Abstract
Objective
To assess the ability of the Age-Adjusted Charlson Comorbidity index (ACCI) to predict perioperative complications and survival in patients undergoing primary debulking for advanced epithelial ovarian cancer (EOC).
Methods
Data were analyzed for all patients with stage IIIB-IV EOC who underwent primary cytoreduction from 1/2001–1/2010 at our institution. Patients were divided into 3 groups based on an ACCI of 0–1, 2–3, and ≥4. Clinical and survival outcomes were assessed and compared.
Results
We identified 567 patients; 199 (35%) had an ACCI of 0–1, 271 (48%) had an ACCI of 2–3, and 97 (17%) had an ACCI of ≥4. The ACCI was significantly associated with the rate of complete gross resection (0–1=44%, 2–3=32%, and ≥4=32%; p=0.02), but was not associated with the rate of minor (47% vs 47% vs 43%, p=0.84) or major (18% vs 19% vs 16%, p=0.8) complications. The ACCI was also significantly associated with progression-free (PFS) and overall survival (OS). Median PFS for patients with an ACCI of 0–1, 2–3, and ≥4 was 20.3, 16, and 15.4 months, respectively (p=0.02). Median OS for patients with an ACCI of 0–1, 2–3, and ≥4 was 65.3, 49.9, and 42.3 months, respectively (p<0.001). On multivariate analysis, the ACCI remained a significant prognostic factor for both PFS (p=0.02) and OS (p<0.001).
Conclusions
The ACCI was not associated with perioperative complications in patients undergoing primary cytoreduction for advanced EOC, but was a significant predictor of PFS and OS. Prospective clinical trials in ovarian cancer should consider stratifying for an age-comorbidity covariate.
Keywords: age-adjusted charlson comorbidity index, ovarian cancer, perioperative complications, progression-free survival, overall survival
Introduction
Of the estimated 21,290 women diagnosed each year with epithelial ovarian, fallopian tube, or peritoneal carcinoma in the United States, the majority present with advanced-stage (International Federation of Gynecology and Obstetrics [FIGO] III/IV) disease [1]. Standard therapy for these patients consists of primary debulking surgery, followed by adjuvant chemotherapy [2]. Numerous studies have shown a survival advantage for patients who undergo ‘optimal’ versus ‘suboptimal’ cytoreduction [3, 4].
In order to achieve optimal surgical outcomes, primary debulking surgery is often lengthy and complex, requiring bowel resection and/or aggressive upper abdominal surgery [5]. Such extensive procedures are commonly associated with significant perioperative complications [6–11]. Given this risk, neoadjuvant chemotherapy followed by interval debulking is offered by certain providers to patients who are poor operative candidates due to age and/or medical comorbidity [12–15]. This is subjective and surgeon dependent, however, and there is no consensus on which comorbid conditions or age render a patient a poor operative candidate.
The Charlson Comorbidity index is a prognostic index that was developed to predict 1-year mortality based on medical comorbidity [16]. It is a score derived by the summation of the weighted scores of 19 medical conditions found to be associated with survival, and has been validated in several populations [17–19]. Age was subsequently found to be predictive of death from comorbid disease by the authors. It was incorporated to create a combined score accounting for both comorbidity and age, the Age-Adjusted Charlson Comorbidity index (ACCI), which has also been validated [20].
Researchers have attempted to predict morbidity and/or survival in patients undergoing primary cytoreduction using a variety of prognostic factors and models [13, 14, 21–26]. However, there are limited data assessing the prognostic significance of a validated comorbidity index on these outcomes. The objective of our study was to assess the ability of the ACCI to predict perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian, fallopian tube, or peritoneal cancer.
Patients and Methods
After obtaining institutional review board approval, we identified all patients with FIGO stage IIIB-IV epithelial ovarian, fallopian tube, and peritoneal cancer who underwent primary cytoreduction at our institution from January 2001 to January 2010. Patients were excluded if they had non-epithelial ovarian cancer, tumors of low-malignant potential, or if they received neoadjuvant chemotherapy. Clinical data, perioperative complications, and survival outcomes were retrospectively reviewed from medical records. Data abstracted included: age, medical comorbidity, body mass index, primary disease site, FIGO stage, histology, tumor grade, preoperative albumin, preoperative platelet count, preoperative CA-125, presence and amount of ascites at surgery, presence of gross residual disease after cytoreductive surgery, time to adjuvant chemotherapy, and intraperitoneal chemotherapy administration.
The ACCI was assigned to all patients using their individual medical conditions and age at the time of primary debulking. The scoring system as described by Charlson et al. is shown in Table 1 [20]. The overall score is calculated based on the total of each patient’s comorbid conditions (weighted according to severity) and age. As all patients had advanced epithelial ovarian cancer, that specific condition was excluded from the scoring system. Patients were categorized into three groups based on an ACCI of 0–1 (low), 2–3 (intermediate), and ≥4 (high).
