Abstract
Introduction
Age itself is not considered a contraindication for high impact surgery. However, the aging process of the liver remains largely unknown. This study evaluates age-dependent changes in liver function using a quantitative liver function test.
Methods
Between January 2005 and December 2014, 508 patients underwent 99mTc-mebrofenin hepatobiliary scintigraphy (HBS) for the assessment of liver function. These included 203 patients with healthy livers (group A) and 57 patients with HCC and Child-Pugh A (group B). 99mTc-mebrofenin-uptake-rate of the whole liver corrected for body surface area (cMUR) was calculated for all patients. Linear regression analysis was performed to assess the relationship between age and cMUR.
Results
The mean cMUR was 8.50 ± 2.05%/min/m2 and 6.94 ± 2.03%/min/m2 in group A and B, respectively. A negative linear correlation was found between patient's age and cMUR in group A, r = 0.244, p = 0.000. In group B, there was no correlation between age and cMUR, however, a trend in decline of liver function with age was noted.
Conclusion
This study shows that liver function deteriorates with age. Since the regenerative capacity of the liver correlates with liver function, this finding should be taken into account when assessing surgical risk in patients considered for major liver resection.
Introduction
In 2012, life expectancy in the Netherlands was 79.1 years for men and 82.8 years for women while the remaining life expectancy for elderly at the age of 65 was +18.3 years for men and +21.2 years for women.1 This corresponds with an increase in life expectancy of 12.6% for males and 14.1% for females in the time period 1950–2012.1 In accordance with this trend, along with expanded possibilities for liver resection, we observe increasing rates of liver resections performed in the elderly. During the last 5 years in our department, approximately 16% of major liver resections were undertaken in patients of ≥75 years old. This percentage increases to nearly 30% when the elderly are defined as patients aged 70 years or older, which is in line with rates reported in literature.2 The described trend underscores the importance of risk assessment associated with major liver resection (≥3 liver segments) in the elderly.
From the oncological point of view, the older could equally benefit from surgical treatment as the younger patients since comparable (disease-free) survival rates are described in patients with colorectal liver metastases (CRLM) and hepatocellular carcinoma (HCC).2, 3, 4, 5 Controversy, however, exists regarding postoperative morbidity and/or mortality in the older patient. Recent studies report that the elderly tolerate liver resection as well as younger patients6, 7, 8, 9 while at the same time, a significantly higher risk of postoperative morbidity and mortality after major liver resections has been reported in the elderly.2, 10 These outcomes often are explained by the increased incidence of comorbidities among aged patients, leading to higher peri- and postoperative risks.
Despite these contradictory reports, it is largely accepted that age itself should not be considered a contraindication provided that a strict preoperative protocol is followed for selection of patients eligible for major liver resection. As with all high impact surgery, screening for comorbidities and evaluation of patient's condition should be undertaken during preoperative work-up. In addition, disease-specific predictors for unfavorable postoperative outcome, e.g. tumor diameter, number and location of lesions, should also be weighed against future remnant liver (FRL) volume and function in the light of the individual risk of postoperative liver failure.
Postoperative liver failure is one of the most severe complications after major liver surgery11 and irrespective of tumor entity, is strongly associated with the extent of resection and underlying parenchymal disease. The occurrence of postoperative liver failure is mainly dictated by the functional capacity of the FRL.12 Accurate preoperative, quantitative assessment of liver function is therefore crucial to prevent this complication by modulating the FRL (Portal vein embolization, ALPPS).13 Increased rates of postoperative liver failure in aged patients after liver surgery have been reported.14 However, the aging process of the human liver and its functional consequences remain largely unknown.
