ABSTRACT
Throughout the epithelial cycle of spermatogenesis, actin microfilaments arranged as bundles near the Sertoli cell plasma membrane at the Sertoli cell-cell interface that constitute the blood-testis barrier (BTB) undergo extensive re-organization by converting between bundled and unbundled/branched configuration to give plasticity to the F-actin network. This is crucial to accommodate the transport of preleptotene spermatocytes across the BTB. Herein, we sought to examine changes in the actin microfilament organization at the Sertoli cell BTB using an in vitro model since Sertoli cells cultured in vitro is known to establish a functional tight junction (TJ)-permeability barrier that mimics the BTB in vivo. Plastin 3, a known actin microfilament cross-linker and bundling protein, when overexpressed in Sertoli cells using a mammalian expression vector pCI-neo was found to perturb the Sertoli cell TJ-barrier function even though its overexpression increased the overall actin bundling activity in these cells. Furthermore, plastin 3 overexpression also perturbed the localization and distribution of BTB-associated proteins, such as occludin-ZO1 and N-cadherin-β-catenin, this thus destabilized the barrier function. Collectively, these data illustrate that a delicate balance of actin microfilaments between organized in bundles vs. an unbundled/branched configuration is crucial to confer the homeostasis of the BTB and its integrity.
keywords: actin bundling activity, actin microfilaments, ectoplasmic specialization, overexpression, plastin 3, spermatogenesis, testis, tight junction barrier
Introduction
In the mammalian testis, including rodents and humans, the onset of meiosis that produces haploid spermatids from spermatocytes in the seminiferous epithelium correlates tightly with the establishment of a functional blood-testis barrier (BTB) which occurs by ∼17–20 dpp (day postpartum) in rats vs. ∼12 years of age in humans (for reviews, see 1,2). A delay of the BTB assembly by 4-wk by treating neonatal rats with 10 µg diethylstilbestrol (a synthetic, non-steroidal estrogen, suspended in olive oil and injected subcutaneously) at 1, 3, 5, 7, 9 and 11 dpp, a total of 6 doses, was also found to delay meiosis by 4-wk since pachytene spermatocytes failed to enter meiosis I/II to produce haploid spermatids but degenerated instead,3 supporting the notion that a functional BTB is necessary to support meiosis. Other studies using toxicants that damaged the BTB irreversibly also led to failure in meiosis and infertility as a result of aspermatogenesis, which included treatment of adult rats with an acute dose of cadmium chloride,4,5 glycerol6,7 or adjudin,8 illustrating the significance of the BTB to support meiosis and spermatogenesis. In rodents and humans, the BTB physically divides the seminiferous epithelium into the basal and the adluminal (apical) compartment, so that meiosis I/II and post-meiotic spermatid development all take place in a specialized microenvironment at the adluminal compartment behind the BTB, and these events are segregated entirely from the systemic circulation (for reviews, see 1,2,9). Interestingly, the BTB is one of the tightest blood-tissue barriers due to the presence of a network of actin filament bundles that is sandwiched in-between the Sertoli cell plasma membrane and the cisternae of endoplasmic reticulum, creating an ultrastructure known as the basal ectoplasmic specialization (ES) because it is found near the basal compartment of the epithelium (for reviews, see 9-12). Since the basal ES coexists with tight junction (TJ), the network of actin filament bundles thus reinforces the TJ ultrastructure, creating an unusual tight barrier at the BTB. Yet the BTB undergoes continuous remodeling, which is necessary to accommodate the transport of preleptotene spermatocytes connected in clones via intercellular bridges across the immunological barrier. As such, actin microfilaments assembled in a bundled configuration at the basal ES that constitute the BTB with TJ must undergo continuous remodeling by re-organizing between a bundled and an unbundled/branched configuration that gives plasticity to the immunological barrier.
Studies have shown that plastins are a family of actin binding proteins (ABPs) that confer actin microfilament bundling by cross-linking individual microfilaments to support cell polarity, cell shape, and cell movement (for reviews, see 13-15). To date, there are 3 known plastins called plastin 1 (I-plastin, and also called fimbrin, mostly expressed in intestine), plastin 2 (L-plastin or L-fimbrin, restrictively expressed by haematopoietic cells), and plastin 3 (T-plastin or T-fimbrin, widely expressed in mammalian cells including epithelial and mesenchymal cells, solid tissues including bone cells, and testis) (for reviews, see 13-15). Interestingly, all 3 plastins have been shown to be expressed by Sertoli and germ cells in the testis. However, plastin 3 is highly expressed by Sertoli and Leydig cells but not germ cells in the rat testis,16 illustrating this is an important regulator to confer actin microfilaments their bundled configuration. A knockdown of plastin 3 in Sertoli cells by RNAi was also found to perturb the TJ-permeability barrier by causing disruption of actin microfilaments in Sertoli cells due to a loss of the plastin 3 bundle-inducing activity.16 We sought to examine if an overexpression of a plastin 3 full-length cDNA in Sertoli cells with an established TJ-permeability barrier would further tighten the barrier function. To our surprise, plastin 3 overexpression led to a TJ-barrier disruption, illustrating the homeostasis of the actin microfilament network, namely the proper ratio of bundled: unbundled/branched actin microfilaments, is crucial to BTB function. These findings led us to formulate a novel hypothesis that by simply altering the steady state level of plastin 3 in Sertoli cells, this provides a unique mechanism to modulate actin filament plasticity. This is the subject of this report.
