Abstract
Background
A brief “Lung Age” feedback intervention has shown promise for personalizing the health impact of smoking and promoting cessation in unselected smokers. Now that many healthcare organizations provide face-to-face cessation services, it is reasonable to ask whether such motivational feedback of lung function tests might improve treatment compliance and cessation rates in smokers wanting to quit. This study assessed effects of baseline motivational spirometry-based “Lung Age” feedback on treatment compliance and tobacco abstinence at 28-day follow-up.
Methods
This randomized controlled pilot study took place in Penn State University-affiliated outpatient medical practices. Participants were 225 adult smokers (≥ 5 cigarettes/day) willing to attend tobacco dependence treatment. At assessment lung function (FEV-1) and exhaled carbon-monoxide (CO) were assessed. The Intervention group (n=120) were randomly allocated to receive motivational “Lung Age” feedback estimated by FEV-1 and on exhaled CO; Control group (n=105) received minimal feedback. Participants were offered 6 weekly group smoking cessation sessions and nicotine patches and followed-up 28 days after target quit date. The primary outcome measure was self-reported 7-day tobacco abstinence, confirmed by CO<10ppm at 28-day follow-up.
Results
Quit rates were similar at follow-up (Intervention 50.8%; Control 52.4%; p=0.65) after controlling for abstinence predictors. Group attendance and patch use were similar. Among those attending follow-up (n=164, 73%), a greater proportion of the Intervention group had improved lung function (67% v. 46%; p=0.0083).
Conclusions
Baseline Lung Age feedback did not improve quit rates or compliance at 28-day follow-up in smokers seeking intensive treatment.
This study was registered at ClinicalTrials.gov (identifier: NCT01980485).
Keywords: smoking, cessation, dependence, spirometry, carbon-monoxide, FEV-1
1. INTRODUCTION
Cigarette dependence is caused by the psychoactive effects of nicotine in the smoke (USDHHS, 1988; RCP, 2000) and is characterized by difficulty quitting smoking despite serious attempts, often despite awareness of serious health impacts. Cigarette smokers are more than 10 times more likely to develop lung diseases such as lung cancer and chronic obstructive pulmonary disease (COPD) as compared to non-smokers (USDHHS, 2004). Smoking also causes serious diseases affecting virtually every organ system in the body, and smokers are more than three times as likely as non-smokers to die of ischemic heart disease before the age of 65 (USDHHS, 2004).
Smoking cessation reverses these risks, such that a smoker who quits by age 50 has one-half the risk of dying in the next 15 years as compared to a continuing smoker (USDHHS, 1990). Some physiological measures such as exhaled carbon monoxide (CO) return to non-smoker levels within a few days of quitting smoking, and lung function improves within months of quitting (Bize et al., 2012; Scanlon et al., 2000; Jang et al., 2010). It has been suggested that providing smokers with feedback on biomedical tests and the possible future effects of smoking and quitting on such test results may be a strategy for increasing smoking cessation rates (Bize et al., 2012).
A meta-analysis of biomedical risk assessment as an aid for smoking cessation (Bize et al., 2012) concluded that, “There is little evidence about the effects of most types of biomedical tests for risk assessment on smoking cessation. Of the fifteen included studies, only two detected a significant effect of the intervention. Spirometry combined with an interpretation of the results in terms of ‘lung age’ had a significant effect in a single good quality trial but the evidence is not optimal.” That trial (Parkes et al., 2008) found that smokers receiving lung age feedback—that is, lung function test results demonstrating lung function in relation to expected performance by age—were more likely to be quit a year later (13.6%) as compared with those who had the measurement carried out and score provided, but not explained (6.4%). Measurement of exhaled CO has also shown effects on smoking cessation in some studies (Jamrozik et al., 1984; Sanders et al., 1989). For example, Sanders et al. (1989) randomized 751 smokers attending a nurse health screening to either brief smoking cessation advice or brief advice plus CO measurement. One month later 11.7% of the CO measurement group had quit, compared with 7.5% in the control group. Risser and Belcher (1990) compared education alone or education plus an additional motivational intervention that contained immediate feedback about the smoker’s exhaled CO, spirometry results, and pulmonary symptoms. They found that 20% versus 7% remained quit 12 months later. This relatively brief intervention (providing feedback on spirometry-based “Lung Age” plus exhaled CO) therefore shows promise as a way to personalize the health impact of smoking and cessation to patients.