99mTc-mebrofenin hepatobiliary scintigraphy (HBS) with SPECT-CT enables quantitative assessment of hepatic uptake function. HBS allows at the same time independent evaluation of total liver function and segmental liver function, i.e. the FRL-function,13 in both patients with diseased or normal liver parenchyma. This functional test has been used for preoperative selection of patients in the setting of major liver surgery and for monitoring of the hypertrophy response after preoperative portal vein embolization (PVE) or ALPPS.12, 15, 16
Apart from measuring base-line liver function, we hypothesize that a quantitative liver function test such as HBS, also indicates the regenerative capacity of the liver, in other words, when age influences the function of the liver, it will also influence the regenerative capacity of the liver remnant after resection. Knowledge of such age-dependent changes of the functional capacity of the liver therefore contributes to accurate preoperative risk assessment in a continuously aging patient population requiring major liver resections. The aim of this study was to evaluate age-dependent changes in total liver function in both patients with normal and affected liver parenchyma.
Methods
Patients
All adult patients with presumed healthy liver parenchyma (group A) or patients with pre-cirrhotic liver parenchyma (group B) who underwent 99mTc-mebrofenin hepatobiliary scintigraphy (HBS) between January 2005 and December 2014 for the assessment of hepatic uptake function were included. Patients diagnosed with hepatic metastases irrespective of origin, or benign tumors were considered to have healthy liver parenchyma. Patients with hepatocellular carcinoma (HCC) and who were classified as Child-Pugh A were considered as pre-cirrhotic.
Patients who were cholestatic or could have been cholestatic prior to HBS were excluded as hyperbilirubinemia may affect HBS results. For this reason, all patients diagnosed with perihilar cholangiocarcinoma or intrahepatic cholangiocarcinoma were excluded. Furthermore, all patients with parenchymal diseases other than those classified as Child-Pugh A were excluded.
Assessment of liver function using HBS
HBS was performed after 4 h fast to standardize the measurements. Patients were positioned supine on the imaging table of a large-field-of-view (FOV) SPECT/CT camera (Symbia T16; Siemens) positioned over the liver and heart region. After intravenous administration of 200 MBq freshly prepared 99mTc-mebrofenin (Bridatec; GE-Amersham Health), dynamic acquisition was obtained (36 frames of 10 s/frame, 128 matrix), which was used for calculation of the hepatic mebrofenin uptake rate (MUR). Data were processed on a Hermes workstation (Hermes Medical Solutions, Sweden).
The following parameters were studied: MUR and MUR corrected for body surface area (cMUR).17, 18, 19 For the calculation of body surface area the formula by Mosteller and colleagues was used. MUR is expressed as %/min while cMUR is expressed as %/min/m2.
Data collection
Patient characteristics were collected form the digital patient records. Diagnosis and status of the liver parenchyma were extracted from the histology reports. cMUR was extracted from nuclear medicine reports.
Study endpoints
The primary endpoint of this study was age-dependent changes in total cMUR defined as correlation between cMUR and age.
The secondary endpoint of this study was differences in the age-dependent changes in total cMUR between patients with presumed healthy liver parenchyma, i.e. group A, and pre-cirrhotic patients, i.e. group B.
Statistical analysis
Statistical analysis was performed with Statistical Package of Social Sciences (SPSS 22.0, IBM Inc., Armonk (NY) USA). Normally distributed continuous data are expressed as mean ± standard deviation (SD), while non-normally distributed data are presented as median along with interquartile range (IQR). Correlation between age and cMUR was studied using linear regression. Continuous data were compared using independent T test or One-way ANOVA test. All statistical tests were two tailed and differences were considered significant at a p-value of <0.05.
Results
Patient characteristics
During the study period, 508 patients underwent HBS for the assessment of hepatic uptake function, 203 of these (40.0%) were included in group A. The majority of these patients, i.e.146 (71.9%), were diagnosed with hepatic metastases of which 129 (63.5%) patients with CRLM and 17 (8.4%) patients with metastases from other origin. Fifty-seven (11.2%) patients were diagnosed with HCC in association with Child-Pugh A score, and were included in group B. Baseline characteristics of the patients included in group A and B are summarized in Table 1.
Table 1.
