranasal, freebase and crack); opiates [heroin, Demerol (meperidine hydrochloride) and morphine]; and hallucinogens (lysergic acid diethylamide, mescaline and phencyclidine). For substances that could be obtained legally, non-medical use was defined as use: (i) without a doctor’s prescription; (ii) in greater amounts or more often than prescribed; or (iii) for any other reason than a doctor said it should be taken. Subjects who endorsed having ‘ever tried’ [DRUG]’ were then asked two questions: “How old were you the first time you took [DRUG]?”; and “How old were you when you used [DRUG] the most?”. A DI score (Yes/No) was then calculated for each substance and scored positive if DI was confirmed by responses to both these items. Because of the need to maintain computational efficiency we analyzed cannabis, stimulants and cocaine as these were the three most commonly initiated illicit substances.
Because the data collected at Wave 3 interview are retrospective, they are potentially contaminated by recall bias and telescoping effects (Pickles et al., 1994). Therefore, our study used a Life History Calendar format (Freedman et al., 1988) to improve the data quality. This method has been shown empirically to improve the accuracy of retrospective reporting by providing multiple cues to improve the chance of accurate recall (Belli, 1998; Freedman et al., 1988). This makes the task more akin to the accurate and well-retained process of recognition than to the less reliable task of free recall.
The 3rd interview included a retrospective assessment of PM between ages 8–17 years using 11 items selected on the basis of previous work examining parental effects on risk of drug use and delinquency (Celdran et al., 1976; Ingram, 1976; Stattin & Kerr, 2000). These items asked subjects questions such as how much their parents or guardians knew who their friends were, how they spent their money, and what they did with their free time, and where they were at night etc. Response options were (their parents) “didn’t know”, “knew a little” or “knew a lot”. PM latent factor scores were estimated for each individual in the Classic Mx software program (Neale, 1999). Again, in order to maintain computational efficiency we restricted our analyses to the three most prevalent PM items: “How much did your parents know about your friends?”; “How much did your parents know about how you spent your money?”; and “How much did your parents know about how you spent your free time?”.
There were 2576 subjects with complete DI, and 1758 subjects with complete PM data. Depending on the substance, this included 1749 to 1754 subjects with both DI and PM data. For each of the three drug classes, there were 694 monozygotic (MZ) and 484 dizygotic (DZ) twin pairs as well as 220 singletons with complete DI data. For PM there were 454 MZ and 280 DZ twin pairs respectively with complete data plus 290 singletons.
Raw Ordinal Data Analysis
All data were analyzed using raw data methods in the OpenMx software implemented in R (Boker et al., 2015). This approach assumes that the observed ordinal categories within each item are an imprecise measure of a latent normal distribution of liability, and that the liability distribution for each variable has one or more thresholds that discriminate between the categories. Thresholds can be conceived of as cut points along a normal distribution which classify individuals in terms of a probability or risk of endorsing one of two or more discrete (ordinal) categories. The DI and PM thresholds were adjusted for the linear effects of age and before fitting univariate and multivariate models were used likelihood ratio chi-square tests to confirm the equality of thresholds within twin pairs and across zygosity. These preliminary tests of threshold homogeneity are analogous to tests of mean and variance homogeneity in the case of continuous data.
Univariate & multivariate genetic analyses
Based on standard biometrical genetic model fitting methods (Neale & Cardon, 1992) which exploit the expected genetic and environmental correlations for MZ and DZ twin pairs, our models assumed that the total variance in each of the observed items and latent constructs can be decomposed into additive (A) genetic, shared environment (C) and non-shared or unique (E) environmental variance components. Because MZ twin pairs are genetically identical, correlations for the A effects are 1.0. For DZ twin pairs who on average share half of their genes, the correlations for the A effects are 0.5. An important assumption of biometrical genetic model is that shared environmental effects (C) are perfectly correlated among MZ and DZ twin pairs alike. Non-shared environmental effects are by definition uncorrelated and also reflect measurement error including short-term fluctuations.
