Abstract
Objective
To investigate the long-term oncological outcome of patients with resectable hepatocellular carcinoma (HCC) undergoing sequential transarterial chemoembolization (TACE) and portal vein embolization (PVE).
Methods
Analysis of all Child A HCC patients who underwent TACE-PVE before major liver resection from 2006 to 2012 was performed according to whether or not they underwent surgical resection as planned.
Results
54 patients (50 men, 93% median 69-years (range 44–87)) were included. Thirty-nine (72%) patients underwent resection, including 19/25, 16/23, and 4/6 of patients with BCLC A, B, and C (p = 0.839). Twenty-two (56%) had tumor recurrence (median delay 10 months) including 9/19, 11/16, and 2/4 of the patients with BCLC A, B, and C (p = 0.430). Survival was significantly better in resected patients as compared to those who were not resected (median overall survival (OS): 44 vs. 18 months; p < 0.001). Recurrence was associated with a poorer prognosis as compared to patients without recurrence (median OS 43 months vs. not reached; p < 0.001). BCLC stage did not influence survival (p = 0.13).
Conclusion
In patients with large unilobar HCC, TACE-PVE leads to resection in most patients, with a good oncological outcome regardless of the tumor burden. When this strategy fails, patients can be managed with TACE despite prior PVE.
Introduction
Despite advances in the management of patients with HCC, large HCCs (i.e. >10 cm) still represent a therapeutic challenge. According to current Western guidelines based on the Barcelona-Clinic Liver Cancer (BCLC) staging system, most of these patients are not considered to be resectable and are candidates for locoregional treatments.1 At the same time, surgery is still the only curative treatment, but is associated with high risk of postoperative liver failure (PLF) and poor clinical outcome because most HCC develop on chronic liver disease.
Preoperative portal vein embolization (PVE) before liver resection has been proposed to induce compensatory contralateral hypertrophy of the future liver remnant (FLR), and prevent PLF, especially for major liver resections, which require the removal of a large quantity of functional liver.2, 3, 4, 5 It has been shown that preoperative sequential selective transarterial chemoembolization (TACE) and PVE before resection increased the rate of FLR hypertrophy and resulted in a high rate of complete tumor necrosis associated with longer recurrence-free survival.6, 7, 8, 9
However, not all patients undergo liver resection mainly because of insufficient liver hypertrophy, and/or tumor progression. There are very little data on the oncological outcome and management of this subgroup of patients. Therefore, a realistic picture of the outcome of patients undergoing sequential TACE-PVE on an intention-to-treat basis is lacking. Thus the purpose of this study was to investigate the long-term oncological outcome of patients with HCC who underwent sequential TACE-PVE on an intention to treat basis.
Patients and methods
Patient's selection
This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the local Ethics Committee. Between March 2006 and December 2012 all patients with HCC who underwent sequential TACE-PVE were studied. Baseline patient (demographic data, underlying liver disease and function) and tumor (size, location, number of lesions, vascular invasion, BCLC stage) characteristics were obtained from the prospective institutional database.
These included surgical data, operative results, tumoral and non-tumoral pathological data, details on additional treatments following recurrence or non-resection and long-term outcome.
Inclusion criteria were 1/HCC diagnosed according to EASL-EORTC recommendations,1 2/no extrahepatic disease based on chest/abdominal-pelvic CT performed within 6 weeks before TACE, 3/indication for sequential TACE-PVE. The decision to treat patients with sequential TACE-PVE to increase the rate of FLR hypertrophy and obtain a higher rate of complete tumor necrosis was made by an institutional multidisciplinary tumor board including hepatologists, oncologists, pathologists, hepatic surgeons, and interventional radiologists. This approach was used in all patients with resectable unilobar HCC and underlying liver disease (i.e. cirrhosis, fibrosis, steatohepatitis or steatosis), who were deemed to require major (>3 Couinaud segments) right-sided or left-sided hepatectomy regardless the volume of future liver remnant (FLR). This approach was indicated in Child A patients with preserved liver function, absence of clinically obvious portal hypertension defined by the presence of esophageal varices (≥grade 2) or ascites or the association of low platelet count (<100.000/mm3) and splenomegaly (largest diameter in transversal plane on CT > 12 cm),10 and with good performance status (ECOG 0–2). Eventually, the decision to operate was not based on the initial volumetry but rather on the regenerative capacity of the liver as assessed by the degree of hypertrophy. In this setting, PVE acted as a stress test of the non-tumoral liver. A degree of hypertrophy of <5% suggested poor regenerative ability, and thus patients who did not reach this cutoff value were not offered resection.11
Imaging work up
All patients underwent a baseline CT examination before the sequential procedure, an intermediate CT between TACE and PVE, and a follow-up CT 4–6 weeks after PVE. All contrast-enhanced multiphased CT of the chest and abdomen were performed on a 64-section multidetector CT scanner (LightSpeed VCT; GE HealthCare, Milwaukee, WI, USA). Following unenhanced abdominal CT, arterial, portal and delayed venous phase acquisitions were obtained 35, 80, and 180 s, respectively following the initiation of contrast injection. All CT examinations were retrospectively reviewed in consensus by two experienced abdominal radiologists (MR and BG).