Most of the trials finding positive effects of motivational lung feedback at baseline were conducted in unselected smokers attending for screenings on other medical assessments. This includes the National Lung Screening Trial (Grannis, 2014), the results of which could be interpreted to indicate that smokers who receive negative (high risk) lung screening results are more likely to quit, or, on the other hand, that smokers receiving neutral or positive (low risk) lung screening results are less likely to quit (Kaminsky et al., 2011). This highlights the need for randomized studies. In addition, now that many healthcare organizations provide face-to-face cessation services, it is reasonable to ask whether addition of such measures and motivational feedback might improve treatment compliance and cessation rates in smokers already wanting to quit. A trial with sufficient statistical power (>80%) to detect a meaningful effect on long-term cessation rates (e.g., 25% v 35% at 6 months) would require over 800 participants. We, therefore, conducted a smaller pilot study that was designed to identify whether there is any evidence that Lung Age and CO feedback at assessment can improve treatment compliance and short-term (28-day) cessation rates.
2. MATERIALS AND METHODS
2.1 Power calculation
This study had 87% power to detect a 40% increase in 28-day abstinence rates (i.e., from 50% to 70%) based on a two-tailed chi-squared test and alpha=0.05. We selected this effect size as being at the lower end of the effect size continuum that would be clinically meaningful at 28 days and have the potential to still be meaningful in the longer term even with similar relapse rates in both groups over subsequent months.
2.2 Recruitment and inclusion criteria
This study was registered at ClinicalTrials.gov. Community smokers were recruited via posters and clinician referrals to attend a smoking cessation group treatment and were offered free group support and a two-week supply of nicotine patches. Participants were eligible if they smoked ≥5 cigarettes per day, were ready to make a quit attempt within the next month, ≥21 years old, willing to attend study visits and able to provide informed consent. Exclusion criteria included contraindications for nicotine patch (allergy, pregnancy, recent cardiac problems) or lung function testing (i.e., recent or planned surgery). Other exclusions included current use of smoking cessation medicines, uncontrolled mental illness or substance use in past 6 months, life expectancy <1 year or unwillingness to quit all tobacco products.
Potential volunteers were screened for eligibility by phone and then an assessment appointment with a Nurse Practitioner (SH) was scheduled. Both the assessments and group support sessions took place at outpatient facilities (primarily Penn State Family Practices based in the community) affiliated with Penn State College of Medicine. Recruitment and follow-up occurred between February, 2012 and November, 2013. Eligible participants provided informed consent and completed a comprehensive baseline assessment (as part of a separate study of predictors of cessation), including a full medical and tobacco use history that included the following measures: Penn State Cigarette Dependence Index (PSCDI; Foulds et al., 2014), Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al., 1991), Hooked on Nicotine Checklist (HONC; DiFranza et al., 2002), Wisconsin Predicting Patients’ Relapse (WI-PREPARE) questionnaire (Bolt et al., 2009), education, sex, age, race, employment status, Body Mass Index (BMI), number of quit attempts in the last year, weight gain on longest previous quit attempt, weight concerns (Borrelli and Mermelstein, 1998), confidence to maintain weight after quitting (Borrelli and Mermelstein, 1998), current dieting status, daily alcoholic beverage servings, caffeine consumption (mg/day), cigarettes per day, dietary measurements, confidence in quitting (Boudreaux et al., 2012), importance of quitting (Boudreaux et al., 2012), Brief Perceived Stress score (Cohen et al., 1983), having previously received substance abuse treatment, history of depression treatment, anxiety or other mental health problem, total Kessler 6 (K6) score (Furukawa et al., 2003), total Patient Health Questionnaire (PHQ-9) score (Kroenke et al., 2001), Alcohol Use Disorders Identification Test (AUDIT) score (Frank et al., 2008), Clinical COPD Questionnaire (CCQ) scores (van der Molen et al., 2003), Pittsburgh Sleep Quality Index (PSQI) score (Buysse et al., 1989), total Minnesota Nicotine Withdrawal Scale (MNWS) score (Hughes and Hatsukami, 1986), history of eating disorders, and smoking mentholated cigarettes. 199 of 225 participants provided blood samples for analysis of nicotine and metabolites. For all participants, assessment included measurement of FEV-1 and Lung Age using the Care Fusion SpiroUSB spirometer and Spirometry PC software (SPCS), which selects the best measure from three valid attempts and compares the patient’s results to NHANES III-based norms adjusting for age, sex, height, weight and race, yielding percent of predicted FEV-1 and “effective lung age”. This spirometer is similar to that used in the original study (Parkes et al., 2008). Exhaled CO was measured using a breath “Smokerlyzer” manufactured by Bedfont Scientific. This type of CO monitor has been validated and used in numerous research studies (Bize et al., 2012).
2.3 Randomization
A CONSORT diagram is included in Figure 1. 373 individuals were assessed for eligibility using a phone screen. Of these 373 individuals, 16 were not interested in participating in the study; 2 did not consent to continue with the phone screen; 40 were found to be ineligible after phone screening; 13 yielded duplicate or incomplete phone screens; 11 did not schedule an assessment visit appointment; 64 did not attend their scheduled assessment visit; and 2 did not consent to participating in the study at their assessment visit. The remaining 225 participants underwent randomization to either the Intervention (n=120) or Control group (n=105). For randomization, the study statistician (AB) prepared a computerized block randomization sequence, with each ascending subject ID number randomly allocated to the Intervention or Control condition. After the lung measures were collected the research clinician opened the randomization envelope for that study ID, which indicated whether to perform the motivational feedback on spirometry and CO (I) or not (C). The Control group was simply informed of their FEV-1 in liters per second and CO in parts per million (ppm), with no additional explanation, and informed of the details of the first group meeting. As the intervention was behavioral this was an unblinded trial.