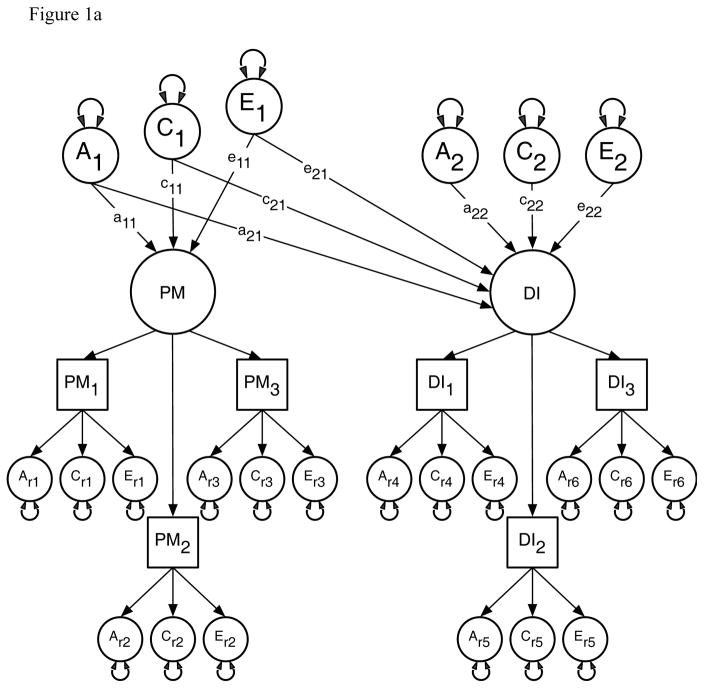
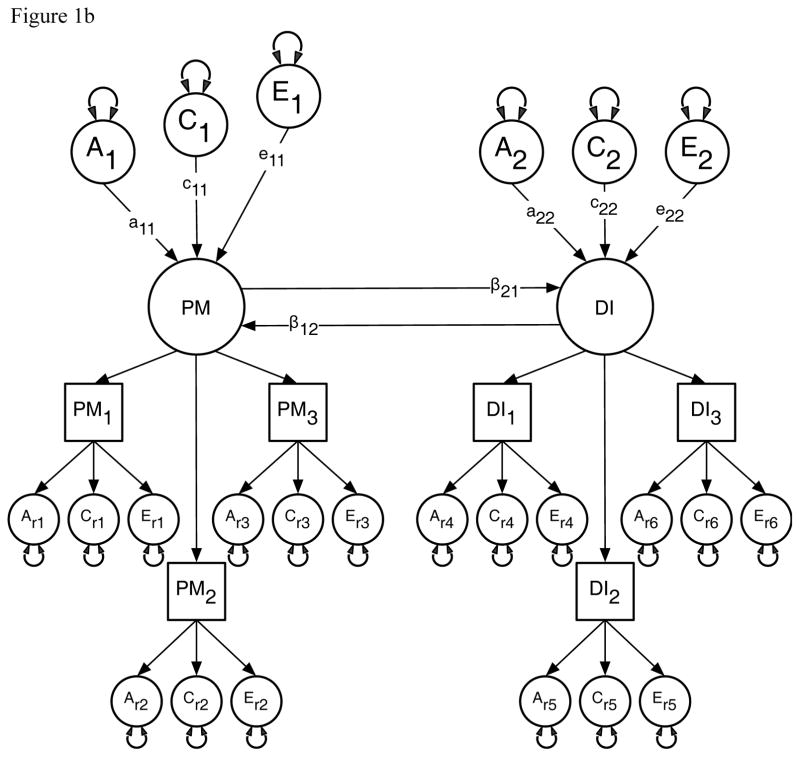
We used this method first to estimate the contribution of genetic and environmental risks (A, C and E) in the univariate analyses of the PM and DI items. In the multivariate analyses we then modeled the direction of causation between the latent factors for PM and DI because measurement error reduces the statistical power for resolving alternative causal hypotheses (Neale et al., 1994a; Neale et al., 1994b). This approach assumes that measurement error occurs at the level of the items or indicator variables and so is uncorrelated across factors (Heath et al., 1993). Specifically, we compared the fit of a correlated liability model against four causal models using Maximum Likelihood estimation in the R based OpenMx software package (Boker et al., 2015). The models were: (i) a comparison or correlated liability model illustrated in Figure 1a; (ii) a reciprocal causation model illustrated in Figure 1b; (iii–iv) two uni-directional causation models. The correlated liability model is identical to bivariate Cholesky decomposition (Neale & Cardon, 1992) and is the null hypothesis because it predicts no causal association between the latent parental monitoring and drug initiation factors. Instead, any phenotypic association is attributable to common or correlated genetic and environmental latent effects i.e. via the a11 and a21, c11 and c21, and e11 and e21 pathways. Under the reciprocal causation and uni-directional models any association between PM and DI arises because of direct phenotypic causality between the latent PM and DI factors via the β12 and β21 pathways.
Figure 1.
Figure 1a: Cholesky decomposition illustrating the association between Parental Monitoring (PM) and Drug Initiation (DI) in which the sources of covariance between PM and DI are decomposed into shared genetic (a2,1) and environmental (c2,1 & e2,1) effects. This model also models the genetic (a2,2) and environmental (c2,2 & e2,2) effects unique to DI (a2,2, c2,2, e2,2).
A1, C1 & E1 = latent additive genetic, shared, and non-shared environmental risks for PM, A2, C2 & E2 = latent additive genetic, shared, and non-shared environmental risks for DI, PM & DI = common factors as indicated by observed phenotypic symptoms PM1–3 and DI1–3 respectively each with their own item specific latent genetic and environmental risk factors, PM1 = Parent’s knowledge of child’s friends, PM2 = Parent’s knowledge of how child spent their money, PM3 = Parent’s knowledge of how child spent their free time, DI1 = Cannabis initiation, DI2 = Stimulant initiation, DI3 = Cocaine initiation.