Table 1.
Age-adjusted charlson comorbidity index (N = 567)
| Score | Comorbidity | n (%) |
|---|---|---|
| 1 | Diabetes mellitus without end-organ damage | 27 (5%) |
| Cerebrovascular disease | 10 (2%) | |
| Myocardial infarction | 14 (2%) | |
| Congestive heart failure | 0 (0%) | |
| Peripheral vascular disease | 9 (2%) | |
| Dementia | 2 (0.4%) | |
| Chronic pulmonary disease | 55 (10%) | |
| Connective tissue disease | 37 (7%) | |
| Peptic ulcer disease | 16 (3%) | |
| Mild liver disease | 5 (1%) | |
| 2 | Diabetes mellitus with end-organ damage | 2 (0.4%) |
| Moderate/severe renal disease | 0 (0%) | |
| Hemiplegia | 0 (0%) | |
| Solid tumor without metastasis (exclude if >5 years from diagnosis) | 32 (6%) | |
| Leukemia | 2 (0.4%) | |
| Lymphoma | 9 (2%) | |
| 3 | Moderate/severe liver disease | 0 (0%) |
| 6 | Metastatic solid tumor | 0 (0%) |
| AIDS (not just HIV positive) | 0 (0%) |
Age adjustment: For each decade after 40 years, add 1 point to total score (i.e. 1 point for age group 50–59 years, 2 points for age group 60–69, etc)
AIDS, Acquired immune deficiency syndrome; HIV, Human immunodeficiency virus
In 2001, our institution established a prospectively maintained adverse events database of all surgical cases. Data on perioperative complications up to 30 days postoperatively are collected for all patients. The database is maintained by a research project manager who reviews the medical record of all surgical patients weekly in that time period, then confirms complications with the patients’ surgeons. Additionally, attending physicians fill out an adverse events sheet at the time of the postoperative visit, and notify the research manager if patients present to the emergency room and are diagnosed with adverse events. Complications are graded for severity on a scale of 1–5 using a standardized institutional grading system: 1 = use of oral medications and/or bedside intervention to treat an event; 2 = use of intravenous medications, parenteral nutrition, enteral nutrition, or blood transfusion to treat an event; 3 = interventional radiology, therapeutic endoscopy, intubation, or operation required to treat an event; 4 = residual and lasting disability requiring major rehabilitation or organ resection; and 5 = event resulting in the death of the patient [27]. This grading system has been validated, with grade 1–2 complications considered minor and grade 3–5 complications considered major [28]. Complications are also classified by system, including but not limited to gastrointestinal, cardiac, pulmonary, and neurologic systems.
The three ACCI groups were assessed for their association with grade 1–2 (minor) and grade 3 (major) perioperative complications. As only one patient each had a grade 4 or 5 complication, those grades were not included in any analysis. Given that the complexity and number of procedures during primary debulking is correlated with the rate and severity of surgical complications [6, 22], we stratified our cohort into three subgroups according to a validated surgical complexity score [22, 29]. As described by Aletti and colleagues [29], that score is calculated based on the specific procedures performed in a cytoreductive case and classifies surgeries into low-, intermediate-, and high-complexity cases. We then performed a secondary analysis, assessing the association between the ACCI and complications within those subgroups. The ACCI was also evaluated for its ability to predict specific systems-based complications.
Progression-free survival (PFS) and overall survival (OS) were additional endpoints in our study. The date of progression was determined by computed tomography (CT) scan and/or CA-125 levels. When determined by CT scan, the progression date was taken as the first appearance of one or more new lesions or increased size of existing lesions. When determined by CA-125 level, the progression date was defined as the first date of the initial CA-125 of greater than or equal to two times the nadir value or upper limit of normal, as applicable [30, 31]. When a subsequent CT scan confirmed that the rise in CA-125 indicated progression, the progression date was defined as the date of CA-125 rise. PFS was defined as the time interval from the date of primary debulking to the date of disease progression, death, or last follow-up. OS was defined as the time interval from the date of surgery to the date of death or last follow-up. OS included death due to comorbid conditions, and was not disease-specific. Patients who were lost to follow-up were censored from the analysis.
Categorical variables were compared using the χ2 test, and continuous variables were compared using the Kruskal-Wallis test. All statistical tests were two-sided, with a p value of <0.05 considered significant. When testing the association between the ACCI and specific systems-based complications, logistic regression analysis was performed adjusting for surgical complexity. The Kaplan–Meier method was used to estimate survival rates. Univariate analysis of all assessed categorical and continuous variables was performed for prognostic significance using the log-rank test and Cox proportional hazards model for significance, respectively. Differences in survival were calculated using the Cox proportional hazards model. Variables with a p value of <0.05 on univariate analysis were then included in a multivariate Cox regression analysis. Statistical analysis was performed using SPSS 22.0 (IBM Corporation, Armonk, NY).