Baseline characteristics of patients with healthy liver parenchyma and patients with cirrhosis/grade Child-Pugh A who underwent HBS for the evaluation of liver function
| Healthy liver, n = 203 | HCC, n = 57 | p | |
|---|---|---|---|
| Age, years [IQR] | 60.0 [49.0–68.0] | 64.0 [57.0–73.0] | 0.062 |
| Age category, n [%] | |||
| [18–39] | 23 [11.3] | – | |
| [40–49] | 28 [13.8] | 3 [5.3] | |
| [50–59] | 48 [23.6] | 15 [26.3] | |
| [60–69] | 66 [32.5] | 18 [31.6] | |
| [70–79] | 38 [18.7] | 21 [36.8] | |
| Male, n [%] | 99 [48.5] | 44 [77.2] | <0.001 |
| Length, cm [IQR] | 175.0 [166.0–176.0] | 172.0 [168.5–176.5] | 0.593 |
| Weight, kg [IQR] | 75.0 [70.0–84.0] | 75.0 [68.0–90.0] | 0.577 |
| BSA, m2[IQR] | 1.91 [1.78–2.03] | 1.91 [1.81–2.1] | 0.697 |
| Diagnosis, n [%] | |||
| CRLM | 129 [63.5] | – | – |
| Other metastases | 17 [8.4] | – | |
| Other malignant tumor | 11 [5.4] | – | |
| Benign lesion | 46 [22.7] | – | |
| HCC | – | 57 [100] | |
HCC, hepatocellular carcinoma; CRLM, colorectal liver metastasis; IQR, interquartile range; BSA, body surface area according to Mesteller's formula
Age dependent changes in total liver function
Group A
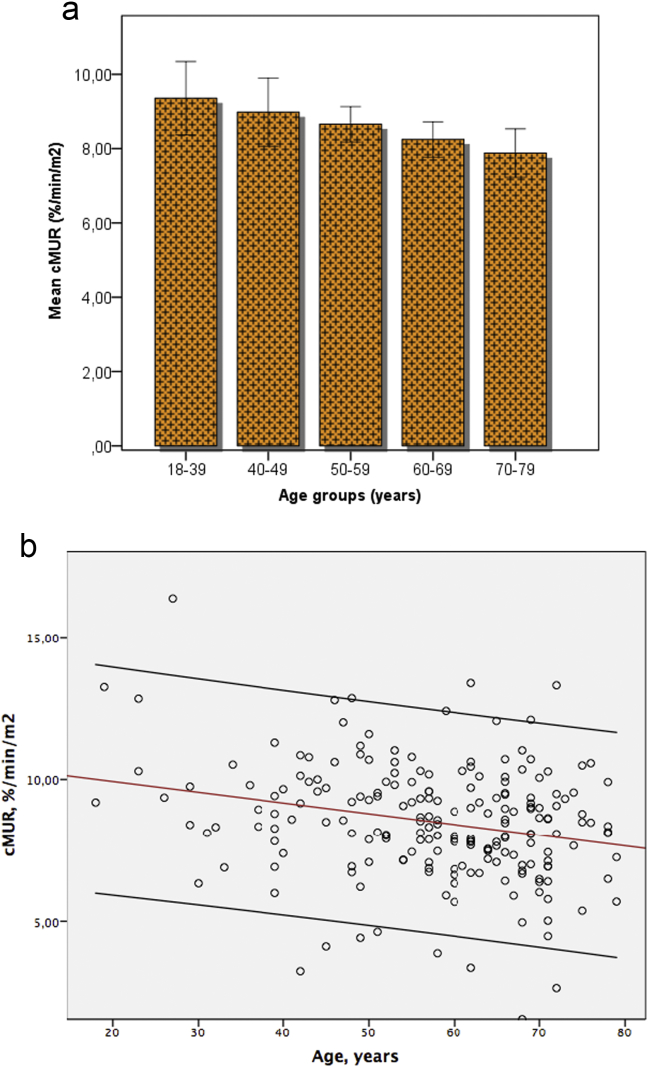
The mean cMUR of all 203 patients included in group A was 8.50 ± 2.05%/min/m2. According to Pearson's correlation coefficient there is a significant negative linear correlation between cMUR and patients' age (r = 0.244, p = 0.000), see Fig. 1. The decline in total cMUR of an adult patient of ≥18 years old can be calculated using the following formula:
| Total cMUR = 10.69%/min/m2 (0.038 × age) |
Figure 1.