Figure 1b: Illustrative model of the direction of causation between the latent factors for Parental Monitoring (PM) and Drug Initiation (DI). This model approach predicts the relationship between PM and DI is explained by a reciprocal interaction, or causal parameters (β21 & β12), at the latent factor level.
A1, C1 & E1 = latent additive genetic, shared, and non-shared environmental risks for PM, A2, C2 & E2 = latent additive genetic, shared, and non-shared environmental risks for DI, PM & DI = common factors as indicated by observed phenotypic symptoms PM1–3 and DI1–3 respectively each with their own item specific latent genetic and environmental risk factors, β21 = causal pathway from PM to DI, β12 = causal pathway from DI to PM, PM1 = Parent’s knowledge of child’s friends, PM2 = Parent’s knowledge of how child spent their money, PM3 = Parent’s knowledge of how child spent their free time, DI1 = Cannabis initiation, DI2 = Stimulant initiation, DI3 = Cocaine initiation.
Model comparisons
The correlated liability model was compared to the reciprocal and uni-directional causal models using likelihood-ratio chi-squared tests with degrees in freedom being equal to the degrees of freedom between the models. The best fitting model was chosen on the basis of parsimony i.e. model with non-significant changes in the chi-square and the smallest number of parameters. To this end, the Akaike Information Criterion (AIC) was calculated for each model and the model with the lowest index value was chosen as the best fitting.
Results
Tests of Threshold Homogeneity & Polychoric Correlations
We found no significant differences in the threshold distribution for the three PM and three DI items within twin pairs or across zygosity. As shown in Table 1 the polychoric correlations between the DI and PM items were all moderate ranging from −0.22 to −0.34 such that lower levels of PM were associated with increased risk of DI. The patterns of MZ and DZ twin pair correlations suggest a pattern of familial aggregation for cannabis initiation attributable to a combination of additive genetic and shared environmental risk factors. For stimulants and cocaine, the twin pair correlations suggest that familial aggregation is more likely explained by additive genetic risks alone. For the three PM items, all DZ twin pair correlations were greater than half the MZ twin pair suggesting a combination of genetic and shared environmental risk factors.
Table 1.
Measures of association (polychoric correlations) between Parental Monitoring and Drug Initiation items along with twin pair (polychoric) correlations and 95% confidence intervals for each item.
| Items | Polychoric correlations | Twin Pair Correlations | ||||||
|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | MZ | DZ | |
| 1. Parents knowledge of child’s friends | 1.00 | 0.47 (0.37–0.56) | 0.39 (0.25–0.51) | |||||
| 2. Parents knowledge of how child spent their money | 0.74 | 1.00 | 0.45 (0.36–0.54) | 0.30 (0.15–0.43) | ||||
| 3. Parents knowledge of how child spent their free time | 0.72 | 0.82 | 1.00 | 0.47 (0.36–0.56) | 0.38 (0.24–0.51) | |||
| 4. Cannabis initiation | −0.24 | −0.31 | −0.34 | 1.00 | 0.81 (0.76–0.85) | 0.55 (0.45–0.64) | ||
| 5. Stimulants initiation | 0.27 | −0.32 | −0.31 | 0.76 | 1.00 | 0.66 (0.53–0.76) | 0.29 (0.10–0.47) | |
| 6. Cocaine initiation | −0.22 | −0.25 | −0.25 | 0.85 | 0.78 | 1.00 | 0.78 (0.68–0.85) | 0.45 (0.25–0.61) |
Note: All correlations adjusted for the effects of age at interview, sex and cohort on item thresholds
Univariate Analyses
Standardized univariate components of variance for PM and DI items are shown in Table 2. Additive genetic risks explained between 17% to 31% of the variance in PM. Although shared environmental risk factors explained 14% to 30% of the variance in the PM items, the 95% confidence intervals spanned zero for all three PM items. Additive genetic risks explained between 42% to 64% of the variance in the DI items. Shared environmental risk factors explained 33% of the variance in the cannabis initiation. Confidence intervals for the shared environmental risks for stimulant and cocaine initiation spanned zero.
Table 2.