Figure 1.
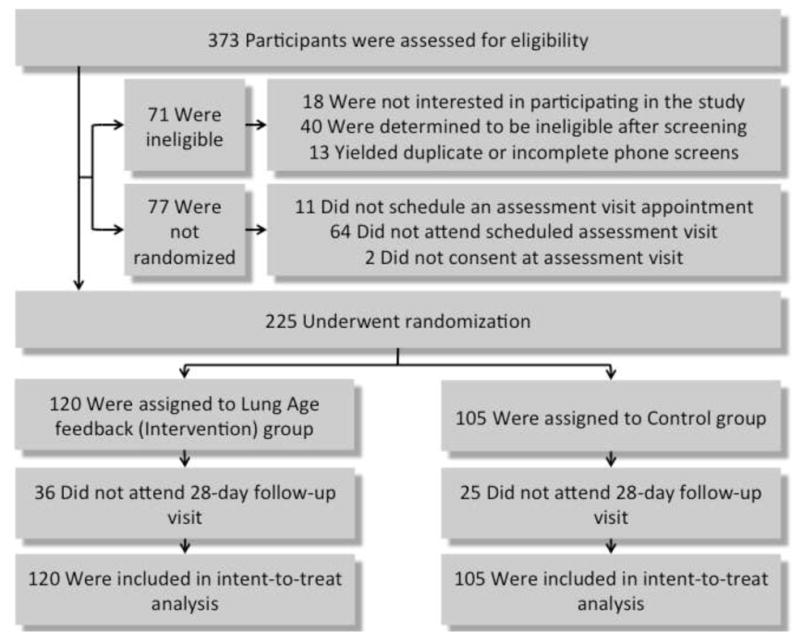
CONSORT diagram for the study. 373 individuals were assessed for eligibility using a phone screen. Of these 373 individuals, 16 were not interested in participating in the study; 2 did not consent to continue with the phone screen; 40 were found to be ineligible after phone screening; 13 yielded duplicate or incomplete phone screens; 11 did not schedule an assessment visit appointment; 64 did not attend their scheduled assessment visit; and 2 did not consent to participating in the study at their assessment visit. The remaining 225 participants underwent randomization to either the Intervention (n=120) or Control group (n=105). Of these groups, 36 and 25 participants did not attend the 28-day follow-up visit, respectively. All 225 were included in the intent-to-treat analysis.
2.4 Intervention
Those allocated to the Intervention group received more detailed feedback on their lung function as follows:
The Intervention group had the effects of smoking on lung function explained to them using a graph (Figure 2) showing examples of changes over time in (a) a never smoker (b) a smoker with rapidly worsening lung function ending up with COPD and (c) a smoker with worsening lung function who stops smoking, experiences an initial improvement in lung function and then a more gradual decline, parallel with that of a never smoker, avoiding severe COPD. The same figure was showed to all Intervention participants, and feedback was personalized by providing participants with their own FEV-1 and CO measurements. These measurements were explained to each participant, and the beneficial effects of quitting smoking were explained using the figure.
Figure 2.
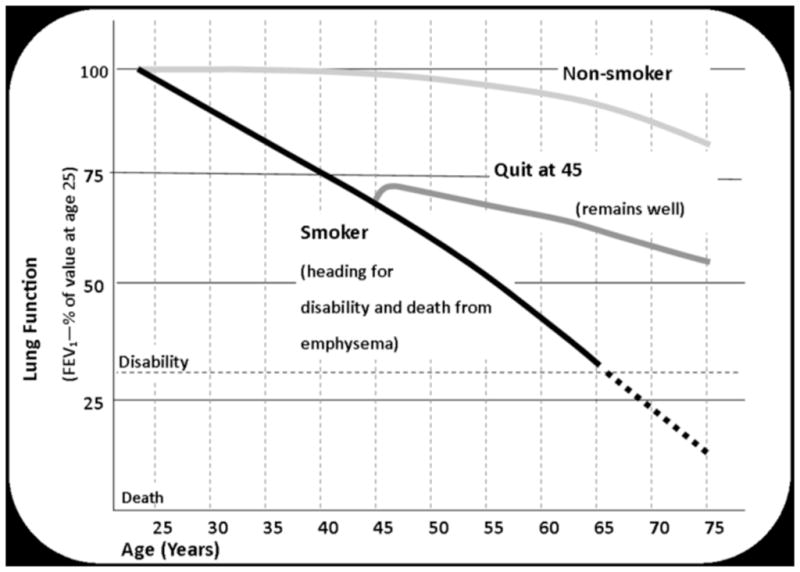
Graphic used to provide “Lung Age” feedback used in the study. Adapted from Fletcher, BMJ 1977; Kohansal, Am J Resp Crit Care Med 2009; and Scanlon, Am J Resp Crit Care Med 2000.