Results
Overexpression of plastin 3 in Sertoli cells in vitro perturbs the TJ-permeability barrier function
Sertoli cells cultured for 2 days with a functional TJ-permeability barrier was transfected with pCI-neo plasmid DNA containing the full-length plastin 3 cDNA clone vs. empty vector (i.e., pCI-neo alone) which served as a control. Thereafter, cells were rinsed twice to remove transfection medium, and cultured in F12/DMEM until day 5 (i.e. ∼3 days after transfection) before cultures were terminated to obtain lysates for immunoblotting as noted in the regimen shown in Figure 1A. Overexpression of plastin 3 was confirmed in these Sertoli cells by immunoblotting (Fig. 1B). However, over-expression of plastin 3 had no effects on the steady-state levels of several BTB-associated proteins when examined by immunoblotting (Fig. 1B). Overexpression of plastin 3 was also confirmed by immunofluorescence microscopy using a specific plastin 3 antibody (Table 1) and shown in Figure 1C. Analysis that monitored changes in plastin 3 fluorescence intensity also supported successful overexpression of plastin 3 in Sertoli cells (see bar graph in Fig. 1C). Interestingly, overexpression of plastin 3, an actin cross-linking and bundling protein, was found to perturb the Sertoli cell TJ-permeability barrier function (Fig. 1D) based on the results of multiple experiments since we had expected that plastin 3 over-expression should strengthen the TJ-barrier, making it tighter since a knockdown of plastin 3 was found to perturb the Sertoli cell TJ-barrier function.16 The findings shown in Figure 1A–D thus support the concept that the actin bundling activity, such as those conferred by plastin 3, is tightly regulated, and any changes in the relative ratio of bundled vs. unbundled/branched actin microfilaments in the microenvironment can perturb the Sertoli cell TJ-barrier function. It was noted that lysates used for IB shown in Figure 1B were harvested from cells on day 5 (see Fig. 1A), and TJ-permeability barrier was disrupted on day 5–7 as noted in Figure 1D. However, lysates harvested from cells on day 7 (with transfection performed on day 2 for 6 hr) for IB displayed similar findings as those shown in Figure 1B.
Figure 1.
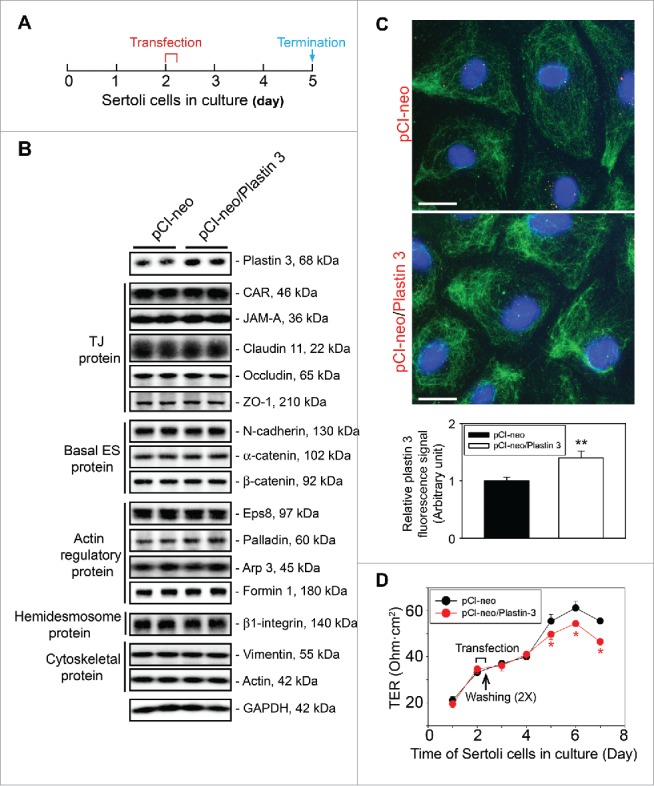
Overexpression of plastin 3, which is known to confer organization of actin microfilaments into bundles, perturbs the Sertoli cell TJ-permeability barrier function. (A) Treatment regimen used to transfect Sertoli cells with a full-length plastin 3 cDNA using the mammalian expression vector pCI-neo (pCI-neo/plastin 3) vs. pCI-neo (i.e., empty vector, control) on day 2 for 6 hr, cells were then rinsed twice to remove the transfection reagent. Thereafter, cells were cultured in fresh F12/DMEM until day 5 (i.e., 3 days after transfection for plastin 3 overexpression) before terminated for immunoblotting, or cell staining by fluorescence microscopy using different marker proteins. TER measurement to assess the TJ-barrier integrity was performed daily until day 8. (B) Immunoblotting that confirmed overexpression of plastin 3 in cultured Sertoli cell in vitro had no effects on the expression of selected BTB-associated proteins. (C) Overexpression of plastin 3 (plasmid DNA, both pCI-neo empty vector vs. vector with full-length plastin 3 cDNA construct, pCI-neo/Plastin 3, labeled with Cy3 as described in Materials and Methods appeared as red fluorescence that confirmed successful transfection) was also confirmed by immunofluorescence microscopy, illustrating the fluorescence intensity of plastin 3 (green fluorescence) was induced in pCI-neo/Plastin 3 vs. pCI-neo empty vector which served as a control. Scale bar, 20 µm. Each bar graph in the histograms shown below is a mean ± SD of n = 3 experiments. To assess the intensity of fluorescence signaling of plastin 3, about 25 Sertoli cells were randomly selected from each experiment with n = 3 experiments. (D) Overexpression of plastin 3 was found to perturb the Sertoli cell TJ-permeability barrier. Each data point is a mean ± SD of quadruplicate bicameral units of a representative experiment from n = 3 experiments which yielded similar results. *, P< 0.05.
Table 1.