Results
Five hundred sixty-seven patients were included over the study period. One hundred ninety-nine patients (35%) had an ACCI of 0–1, 271 patients (48%) had an ACCI of 2–3, and 97 patients (17%) had an ACCI of ≥4. The most common comorbid conditions were ‘chronic pulmonary disease’ (n = 55, 10%), ‘connective tissue disease’ (n = 37, 7%), ‘other solid tumors’ (n = 32, 6%), and ‘diabetes mellitus without end-organ damage’ (n = 27, 5%) (Table 1). Patient and tumor characteristics are shown in Table 2. The ACCI ≥4 group had the highest median age, while the ACCI 0–1 group had the highest proportion of patients with stage IV disease and the highest median preoperative platelet count. The ACCI was significantly associated with both the rate of optimal debulking (≤1 cm residual disease) (0–1 = 82%, 2–3 = 71%, and ≥4 = 70%; p=0.01) and the rate of complete gross resection (0–1 = 44%, 2–3 = 32%, and ≥4 = 32%; p=0.02).
Table 2.
Patient and tumor characteristics (N = 567)
| Characteristic | ACCI 0–1 (Low) n = 199 (35%) |
ACCI 2–3 (Intermediate) n = 271 (48%) |
ACCI ≥4 (High) n = 97 (17%) |
p |
|---|---|---|---|---|
|
| ||||
| Median age (range) | 51 years (23 – 59) | 65 years (43 – 79) | 74 years (60 – 96) | <0.001 |
|
| ||||
| Median body mass index (range) | 25.2 kg/m2 (16.3 – 43.7) | 25.7 kg/m2 (17.6 – 54.6) | 25 kg/m2 (18.3 – 50.1) | 0.49 |
|
| ||||
| FIGO Stage | ||||
| IIIB | 10 (5%) | 8 (3%) | 5 (5%) | 0.01 |
| IIIC | 148 (74%) | 226 (83%) | 86 (89%) | |
| IV | 41 (21%) | 37 (14%) | 6 (6%) | |
|
| ||||
| Primary disease site | ||||
| Ovary | 167 (84%) | 204 (75%) | 74 (76%) | 0.19 |
| Fallopian tube | 16 (8%) | 28 (10%) | 9 (9%) | |
| Peritoneum | 16 (8%) | 39 (15%) | 14 (15%) | |
|
| ||||
| Histology | ||||
| Serous | 181 (91%) | 249 (92%) | 81 (84%) | 0.1 |
| Endometrioid | 1 (1%) | 2 (1%) | 2 (2%) | |
| Clear cell | 2 (1%) | 0 (0%) | 0 (0%) | |
| Mixed/Other | 15 (8%) | 20 (7%) | 14 (14%) | |
|
| ||||
| Tumor grade | ||||
| 1 | 8 (4%) | 7 (3%) | 3 (3%) | 0.6 |
| 2 | 16 (8%) | 14 (5%) | 5 (5%) | |
| 3 | 175 (88%) | 250 (92%) | 89 (92%) | |
|
| ||||
| Median preoperative albumin (range) * | 4.1 g/dL (2.5 – 4.8) | 4.1 g/dL (2.1 – 5) | 4.1 g/dL (2.4 – 4.8) | 0.97 |
|
| ||||
| Median preoperative platelet count (range) | 367 K/μl (204 – 1067) | 366 K/μl (175 – 920) | 314 K/μl (113 – 1067) | <0.001 |
|
| ||||
| Median preoperative CA-125 (range) † | 681 U/mL (3 – 28,503) | 496 U/mL (3 – 38,100) | 444 U/mL (9 – 24,500) | 0.05 |
|
| ||||
| Ascites | ||||
| None | 50 (25%) | 74 (27%) | 38 (39%) | 0.09 |
| 1 – 1000 ml | 57 (29%) | 71 (26%) | 24 (25%) | |
| 1001 – 5000 ml | 73 (37%) | 92 (34%) | 31 (32%) | |
| >5000 ml | 19 (9%) | 34 (13%) | 4 (4%) | |
|
| ||||
| Residual disease | ||||
| None | 87 (44%) | 86 (32%) | 31 (32%) | 0.02 |
| ≤1 cm | 77 (39%) | 107 (39%) | 37 (38%) | |
| >1 cm | 35 (17%) | 78 (29%) | 29 (30%) | |
|
| ||||
| Median time to adjuvant chemotherapy (range) ‡ | 32 days (4 – 90) | 31 days (2 – 93) | 35 days (4 – 72) | 0.27 |
|
| ||||
| Intraperitoneal chemotherapy administration (optimally debulked patients 2005 – 2010) | 64/101 (63%) | 60/124 (48%) | 20/36 (56%) | 0.08 |
ACCI: Age-adjusted charlson comorbidity index; FIGO, International Federation of Gynecology and Obstetrics
Data missing for:
8,
24, and
20 patients.