In the Intervention group, if an individual’s Lung Age was equal to or less than their chronological age, they were briefly informed that the test result was normal and that it was important to avoid potential future lung problems by stopping smoking. For those in the Intervention group with a “normal” FEV-1, the intervention focused on their exhaled CO (described below).
If their Lung Age was greater than their chronological age, they were given their “Lung Age” in years. For example, a 45-year-old who had the FEV-1 of a 50-year-old was told, “Now I want to give you some important information about the results of your lung function tests. The test found that you have a “Lung Age” of 50 years. This means that you have the lung function of someone 5 years older than you. As we showed you on the graph, smoking causes lung function to get worse at a much faster rate, and it is very likely that your lung function is worse than it should be because of your smoking. It is also likely that it will continue to worsen if you keep smoking. However, if you quit smoking, we would expect improvement of your lung function. So it is really important that you attend all the stop-smoking group meetings, use the nicotine patches and succeed in stopping smoking. We will measure your lung function again at the last group meeting, a month after you quit smoking, so we can measure any improvement.”
In addition, our Institutional Review Board required that all participants (both Intervention and Control) with a baseline FEV-1 <80% of predicted (on the basis of national norms) had a letter sent to their family doctor informing them, and the participant was also informed that “Your Forced Expiratory Volume in one second (FEV-1) was x liters per second. This kind of lung function test does not itself allow us to make any diagnosis. Your score was lower than we would expect for someone of your age and we recommend that you see your doctor to discuss whether further diagnostic testing and treatment would be appropriate.”
Those in the Intervention Group had their exhaled CO result explained in more detail. Non-smokers typically have an exhaled CO of 0–4ppm, whereas smokers typically have a CO of 10–50ppm. Because of its relatively short half-life (around 4–5 hours), CO levels return to normal within a few days of stopping smoking. So a smoker in the Intervention group with a typical baseline CO of 20ppm was informed as follows:
“Your exhaled carbon-monoxide reading was 20. This is much higher than that of a non-smoker (typically 0–4). It means that the carbon-monoxide from inhaled cigarette smoking is binding to the red blood cells that carry oxygen in your blood and displacing oxygen. This means that your heart has to do more work to supply oxygen to your body, and it is part of the reason why smoking causes serious cardiovascular diseases such as heart attack or a stroke. It is very important to get that number down. The good news is that when you stop smoking the concentration of carbon-monoxide in your body will return to that of a never-smoker within a week. So it is really important that you attend all the stop-smoking group meetings, use the nicotine patches and succeed in stopping smoking. We will measure your CO levels at every appointment and you will be able to see the improvement when you quit smoking.”
2.5 Smoking cessation treatment provided to all participants
All participants were invited to attend 6 weekly sessions of group smoking cessation support, with the Target Quit Date (TQD) being the day of the second group meeting (visit 3) and with the sessions aiming to maximize group cohesion and support for achieving complete tobacco abstinence. Each participant was seen very briefly (1–2 minutes) individually prior to each group meeting to have their CO measured, and to hand in their completed questionnaires. Participants each reported to the group whether they had smoked in the previous week at the start of each meeting, and made a promise to the group to attend the next session without smoking, at the end of each session. Average group size was 10 participants (range =5–24) across 22 closed groups. This smoking cessation group format has been used in previous studies and found to be at least as effective as individual counseling (Foulds et al., 2006; McEwan et al., 2006). All participants were provided with a 14-day supply of 21mg, 24-hour transdermal nicotine patches at no cost, and were also provided with assistance (i.e., a prescription) to obtain additional patch supplies for the lowest cost available from insurance or other community resources.
Group session attendance, exhaled CO, nicotine withdrawal symptoms, adverse events and cigarette and nicotine replacement therapy (NRT) use were recorded at each weekly visit. Participants were treated in mixed groups, but none of the individual baseline or ongoing Lung Age or CO measures were discussed in detail with participants after the assessment session. As this study was designed to look at short-term effects of the Intervention, the primary outcome measure was intent-to-treat self-reported 7-day point prevalence tobacco abstinence (answering “no” to the question, “Have you smoked or used any tobacco in the past 7 days?”), confirmed by CO<10ppm at the 28-day follow-up (visit 7). Secondary outcomes included measures of treatment compliance (attendance and patch use) and change in lung function. The primary hypothesis was that smokers provided with motivational lung function feedback at assessment would be more likely to be abstinent 28 days after the TQD.