Antibodies used for different experiments in this report.
| Dilution |
|||||
|---|---|---|---|---|---|
| Antibodies | Host species | Vendor | Catalog number | IB | IF/IHC |
| Actin | Goat | Santa Cruz Biotechnology | sc-1616 | 1:200 | |
| Arp3 | Mouse | Sigma-Aldrich | A5979 | 1:3000 | 1:100 |
| α-catenin | Rabbit | Santa Cruz Biotechnology | sc-7894 | 1:200 | |
| β-catenin | Rabbit | Invitrogen | 71–2700 | 1:1000 | 1:500 |
| β1-integrin | Rabbit | Santa Cruz Biotechnology | sc-8978 | 1:200 | |
| CAR | Rabbit | Santa Cruz Biotechnology | sc-15405 | 1:200 | |
| Claudin-11 | Rabbit | Invitrogen | 36–4500 | 1:500 | 1:100 |
| Eps8 | Mouse | BD Biosciences | 610143 | 1:500 | 1:100 |
| Formin 1 | Mouse | Abcam | ab68058 | 1:500 | 1:100 |
| GAPDH | Mouse | Abcam | ab8245 | 1:1000 | |
| JAM-A | Rabbit | Invitrogen | 36–1700 | 1:250 | |
| N-cadherin | Mouse | Invitrogen | 33–3900 | 1:500 | 1:100 |
| Occludin | Rabbit | Invitrogen | 71–1500 | 1:250 | |
| Palladin | Rabbit | Proteintech | 10853–1-AP | 1:1000 | |
| Plastin-3 | Rabbit | Abcam | ab137585 | 1:500 | |
| Plastin-3 | Mouse | Santa Cruz Biotechnology | sc-166208 | 1:100 | |
| Vimentin | Mouse | Santa Cruz Biotechnology | sc-6260 | 1:1000 | |
| ZO-1 | Rabbit | Invitrogen | 61–7300 | 1: 500 | 1:100 |
Overexpression of plastin 3 that reduces actin plasticity perturbs localization of TJ and basal ES proteins at the Sertoli cell-cell interface
We next examined changes in the distribution of BTB-associated proteins at the Sertoli cell-cell interface following overexpression of plastin 3 in Sertoli cells (Fig. 2). When plastin 3 expression was considerably induced through its overexpression in Sertoli cells and cells were harvested on day 5 and processed for immunofluorescence microscopy (Fig. 2A, top panel), both TJ (e.g., claudin 11, ZO-1) and basal ES (e.g., N-cadherin, ß-catenin) proteins that were tightly localized at the Sertoli cell cortical zone in control Sertoli cells, were found to be re-distributed from the cell-cell interface, appeared to be either internalized into the cell cytosol (e.g., TJ proteins claudin 11 and ZO-1) or no longer tightly localized, but diffusely localized, at the Sertoli cell-cell interface (e.g., basal ES proteins N-cadherin, ß-catenin) (Fig. 2A). Detailed analysis of these changes shown in Figure 2B further support the notion that overexpression of plastin 3 in Sertoli cells perturbs F-actin organization by disrupting the homeostasis of bundled vs. unbundled/branched actin microfilaments. These findings are in agreement with the physiological data shown in Figure 1D that the Sertoli cell TJ-permeability barrier was perturbed following overexpression of plastin 3. We next investigated if overexpression of plastin 3 indeed caused changes in F-actin organization in Sertoli cells.
Figure 2.
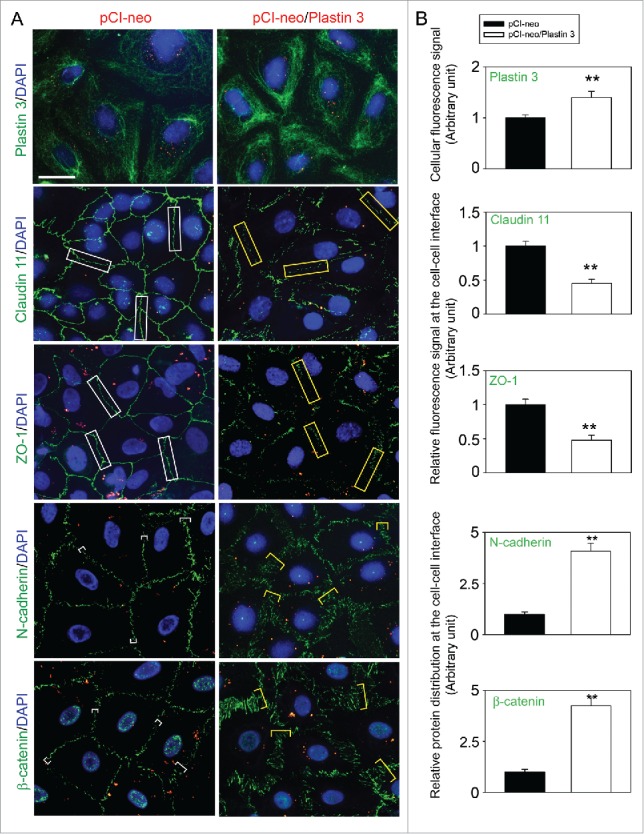
Overexpression of plastin 3 in Sertoli cell epithelium impedes the localization of TJ and basal ES proteins at the cell-cell interface. (A) Immunofluorescence microscopy confirmed overexpression of plastin 3 in Sertoli cells when cells were harvested to assess changes in the plastin 3 (green fluorescence) signals by Image J as described in Materials and Methods. Furthermore, the localization of TJ proteins claudin 11 and ZO-1 and basal ES proteins N-cadherin and ß-catenin was found to be impeded, in which they either rapidly internalized, reducing their levels at the cell-cell interface (e.g., claudin 11 and ZO-1; see white rectangle boxes in control vs. yellow rectangle boxes in plastin 3 overexpressed cells) or no longer tightly but diffusely localized at the cell-cell interface (e.g., N-cadherin and ß-catenin; see white brackets in control vs. yellow brackets in plastin 3 overexpressed cells). These changes thus destabilized the Sertoli cell TJ-permeability barrier as noted in Figure 1D. Scale bar, 20 µm, which applies to all micrographs shown here. These images are representative findings of an experiment from n = 3 independent experiments using different batches of Sertoli cells which yielded similar results. (B) Histograms are either fluorescence signals of plastin 3 across the Sertoli cell (to confirm overexpression of plastin 3), changes in fluorescence signals of claudin 11 and ZO-1 at cell-cell interface between adjacent Sertoli cells (see white or yellow rectangle boxes with signals from 2 opposite ends were scored), or changes in localization of N-cadherin and ß-catenin at cell-cell interface between adjacent cells (bracketed in white or yellow with signals from 2 opposite ends were scored in control (pCI-neo) vs. plastin 3 overexpressed (pCI-neo/Plastin 3) Sertoli cells. Each bar is a mean ± SD of 25 randomly selected Sertoli cells from each experiment with n = 3 experiments were scored (i.e., a total of ∼70 Sertoli cells). **, P < 0.01.