Variance components (standardized) in the Parental Monitoring and Drug Initiation items attributed to additive genetic (A), shared environment (C), and non-shared environmental (E) effects along with their 95% confidence intervals.
| Items | A | C | E |
|---|---|---|---|
| 1. Parents knowledge of child’s friends | 0.17 (0.00–0.51) | 0.30 (0.00–0.50) | 0.53 (0.43–0.63) |
| 2. Parents knowledge of how child spent their money | 0.31 (0.00–0.54) | 0.14 (0.00–0.43) | 0.55 (0.46–0.65) |
| 3. Parents knowledge of how child spent their free time | 0.17 (0.00–0.49) | 0.30 (0.00–0.49) | 0.53 (0.44–0.64) |
| 4. Cannabis initiation | 0.42 (0.25–0.60) | 0.33 (0.16–0.49) | 0.25 (0.21–0.30) |
| 5. Stimulants initiation | 0.57 (0.26–0.74) | 0.09 (0.00–0.36) | 0.34 (0.26–0.43) |
| 6. Cocaine initiation | 0.64 (0.34–0.79) | 0.08 (0.00–0.35) | 0.28 (0.21–0.36) |
Multivariate Analysis
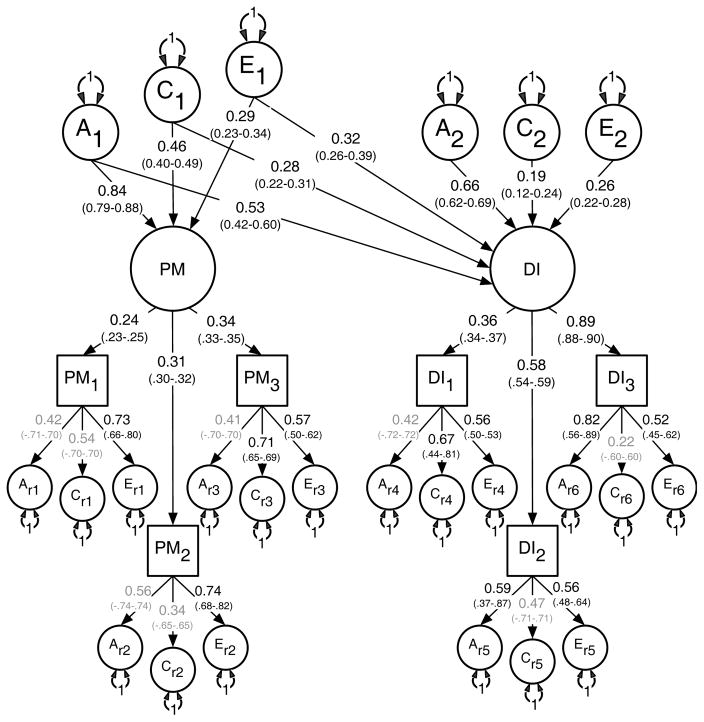
Results for the multivariate modeling fitting are shown in Table 3. The two uni-directional and the reciprocal causation models deteriorated significantly when compared to the (null) correlated liability model. Based on these results and the lowest AIC, the correlated liability was therefore chosen as the best fitting and most parsimonious. Standardized path coefficients for the correlated liability model appear in Figure 2.
Table 3.
Multivariate model fitting results for the (i) non-causal correlated liability, (ii) reciprocal causation (PM↔DI), (iii) uni-directional Parental Monitoring causes Drug Initiation (PM→DI), and (iv) uni-directional Drug Initiation causes Parental Monitoring (DI→PM) models.
| −2LL | df | Δ −2LL | Δdf | p | AIC | |
|---|---|---|---|---|---|---|
| Correlated Liability | 14548.68 | 12950 | - | - | - | −11351.32 |
| Reciprocal Causation | 14552.96 | 12951 | 4.28 | 1 | 0.04 | −11349.04 |
| PM→DI | 14596.70 | 12952 | 48.03 | 2 | *** | −11307.30 |
| DI→PM | 14567.76 | 12952 | 19.08 | 2 | *** | −11336.24 |
Note: All PM & DI thresholds are adjusted for the linear effects of age at interview. −2LL = log-likelihood, Δ−2LL=change in log-likelihood which is asymptotically distributed as a chi-square, AIC=Akaike Information Criteria,
= p < 0.001
Figure 2.
Best fitting non-causal correlated liability model between the latent factors for Parental Monitoring (PM) and Drug Initiation (DI) with standardized path coefficients and 95% confidence intervals.
PM1 = Parent’s knowledge of child’s friends, PM2 = Parent’s knowledge of how child spent their money, PM3 = Parent’s knowledge of how child spent their free time, DI1 = Cannabis initiation, DI2 = Stimulant initiation, DI3 = Cocaine initiation.
Discussion
Parental monitoring (PM) has been assumed to be an environmental, causal risk factor for drug initiation (DI). Although we found a significant phenotypic association between PM and measures of DI, our data revealed that the liability to DI was not consistent with any causal hypothesis. Instead, a correlated liability model in which PM and risk of DI share common genetic and environmental risks provided a better fit to the cannabis, stimulants and cocaine data.
Our univariate point estimates for the genetic and environmental risks in drug use were comparable to those reported elsewhere (Gillespie et al., 2013; Kendler et al., 2012; Verweij et al., 2010). Our analyses also showed that familial aggregation in PM could be explained by a combination of additive genetic effects and environmental risks. This is consistent with previous analyses based on phenotypic correlates of PM such as Parker’s Parental Bonding Instrument (PBI) (Parker et al., 1979) that have found that, depending on the informant, parenting behaviors are best explained by a combination of genetic and environmental risk factors (Gillespie et al., 2003; Kendler, 1996; Kendler et al., 1997).