a Patients with normal liver parenchyma (group A) divided in five age groups. The mean liver function corrected for body surface area (cMUR) declines significantly with age, with the differences being most pronounced between the very old and very young patients. b Scatter plot illustrating the linear correlation between patient's age and cMUR
The relationship between age and cMUR was further studied by dividing patients in five different age groups, i.e. [18–39], [40–49], [50–59], [60–69], and [70–79] years. Patients up to the age of 39 years were pooled to ensure comparability of the sample size. The remaining patients were divided in groups with time intervals of 10 years. The mean cMUR in the five age groups was 9.36 ± 2.37%/min/m2, 8.98 ± 2.43%/min/m2, 8.66 ± 1.65%/min/m2, 8.25 ± 1.93%/min/m2 and 7.88 ± 2.01%/min/m2, respectively. Comparison of the mean cMUR between the age groups revealed significant differences (p = 0.033) with most pronounced differences between the youngest and oldest patients, i.e. differences in mean cMUR reached significance when comparing the youngest [18–39] age group with the older age groups [60–69] and [70–79] years (Fig. 1). The differences however are not significant when comparing the [40–49] and [50–59] age groups with the [18–39] group.
Group B
The mean cMUR of the 57 patients included in group B was 7.11 ± 2.03%/min/m2. In contrast to patients in group A, no linear relation was found between age and total cMUR among patients in group B.
None of the patients in group B was under the age of 39 years and only 3 patients were younger than 49 years. For this reason, the mean cMUR could not be calculated in the age groups [18–39] and [40–49] among patients with HCC and Child-Pugh A. For the patients in age groups [50–59] and [60–69], the mean cMUR was 6.96 ± 1.44%/min/m2 and 6.80 ± 1.83%/min/m2, respectively. The cMUR in the oldest [70–79] age group was relatively high, i.e. 7.72 ± 2.21%/min/m2. There were no differences in the WHO performance status or the Charlson comorbidity index between the three age groups. Significantly less chronic liver disease defined as mild cirrhotic changes or chronic hepatitis B and/or C infection was found in the oldest [70–79] age group; n = 13, n = 11 and n = 2 for the age groups [50–59], [60–69] and [70–79], respectively (p < 0.001).
Influence of age on different type of liver parenchyma
The cMUR was compared in patients with healthy liver parenchyma in group A and the pre-cirrhotic livers in group B to assess the impact of age on liver parenchyma of different quality. This comparison was performed for patients from the age groups [50–59], [60–69] and [70–79], respectively, considering the lack of younger patients in group B as mentioned above.
There were no unexpected differences in the main baseline characteristics between patients in group A and group B. There were significantly more male patients in group B, which is in accordance with epidemiological data on HCC (Table 1). The mean cMUR was significantly higher in group A as compared to group B in the age groups [50–59] and [60–69], p < 0.001 and p = 0.006, respectively. There were however no differences between mean cMUR in patients aged [70–79] years of group A and B, p = 0.464.
Discussion
The consequences of aging on liver function are largely unknown. Except for animal studies, the greater part of our clinical knowledge regarding this topic derives from transplantation surgery. Due to the shortage of donor organs, donor livers of older patients are often accepted for transplantation purposes. Comparable outcomes of liver grafts from older donors have been described in terms of post-transplant liver graft quality, post-transplant rejection episodes and survival.20 However, we need to be aware of possible difficulties in translating these results to the field of major liver resection as in the latter, a substantial part of total liver mass is removed.
At the same time, available evidence shows alternation of liver processes with age. Post-transplantation deterioration of conventional liver function tests and regeneration have been described in humans after partial liver graft transplantation21 and in animals after resection.22 Abnormalities in protein synthesis21, 23 and prolonged intrahepatic cholestasis24, 25 after transplantation have been reported as well, all of which lead to worse long-term outcomes in older patients.