Overexpression of plastin 3 in Sertoli cells disrupts F-actin organization in Sertoli cells, thereby impeding the TJ-barrier function
When F-actin distribution in Sertoli cells following overexpression of plastin 3 was examined and compared to control cells transfected with empty pCI-neo plasmid DNA, the organization of actin microfilaments was grossly affected in cells overexpressed with plastin 3 (Fig. 3A). For instance, microfilaments no longer evenly aligned and stretched across the Sertoli cell cytosol as seen in control cells, instead, these microfilaments aligned in a disorganized fashion, somewhat clustered in specific domains in the cell cytosol such as near the cell-cell interface, supporting the concept that changes in the overall actin bundling activity in the Sertoli cell epithelium by overexpressing plastin 3 to increase the overall cellular intrinsic actin bundling activity would tip the homeostatic balance between bundled and unbundled/branched actin microfilaments to maintain the Sertoli cell BTB function. An increase in the overall cellular intrinsic actin bundling activity in Sertoli cells following overexpression of plastin 3 was also confirmed using an actin bundling biochemical assay (Fig. 3B).
Figure 3.
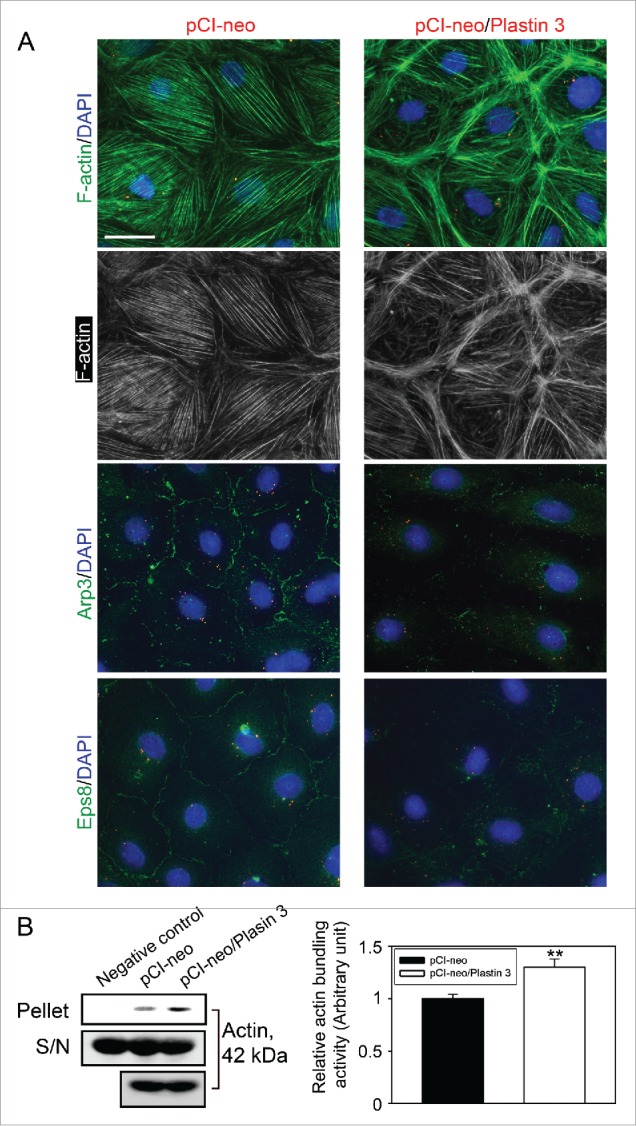
Overexpression of plastin 3 in Sertoli cell epithelium that induces the overall intrinsic actin bundling activity leads to disorganization of actin microfilaments through changes in the spatial expression of Arp3 and Eps8. (A) Overexpression of plastin 3 in Sertoli cells was found to cause dis-organization of actin microfilaments in Sertoli cells in which actin microfilament no longer stretched all across the cell cytosol evenly, instead, they were shown to have actin filaments being bundled at or near the cell cortical zone. These changes appeared to be modulated via changes in the spatial expression of Arp3 (a branched actin-inducing protein) and Eps8 (an actin barbed end capping and bundling protein). However, these changes offset the balance of bundled vs. unbundled/branched actin microfilaments, failing to support F-actin plasticity in the Sertoli cell microenvironment, thereby causing a disruption of the Sertoli cell TJ-permeability barrier as noted in Figure 1D. Scale bar, 20 µm, which applies to all other micrographs. Data shown herein are representative micrographs from a single experiment of n = 3 independent experiments which yielded similar results. (B) Biochemical actin bundling assay using lysates of Sertoli cells overexpressed with plastin 3 (pCI-neo/Plastin 3) vs. control (pCI-neo vector alone) also illustrated an increase in intrinsic actin bundling activity following overexpression of plastin 3 detected in the pellet containing bundled microfilaments vs. supernatant (S/N) containing free microfilaments (see Materials and Methods for details). These findings are representative data of a single experiment with n = 3 independent experiment which yielded similar results as shown in the bar graph on the right panel. Each bar is a mean ± SD of n = 3 independent assays. **, P< 0.01.