Previous reports have shown how family involvement is associated with levels of drug use even after controlling for peer influences (Chassin et al., 1993). In genetically informative studies, PM has been shown to attenuate the impact of genetic risks on other substances such as alcohol (Dick et al., 2009; Dick et al., 2011), and nicotine consumption (Dick et al., 2007). And although the genetic risks for alcohol and nicotine use appear to be maximized under conditions of low social constraint (Dick et al., 2011; Latendresse et al., 2008, 2009) we found no causal impact of the PM on DI or DI on PM. Instead, covariation between self-reports of PM and the liability to DI appears to be attributable to shared, non-causal genetic and environmental risk factors
The idea of correlated genetic and environmental risks indexing both PM and DI is consistent with Scarr and McCartney’s active model which predicts that individuals seek out environments that they find compatible and stimulating based on motivational, personality, and intellectual aspects of their genotype (Scarr & McCartney, 1983). Our results are also consistent with genotype–environment correlations (CorGE) which describe situations in which an individual’s environment is unlikely to be entirely random, but partly caused or correlated with his or her genotype (Scarr & McCartney, 1983). Active genotype–environment correlations describe cases whereby individuals ‘select’ or ‘create’ environments that are a function of their genotypes, whereas evocative genotype–environment correlations describe cases whereby an individual’s heritable behavior ‘evokes’ an environmental response (Neale & Cardon, 1992). As an example of the later, adoptees at high genetic risk for antisocial behavior have been found to be more likely to elicit more negative parenting from their adoptive parents (O’Connor et al., 1998). Both active and evocative CorGE are analogous to the uni-directional “drug use causes PM” hypothesis that our data do not support. If parental knowledge derives primarily from voluntary disclosures rather than active parental surveillance (Stattin & Kerr, 2000), then our results suggest that adolescents’ tendency to avoid telling their parents about their activities, and whereabouts when they are engaged in drug use, or other forms of delinquent behavior may be a function of their genetic predisposition to drug use. Another form of CorGE arises because environments in which individuals develop are provided by their biological relatives, and are known as instances of passive CorGE (Neale & Cardon, 1992). For example, children who are genetically predisposed to drug use may also experience a pathogenic environment whereby the tendency of parents to not monitor their children may be attributable the genes also increasing the risk of DI. Therefore a high genetic predisposition to DI is correlated with exposure to an adverse environment e.g. low PM, because both genes and environment originally derive from the parents (Neale & Cardon, 1992). This is consistent with our best fitting correlated liability results. Children who are genetically predisposed to DI may live in a drug tolerant home wherein the behavioral tendency of parents to be less attentive of their children’s whereabouts are underpinned by the same genes.
Finally, a third form of CorGE arises because
Limitations
Our findings must be interpreted in the context of five potential limitations. First is the representativeness of our twin data drawn from Caucasian Virginian males. Nevertheless, our results are likely generalizable to the population at large. Although males have a higher prevalence of drug use (Kendler et al., 2002), previous analyses using the same data suggest that it is broadly representative of US males and does not differ from the general population in rates of psychopathology, drug use and abuse (Kendler et al., 2000b). Second, because the difference in fit between the reciprocal causation and the correlated liability model was marginal the power to rule out this competing hypothesis may be insufficient. Third, our modeling was not exhaustive, and since the confidence intervals on the item-specific shared environmental factors spanned zero, these parameters were dropped in order to identify a model that simultaneously fitted both the correlated liability and the reciprocal parameters to the data. This hybrid model provided a very poor fit to the data when compared to the correlated liability model (Δ-2LL=−1.93, Δdf = 2, p<0.001). Fourth, because we relied on self-reports of PM it is unclear if drug use was associated with actual versus perceived changes in PM. Finally, demands on computational efficiency imposed by raw ordinal data analyses meant that our analyses were necessarily restricted to three PM and three DI items. We analyzed ten additional three-item combinations from the eleven PM as well as three-items combinations of the illicit substance initiation items. In all cases, we found irrespective of which combination of PM and DI items were used, that the non-causal, correlated liability model provided the best fit to the data.
Acknowledgments
Funding was received from the US National Institute on Drug Abuse (R00DA023549 & DA-018673). This work was also supported by NIH grants AA07535, and AA07728. We also thank the twins for their cooperation.
Footnotes
Conflict of interest
None
References
- Belli RF. The structure of autobiographical memory and the event history calendar: potential improvements in the quality of retrospective reports in surveys. Memory. 1998;6(4):383–406. doi: 10.1080/741942610. [DOI] [PubMed] [Google Scholar]
- Boker SM, Neale MC, Maes HH, Wilde MJ, Spiegel M, Brick TR, et al. Multipurpose Software for Statistical Modeling. 2015 (Producer) Retrieved from http://openmx.psyc.virginia.edu.