Among the entire cohort, 261 patients (46%) had a grade 1–2 (minor) complication, and 101 patients (18%) had a grade 3 (major) complication. One patient with an ACCI of 1 had a grade 4 complication, and one patient with an ACCI of 4 had a grade 5 complication. The ACCI was not associated with the overall rate of grade 1–2 or grade 3 complications. For patients with an ACCI of 0–1, 2–3, and ≥4, the overall rate of grade 1–2 complications was 47% (n = 93/199), 47% (n = 126/271), and 43% (n = 42/97), respectively (p=0.84). Similarly, the overall rate of grade 3 complications was 18% (n = 36/199), 19% (n = 50/271), and 16% (n = 15/97), respectively (p=0.8). When modeled as a continuous variable, there was also no association between the ACCI and the overall rate of grade 1–2 or grade 3 complications (Table S1).
As the extent of a debulking procedure is related to perioperative complications, we stratified our cohort into three subgroups based on a surgical complexity score [29]. One hundred fifty patients (27%) had a surgery of low complexity, 255 patients (45%) had a surgery of intermediate complexity, and 162 patients (29%) had a surgery of high complexity (Table 3). After this stratification, the ACCI was still not associated with the overall rate of grade 1–2 or grade 3 complications.
Table 3.
Age-adjusted charlson comorbidity index and perioperative complications based on surgical complexity
| Surgical Complexity Score | Total Patients n (%) |
ACCI | Patients n (%) |
Grade 1–2 (Minor) Complications n (%) |
p | Grade 3 (Major) Complications n (%) |
p |
|---|---|---|---|---|---|---|---|
| ≤3 (Low) | 150 (27%) | 0 – 1 | 40 (27%) | 21/40 (52%) | 0.64 | 6/40 (15%) | 0.29 |
| 2 – 3 | 70 (46%) | 35/70 (50%) | 6/70 (9%) | ||||
| ≥4 | 40 (27%) | 17/40 (43%) | 2/40 (5%) | ||||
| 4 – 7 (Intermediate) | 255 (45%) | 0 – 1 | 104 (41%) | 48/104 (46%) | 0.61 | 11/104 (11%) | 0.49 |
| 2 – 3 | 112 (44%) | 56/112 (50%) | 14/112 (13%) | ||||
| ≥4 | 39 (15%) | 16/39 (41%) | 7/39 (18%) | ||||
| ≥8 (High) | 162 (29%) | 0 – 1 | 55 (34%) | 24/55 (44%) | 0.67 | 19/55 (35%) | 0.99 |
| 2 – 3 | 89 (55%) | 35/89 (39%) | 30/89 (34%) | ||||
| ≥4 | 18 (11%) | 9/18 (50%) | 6/18 (33%) |
ACCI: Age-adjusted charlson comorbidity index
The most common complications were gastrointestinal (n = 146, 26%), followed by wound (n = 131, 23%), infectious (n = 116, 21%), and pulmonary complications (n = 90, 16%) (Table 4). Among the 12 different system-based complications, the ACCI was only significantly associated with cardiovascular complications on univariate analysis: the rate was 3% (n = 6/199), 5% (n = 13/271), and 10% (n = 10/97) for patients with an ACCI of 0–1, 2–3, and ≥4, respectively (p=0.03). However, after adjusting for surgical complexity on logistic regression analysis, that difference was no longer significant (p=0.06).
Table 4.