Discussion
Following overexpression of plastin 3 in Sertoli cells, we initially expected that an increase in the endogenous intrinsic actin bundling activity conferred by plastin 3 would promote the Sertoli cell TJ-barrier function since more actin microfilaments were available to maintain the integrity of the actin network at the basal ES/BTB. Also, a knockdown of plastin 3 was earlier reported to perturb the Sertoli cell TJ-barrier function due to reduced intrinsic actin bundling activity in which actin microfilaments in Sertoli cells were found to be truncated.16 As reported here, the Sertoli cell barrier function was found to be perturbed as well, based on multiple experiments when plastin 3 was overexpressed. These findings thus clearly demonstrate the importance of a correct balance between the actin bundling vs. unbundling/branching activity conferred by bundling proteins (e.g., plastin 3, palladin, Eps8) and branched actin nucleation protein (e.g., Arp2/3 complex), respectively (for reviews, see 17,18). These also confer plasticity to the F-actin network at the ES to support continuous remodeling of the Sertoli cell BTB to accommodate the transport of preleptotene spermatocytes across the immunological barrier at stage VIII of the epithelial cycle. These findings are also consistent with an earlier report in which overexpression of plastin 2 (L-plastin) in a human colon adenocarcinoma cell line SW480 was found to cause a loss of E-cadherin expression, thereby perturbing its cell adhesive function, inducing proliferation and invasiveness,19 illustrating an increase in intrinsic actin bundling activity can offset the balance of F-actin plasticity by increasing the metastatic activity of these cancer cells. These findings are also supported by recent studies in mice when Arp3 was inactivated via specific deletion of N-WASP (neuronal Wiskott-Aldrich syndrome protein, an upstream activator of the Arp2/3 complex) in Sertoli cells, causing actin microfilaments fail to undergo unbundling and branching; and these mice were found to be infertile due to failure in spermiogenesis.20 A more detailed analysis of these Sertoli cell-specific conditional N-WASP KO mice to inactivate the Arp2/3 complex have shown that the BTB integrity of these mice were disrupted, while meiosis was detected in some tubules, none of the round spermatids were found to proceed to spermiogenesis to form elongating spermatids21 due to defects in F-actin organization because of the lack of the Arp2/3 intrinsic activity to convert actin filament bundles into a unbundled/branched network to confer F-actin plasticity. As such, BTB-associated proteins, including TJ proteins occludin and CAR (coxsackievirus and adenovirus receptor), basal ES protein N-cadherin, and GJ protein connexin 43, failed to be recruited to the BTB to assemble a functional TJ-barrier.21 Taking these data collectively, coupled with findings from a recent report that illustrate a knockdown of plastin 3 in Sertoli cell epithelium in vitro with an established TJ-permeability barrier impedes Sertoli cell TJ-barrier function and also impairs the recruitment of adhesion proteins to the BTB,16 clearly support the concept that the correct balance between intrinsic actin bundling and unbundling activity conferred by ABPs including plastin 3, palladin, Eps8, Arp2/3 complex and others in Sertoli cells is necessary to support BTB function.
In this context, it is of interest to note that BTB remodeling that supports preleptotene spermatocyte transport is also conferred by microtubule (MT) dynamics in which polarized MTs constituted by polymerization of dimers of α- and ß-tubulins are known to play a crucial role even though the underlying molecular mechanism(s) are less known (for reviews, see 22-24). However, studies have shown that the Sertoli cell-specific AKAP9 (A-kinase anchor protein 9 is a member of the AKAP family known to bind to the regulatory subunit of protein kinase A to modulate MT dynamics (for reviews, see 25,26) KO mice are infertile due to meiotic arrest.27,28 Even though round spermatids were found in a few tubules, none was capable of entering spermiogenesis to develop into elongating spermatids.28 Furthermore, the BTB of these mice is disrupted even though F-actin apparently remains relatively intact.28 Studies using these genetic models thus confirm the notion that the BTB is structurally and functionally supported by F-actin- and MT-based cytoskeletons. Thus, it will be of interest in future studies to assess if a disruption of plastin 3-conferred intrinsic actin bundling activity would perturb MT organization since a recent report has shown that a knockdown of EB1 (end binding protein 1, a MT plus (+) end binding protein, +TIP, known to stabilize MT) that perturbs MT organization also disrupts actin microfilament organization,29 illustrating the actin- and MT-based cytoskeletons are functionally integrated. Furthermore, it is noted herein that overexpression of plastin 3 that impedes the Sertoli cell TJ-barrier function through disruptive changes in actin microfilament organization in Sertoli cells are mediated by changes in the spatial expression of Arp3 and Eps8. These findings also illustrate these ABPs are tightly networked and are working in concert to modulate F-actin organization, likely through a signaling pathway, which may involve p-FAK-Tyr407 since this activated FAK isoform is known to modulate F-actin polymerization by regulating the spatiotemporal activation of Arp2/3 complex via its activation by N-WASP.30
In summary, we have demonstrated unequivocally that a delicate homeostatic level of plastin 3 is necessary to be maintained at the Sertoli cell BTB microenvironment to generate the necessary plasticity to F-actin network to confer proper function of the immunological barrier.
Materials and methods
Animals and antibodies
The use of rats for different experiments reported herein was approved by the Rockefeller University Institutional Animal Care and Use Committee (IACUC) with Protocol Numbers 12–506 and 15-780H. Sprague-Dawley rats were purchased from Charles River Laboratories (Kingston, NY) and housed at the Rockefeller University Comparative Bioscience Center (CBC). Every 10 pups were housed with a foster mother. Rats were euthanized by CO2 asphyxiation using slow (20%–30%/min) displacement of chamber air from compressed CO2 with a built-in regulator approved by the Rockefeller University Lab Safety and CBC. Antibodies that used in this report were obtained from different vendors and listed in Table 1.