Transarterial chemoembolization
TACE procedures were performed before PVE. The procedure was performed under local anesthesia as selectively as possible depending on tumor distribution reserve. A conventional approach (cTACE), or drug-eluting beads (DEB-TACE) were used, with the latter in more recent patients. TACE procedures were performed by a team of experienced interventional radiologists.
cTACE included an intraarterial injection of a mixture of chemotherapy (150 mg of doxorubicin; Adriamycin; Pharmacia Upjohn, Kalamazoo, MI, USA), emulsified in iodized oil (Lipiodol, Gerbet, Aulnay-sous-Bois, France). Embolization was achieved by injection of gelatin sponge (Gelitaspon, Gelita Medical B.V., Amsterdam, Netherlands) or polyvinyl alcohol particles (Bead Block, Biocompatibles, Farnham, UK).
The drug-eluting beads procedure included 100–300 μm and/or 300–500 μm sized particles (Biocompatibles, Farnham, UK), as described in the guidelines.12 Bead loading was performed with an intended dose of 150 mg/patient. In the absence of adverse effects or complications, patients were discharged 24–48 h after the procedure.
Portal vein embolization
Procedures were performed under general anesthesia, as previously reported.13 PVE was performed without embolization of the portal branches supplying segment 4 with a mixture of N-butyl-2-cyanoacrylate and iodized oil (Lipiodol, Guerbet, Aulnay-sous-Bois, France). Embolization was completed with 0.035-inch coils (Tornado 0.035, Cook, Limerick, Ireland), and polyvinyl alcohol particles (Beadblock, Biocompatibles, Farnham, UK), when necessary. In the absence of adverse effects or complications, patients were discharged 24–48 h after the procedure.
Patient follow-up and management
Resected patients
Resected liver specimens were retrospectively reviewed by a liver pathologist blinded to imaging data. The amount of residual tumor cells (RT) was quantitatively assessed for each lesion as the ratio of the residual tumor cells compared to the total surface volume of the lesion. Lesions were classified into 3 groups: 1/major histological response (RT <10%), 2/partial histological response (RT from 10% to 50%), and 3/no histological response (RT > 50%).14, 15, 16
Patients underwent follow-up CT examinations every four months the first year after liver resection and every six months thereafter. Patients with recurrent HCC were identified. The site and delay of recurrence were noted.
Non-resected patients
For patients who did not undergo resection, the reason was noted as follows: 1/tumor progression, 2/insufficient FLR hypertrophy, as defined by a degree of hypertrophy <5%,17 3/liver failure and 4/others. In case of tumor progression, the site of progression was recorded.
Further treatments
Treatment of patients without liver resection as well as those with recurrence following surgical treatment were discussed in the multidisciplinary tumor board. Additional therapeutic management was described for each patient.
Statistical analysis
Continuous data are expressed as median (range) and categorical data as number (frequency). Comparisons were performed with Fisher exact test for frequencies, and Mann–Whitney test for continuous variables. Overall survival (OS) was measured from the date of the beginning of sequential treatment to the date of death, whatever the cause, or censured at the time of the last follow-up visit (date of end of study December 31 2014). Recurrence free survival in patients undergoing resection was measured from the date of resection to the date of identified tumor recurrence following exclusion of postoperative deaths. Survival curves were prepared using the Kaplan–Meier method and compared using the log-rank test. All statistical tests were two-tailed. A p-value of 0.05 was considered to be significant. All analyses were performed using the Statistical Package for Social Sciences (SPSS) software (version 20.0. SPSS Inc., Chicago, IL, USA).