- Boyle MH, Offord DR, Racine YA, Szatmari P, Fleming JE, Links PS. Predicting Substance Use in Late Adolescence - Results from the Ontario Child Health Study Follow-Up. American Journal of Psychiatry. 1992;149(6):761–767. doi: 10.1176/ajp.149.6.761. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53(11):1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Casswell S, Pledger M, Pratap S. Trajectories of drinking from 18 to 26 years: identification and prediction. Addiction. 2002;97(11):1427–1437. doi: 10.1046/j.1360-0443.2002.00220.x. [DOI] [PubMed] [Google Scholar]
- Celdran E, Bekett PR, Roberts DJ. An experimental and clinical pharmacological study of the influence of triamterene on the diuretic and saluretic properties of furosemide xantinol. Arzneimittel-Forschung. 1976;26(11):2073–2076. [PubMed] [Google Scholar]
- Chassin L, Pillow DR, Curran PJ, Molina BS, Barrera MJ. Relation of parental alcoholism to early adolescent substance use: a test of three mediating mechanisms. Journal of Abnormal Psychology. 1993;102(1):3–19. doi: 10.1037//0021-843x.102.1.3. [DOI] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: predictors and substance abuse outcomes. Journal of Consulting and Clinical Psychology. 2002;70(1):67–78. [PubMed] [Google Scholar]
- Colder CR, Campbell RT, Ruel E, Richardson JL, Flay BR. A finite mixture model of growth trajectories of adolescent alcohol use: predictors and consequences. Journal of Consulting and Clinical Psychology. 2002;70(4):976–985. doi: 10.1037//0022-006x.70.4.976. [DOI] [PubMed] [Google Scholar]
- Dick DM, Latendresse SJ, Lansford JE, Budde JP, Goate A, Dodge KA, et al. Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Archives of General Psychiatry. 2009;66(6):649–657. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Meyers JL, Latendresse SJ, Creemers HE, Lansford JE, Pettit GS, et al. CHRM2, parental monitoring, and adolescent externalizing behavior: evidence for gene-environment interaction. Psychol Sci. 2011;22(4):481–489. doi: 10.1177/0956797611403318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. Journal of Abnormal Psychology. 2007;116(1):213–218. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Loeber R. Adolescent Marijuana and Alcohol-Use - the Role of Parents and Peers Revisited. American Journal of Drug and Alcohol Abuse. 1985;11(1–2):11–25. doi: 10.3109/00952998509016846. [DOI] [PubMed] [Google Scholar]
- Dubow EF, Boxer P, Huesmann LR. Childhood and adolescent predictors of early and middle adulthood alcohol use and problem drinking: the Columbia County Longitudinal Study. Addiction. 2008;103(Suppl 1):36–47. doi: 10.1111/j.1360-0443.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Martin NG. Inferring the direction of causation in cross-sectional twin data: theoretical and empirical considerations [see comments] Genetic Epidemiology. 1994;11(6):483–502. doi: 10.1002/gepi.1370110606. [DOI] [PubMed] [Google Scholar]
- Ellickson PL, Martino SC, Collins RL. Marijuana use from adolescence to young adulthood: Multiple developmental trajectories and their associated outcomes. Health Psychology. 2004;23(3):299–307. doi: 10.1037/0278-6133.23.3.299. [DOI] [PubMed] [Google Scholar]
- Englund MM, Egeland B, Oliva EM, Collins WA. Childhood and adolescent predictors of heavy drinking and alcohol use disorders in early adulthood: a longitudinal developmental analysis. Addiction. 2008;103(Suppl 1):23–35. doi: 10.1111/j.1360-0443.2008.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT. Conduct problems in childhood and psychosocial outcomes in young adulthood: A prospective study. Journal of Emotional and Behavioral Disorders. 1998;6(1):2–18. [Google Scholar]
- Freedman D, Thornton A, Camburn D, Alwin D, Young-demarco L. The life history calendar: a technique for collecting retrospective data. Sociological Methodology. 1988;18:37–68. [PubMed] [Google Scholar]
- Freisthler B, Gruenewald PJ, Johnson FW, Treno AJ, Lascala EA. An exploratory study examining the spatial dynamics of illicit drug availability and rates of drug use. Journal of Drug Education. 2005a;35(1):15–27. doi: 10.2190/25QY-PBC3-B1EB-JB5Y. [DOI] [PubMed] [Google Scholar]
- Freisthler B, Needell B, Gruenewald PJ. Is the physical availability of alcohol and illicit drugs related to neighborhood rates of child maltreatment? Child Abuse and Neglect. 2005b;29(9):1049–1060. doi: 10.1016/j.chiabu.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Gfroerer J. Correlation between drug use by teenagers and drug use by older family members. American Journal of Drug and Alcohol Abuse. 1987;13(1–2):95–108. doi: 10.3109/00952998709001502. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Gehrman P, Byrne EM, Kendler KS, Heath AC, Martin NG. Modeling the direction of causation between cross-sectional measures of disrupted sleep, anxiety and depression in a sample of male and female Australian twins. Journal of Sleep Research. 2012a;21(6):675–683. doi: 10.1111/j.1365-2869.2012.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Lichtman AH, Kendler KS. The Genetics of Cannabis Use and Cannabis Use Disorders. In: Miller P, editor. Biological Research on Addiction, Comprehensive Addictive Behaviors and Disorders. Vol. 2. Amsterdam: Elsevier; 2013. pp. 523–529. [Google Scholar]