Primary Sertoli cell cultures
Sertoli cells were isolated from testes of 20-day-old pups and cultured in serum-free F12/DMEM medium (Sigma-Aldrich, St. Louis, MO), supplemented with growth factors and gentamicin at 35°C with 95% air-5% CO2 (vol/vol) in a humidified CO2 incubator as described.31 Freshly isolated Sertoli cells were plated on Matrigel (BD Biosciences, Billerica, MA, diluted 1:7 with F12/DMEM medium)-coated: (i) coverslips at 0.04 to 0.08 × 106 cell/cm2 which were then placed in 12-well dishes containing 2 ml F12/DMEM to be used for immunofluorescence staining; (ii) 6-well dishes at 0.4×106 cell/cm2 containing 5 ml F12/DMEM per well to be used for lysate preparation for immunoblotting or for overexpression experiments. About 36 hr thereafter, cultures were subjected to a brief hypotonic treatment using 20 mM Tris, pH 7.4, at 22°C for 2.5 min as described32 to lyse residual germ cells. Sertoli cells were then rinsed twice before cultured in F12/DMEM containing growth factors and other supplements31, and the purity of Sertoli cells used in our studies was >98% with negligible contaminations of peritubular myoid cells, Leydig cells and germ cells using specific markers for these cells by immunoblotting or RT-PCR as described.33
Cloning of plastin 3 into pCI-neo mammalian expression vector
The ORF (open reading frame) of the full-length plastin 3 cDNA clone was amplified from rat Sertoli cell cDNAs reversed transcribed from total RNAs using AccuPrime Pfx DNA polymerase (Invitrogen, Carlsbad, CA), and was cloned into the pCI-neo mammalian expression vector (Promega, Madison, WI) by PCR using the primer pair listed in Table 2, including the corresponding Mul I and Sal I restriction enzyme recognition sites as described.30,34 This cDNA clone of 1893 bp with start and stop codons was obtained using Sertoli cells total cDNAs, and its identity was confirmed by direct nucleotide sequence analysis at Genewiz, which was identical to GenBank Accession Number: NM_031084.1 for plastin 3 except that nucleotides at 622–624 GGG encoding Gly was GGA (also encoding Gly) in this rat testis clone. The authenticity of this cDNA clone was confirmed in 4 separate cloning and sequencing experiments. pCI-neo plasmid DNA (empty vector) and pCI-neo/Plastin 3 plasmid DNA was purified using the EndoFree Plasmid Mega Kit (Qiagen, Germantown, MD) to remove possible contamination of endotoxins prior to their use for transfection to overexpress plastin 3 in Sertoli cells.
Table 2.
Primers used for cloning of the full-length rat plastin 3 cDNA.
| Target gene | GenBank accession number | Primer sequence (5′−3′) | Nucleotide position | Expected size in b.p. |
|---|---|---|---|---|
| Plastin 3 | NM_031084.1 | Sense: acACGCGTATGGACGAGATGGCAACCAC | 85–104 | 1893 |
| Antisense: acGTCGACTCACACTCTCTTCATCCCTCTGC | 1955–1977 |
Note. The bold letter indicated that the restriction enzyme recognition sites of Mul I and Sal I, respectively, were added into the full-length plastin 3 cDNA clone.
Overexpression of plastin 3 in Sertoli cells cultured in vitro
Sertoli cells were transfected with pCI-neo empty vector vs. pCI-neo containing plastin 3 full-length clone (pCI-neo/Plastin 3) using Effectene Transfection Reagent (Qiagen) for 6 hr on day 2. In studies to be used for immunoblotting at 0.4 × 106 cells/cm2, cells in each well of a 6-well dish were transfected with 1.6 μg plasmid DNA with 200 μl EC (Enhancer and DNA condensation) buffer, 12.8 μl Enhancer, 24 μl Effectene in 2 ml F12/DMEM with supplements; for immunofluorescence staining (0.04–0.08 × 106 cells/cm2 on coverslips which were placed in 12-well dishes) and TJ-permeability barrier assay (1.2 × 106 cells/cm2 on bicameral units which were placed in 24-well dishes), Sertoli cells were transfected with 0.2 μg plasmid DNA with 25 μl EC buffer, 1.6 μl Enhancer, 3 μl Effectene in 0.5 ml F12/DMEM with supplements. For immunofluorecence staining, plasmid DNA was labeled with Cy3 by using a Label IT Tracker Intracellular Nucleic Acid Localization Kit (Mirus) to monitor successful transfection.
Assessment of Sertoli cell TJ-permeability barrier function in vitro
Sertoli cells at 1.2 × 106 cells/cm2 were plated on Matrigel (1:5 freshly diluted with F12/DMEM)-coated Millicell HA (mixed cellulose esters) cell culture inserts (diameter, 12 mm; pore size, 0.45 μm, effective surface area, ∼0.6 cm2; EMD Millipore, Billerica, MA). Bicameral units were then placed in 24-well dishes with each insert contained 0.5 ml F12/DMEM in the apical and basal compartments, respectively. Sertoli cell TJ-permeability barrier was quantified daily by measuring transepithelial electrical resistance (TER) across the cell epithelium in cultures transfected with pCI-neo/Plastin 3 vs. pCI-neo empty vector as described.31 Each treatment group had quadruplicate inserts and each experiment was repeated at least 3 times using different batches of Sertoli cells that yielded similar results.
Actin bundling assay
Actin bundling assay was prepared as detailed elsewhere.29,35 In brief, F-actin was obtained from G-actin using an Actin Binding Protein Biochem Kit (Cytoskeleton, Cat #: BK013) according to the manufacturer's protocol. These actin microfilaments served as the substrate to assess the ability of Sertoli cell lysates to induce actin microfilament bundling. Cell lysates were obtained from Sertoli cells previously transfected with pCI-neo/plastin 3 vs. pCI-neo empty vector plasmid DNA using 100 μl Tris lysis buffer [20 mM Tris, pH 7.5 at 22°C, containing 20 mM NaCl, 0.5% Triton X-100 (vol/vol), freshly supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich) at a 1:100 dilution (vol/vol)] and cellular debris was removed by centrifugation at 20,800 g at 4°C for 1 hr. 10 μl of cell lysates containing ∼20–30 µg protein from both groups (clear supernatant from the above step) containing equal amount of protein (based on estimation using a BioRad DC Protein Assay kit with BSA served as a standard) vs. 10 μl Tris lysis buffer (served as a negative control) were then added into 40 μl of freshly prepared F-actin obtained above. This mixture was incubated for 1 hr at room temperature to allow actin bundling, and then centrifuged at 14,000 g for 5 min at 24°C to pellet bundled F-actin, whereas unbundled actin microfilaments remained in the supernatant. The whole pellet and 5 μl supernatant of each sample from both groups vs. negative control were analyzed by immunoblotting. Cell lysate (∼20 µg protein) from each sample was also analyzed by immunoblotting to serve as a protein loading control. This experiment was repeated 3 times using different cell preparations.