Results
Patients and tumors
A total of 54 Child A patients underwent TACE-PVE during the study period. Table 1 summarizes their characteristics. The median delay between baseline CT and the first TACE session was 8 days [interquartile range 3–22].
Table 1.
Baseline patients and tumors characteristics
| Patients (n = 54) | |
| Sex (M/F) | 50/4 (93/7%) |
| Age (median) | 69 (range 44–87) |
| Etiologies of chronic liver disease | |
| HCV | 14 (24%) |
| HBV | 16 (28%) |
| NASH | 17 (31%) |
| Alcohol consumption | 9 (17%) |
| Unknown | 2 (4%) |
| Child Pugh Score A | 54 (100%) |
| BCLC A/B/C | 25/23/6 (46%/43%/11%) |
| AFP serum level mg/dL | |
| <100 | 35 (65%) |
| 100–1000 | 5 (9%) |
| >1000 | 8 (15%) |
| unknown | 6 (11%) |
| Blood tests (median) | |
| Serum bilirubin (μmol/l) | 15 (4–150) |
| PT (%) | 93 (48–116) |
| AST (xUNL) | 1.7 (0.5–11) |
| ALT (xUNL) | 1.6 (0.5–8) |
| Creatinine (μmol/l) | 88 (59–265) |
| Tumors (n = 109) | |
| Number/patient (median) | 2 (1–8) |
| 1 | 26 (48%) |
| ≤3 | 22 (41%) |
| >3 | 6 (11%) |
| Size of the largest lesion (median) | 7.3 (4–17) |
| ≤5 cm | 18 (33%) |
| 5–10 cm | 21 (39%) |
| >10 cm | 15 (27%) |
| Location | |
| Right liver lobe | 53 (98%) |
| Left liver lobe | 1 (2%) |
| Portal vein invasion | |
| Segmental intrahepatic | 6 (11%) |
| Extrahepatic | 0 |
Table 2 reports the details of the sequential combined treatment procedures.
Table 2.
Sequential TACE-PVE procedural details
| PVE (n = 54) | |
| Type of PVE | |
| Right portal vein | 51 (94%) |
| Left portal vein | 1 (2%) |
| Right portal vein + left segment IV | 2 (4%) |
| Portal vein access | |
| Contralateral | 52 (96%) |
| Ipsilateral | 2 (4%) |
| Embolization material | |
| HLUF | 54 (100%) |
| Microspheres | 17 (31%) |
| Coils | 7 (13%) |
| TACE (n = 66) | |
| Number/patient (median and range) | 1 (1–2) |
| 1 procedure | 42 (78%) |
| 2 procedures | 12 (22%) |
| Type of TACE | |
| Conventional | 48 (73%) |
| DC Beads | 18 (27%) |
| Embolic agenta | |
| Gelfoam | 46 (70%) |
| Micro-particules | 16 (24%) |
| Coils | 3 (6%) |
| Selectivity | |
| Non selective | 31 (47%) |
| Selective | 35 (53%) |
| Delay TACE-PVE | |
| Median (range) | 30 (11–209) |
| Delay PVE-Surgery | |
| Median (range) | 53 (9–306) |
The sum exceeds 66 because some procedures included several agents.
No complications were observed following TACE. One patient experienced a PVE related complication involving contralateral embolic agent migration with no clinical consequences.
Table 3 summarizes the data on patients who underwent liver resection. The BCLC stage did not influence the feasibility of the strategy as resections were performed in 19/25, 16/23, and 4/6 of BCLC A, B, and C patients, respectively (p = 0.839). The resection rate was not different between patients who underwent conventional TACE, and those who underwent DEB-TACE (32/42 with cTACE vs. 7/12 with DEB-TACE, p = 0.195).
Table 3.