Age-adjusted charlson comorbidity index and complication type (N = 567)
| Complication Type | Total Patients n (%) |
ACCI 0–1 (Low) n = 199 (35%) |
ACCI 2–3 (Intermediate) n = 271 (48%) |
ACCI ≥4 (High) n = 97 (17%) |
p | Adjusted p* |
|---|---|---|---|---|---|---|
| Infectious | 116 (21%) | 43 (22%) | 55 (20%) | 18 (19%) | 0.83 | 0.88 |
| Venous thromboembolism | 76 (13%) | 18 (9%) | 44 (16%) | 14 (14%) | 0.07 | 0.1 |
| Hematologic | 72 (13%) | 21 (11%) | 41 (15%) | 10 (10%) | 0.25 | 0.47 |
| Gastrointestinal | 146 (26%) | 52 (26%) | 77 (28%) | 17 (18%) | 0.11 | 0.14 |
| Genitourinary | 46 (8%) | 16 (8%) | 23 (9%) | 7 (7%) | 0.93 | 0.99 |
| Cardiovascular | 29 (5%) | 6 (3%) | 13 (5%) | 10 (10%) | 0.03 | 0.06 |
| Pulmonary | 90 (16%) | 35 (18%) | 42 (16%) | 13 (13%) | 0.63 | 0.59 |
| Wound | 131 (23%) | 39 (20%) | 67 (25%) | 25 (26%) | 0.34 | 0.28 |
| General | 14 (3%) | 5 (3%) | 6 (2%) | 3 (3%) | 0.89 | 0.85 |
| Endocrine | 2 (0.5%) | 0 (0%) | 2 (1%) | 0 (0%) | 0.33 | 0.99 |
| Musculoskeletal | 1 (0.2%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.09 | 0.99 |
| Neurologic | 14 (3%) | 5 (3%) | 4 (2%) | 5 (5%) | 0.13 | 0.1 |
ACCI: Age-adjusted charlson comorbidity index
P value adjusting for surgical complexity score
The median PFS and OS for the entire study population were 17.1 months (95% CI, 15.7 – 18.5) and 52.1 months (95% CI, 47.6 – 56.6), respectively, with a median follow-up of 68.1 months (range, 1 – 147.3) for the 181 survivors. The ACCI was significantly associated with PFS and OS (Figures 1 & 2). Median PFS for patients who had an ACCI of 0–1, 2–3, and ≥4 was 20.3 months (95% CI, 16.6 – 24), 16 months (95% CI, 14.5 – 17.6), and 15.4 months (95% CI, 13 – 17.8), respectively (p=0.02). Median OS for patients who had an ACCI of 0–1, 2–3, and ≥4 was 65.3 months (95% CI, 54.7 – 75.8), 49.9 months (95% CI, 42.9 – 57), and 42.3 months (95% CI, 29.9 – 54.8), respectively (p<0.001). On multivariate analysis, after adjusting for stage, histology, preoperative albumin, ascites volume, residual disease, and intraperitoneal chemotherapy administration, both PFS (p=0.02) and OS (p<0.001) remained significant.
Figure 1.
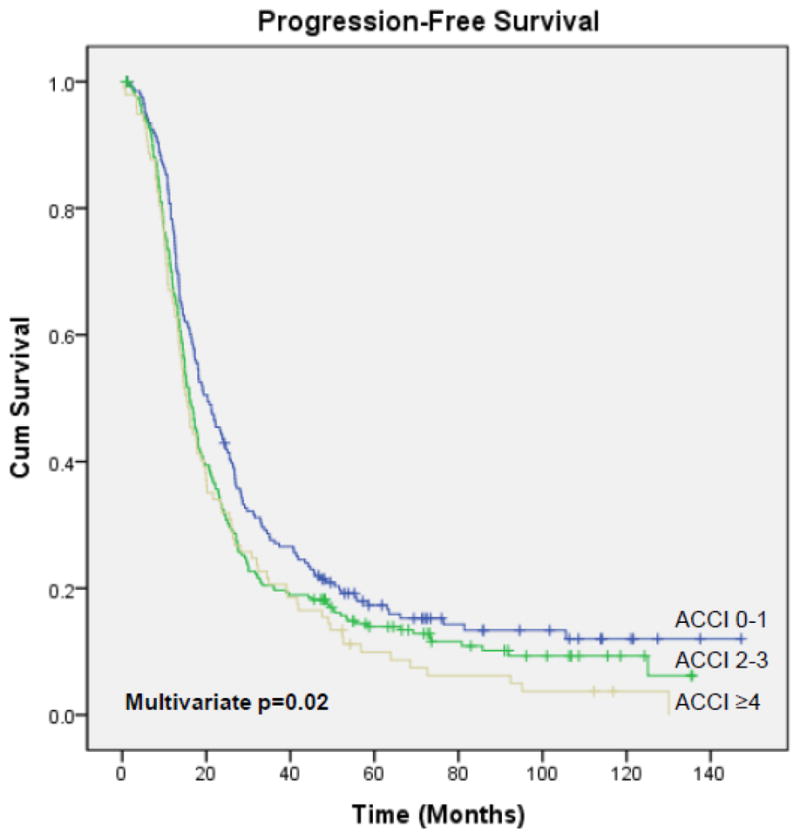
Progression-Free Survival: ACCI 0–1 vs 2–3 vs ≥4
Figure 2.