Two additional remarks can be made regarding the available evidence. Firstly, clinical studies reporting on the influence of age on liver function use empirically determined and not validated cut-off values for age to discriminate the elderly from the young patients. This creates a practical difficulty in interpretation of the results as the age at which patients are considered to be elderly varies. In this study we have shown that the impact of age on liver function is continuous and progressive. The decline of liver function becomes more pronounced as the patient ages. Secondly, the parameters that are frequently used to assess liver function such as bilirubin, transaminases, alkaline phosphatase and gamma GT, do not represent real liver function: These conventional blood tests termed ‘liver function tests’ represent the (side) products of the metabolic processes performed by the liver rather than liver function per se, and therefore are surrogate markers of liver function or damage.12 In the present study we used a quantitative dynamic liver function test which enables assessment of one of the key processes performed by the liver itself, i.e. the uptake function of the hepatocytes, instead of measuring a substitute or surrogate parameter.12, 13
We were not able to show the same linear decrease of liver function with age in patients with potentially compromised liver parenchyma. A trend in decline of liver function was however seen, but this did not reach statistical significance. This may have been caused by the surprisingly good liver function found among the [70–79] years old patients in the HCC group. None of the 57 patients in the HCC group had clinical evidence of cirrhosis and all of them complied with Child-Pugh A score. However, the mean cMUR in the [70–79] patients exceeded the mean cMUR of younger patients in the HCC group and was comparable with the cMUR of the [70–79] years old patients with healthy liver parenchyma of group A. A plausible explanation lies in the preoperative selection of patients diagnosed with HCC in the [70–79] years old patients. Only those in excellent condition were considered candidates for surgery and underwent assessment of liver function using HBS. This notion is corroborated by the finding that the oldest HCC patients were least affected by chronic liver disease, with less patients with fibrotic changes and/or chronic hepatitis B and/or C, while their physical performance status and comorbidity index did not differ from the younger HCC patients. This explains the relatively higher cMUR in the [70–79] group with HCC.
In this study patients diagnosed with hepatic metastases were included in the group of patients considered to have normal liver parenchyma. Although in these patients the liver usually is not compromised, liver function may be temporarily reduced by previous chemotherapy. The degree of this impairment of liver function and the time the liver needs to recover after the last administration of chemotherapy are largely uncertain and have not been substantiated using functional methods. We have therefore, not included this data in our analysis which is a limitation of this study.
The main question is how age influences the capacity of the liver to up-regulate its function and to regenerate when necessary, i.e. when the liver is damaged or partially resected. Although largely unknown in humans, experimental studies in rats and mice have shown impaired proliferation of hepatocytes in older animals.26, 27 Both the ability to upregulate function and to respond with regeneration likely depend on base-line functional status of the remaining hepatocytes. Results of this study show that liver function does decline with age, however, the decision to perform a large resection in the elderly depends on how much function will remain in the FRL. Using CT-volumetry only, calculation of the volume of the FRL may lead to overestimation of the functional capacity of the FRL. The advantage of HBS as a quantitative liver function test is that it provides a calculated cut-off value for sufficient function of the fraction of FRL, individualized for each patient based on body surface area, independently of pre-existing parenchymal disease. Thus, an old patient may have an overall decreased liver function which may further be diminished when there is additional parenchymal disease, but in the end, the function measured in the FRL determines whether the FRL will provide sufficient function to support the postoperative metabolic needs of the patient. Preoperative evaluation of patients requiring major liver resection should preferably be performed using functional liver function tests.
In conclusion, we have shown that hepatic uptake function of the liver is negatively correlated with patients' age in a series of patients with normal liver parenchyma while a trend is apparent among patients with HCC and Child-Pugh A score. Since the regenerative capacity of the liver correlates with liver function, these age-dependent changes in liver function should be taken into account when assessing surgical risk in patients considered for resection, especially when volumetric measurements are used for preoperative assessment.