2.6 Statistical analysis
All 225 randomized participants (n=105 in Control group; n=120 in Intervention group) were included in the primary outcome analysis, as those who did not attend the 28-day follow-up or who had an exhaled CO>9ppm were counted as continuing smokers. Univariate tests were performed to examine the effect of baseline covariates on 28-day abstinence. Covariates that predicted abstinence with univariate p-value <0.20 were included in a multivariable logistic regression model alongside the randomization group. Statistical analyses were performed in SAS 9.4 (SAS Institute, Inc, Cary, NC, USA).
3. RESULTS
The baseline characteristics of the two groups are provided in Table 1. The two groups were well matched on most key variables, as evidenced by statistical comparisons between the two groups, except that those in the Control group rated themselves significantly less confident in their ability to quit (note this was assessed prior to the Lung Age feedback). Overall, they were fairly typical of smokers seeking help to quit smoking in the United States and rated the importance of quitting smoking as 9.3 on a 1–10 scale. Overall, 41% had a baseline FEV-1 <80% of their predicted FEV-1. 8.6% of participants in the Control group and 12.5% of participants in the Intervention group had Lung Ages less than or equal to their chronological age, and therefore may not have received detailed Lung Age feedback (Chi-square p=0.34).
Table 1.
Baseline cohort demographics.
| Variable | Overall (n=225) | Control (n=105) | Intervention (n=120) | p Value |
|---|---|---|---|---|
| Age (years), mean (SD) | 48.5 (12.5) | 49.8 (12.5) | 47.5 (12.5) | 0.16 |
| Female (n, %) | 136 (60.4%) | 64 (61.0%) | 72 (60.0) | 0.88 |
| White (n, %) | 196 (87.1%) | 95 (90.5%) | 101 (84.2%) | 0.16 |
| College degree or higher, n (%) | 58 (25.8%) | 22 (21.0%) | 36 (30.0%) | 0.12 |
| Cigarettes per day, mean (SD) | 17.6 (7.35) | 17.6 (6.82) | 17.6 (7.80) | 0.96 |
| Fagerstrom Test for Nicotine Dependence (FTND) score,15 mean (SD) | 4.58 (1.87) | 4.58 (1.88) | 4.58 (1.87) | 0.99 |
| Penn State Cigarette Dependence Index (PSCDI) score,14 mean (SD) | 12.4 (3.06) | 12.6 (3.14) | 12.3 (3.00) | 0.43 |
| WI-PREPARE score,17 mean (SD) | 5.18 (1.99) | 5.25 (2.09) | 5.13 (1.89) | 0.65 |
| Plasma nicotine (ng/mL), mean (SD) | 13.1 (7.19) | 13.1 (6.70) | 13.1 (7.65) | 0.99 |
| Plasma cotinine (ng/mL), mean (SD) | 241 (124) | 247 (137) | 235 (110) | 0.51 |
| Current smoking-related symptoms/disease, n (%) | 108 (48.0%) | 50 (47.6%) | 58 (48.3%) | 0.97 |
| FEV-1 percent of predicted, mean (SD) | 79.7 (19.1) | 79.3 (17.8) | 80.0 (20.2) | 0.80 |
| CO (ppm), mean (SD) | 20.6 (11.1) | 19.9 (10.4) | 21.3 (11.7) | 0.35 |
| Lung Age (years), mean (SD) | 63.1 (17.8) | 65.2 (15.9) | 61.3 (19.2) | 0.095 |
| Importance of quitting (range 1–10), mean (SD) | 9.25 (1.18) | 9.14 (1.25) | 9.34 (1.11) | 0.21 |
| Confidence in quitting (range 1–10), mean (SD) | 7.56 (2.23) | 7.20 (2.28) | 7.88 (2.15) | 0.024* |
| Weight concerns related to quitting score,18 mean (SD) | 4.96 (2.34) | 4.92 (2.36) | 5.01 (2.33) | 0.78 |
| Confidence to maintain weight after quitting score,18 mean (SD) | 6.52 (2.09) | 6.43 (2.08) | 6.59 (2.10) | 0.55 |
| Brief Perceived Stress score,19 mean (SD) | 5.98 (2.99) | 6.01 (3.02) | 5.96 (2.97) | 0.90 |
| Total Kessler 6 (K6) score20 >12, n (%) | 23 (10.2%) | 8 (7.62%) | 15 (12.5%) | 0.23 |
| Previous treatment for substance abuse, n (%) † | 44 (19.8%) | 17 (16.7%) | 27 (22.5%) | 0.28 |
| Smokes mentholated cigarettes, n (%) | 105 (46.7%) | 51 (48.6%) | 54 (45.0%) | 0.59 |
indicates p<0.05;
3 participants missing substance abuse data.