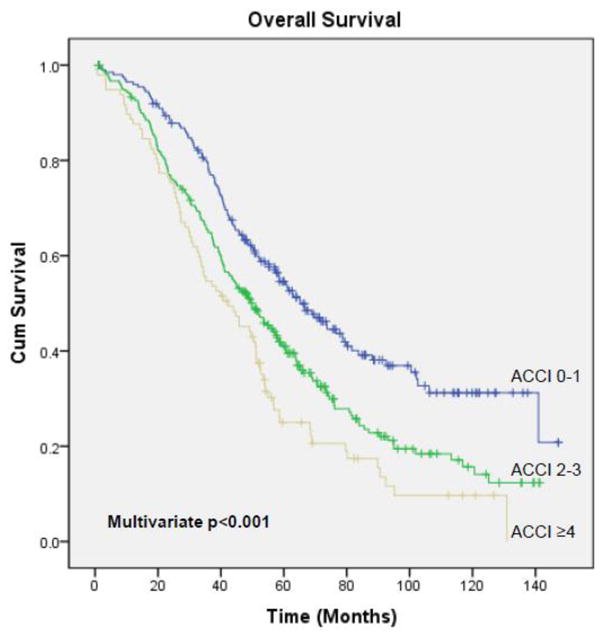
Overall Survival: ACCI 0–1 vs 2–3 vs ≥4
Discussion
In this large institutional cohort of patients undergoing primary cytoreduction for advanced epithelial ovarian, fallopian tube, and peritoneal cancer, the ACCI was a significant predictor of both PFS and OS. It was not associated with minor or major perioperative complications.
Previous investigators have attempted to predict survival outcomes in patients undergoing debulking for ovarian cancer using different prognostic criteria and models [13, 14, 22, 23, 25, 26]. Few have used a validated comorbidity score, and we could not identify any studies that used the ACCI. Using a Danish cancer registry, Tetsche et al. evaluated the original Charlson Comorbidity index in patients with stage I-IV disease and found it to be significantly associated with 1- and 5-year survival [25]. Sperling and colleagues also used a Danish national clinical database to assess patients with all-stage disease and reported a significant association between the Charlson Comorbidity index and OS [26]. Both authors acknowledged the limitations of using administrative databases, including the potential underreporting of comorbidity and misclassification due to reliance on ICD-10 codes. Our study is consistent with those results but differs in several ways. We used a validated index that not only assessed comorbidity but also took age into account. This is important as age has been found to be an independent prognostic factor for survival [14, 32, 33]. We considered that the ACCI, a tool that combines both age and comorbidity and takes into account the interplay between both, was an ideal instrument to evaluate this outcome. In addition, we only assessed women with advanced-stage disease, a group that has significantly worse survival outcomes compared to those with early-stage cancer. We also extracted data directly from medical records, and evaluated both PFS and OS.
It is interesting to note that more patients in the low ACCI group (0–1) had both an optimal debulking outcome and complete gross resection. This suggests that physicians may be more likely to subject patients to an extensive procedure to achieve those outcomes if those patients are younger and/or have less comorbidity. Indeed, patients in the high ACCI group (≥4) comprised 27% of those who underwent a low-complexity surgery, compared to 11% of those who underwent a high-complexity surgery (Table 3). Furthermore, better debulking outcomes were seen in the low ACCI group (0–1) despite that group having more patients with stage IV disease. The difference in stage distribution also suggests that patients with a high ACCI (≥4) who were suspected of having Stage IV disease may have been more likely to get neoadjuvant chemotherapy, an outcome not assessed in our study. In addition, the ACCI was not associated with the rate of intraperitoneal chemotherapy administration when only assessing optimally debulked patients. Importantly, the discrepancies mentioned did not affect PFS and OS, as the differences in survival persisted in a multivariate model adjusting for them.
Researchers have also attempted to predict perioperative morbidity in patients undergoing primary debulking [14, 21, 22, 24]. As with survival, there are few reports of a validated comorbidity score being employed in this setting. Using claims data from the Nationwide Inpatient Sample database, Wright et al. showed that the Charlson Comorbidity index was associated with surgical-site, medical, and infectious complications [21]. The authors recognized that not being able to account for the ‘degree’ of cytoreduction was an important limitation of their study. On the other hand, our data showed no association between the ACCI and either minor or major perioperative complications. Our stratification by surgical complexity is a major strength, as the extent of a cytoreductive procedure is correlated with surgical complications, especially when upper abdominal procedures are employed [6, 22]. After this stratification, the ACCI was still not associated with either minor or major complications. We also found no association between the ACCI and separate systems-based complications. The use of our adverse events database for our analysis is another strength, in which complications are prospectively reported, classified by system, and graded for severity, which adds to the quality of our data. Evaluation of the broad surgical literature reveals conflicting results with regards to the association between comorbidity and perioperative complications. In a cohort of patients undergoing radical prostatectomy, comorbidity was found to be predictive of operative morbidity [34], while another study of patients undergoing treatment for breast cancer found no association between the two [35]. It is important to note that the ACCI was originally developed to predict the risk of mortality, and was not specifically designed to predict perioperative complications in patients undergoing surgery. This may explain why no association between the index and perioperative complications was found in our population. Additionally, it is possible that when taking age into account, comorbidity may not be as predictive of complications as other studies have suggested [21]. As with our survival analysis, we consider that using a comorbidity index that accounts for age to also be an advantage when assessing perioperative complications. Many providers prefer to administer neoadjuvant chemotherapy to elderly patients, due to a concern for complications and those patients’ ability to tolerate them [12, 14]. One can hypothesize, however, that a healthy 75-year-old woman with no comorbidity may be more likely to tolerate an extensive debulking procedure than a 60-year-old with significant medical conditions. The rates of both minor and major complications were similar in all ACCI groups in our data, suggesting that patients who are older but have less comorbidity have similar outcomes to those who are younger but have more medical problems. This might reinforce the case that healthy elderly women should not be denied the possible benefits of optimal primary debulking and subsequent intraperitoneal therapy based on age alone [14].