Funding sources
None of the authors has received funding related to this subject from the following organizations: National Institutes of Health; Wellcome Trust; Howard Hughes Medical Institute (HHMI); and other(s).
Conflict of interest
None declared.
Footnotes
This study was presented at the 12th Congress of the IHPBA in Brazil, in the “Best of the Best” plenary session on April 23rd, 2016.
Contributor Information
Kasia P. Cieslak, Email: k.p.cieslak@amc.uva.nl.
Thomas M. van Gulik, Email: t.m.vangulik@amc.uva.nl.
References
- 1.Poos MJJC. Wat is in Nederland de levensverwachting? Volksgezondheid Toekomst Verkenning, Nationaal Kompas Volksgezondheid. Bilthoven: RIVM. 17-3-2014 BC. Available at: http://www.nationaalkompas.nl. (last accessed 24 December 2015).
- 2.Adam R., Frilling A., Elias D., Laurent C., Ramos E., Capussotti L. Liver resection of colorectal metastases in elderly patients. Br J Surg. 2010;97:366–376. doi: 10.1002/bjs.6889. [DOI] [PubMed] [Google Scholar]
- 3.Ferrero A., Vigano L., Polastri R., Ribero D., Lo T.R., Muratore A. Hepatectomy as treatment of choice for hepatocellular carcinoma in elderly cirrhotic patients. World J Surg. 2005;29:1101–1105. doi: 10.1007/s00268-005-7768-2. [DOI] [PubMed] [Google Scholar]
- 4.Huang J., Li B.K., Chen G.H., Li J.Q., Zhang Y.Q., Li G.H. Long-term outcomes and prognostic factors of elderly patients with hepatocellular carcinoma undergoing hepatectomy. J Gastrointest Surg. 2009;13:1627–1635. doi: 10.1007/s11605-009-0933-4. [DOI] [PubMed] [Google Scholar]
- 5.Zacharias T., Jaeck D., Oussoultzoglou E., Bachellier P., Weber J.C. First and repeat resection of colorectal liver metastases in elderly patients. Ann Surg. 2004;240:858–865. doi: 10.1097/01.sla.0000143272.52505.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cescon M., Grazi G.L., Del G.M., Ercolani G., Ravaioli M., Nardo B. Outcome of right hepatectomies in patients older than 70 years. Arch Surg. 2003;138:547–552. doi: 10.1001/archsurg.138.5.547. [DOI] [PubMed] [Google Scholar]
- 7.Ettorre G.M., Sommacale D., Farges O., Sauvanet A., Guevara O., Belghiti J. Postoperative liver function after elective right hepatectomy in elderly patients. Br J Surg. 2001;88:73–76. doi: 10.1046/j.1365-2168.2001.01629.x. [DOI] [PubMed] [Google Scholar]
- 8.Shirabe K., Kajiyama K., Harimoto N., Gion T., Tsujita E., Abe T. Early outcome following hepatic resection in patients older than 80 years of age. World J Surg. 2009;33:1927–1932. doi: 10.1007/s00268-009-0122-3. [DOI] [PubMed] [Google Scholar]
- 9.Menon K.V., Al-Mukhtar A., Aldouri A., Prasad R.K., Lodge P.A., Toogood G.J. Outcomes after major hepatectomy in elderly patients. J Am Coll Surg. 2006;203:677–683. doi: 10.1016/j.jamcollsurg.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Reddy S.K., Barbas A.S., Turley R.S., Gamblin T.C., Geller D.A., Marsh J.W. Major liver resection in elderly patients: a multi-institutional analysis. J Am Coll Surg. 2011;212:787–795. doi: 10.1016/j.jamcollsurg.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 11.van den Broek M.A., Olde Damink S.W., Dejong C.H., Lang H., Malago M., Jalan R. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767–780. doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 12.Cieslak K.P., Runge J.H., Heger M., Stoker J., Bennink R.J., van Gulik T.M. New perspectives in the assessment of future remnant liver. Dig Surg. 2014;31:255–268. doi: 10.1159/000364836. [DOI] [PubMed] [Google Scholar]