Details on surgical resection
| Resected patients (n = 39) | |
| Type of operation | |
| Right hepatectomy | 28 |
| Extended right hepatectomy | 11 |
| Margin status | |
| R0 | 34 |
| R1 | 5 |
| Patients not resected (n = 15) | |
| Cause of non-resectiona | |
| Insufficient FRL hypertrophy | 4 |
| Tumor progression | 11 |
| - Liver | 10 |
| - Lymph node | 1 |
| - Bone | 1 |
| Liver failure | 1 |
| Major fatigue | 1 |
Two patients had more than one reason for the absence of resection.
Of the 10 patients who developed tumor progression, it was ipsilateral in 3, contralateral in 3, or bilateral in 4 patients. The size of the lesions at baseline was not significantly different between patients with tumor progression than those without progression (median 90 mm (range 40–170 mm) vs. 70 mm (range 40–140 mm), p = 0.302). Patient with progression had significantly poorer liver function tests, in particular higher serum bilirubin levels (median 19 μmol/L (range 6–150) vs. 14 (range 4–29) μmol/L, p = 0.002), and AST level (median 2.4N (range 0.8–11.1) vs. 1.5N (range 0.5–7.1), p = 0.03).
The median tumor necrosis rate was 70% (range 20–100%). Major, partial and absence of histological response were present in 14, 19, and 6 lesions, respectively. Twenty-three HCCs showed intermediate differentiation. Peritumoral microscopic vascular invasion was identified in 20 patients. None of the patients had a normal liver parenchyma. Patients had cirrhosis (n = 18) or advanced fibrosis (n = 9). Steatosis was found in 17 patients, and was mild (<30%) in most patients (n = 14). The tumor necrosis was not affected by the type of chemoembolization (median 75% (range 20–100%) for cTACE vs. 60% (range 30–90%) for DEB-TACE, p = 0.530).
Patient outcome and survival analysis
Median follow-up was 31 months (1–108 months). Median OS was 41 months for the entire population with 1, 3, and 5 year-survival rates of 76%, 55%, and 22%, respectively.
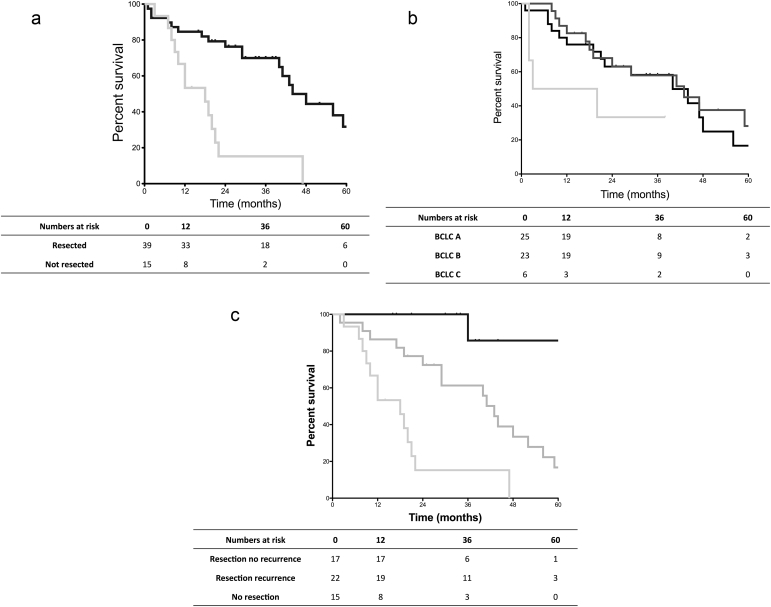
OS rates were significantly better in patients who underwent resection compared to patients who did not (p < 0.001) (Fig. 1a). The median OS was not significantly different according to the BCLC stage (p = 0.134) (Fig. 1b).