- Gillespie NA, Lubke GH, Gardner CO, Neale MC, Kendler KS. Two-part random effects growth modeling to identify risks associated with alcohol and cannabis initiation, initial average use and changes in drug consumption in a sample of adult, male twins. Drug and Alcohol Dependence. 2012b;123(1–3):220–228. doi: 10.1016/j.drugalcdep.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: a multi-stage model from cannabis availability, cannabis initiation and progression to abuse. Addiction. 2009;104(3):430–438. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Zhu G, Neale MC, Heath AC, Martin NG. Direction of causation modeling between cross-sectional measures of parenting and psychological distress in female twins. Behavior Genetics. 2003;33(4):383–396. doi: 10.1023/a:1025365325016. [DOI] [PubMed] [Google Scholar]
- Glaser CB, Karic L, Cohen AB. Low pH stability of alpha-1-antititrypsin. Biochimica et Biophysica Acta. 1977;491(1):325–330. doi: 10.1016/0005-2795(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing Hypotheses About Direction of Causation Using Cross-Sectional Family Data. Behavior Genetics. 1993;23(1):29–50. doi: 10.1007/Bf01067552. [DOI] [PubMed] [Google Scholar]
- Helzer JE, Bucholz KK, Robins LN. Results of the epidemiologic catchment area survey. In: Helzer JE, Canino GJ, editors. Alcoholism in North America Europe and Asia. New York: Oxford University Press; 1992. [Google Scholar]
- Ingram M. The Tom Gibson Memorial Lecture. The microbiological role of nitrite in meat products. Society for Applied Bacteriology Symposium Series. 1976;4(3):1–18. [PubMed] [Google Scholar]
- Jackson KM, Sher KJ. Comparison of longitudinal phenotypes based on number and timing of assessments: a systematic comparison of trajectory approaches II. Psychol Addict Behav. 2006;20(4):373–384. doi: 10.1037/0893-164X.20.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MM, Ogilvie JW, Ackers GK. Active enzyme gel chromatography. I. Experimental aspects. Biophysical Chemistry. 1976;5(3):339–350. doi: 10.1016/0301-4622(76)80045-2. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Parenting: a genetic-epidemiologic perspective. American Journal of Psychiatry. 1996;153(1):11–20. doi: 10.1176/ajp.153.1.11. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women - An epidemiological and Cotwin control analysis. Archives of General Psychiatry. 2000a;57(10):953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, et al. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nature Neuroscience. 2012;15(2):181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Dependent stressful life events and prior depressive episodes in the prediction of major depression: the problem of causal inference in psychiatric epidemiology. Archives of General Psychiatry. 2010;67(11):1120–1127. doi: 10.1001/archgenpsychiatry.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000b;57(3):261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J. A developmental twin study of church attendance and alcohol and nicotine consumption: a model for analyzing the changing impact of genes and environment. American Journal of Psychiatry. 2009;166(10):1150–1155. doi: 10.1176/appi.ajp.2009.09020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Thornton LM, Aggen SH, Gilman SE, Kessler RC. Cannabis use in the last year in a US national sample of twin and sibling pairs. Psychological Medicine. 2002;32(3):551–554. doi: 10.1017/S0033291701004950. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. 1. New York: The Guilford Press; 2006. [Google Scholar]
- Kendler KS, Sham PC, MacLean CJ. The determinants of parenting: an epidemiological, multi-informant, retrospective study. Psychological Medicine. 1997;27(3):549–563. doi: 10.1017/s0033291797004704. [DOI] [PubMed] [Google Scholar]