The main limitation of our study is the retrospective nature of our analysis. The ACCI was calculated based on comorbidity reported by patients at the initial visit with the gynecologic oncologist. It is possible that some comorbid conditions may have been underreported by patients at that visit or undiagnosed at that time. We addressed this by reviewing records from medical clearance notes, outside referring provider documentation, and interdisciplinary consultations, with minimal discrepancy found. We also did not take into account subsequent medical conditions that patients developed during their follow-up course, which may have ultimately impacted their survival. We chose not to, as the main aim of our study was to predict perioperative complications and survival based on the ACCI at the time of debulking. Our study is also limited by the smaller number of patients with an ACCI ≥4 (97 patients), and therefore it is possible that a greater number of patients with a high ACCI was needed to detect a difference in complications. Despite its validation in numerous populations, the use of the ACCI has its own limitations. Owing to advances in medical treatment of different conditions, the survival impact of the comorbid conditions included in its summation may be different today than when the index was developed [16, 20].
In addition to being a prognostic factor for survival, our results suggest that the ACCI may have important implications in ovarian cancer research as well. As demonstrated in our data, median PFS decreased from 20.3 months to 15.4 months for patients who had an ACCI of 0–1 and ≥4, respectively. Median OS also decreased from 65.3 months to 42.3 months, respectively. Not accounting for these differences in survival or considering age or comorbidity alone when designing a trial may lead to an imbalance of patients in different arms and significant confounding of treatment effects.
In conclusion, the ACCI was significantly associated with survival outcomes in patients undergoing primary debulking for advanced epithelial ovarian, fallopian tube, and peritoneal cancer. It was not predictive of minor or major perioperative complications. Further investigation is needed to identify women who are at high risk for operative morbidity. Prospective clinical trials in ovarian cancer should consider stratifying for an age-comorbidity covariate.
Supplementary Material
Research Highlights.
The Age-Adjusted Charlson Comorbidity index was a significant predictor of survival in patients undergoing primary cytoreduction for ovarian cancer.
The index was not associated with minor or major perioperative complications at primary debulking.
Acknowledgments
This study was supported by the Roy M. Speer Foundation.
Footnotes
Presented at the 46th Annual Meeting of the Society of Gynecologic Oncologists, Chicago, IL, March 28–31, 2015
Conflict of Interest Statement:
Dr. Mary E. Charlson reports that Cornell University has applied for a patent for the use of the comorbidity index to predict future utilization. The other authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 3.Winter WE, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 4.Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol. 2013;130:493–8. doi: 10.1016/j.ygyno.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 5.Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94:650–4. doi: 10.1016/j.ygyno.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Chi DS, Zivanovic O, Levinson KL, Kolev V, Huh J, Dottino J, et al. The incidence of major complications after the performance of extensive upper abdominal surgical procedures during primary cytoreduction of advanced ovarian, tubal, and peritoneal carcinomas. Gynecol Oncol. 2010;119:38–42. doi: 10.1016/j.ygyno.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Dowdy SC, Loewen RT, Aletti G, Feitoza SS, Cliby W. Assessment of outcomes and morbidity following diaphragmatic peritonectomy for women with ovarian carcinoma. Gynecol Oncol. 2008;109:303–7. doi: 10.1016/j.ygyno.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenhauer EL, D’Angelica MI, Abu-Rustum NR, Sonoda Y, Jarnagin WR, Barakat RR, et al. Incidence and management of pleural effusions after diaphragm peritonectomy or resection for advanced mullerian cancer. Gynecol Oncol. 2006;103:871–7. doi: 10.1016/j.ygyno.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Magtibay PM, Adams PB, Silverman MB, Cha SS, Podratz KC. Splenectomy as part of cytoreductive surgery in ovarian cancer. Gynecol Oncol. 2006;102:369–74. doi: 10.1016/j.ygyno.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Eisenkop SM, Spirtos NM, Lin WC. Splenectomy in the context of primary cytoreductive operations for advanced epithelial ovarian cancer. Gynecol Oncol. 2006;100:344–8. doi: 10.1016/j.ygyno.