Figure 1.
a. Overall survival in patients undergoing resection compared to non resected patients. Resected patients (black line) had significantly better survival compared to patients who did not undergo resection (grey line) (median 44 months, vs. 18 months). b. Overall survival in patients according to BCLC stage. BCLC A (black line, median 40 months) and BCLC B (dark grey line, median 43 months) had comparable survival, better than that of BCLC C (light grey line, median 11.5 months). c. Survival comparison of patients undergoing resection without recurrence, undergoing resection with recurrence and not undergoing resection. The figure shows a statistically significant difference between median overall survival in resected patients without recurrence (black line, not reached), compared to resected patients with recurrence (dark grey line, 43 months), and with non-resected patients (light grey line, 18 months)
After excluding the three postoperative deaths (two from liver failure, and one from pulmonary infection), tumor recurrence occurred in 22/36 patients who underwent surgical resection after a median of 10 months (range 1–60). Recurrence occurred in 9/19, 11/16, and 2/4 resected patients with BCLC A, B, and C HCC, respectively (p = 0.430). The recurrence rate was not affected by the type of chemoembolization (18/32 for cTACE vs. 4/7 for DEB-TACE, p = 0.650). Recurrences occurred in the liver (n = 16), the lung (n = 4), peritoneum (n = 2), adrenal gland (n = 2), abdominal wall (n = 1) and bone (n = 1). Recurrences were multifocal at diagnosis in 4 patients.
The prognosis of patients with recurrence who underwent resection was poorer than in those without recurrence (p < 0.001) (Fig. 1c). After resection, the median recurrence free survival was 34 months.
Further treatment and patient management
Table 4 shows that the management of non-resected patients as well as those with recurrence following resection substantially differed. None of the patients who did not undergo liver resection received other curative treatments. On the other hand, 10/22 patients with tumor recurrence following resection received curative treatments (according to the BCLC classification, i.e. salvage liver transplantation, liver resection and percutaneous ablation).
Table 4.
Details on further treatment after recurrence or non-resection
| No resection (n = 15) | Resection and recurrence (n = 22) | P-value | |
|---|---|---|---|
| Curative treatment | 0 | 10 | 0.002 |
| Liver resection | 0 | 7 | 0.028 |
| Liver transplantation | 0 | 1 | 1.000 |
| Percutaneous ablation | 0 | 2 | 0.505 |
| - Number of sessions | 0 | 3 | 1.000 |
| - Median number/patient (range) | 0 | 1.5 (1–2) | 1.000 |
| Non curative treatment | 10 | 20 | 0.095 |
| TACE | 8 | 14 | 0.734 |
| - Number of TACE sessions | 19 | 31 | 0.850 |
| - Median number/patient (range) | 2 (1–5) | 2 (1–4) | 0.850 |
| Systemic treatment | |||
| - Sorafenib | 6 | 13 | 0.325 |
| Second line systemic therapy | 1 | 5 | 0.370 |
| - Sunitinib | 1 | 1 | 1.000 |
| - TGF beta inhibitors | 0 | 1 | 1.000 |
| - mTOR inhibitors | 0 | 1 | 1.000 |
| - Gemcitabin-Oxaliplatin | 0 | 2 | 0.505 |
| Radioembolization | 1 | 0 | 0.405 |
| Best Supportive Care | 5 | 2 | 0.095 |
Repeat TACE was performed in 8 patients without resection, and in 14 patients with tumor recurrence following resection. No complications were observed following the TACE sessions. Treatment was lobar in three patients, and selective in the remaining five. TACE was performed ipsilateral to PVE in six patients, and contralateral in the remaining two.
Discussion
The present series reports the results of sequential TACE and PVE treatment as a first step towards oncological liver resection in patients with large HCC on an intention to treat basis. In this study 72% of the patients were able to undergo the entire treatment protocol. Although as many as 43% of patients were classified as BCLC stage B and did not theoretically qualify for resection according to current recommendations,1 this did not significantly influence the feasibility or results of this approach. On the other hand while as many as 28% of the patients could not undergo resection, results showed that alternative therapeutic options including TACE could be safely performed and resulted in reasonable survival.