The seven-day biochemically-confirmed tobacco abstinence rates at 28 days (visit 7) were similar in the Intervention (61/120=50.8%) and Control groups (55/105=52.4%, p=0.65), even after controlling for other significant predictors of abstinence (dependence, confidence in quitting, weight concerns related to quitting, confidence to maintain weight after quitting, baseline stress, and smoking mentholated cigarettes; see Table 2).
Table 2.
Model of cessation outcomes at 28-day follow-up. Multivariable logistic regression model controlled for potential confounders demonstrating no association between study intervention and 28-day abstinence
| Covariate | Odds Ratio (95% CI) | p Value |
|---|---|---|
|
| ||
| Study randomization | ||
| Control | Referent | 0.65 |
| Intervention | 0.863 (0.458 – 1.63) | |
|
| ||
| Penn State Cigarette Dependence Index (PSCDI) | 0.824 (0.707 – 0.961) | 0.014* |
|
| ||
| WI-PREPARE | 0.843 (0.673 – 1.06) | 0.14 |
|
| ||
| Confidence in quitting (baseline) | 1.18 (1.00 – 1.38) | 0.044* |
|
| ||
| Smokes mentholated cigarettes | ||
| No | Referent | 0.025* |
| Yes | 0.473 (0.247 – 0.908) | |
|
| ||
| Weight concerns related to quitting (baseline) | 1.24 (1.08 – 1.43) | 0.0028* |
|
| ||
| Confidence to maintain weight after quitting (baseline) | 0.827 (0.700 – 0.976) | 0.024* |
|
| ||
| Total stress (baseline) | 0.877 (0.783 – 0.982) | 0.022* |
|
| ||
| Cigarettes per day (baseline) | 0.954 (0.903 – 1.01) | 0.091 |
|
| ||
| Received treatment for substance abuse † | ||
| No | Referent | 0.098 |
| Yes | 0.496 (0.216 – 1.14) | |
indicates p<0.05;
3 participants missing substance abuse data.
Attendance of the last group session was similar in the Intervention group (84/120=70%) and the Control group (80/105=76%, chi-squared=1.09, p=0.30), as was the mean number of total patches used (16.1 v. 18.0 days, p=0.12).
Among those who attended the 28-day follow-up (n=164) there was a significantly greater pre-post increase in FEV-1 in the Intervention than the Control group (p=0.027). In the Intervention and Control groups, mean FEV-1 scores at baseline in liters were 2.64 and 2.57 (p=0.60), respectively; at 28-day follow-up the means were 2.73 and 2.58 (p=0.28), respectively. When an improvement in lung function was defined as “FEV-1 at 28 days> FEV-1 at baseline”, a greater proportion of the Intervention group had an improved lung function as compared to Control (67% v. 46%, p=0.0083). However, we could find no evidence that this was related to any greater reduction in cigarette smoke exposure as the exhaled CO at the 28-day visit was similar (6.8 v. 6.1, p=0.53), as was the mean number of cigarettes smoked per day in the prior week among those who had smoked (8.8 v. 5.8, p=0.45).
Figure 3 shows (a) percent attendance at each group meeting; (b) percent using the transdermal nicotine patches in the previous week at each visit; (c) mean exhaled CO among those attending each visit; and (d) percent (ITT) attending with a CO <10ppm and self-report of no tobacco use in the prior week, in the Intervention and Control groups. Attendance increased at visit 7 (the last group meeting) as research staff emphasized the importance of attending the last group meeting. There was no evidence that participants in the Intervention group had better rates of attendance, patch use, exhaled CO, or abstinence than the Control group; in fact, most of the measures favored the Control group.
Figure 3.
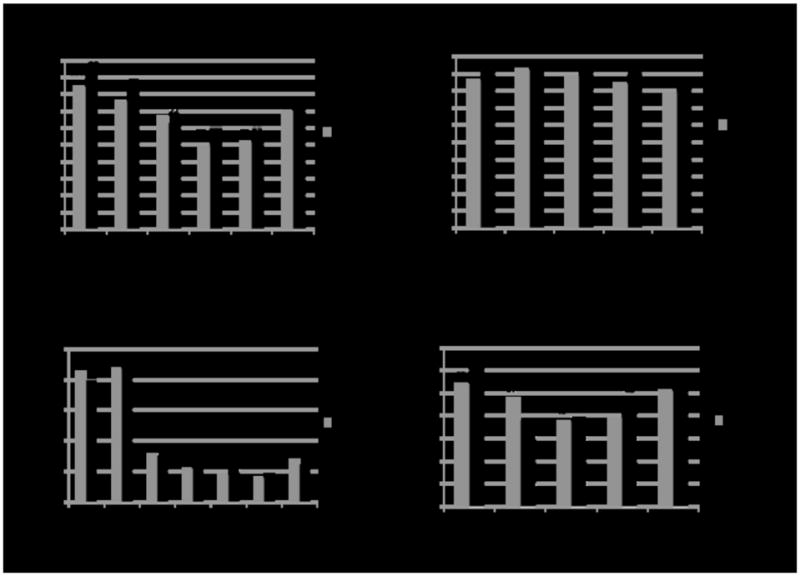
Summary of study outcomes. Figure 3 depicts the following measures in the Intervention and Control groups: (a) percent attendance at each group meeting (out of a total of n=120 participants in the Intervention group and n=105 in the Control group; raw number of attending participants given above each bar); (b) percent using the transdermal nicotine patches in the previous week at each visit (as a percentage of the number of participants attending each group meeting; see (a)); (c) mean exhaled CO among those attending at each visit (see (a) for raw number of participants attending each group meeting); and (d) intent-to-treat percent attending with a CO<10ppm and self-report of no tobacco use in the prior week (out of a total of n=120 participants in the Intervention group and n=105 in the Control group; raw number of abstinent participants given above each bar).