Immunoblotting and immunofluorescence analysis
Sertoli cell lysates were obtained by using IP lysis buffer [10 mM Tris, pH 7.4, at 22°C, containing 0.15 M NaCl, 1% NP-40 (vol/vol), and 10% glycerol (vol/vol)] freshly supplemented with protease and phosphatase inhibitor cock-tails (Sigma-Aldrich) at a 1:100 dilution (vol/vol) as described.30,36 About 20 μg protein was subjected to SDS-PAGE, and immunoblotting was performed as described 30,36 using corresponding specific antibodies as listed in Table 1, and chemiluminescence was performed using kits prepared in-house as detailed elsewhere.37 Cell immunofluorescence analysis was performed as earlier described.30,36 In brief, cells were fixated by 4% (wt/vol) paraformaldehyde (PFA) or cold methanol and permeabilized by 0.1% (vol/vol) Triton X-100, blocked with 4% (wt/vol) BSA in PBS, thereafter cells were incubated with corresponding primary antibodies listed in Table 1 at 4˚C overnight, to be followed by visualizing with Alexa Fluor-conjugated secondary antibodies (Alexa Fluor 488 for green fluorescence, Invitrogen) at a dilution of 1:250. For F-actin staining, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma-Aldrich) at a dilution of 1:50.38 Cells were then mounted in Prolong Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI, a blue fluorescence nuclei stain, Invitrogen). Fluorescence images were acquired using an Olympus BX61 fluorescence microscope with a built-in Olympus DP70 12.5-MPx digital camera and analyzed using the Olympus MicroSuite Five software (Version 1.224, Olympus Soft Imaging Solution Corp, Tokyo, Japan). Adobe Photoshop in Adobe Creative Suite (Version 3.0, San Jose, CA) was used for image overlay.
Image analysis
For Sertoli cells following overexpression of plastin 3, at least 200 cells were randomly selected and examined in experimental vs. control groups with n = 3 experiments. Fluorescence intensity of a target protein in Sertoli cells was quantified using Image J (Version 1.45, US. National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij).
Statistical analysis
For studies using Sertoli cell cultures, triplicate coverslips and dishes were used, whereas quadruplicate bicameral units were used to assess the Sertoli cell TJ-permeability barrier function. Each data point (or bar graph) is a mean±SD of n = 3 to 5 experiments (or n = 3 rats). For each experiment, data in treatment groups were normalized against the corresponding control, which was arbitrarily set at 1. Statistical analysis was performed using the GB-STAT software package (Version 7.0; Dynamic Microsystems, Silver Spring, MD). Statistical analysis was performed by 2-way analysis of variance (ANOVA) followed by Dunnett's test. In selected experiments, Student's t-test was used for paired comparisons.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National Institutes of Health (NICHD R01 HD056034 to C.Y.C; U54 HD029990, Project 5, to C.Y.C.); National Natural Science Foundation of China (NSFC)/Hong Kong Research Grants Council (RGC) Joint Research Scheme Grant N_HKU 717/12 (to W.M.L.), Hong Kong Research Grants Council General Research Fund Grant 771513 (to W.M.L.) and The Committee for Research and Conference Grants (CRCG) of the University of Hong Kong Seed Funding Grant (to W.M.L.).
References
- [1].Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev 2012; 64:16-64; PMID:22039149; http://dx.doi.org/ 10.1124/pr.110.002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol 2012; 763:237-59; PMID:23397628 [PubMed] [Google Scholar]
- [3].Toyama Y, Ohkawa M, Oku R, Maekawa M, Yuasa S. Neonatally administered diethylstilbestrol retards the development of the blood-testis barrier in the rat. J Androl 2001; 22:413-23; PMID:11330641 [PubMed] [Google Scholar]
- [4].Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod 1993; 49:840-9; PMID:8218650; http://dx.doi.org/ 10.1095/biolreprod49.4.840 [DOI] [PubMed] [Google Scholar]
- [5].Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol 1970; 47:81-6; PMID:5428920; http://dx.doi.org/ 10.1677/joe.0.0470081 [DOI] [PubMed] [Google Scholar]
- [6].Wiebe J, Kowalik A, Gallardi R, Egeler O, Clubb B. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl 2000; 21:625-35; PMID:10975408 [PubMed] [Google Scholar]
- [7].Wiebe J, Barr K. Suppression of spermatogenesis without inhibition of steroidogenesis by 1,2,3-trihydroxypropane solution. Life Sci 1984; 34:1747-54; PMID:6427545; http://dx.doi.org/ 10.1016/0024-3205(84)90574-5 [DOI] [PubMed] [Google Scholar]
- [8].Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl 2012; 35:86-101; PMID:21696392; http://dx.doi.org/ 10.1111/j.1365-2605.2011.01183.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem 2011; 46:49-127; PMID:21705043; http://dx.doi.org/ 10.1016/j.proghi.2011.05.001 [DOI] [PubMed] [Google Scholar]
- [10].Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 2002; 82:825-74; PMID:12270945; http://dx.doi.org/ 10.1152/physrev.00009.2002 [DOI] [PubMed] [Google Scholar]
- [11].Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol 2008; 636:186-211; PMID:19856169; http://dx.doi.org/ 10.1007/978-0-387-09597-4_11 [DOI] [PubMed] [Google Scholar]
- [12].Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985; 94:177-211; PMID:3894273; http://dx.doi.org/ 10.1016/S0074-7696(08)60397-6 [DOI] [PubMed] [Google Scholar]
- [13].Delanote V, Vandekerckhove J, Gettemans J. Plastins: versatile modulators of actin organization in (patho)physiological cellular processes. Acta Pharmacol Sin 2005; 26:769-79; PMID:15960882; http://dx.doi.org/ 10.1111/j.1745-7254.2005.00145.x [DOI] [PubMed] [Google Scholar]