Several studies have shown that sequential TACE-PVE is associated with increased FLR hypertrophy, higher tumor necrosis rate, and improved survival in HCC patients.8, 9 This confirms the interest of combination therapy in large HCCs and could provide a strong argument for more aggressive management of these lesions. Because non-alcoholic fatty liver diseases (NAFLD) is becoming a major risk factor for the development of HCC, which are larger when they are diagnosed than in patients with alcoholic or viral hepatitis,18 sequential TACE-PVE will probably be performed more often. Indeed, there is an ongoing debate in the surgical and oncological communities on the extent of surgical management required in these lesions. Recently, it was suggested that patients with large HCCs could benefit from more aggressive treatment, mainly surgical resection.19 The current results support this strategy, because the resection rate between patients with BCLC A tumors and those with BCLC B or C was comparable. This resulted in comparable survival in BCLC A and B patients, which further suggests that the BCLC staging system may be too restrictive.20
Although most patients underwent resection as planned, resection could not be performed in 28%, which is similar to published series.6, 8 The fact that most patients did not qualify for surgery because of tumor progression shows that sequential TACE-PVE could also provide a good evaluation of tumor aggressiveness and allow better patient selection. Indeed, and as expected, patients who did not undergo liver resection as planned had the worst outcome. Nevertheless, the median overall survival of 18 months in this group was acceptable considering the initial tumor stage (size and number of tumors) and was probably the consequence of an aggressive management using other treatments, especially TACE. This could be considered surprising due to the portal vein occlusion induced by PVE. In this setting, one might expect TACE to lead to marked toxicity, parenchymal necrosis, preventing the use of intra-arterial procedures. In fact one article and one case report found that TACE was feasible and well tolerated after PVE.21, 22 Nevertheless, Kang et al. reported the formation of three abscesses and Wallace et al. one biliary complication.21, 22 One could also hypothesize that TACE ipsilateral to prior PVE may be less well tolerated than contralateral to PVE. Results show that TACE was very well tolerated in these patients (including 75% of the sessions ipsilateral to PVE). Therefore, TACE should not be contraindicated in patients who undergo initial sequential TACE and PVE but this approach should be discussed during multidisciplinary tumor boards along with other treatments. Wallace et al. recommend dose reduction and the reduction of arterial flow without stasis.22 In the current study, regular techniques and dose administration were used.
Tumor recurrence occurred in most of the patients in this study who underwent resection like in the literature.8, 23 Even after curative hepatectomy the risk of tumor recurrence is extremely high and even higher in large tumors with vascular invasion. Most recurrences were intrahepatic but a few patients also developed distant recurrence, emphasizing the importance of whole body imaging during follow-up.
Overall, the current survival curves are more complete and realistic for patients undergoing sequential treatment. Published data by other teams mainly focus on the subgroup of patients who undergo resection. In this setting 5-year overall survival rates ranged from 43 to 72%,8, 9, 24 which is slightly more than the 36% was observed in the present study. However, it is difficult to compare these data because the recurrence rate can vary among populations. Nevertheless, it should be noted that the subgroup of resected patients free of recurrence had a very good prognosis, with a 5-year survival rate of 71%.
Besides its retrospective design this study has certain limitations. First, a calculation of liver volumes was not performed. This has been clearly shown in several publications, and the present study aimed at focusing on the oncological outcome of the entire population. Second, factors associated with recurrence or tumor progression following sequential treatment were not analyzed. Once again, this has already been addressed in several studies, and was not the aim of the present study.
In conclusion, sequential TACE and PVE is a valid oncologic strategy in patients with large unilobar HCC because it results in surgical resection in most patients, with a good oncological outcome. Patients who did not undergo resection can nevertheless be managed with the usual antitumoral treatments, including TACE despite previous PVE.
Conflict of interest
None declared.