4. DISCUSSION
Overall, despite the fact that the smoking cessation treatment had good compliance, with 73% attending the last group session, and an impressive 28-day biochemically verified abstinence rate of 52%, we found no evidence to suggest that the motivational lung function and CO feedback at assessment improved treatment compliance or short-term smoking cessation outcomes. The fact that for all of these the outcome was slightly (non-significantly) better in the Control group suggests that this finding was unlikely to be due to lack of statistical power to detect a clinically meaningful positive effect.
One possible explanation for the lack of effect could be that among smokers who volunteer for a relatively intensive smoking cessation treatment (involving attendance at six weekly one-hour group support sessions) the motivation to quit is already so high that a fairly brief motivational intervention has little opportunity to make a meaningful difference. This is supported by our finding of a near ceiling effect on the 0–10 point scale (Boudreaux et al., 2012) on which smokers rated how important it was for them to quit, at baseline, with both groups rating a mean score >9.
Another possible explanation stems from the fact that we performed the lung function and CO measurement in both groups, with only the feedback being different. Our scientific review committee recommended that all participants with an FEV-1 lower than 80% of predicted should be informed and have a letter sent to their family doctor, regardless of group allocation. It is possible that this may have diluted between group differences. When we restricted analyses only to those within each group with an FEV-1 >79% of predicted, (n=70 in the Intervention group; n=63 in the Control group), the overall pattern of results remained similar, with no sign that the lung age or CO feedback boosted compliance or quit rates. One potential weakness of this study is that it only examined short-term outcomes during the first month after a quit attempt. While it is possible that the Lung Age feedback may have a “sleeper effect”—a psychological effect on longer term smoking cessation, when we designed this study we felt it was much more likely that the intervention would initially affect short-term cessation (Paek et al., 2014), via better compliance with treatment, and this is what the study was designed to detect. No such effects were detected. The previous randomized trial reporting a significant positive effect of Lung Age feedback on smoking cessation (Parkes et al., 2008) studied smokers recruited in family medicine practices for research on lung function, whereas the current study recruited smokers for help to quit smoking. We suspect that the different results may relate to the different baseline motivational states of the participants, with our participants already having a high level of motivation to quit smoking. Lung Age feedback may still be helpful for unselected smokers in general medical settings.
The finding of a significantly greater improvement in FEV-1 in the Intervention than the Control group was unexpected, given a lack of effect on smoking cessation or cigarette consumption. This could be simply a chance finding. Although all participants were encouraged to give their best effort on the lung function tests at baseline and follow-up, it is possible that the feedback provided to the Intervention group after baseline testing provided them with extra motivation and effort on the follow-up lung function tests, although it did not directly affect smoking cessation behavior.
This study has observed that, among individuals already highly motivated to quit, an additional motivator in the form of Lung Age feedback does not appear to enhance 28-day biochemically-verified tobacco abstinence rates. Nicotine dependence, as measured by the Penn State Cigarette Dependence Index (PSCDI), may be a more important predictor of 28-day abstinence in this population of already highly motivated individuals. Although spirometry, Lung Age and CO feedback at assessment may provide useful biomedical information to smokers about to embark on a smoking cessation attempt using intensive behavioral support, that feedback delivered at baseline assessment does not appear to improve treatment compliance or short-term smoking cessation in smokers already motivated to quit.
Supplementary Material
Highlights.
This study assessed baseline “Lung Age” feedback effects on tobacco abstinence.
Intervention group received “Lung Age” feedback; Control received minimal feedback.
Primary outcome was self-reported 7-day tobacco abstinence confirmed by CO<10ppm.
Quit rates were similar at 28-day follow-up (Intervention 50.8%; Control 52.4%).
Acknowledgments
ROLE OF FUNDING SOURCE. This work was supported by internal funding from Penn State Cancer Institute (PI: JF). JF, SV, SH, JY & JM are primarily funded by the National Institute on Drug Abuse of the National Institutes of Health (NIH) and the Center for Tobacco Products of the U.S. Food and Drug Administration (FDA) (grant numbers P50-DA-036107-01, P50-DA-036105). This study was not funded by that grant.