- [14].Li N, Wong CK, Cheng CY. Plastins regulate ectoplasmic specialization via its actin bundling activity on microfilaments in the rat testis. Asian J Androl 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: Regulation of cytoskeletal microfilaments. Physiol Rev 2003; 83:433-73; PMID:12663865; http://dx.doi.org/ 10.1152/physrev.00026.2002 [DOI] [PubMed] [Google Scholar]
- [16].Li N, Mruk DD, Wong CK, Lee WM, Han D, Cheng CY. Actin-bundling protein plastin 3 is a regulator of ectoplasmic specialization dynamics during spermatogenesis in the rat testis. FASEB J 2015; 29:3788-805; PMID:26048141; http://dx.doi.org/ 10.1096/fj.14-267997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Qian X, Mruk DD, Cheng YH, Tang EI, Han D, Lee WM, Wong EW, Cheng CY. Actin binding proteins, spermatid transport and spermiation. Semin Cell Dev Biol 2014; 30:75-85; PMID:24735648; http://dx.doi.org/ 10.1016/j.semcdb.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cheng CY, Mruk DD. Actin binding proteins and spermiogenesis. Some unexpected findings. Spermatogenesis 2011; 1:99-104; PMID:22319657; http://dx.doi.org/ 10.4161/spmg.1.2.16913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Foran E, McWilliam P, Kelleher D, Croke DT, Long A. The leukocyte protein L-plastin induces proliferation, invasion and loss of E-cadherin expression in colon cancer cells. Int J Cancer 2006; 118:2098-104; PMID:16287074; http://dx.doi.org/ 10.1002/ijc.21593 [DOI] [PubMed] [Google Scholar]
- [20].Rotkopf S, Hamberg Y, Aigaki T, Snapper SB, Shilo BZ, Shejter ED. The WASp-based actin polymerization machinery is required in somatic support cells for spermatid maturation and release. Development 2011; 138:2729-39; PMID:21652648; http://dx.doi.org/ 10.1242/dev.059865 [DOI] [PubMed] [Google Scholar]
- [21].Xiao X, Mruk DD, Tang EI, Massarwa R, Mok KW, Li N, Wong CK, Lee WM, Snapper SB, Shilo BZ, et al.. N-WASP is required for structural integrity of the blood-testis barrier. PLoS genetics 2014; 10:e1004447; PMID:24967734; http://dx.doi.org/ 10.1371/journal.pgen.1004447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].O'Donnell L, O'Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol 2014; 30:45-54; PMID:24440897; http://dx.doi.org/ 10.1016/j.semcdb.2014.01.003 [DOI] [PubMed] [Google Scholar]
- [23].Tang EI, Mruk DD, Cheng CY. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Sem Cell Dev Biol (in press) 2016; PMID:26791048; http://dx.doi.org/11282019 10.1016/j.semcdb.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tang EI, Mruk DD, Lee WM, Cheng CY. Cell-cell interactions, cell polarity, and the blood-testis barrier In: Cell Polarity 1, 2015; Ed Ebnet K; Geneva, Switzerland: Springer International Publishing; pp 303-326; http://dx.doi.org/101007/978-3-319-14463-4_13. [Google Scholar]
- [25].Diviani D, Scott JD. AKAP signaling complexes at the cytoskeleton. J Cell Sci 2001; 114:1431-7; PMID:11282019 [DOI] [PubMed] [Google Scholar]
- [26].Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol 2004; 5:959-70; PMID:15573134; http://dx.doi.org/ 10.1038/nrm1527 [DOI] [PubMed] [Google Scholar]
- [27].Schimenti KJ, Feuer SK, Griffin LB, Graham NR, Bovet CA, Hartford S, Pendola J, Lessard C, Schimenti JC, et al.. AKAP9 is essential for spermatogenesis and Sertoli cell maturation in mice. Genetics 2013; 194:447-57; PMID:23608191; http://dx.doi.org/ 10.1534/genetics.113.150789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Venkatesh D, Mruk D, Herter JM, Cullere X, Chojnacka K, Cheng CY, Mayadas TN. AKAP9, a regulator of microtubule dynamics, contributes to blood-testis barrier function. Am J Pathol 2015, 186:270-84; PMID:26687990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the Sertoli cell blood-testis barrier in male rats - an in vitro study. Endocrinology 2015; 156:680-93; PMID:25456071; http://dx.doi.org/ 10.1210/en.2014-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA 2012; 109:12562-7; PMID:22797892; http://dx.doi.org/ 10.1073/pnas.1207606109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol 2011; 763:237-52; PMID:21874456; http://dx.doi.org/ 10.1007/978-1-61779-191-8_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl 1981; 2:249-54; http://dx.doi.org/ 10.1002/j.1939-4640.1981.tb00625.x [DOI] [Google Scholar]
- [33].Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl 2004; 25:200-15; PMID:14760006; http://dx.doi.org/ 10.1002/j.1939-4640.2004.tb02780.x [DOI] [PubMed] [Google Scholar]
- [34].Li N, Mruk DD, Mok KW, Li MWM, Wong CKC, Lee WM, Han D, Silvestrini B, Cheng CY.. Connexin 43 reboots meiosis and reseals blood-testis barrier following toxicant-mediated aspermatogenesis and barrier disruption. FASEB J 2016; 30:1436-52; PMID:26678449; http://dx.doi.org/101096/fj.15-276527.25714812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mok KW, Chen H, Lee WM, Cheng CY. rpS6 regulates blood-testis barrier dynamics through Arp3-mediated actin microfilament organization in rat Sertoli cells. An in vitro study. Endocrinology 2015; 156:1900-13; PMID:25714812; http://dx.doi.org/ 10.1210/en.2014-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wan HT, Mruk DD, Wong CKC, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr407 - an in vitro study. Endocrinology 2014; 155:249-62; PMID:24169556; http://dx.doi.org/ 10.1210/en.2013-1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting. An inexpensive alternative to commercially available kits. Spermatogenesis 2011; 1:121-2; PMID:22319660; http://dx.doi.org/ 10.4161/spmg.1.2.16606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol 2011; 43:651-65; PMID:21256972; http://dx.doi.org/ 10.1016/j.biocel.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