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Kehoe SM, Eisenhauer EL, Abu-Rustum NR, Sonoda Y, D’Angelica M, Jarnagin WR, et al. Incidence and management of pancreatic leaks after splenectomy with distal pancreatectomy performed during primary cytoreductive surgery for advanced ovarian, peritoneal and fallopian tube cancer. Gynecol Oncol. 2009;112:496–500. doi: 10.1016/j.ygyno.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Schorge JO, Clark RM, Lee SI, Penson RT. Primary debulking surgery for advanced ovarian cancer: Are you a believer or a dissenter? Gynecol Oncol. 2014;135:595–605. doi: 10.1016/j.ygyno.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen TL, Teiblum S, Paludan M, Poulsen LØ, Jorgensen AY, Bruun KH, et al. Significance of age and comorbidity on treatment modality, treatment adherence, and prognosis in elderly ovarian cancer patients. Gynecol Oncol. 2012;127:367–74. doi: 10.1016/j.ygyno.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Langstraat C, Aletti GD, Cliby WA. Morbidity, mortality and overall survival in elderly women undergoing primary surgical debulking for ovarian cancer: A delicate balance requiring individualization. Gynecol Oncol. 2011;123:187–91. doi: 10.1016/j.ygyno.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Wright JD, Ananth CV, Tsui J, Glied SA, Burke WM, Lu YS, et al. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer. 2014;120:1246–54. doi: 10.1002/cncr.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–7. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110:593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–7. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 20.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 21.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Neugut AI, et al. Defining the limits of radical cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2011;123:467–73. doi: 10.1016/j.ygyno.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197:1–7. doi: 10.1016/j.ajog.2007.10.495. [DOI] [PubMed] [Google Scholar]
- 23.Barlin JN, Yu C, Hill EK, Zivanovic O, Kolev V, Levine DA, et al. Nomogram for predicting 5-year disease-specific mortality after primary surgery for epithelial ovarian cancer. Gynecol Oncol. 2012;125:25–30. doi: 10.1016/j.ygyno.2011.12.423. [DOI] [PubMed] [Google Scholar]
- 24.Clark RM, Lee MS, Alejandro Rauh-Hain J, Hall T, Boruta DM, Del Carmen MG, et al. Surgical Apgar Score and prediction of morbidity in women undergoing hysterectomy for malignancy. Gynecol Oncol. 2015;136:516–20. doi: 10.1016/j.ygyno.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Tetsche MS, Dethlefsen C, Pedersen L, Sorensen HT, Norgaard M. The impact of comorbidity and stage on ovarian cancer mortality: a nationwide Danish cohort study. BMC Cancer. 2008;8:31. doi: 10.1186/1471-2407-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sperling C, Noer MC, Christensen IJ, Nielsen MLS, Lidegaard Ø, Høgdall C. Comorbidity is an independent prognostic factor for the survival of ovarian cancer: a Danish register-based cohort study from a clinical database. Gynecol Oncol. 2013;129:97–102. doi: 10.1016/j.ygyno.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Martin RC, 2nd, Jaques DP, Brennan MF, Karpeh M. Achieving R0 resection for locally advanced gastric cancer: Is it worth the risk of multiorgan resection? J Am Coll Surg. 2002;194:568–77. doi: 10.1016/s1072-7515(02)01116-x. [DOI] [PubMed] [Google Scholar]
- 28.Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204:356–64. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope RC, Wilson TO, et al. Quality improvement in the surgical approach to advanced ovarian cancer: the Mayo Clinic experience. J Am Coll Surg. 2009;208:614–20. doi: 10.1016/j.jamcollsurg.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 31.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. 2001;19:4054–7. doi: 10.1200/JCO.2001.19.20.4054. [DOI] [PubMed] [Google Scholar]
- 32.Markman M, Lewis JL, Saigo P, Hakes T, Rubin S, Jones W, et al. Impact of age on survival of patients with ovarian cancer. Gynecol Oncol. 1993;49:236–9. doi: 10.1006/gyno.1993.1113. [DOI] [PubMed] [Google Scholar]
- 33.Matthews KS, Hoskins KE, Kemper MK, Wang W, Straughn JM, Rocconi RP. The impact of age on the treatment and survival of ovarian cancer patients. Clin Ovarian Cancer. 2010;3:122–5. [Google Scholar]
- 34.Alibhai SMH, Leach M, Tomlinson G, Krahn MD, Fleshner N, Holowaty E, et al. 30-day mortality and major complications after radical prostatectomy: influence of age and comorbidity. J Natl Cancer Inst. 2005;97:1525–32. doi: 10.1093/jnci/dji313. [DOI] [PubMed] [Google Scholar]
- 35.Houterman S, Janssen-Heijnen MLG, Verheij CDGW, Louwman WJ, Vreugdenhil G, van der Sangen MJC, et al. Comorbidity has negligible impact on treatment and complications but influences survival in breast cancer patients. Br J Cancer. 2004;90:2332–7. doi: 10.1038/sj.bjc.6601844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