- Latendresse SJ, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Parenting mechanisms in links between parents’ and adolescents’ alcohol use behaviors. Alcoholism: Clinical and Experimental Research. 2008;32(2):322–330. doi: 10.1111/j.1530-0277.2007.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latendresse SJ, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Parental socialization and adolescents’ alcohol use behaviors: predictive disparities in parents’ versus adolescents’ perceptions of the parenting environment. J Clin Child Adolesc Psychol. 2009;38(2):232–244. doi: 10.1080/15374410802698404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FZ, Duncan TE, Hops H. Examining developmental trajectories in adolescent alcohol use using piecewise growth mixture modeling analysis. Journal of Studies on Alcohol. 2001;62(2):199–210. doi: 10.15288/jsa.2001.62.199. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM. Childhood Conduct Problems, Attention-Deficit Behaviors, and Adolescent Alcohol, Tobacco, and Illicit Drug-Use. Journal of Abnormal Child Psychology. 1995;23(3):281–302. doi: 10.1007/Bf01447558. [DOI] [PubMed] [Google Scholar]
- Maggs JL, Patrick ME, Feinstein L. Childhood and adolescent predictors of alcohol use and problems in adolescence and adulthood in the National Child Development Study. Addiction. 2008;103(Suppl 1):7–22. doi: 10.1111/j.1360-0443.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- Manzardo AM, Penick EC, Knop J, Nickel EJ, Hall S, Jensen P, et al. Developmental differences in childhood motor coordination predict adult alcohol dependence: proposed role for the cerebellum in alcoholism. Alcoholism: Clinical and Experimental Research. 2005;29(3):353–357. doi: 10.1097/01.alc.0000156126.22194.e0. 00000374-200503000-00009 [pii] [DOI] [PubMed] [Google Scholar]
- Mcdermott D. The Relationship of Parental Drug-Use and Parents Attitude Concerning Adolescent Drug-Use to Adolescent Drug-Use. Adolescence. 1984;19(73):89–97. [PubMed] [Google Scholar]
- McGue M, Slutske W, Iacono WG. Personality and substance use disorders: II. Alcoholism versus drug use disorders. Journal of Consulting and Clinical Psychology. 1999;67(3):394–404. doi: 10.1037/0022-006x.67.3.394. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical Modelling. 5. Box 126 MCV, Richmond, VA 23298: Department of Psychiatry; 1999. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. 1. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Duffy DL, Martin NG. Direction of Causation - Reply to Commentaries. Genetic Epidemiology. 1994a;11(6):463–472. doi: 10.1002/gepi.1370110603. [DOI] [PubMed] [Google Scholar]
- Neale MC, Walters E, Health AC, Kessler RC, Perusse D, Eaves LJ, et al. Depression and parental bonding: cause, consequence, or genetic covariance? Genetic Epidemiology. 1994b;11(6):503–522. doi: 10.1002/gepi.1370110607. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Deater-Deckard K, Fulker D, Rutter M, Plomin R. Genotype-environment correlations in late childhood and early adolescence: antisocial behavioral problems and coercive parenting. Developmental Psychology. 1998;34(5):970–981. doi: 10.1037//0012-1649.34.5.970. [DOI] [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown LB. A parental bonding instrument. British Journal of Medical Psychology. 1979;52:1–10. [Google Scholar]
- Pickles A, Neale M, Simonoff E, Rutter M, Hewitt J, Meyer J, et al. A simple method for censored age-of-onset data subject to recall bias: mothers’ reports of age of puberty in male twins. Behavior Genetics. 1994;24(5):457–468. doi: 10.1007/BF01076181. [DOI] [PubMed] [Google Scholar]
- Pitkanen T, Kokko K, Lyyra AL, Pulkkinen L. A developmental approach to alcohol drinking behaviour in adulthood: a follow-up study from age 8 to age 42. Addiction. 2008;103(Suppl 1):48–68. doi: 10.1111/j.1360-0443.2008.02176.x. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Development. 1983;54(2):424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Stattin H, Kerr M. Parental monitoring: a reinterpretation. Child Development. 2000;71(4):1072–1085. doi: 10.1111/1467-8624.00210. [DOI] [PubMed] [Google Scholar]
- Szobot CM, Bukstein O. Attention deficit hyperactivity disorder and substance use disorders. Child and Adolescent Psychiatric Clinics of North America. 2008;17(2):309–323. viii. doi: 10.1016/j.chc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Verweij KJ, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010;105(3):417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner M, Weichold K, Silbereisen RK. Trajectories of alcohol use among adolescent boys and girls: Identification, validation, and sociodemographic characteristics. Psychology of Addictive Behaviors. 2007;21(1):62–75. doi: 10.1037/0893-164x.21.1.62. [DOI] [PubMed] [Google Scholar]
- Windle M, Mun EY, Windle RC. Adolescent-to-young adulthood heavy drinking trajectories and their prospective predictors. Journal of Studies on Alcohol. 2005;66(3):313–322. doi: 10.15288/jsa.2005.66.313. [DOI] [PubMed] [Google Scholar]
- Young SE, Mikulich SK, Goodwin MB, Hardy J, Martin CL, Zoccolillo MS, et al. Treated Delinquent Boys Substance Use - Onset, Pattern, Relationship to Conduct and Mood Disorders. Drug and Alcohol Dependence. 1995;37(2):149–162. doi: 10.1016/0376-8716(94)01069-W. [DOI] [PubMed] [Google Scholar]