Footnotes
CONSORT 2010 Checklist appears as Supplementary Material and can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
CONTRIBUTORS. All authors have contributed equally to this manuscript and accept responsibility for its contents.
CONFLICT OF INTEREST. JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bize R, Burnand B, Mueller Y, Rège-Walther M, Camain JY, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2012;12:CD004705. doi: 10.1002/14651858.CD004705.pub4. [DOI] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, McCarthy DE, Japuntich SJ, Fiore MC, Smith SS, Baker TB. The Wisconsin Predicting Patients’ Relapse questionnaire. Nicotine Tob Res. 2009;11:481–492. doi: 10.1093/ntr/ntp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based cessation program. Addict Behav. 1998;23:6096–22. doi: 10.1016/s0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Boudreaux ED, Sullivan A, Abar B, Bernstein SL, Ginde AA, Camargo CA., Jr Motivation rulers for smoking cessation: a prospective observational examination of construct and predictive validity. Addict Sci Clin Pract. 2012;7:8. doi: 10.1186/1940-0640-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397–403. doi: 10.1001/archpedi.156.4.397. [DOI] [PubMed] [Google Scholar]
- Foulds J, Gandhi KK, Steinberg MB, Richardson DL, Williams JM, Burke MV, Rhoads GG. Factors associated with quitting smoking at a tobacco dependence treatment clinic. Am J Health Behav. 2006;30:400–412. doi: 10.5555/ajhb.2006.30.4.400. [DOI] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a questionnaire to assess dependence on electronic cigarettes in a large sample of ex-smoking e-cig users. Nicotine Tob Res. 2015;17:186–92. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, DeBenedetti AF, Volk RJ, Williams EC, Kivlahan DR, Bradley KA. Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J Gen Intern Med. 2008;23:781–787. doi: 10.1007/s11606-008-0594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa TA, Kessler RC, Slade T, Andrews G. The performance of the K6 and K10 screening scales for psychological distress in the Australian National Survey of Mental Health and Well-Being. Psychol Med. 2003;33:357–362. doi: 10.1017/s0033291702006700. [DOI] [PubMed] [Google Scholar]
- Grannis FW., Jr National Lung Screening Trial limitations and public health policy. Oncology (Williston Park) 2014;28(11) pii: 202326. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jamrozik K, Vessey M, Fowler G, Wald N, Parker G, Van Vunakis H. Controlled trial of three different antismoking interventions in general practice. Br Med J (Clin Res Ed) 1984;288:1499–1503. doi: 10.1136/bmj.288.6429.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang AS, Park SW, Kim DJ, Uh S, Kim YH, Whang HG, Lim GI, Park CS. Effects of smoking cessation on airflow obstruction and quality of life in asthmatic smokers. Allergy Asthma Immunol Res. 2010;2:254–259. doi: 10.4168/aair.2010.2.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky DA, Marcy T, Dorwaldt A, Pinckney R, DeSarno M, Solomon L, Hughes JR. Motivating smokers in the hospital pulmonary function laboratory to quit smoking by use of the lung age concept. Nicotine Tob Res. 2011;13:1161–1166. doi: 10.1093/ntr/ntr096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen A, West R, McRobbie H. Effectiveness of specialist group treatment for smoking cessation vs. one-to-one treatment in primary care. Addict Behav. 2006;31:1650–1660. doi: 10.1016/j.addbeh.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598–600. doi: 10.1136/bmj.39503.582396.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek YJ, Lee S, Kim YH, Lee KS, Yim HW, Kim MS, Kim CH, Jeung O. Effect on smoking quit rate of telling smokers their health risk appraisal in terms of health age: a randomized control trial. Asian Pac J Cancer Prev. 2014;15:4963–4968. doi: 10.7314/apjcp.2014.15.12.4963. DOI: http://dx.doi.org/10.7314/APJCP.2014.15.12.4963. [DOI] [PubMed] [Google Scholar]
- Risser NL, Belcher DW. Adding spirometry, carbon monoxide, and pulmonary symptom results to smoking cessation counseling: a randomized trial. J Gen Intern Med. 1990;5:16–22. doi: 10.1007/BF02602303. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians. Nicotine Addiction in Britain: A Report of the Tobacco Advisory Group of the Royal College of Physicians. RCP; London: 2000. [Google Scholar]
- Sanders D, Fowler G, Mant D, Fuller A, Jones L, Marzillier J. Randomized controlled trial of anti-smoking advice by nurses in general practice. J R Coll Gen Pract. 1989;39:273–276. [PMC free article] [PubMed] [Google Scholar]
- Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS, Tashking DP Lung Health Study Research Group. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161:381–390. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: Nicotine Addiction A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 1988. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 1990. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2004. [Google Scholar]
- van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes. 2003;1:13. doi: 10.1186/1477-7525-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