References
- 1.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.de Baere T., Roche A., Vavasseur D., Therasse E., Indushekar S., Elias D. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology. 1993;188:73–77. doi: 10.1148/radiology.188.1.8511321. [DOI] [PubMed] [Google Scholar]
- 3.de Baere T., Roche A., Elias D., Lasser P., Lagrange C., Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386–1391. doi: 10.1053/jhep.1996.v24.pm0008938166. [DOI] [PubMed] [Google Scholar]
- 4.Farges O., Belghiti J., Kianmanesh R., Regimbeau J.M., Santoro R., Vilgrain V. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abulkhir A., Limongelli P., Healey A.J., Damrah O., Tait P., Jackson J. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 6.Imamura H., Seyama Y., Makuuchi M., Kokudo N. Sequential transcatheter arterial chemoembolization and portal vein embolization for hepatocellular carcinoma: The University of Tokyo experience. Semin Interv Radiol. 2008;25:146–154. doi: 10.1055/s-2008-1076683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilgrain V., Sibert A., Zappa M., Belghiti J. Sequential arterial and portal vein embolization in patients with cirrhosis and hepatocellular carcinoma: The Hospital Beaujon experience. Semin RI. 2008:1–7. doi: 10.1055/s-2008-1076689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo H., Kim J.H., Ko G.Y., Kim K.W., Gwon D.I., Lee S.G. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:1251–1257. doi: 10.1245/s10434-010-1423-3. [DOI] [PubMed] [Google Scholar]
- 9.Ogata S., Belghiti J., Farges O., Varma D., Sibert A., Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–1098. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 10.Faitot F., Allard M.A., Pittau G., Ciacio O., Adam R., Castaing D. Impact of clinically evident portal hypertension on the course of hepatocellular carcinoma in patients listed for liver transplantation. Hepatology. 2015;62:179–187. doi: 10.1002/hep.27864. [DOI] [PubMed] [Google Scholar]
- 11.Ribero D., Abdalla E.K., Madoff D.C., Donadon M., Loyer E.M., Vauthey J.N. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 12.Lencioni R., De Baere T., Burrel M., Caridi J.G., Lammer J., Malagari K. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Interv Radiol. 2011;35:980–985. doi: 10.1007/s00270-011-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Baere T., Denys A., Madoff D.C. Preoperative portal vein embolization: indications and technical considerations. Tech Vasc Interv Radiol. 2007;10:67–78. doi: 10.1053/j.tvir.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Kwan S.W., Fidelman N., Ma E., Kerlan R.K., Jr., Yao F.Y. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl. 2012;18:727–736. doi: 10.1002/lt.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kornberg A., Witt U., Kupper B., Wildgruber M., Friess H. Postinterventional tumor necrosis predicts recurrence-free long-term survival in liver transplant patients with advanced hepatocellular carcinoma. Transpl Proc. 2013;45:1913–1915. doi: 10.1016/j.transproceed.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Bryant M.K., Dorn D.P., Zarzour J., Smith J.K., Redden D.T., Saddekni S. Computed tomography predictors of hepatocellular carcinoma tumour necrosis after chemoembolization. HPB. 2014;16:327–335. doi: 10.1111/hpb.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sangro B., Carpanese L., Cianni R., Golfieri R., Gasparini D., Ezziddin S. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 18.Cauchy F., Zalinski S., Dokmak S., Fuks D., Farges O., Castera L. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013;100:113–121. doi: 10.1002/bjs.8963. [DOI] [PubMed] [Google Scholar]
- 19.Yau T., Tang V.Y., Yao T.J., Fan S.T., Lo C.M., Poon R.T. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691–1700 e1693. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Torzilli G., Belghiti J., Kokudo N., Takayama T., Capussotti L., Nuzzo G. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929–937. doi: 10.1097/SLA.0b013e31828329b8. [DOI] [PubMed] [Google Scholar]
- 21.Kang B.K., Kim J.H., Kim K.M., Ko G.Y., Yoon H.K., Gwon D.I. Transcatheter arterial chemoembolization for hepatocellular carcinoma after attempted portal vein embolization in 25 patients. AJR Am J Roentgenol. 2009;193:W446–W451. doi: 10.2214/AJR.09.2479. [DOI] [PubMed] [Google Scholar]
- 22.Wallace M.J., Ahrar K., Madoff D.C. Chemoembolization of the liver after portal vein embolization: report of three cases. J Vasc Interv Radiol. 2008;19:1513–1517. doi: 10.1016/j.jvir.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Naito S., Imamura H., Tukada A., Matsuyama Y., Yoshimoto J., Sugo H. Postoperative recurrence pattern and prognosis of patients with hepatocellular carcinoma, with particular reference to the hepatitis viral infection status. Liver Int. 2014;34:802–813. doi: 10.1111/liv.12447. [DOI] [PubMed] [Google Scholar]
- 24.Aoki T., Imamura H., Hasegawa K., Matsukura A., Sano K., Sugawara Y. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766–774. doi: 10.1001/archsurg.139.7.766. [DOI] [PubMed] [Google Scholar]