- 13.de Graaf W., van Lienden K.P., Dinant S., Roelofs J.J., Busch O.R., Gouma D.J. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010;14:369–378. doi: 10.1007/s11605-009-1085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiergens T.S., Stielow C., Schreiber S., Hornuss C., Jauch K.W., Rentsch M. Liver resection in the elderly: significance of comorbidities and blood loss. J Gastrointest Surg. 2014;18:1161–1170. doi: 10.1007/s11605-014-2516-2. [DOI] [PubMed] [Google Scholar]
- 15.de Graaf W., van Lienden K.P., van den Esschert J.W., Bennink R.J., van Gulik T.M. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg. 2011;98:825–834. doi: 10.1002/bjs.7456. [DOI] [PubMed] [Google Scholar]
- 16.Cieslak KP, Olthof PB, van Lienden KP, Besselink MG, Busch OR, van Gulik TM et al. Assessment of liver function using (99m)Tc-mebrofenin hepatobiliary scintigraphy in ALPPS (associating liver partition and portal vein ligation for staged hepatectomy). Case Rep Gastroenterol 9:353–360. [DOI] [PMC free article] [PubMed]
- 17.Bennink R.J., Dinant S., Erdogan D., Heijnen B.H., Straatsburg I.H., van Vliet A.K. Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J Nucl Med. 2004;45:965–971. [PubMed] [Google Scholar]
- 18.Ekman M., Fjalling M., Friman S., Carlson S., Volkmann R. Liver uptake function measured by IODIDA clearance rate in liver transplant patients and healthy volunteers. Nucl Med Commun. 1996;17:235–242. doi: 10.1097/00006231-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Erdogan D., Heijnen B.H., Bennink R.J., Kok M., Dinant S., Straatsburg I.H. Preoperative assessment of liver function: a comparison of 99mTc-mebrofenin scintigraphy with indocyanine green clearance test. Liver Int. 2004;24:117–123. doi: 10.1111/j.1478-3231.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- 20.Borchert D., Glanemann M., Mogl M., Langrehr J.M., Neuhaus P. Older liver graft transplantation, cholestasis and synthetic graft function. Transpl Int. 2005;18:709–715. doi: 10.1111/j.1432-2277.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 21.Ikegami T., Nishizaki T., Yanaga K., Shimada M., Kishikawa K., Nomoto K. The impact of donor age on living donor liver transplantation. Transplantation. 2000;70:1703–1707. doi: 10.1097/00007890-200012270-00007. [DOI] [PubMed] [Google Scholar]
- 22.Tsukamoto I., Nakata R., Kojo S. Effect of ageing on rat liver regeneration after partial hepatectomy. Biochem Mol Biol Int. 1993;30:773–778. [PubMed] [Google Scholar]
- 23.Anantharaju A., Feller A., Chedid A. Aging liver. A review. Gerontology. 2002;48:343–353. doi: 10.1159/000065506. [DOI] [PubMed] [Google Scholar]
- 24.Washburn W.K., Johnson L.B., Lewis W.D., Jenkins R.L. Graft function and outcome of older (>or = 60 years) donor livers. Transplantation. 1996;61:1062–1066. doi: 10.1097/00007890-199604150-00013. [DOI] [PubMed] [Google Scholar]
- 25.Yersiz H., Shaked A., Olthoff K., Imagawa D., Shackleton C., Martin P. Correlation between donor age and the pattern of liver graft recovery after transplantation. Transplantation. 1995;60:790–794. [PubMed] [Google Scholar]
- 26.Enkhbold C, Morine Y, Utsunomiya T, Imura S, Ikemoto T, Arakawa Y et al. Dysfunction of liver regeneration in aged liver after partial hepatectomy. J Gastroenterol Hepatol 30:1217–1224. [DOI] [PubMed]
- 27.Serra MP, Marongiu F, Marongiu M, Contini A, Laconi E. Cell-autonomous decrease in proliferative competitiveness of the aged hepatocyte. J Hepatol 62:1341–1348. [DOI] [PubMed]