Abstract
Background
Postcholecystectomy pain (PCP) is characterized by abdominal pain after cholecystectomy. However, prevention of PCP is not well known yet. The purpose of this study was to determine whether Rowachol might be useful in preventing PCP.
Methods
Between May 2013 and January 2014, a total of 138 patients with gallbladder disease who were scheduled to undergo laparoscopic cholecystectomy were randomly assigned to orally receive 100 mg Rowachol or placebo three times daily for 3 months after surgery. Abdominal pain was assessed using the European Organization for Research and Treatment of Cancer QLQ-C30 questionnaire.
Results
Incidence of PCP in the placebo group (n = 9, 14.3%) was higher than that in the Rowachol group (n = 3, 4.7%) with statistically marginal significance (P = 0.08). Risk factor analysis implicated PCP with increased difficulty in performing LC, more frequent pathology with acute cholecystitis, and absence of postoperative Rowachol treatment. Multivariate analysis revealed that greater difficulty of laparoscopic cholecystectomy (HR = 5.78, 95% CI 1.36–24.40, P < 0.05), and absence of postoperative Rowachol treatment (HR = 2.54, 95% CI 1.10–10.39, P < 0.05) were independent risk factors for development of PCP.
Conclusion
Rowachol might be beneficial for prevention of PCP after laparoscopic cholecystectomy.
Introduction
Since its introduction in 1986, laparoscopic cholecystectomy (LC) has become more widely used and is now considered the treatment of choice for various gallbladder (GB) diseases.1, 2, 3, 4, 5 However, many patients remain symptomatic after GB removal. Approximately a third of all patients who undergo cholecystectomy complain of persistent or recurrent pain after surgery, and this phenomenon is referred to as postcholecystectomy pain (PCP).1, 6, 7, 8, 9, 10, 11
Anatomical factors may contribute to PCP and these include sphincter of Oddi dysfunction (SOD),12, 13 cystic-duct remnant neuroma,14 and retained cystic-duct remnant calculi.15 However, the etiology for most of PCP remains unclear. As a result, the clinical management of these patients is frequently without an evidence-based approach.
Rowachol is a terpene mixture that enhances the solubility of cholesterol, calcium carbonate, and calcium phosphate, which makes it a potent choleretic agent.16, 17, 18, 19 As a result, terpene preparations are effective in resolving biliary stones.19 However, the preventive effect of Rowachol as a choleretic drug for the patients with PCP is unclear.16, 20, 21
This clinical trial was designed to determine whether Rowachol is useful in the prevention of PCP and for the improvement of symptoms after LC using a validated questionnaire. We also explored the possible risk factors for PCP in the study.
Methods
Patients
Male or female patients between 18 and 85 years of age with suspected GB diseases were evaluated at the outpatient clinic or emergency room. After a thorough examination that included a physical examination, laboratory testing, and abdominal imaging such as USG, or CT scan, the patients who were diagnosed with symptomatic gallstone disease, cholecystitis, GB polyp, or early GB cancer with LC were enrolled.
Patients diagnosed with severe psychiatric or neurologic diseases were excluded, as were the patients who had received immunosuppressive therapy days prior to enrollment, the patients having undergone chemotherapy or radiotherapy within 4 weeks prior to their operation, or those who were unable to follow the instructions given by the investigator. Addition exclusions were pregnant or lactating female patients and patients who had a history of drug- and/or alcohol-abuse according to the local standards.
Study design
This prospective, multicenter, randomized, single-blind (participants), placebo-controlled study compared the efficacy of Rowachol versus placebo as prophylaxis for the prevention of PCP in patients receiving LC. Between May 2013 and January 2014, 160 patients were assessed for eligibility at Dongguk University Ilsan Hospital and Chung-Ang University Hospital. Both are tertiary referral centers in South Korea. The Institutional Review Board (IRB) at each hospital approved the study protocol, and written informed consent was obtained from all patients before enrollment. The trial was also registered under clinicaltrials.gov (trial no. NCT01765465) before the patient recruitment commenced. The treatment assigned to each patient was chosen randomly by the investigator using computer software that incorporated a standard procedure for generating random numbers. The assignments for each center were in balanced blocks to ensure that approximately equal number of patients was allocated to each treatment arm across the total study population. To control or minimize potential bias in patients selection in using criteria such as technical difficulties for a given LC, a study nurse performed randomization at postoperative 1–2 days, and as a result, none of the surgeons involved could know the allocation of patients before or at the time of surgery.
Rowachol (Rowa Pharma, Cork, U.K.), as a mixture of terpene (pinene 17 mg, camphene 5 mg, cineol 2 mg, menthone 6 mg, menthol 32 mg, and borneol 5 mg), and administration-matched placebo (supplied by the onsite pharmacy) were orally administered at a dose of 100 mg three times a day for 3 months. Rowachol has been widely used in more than 50 countries in Europe, the Middle East, East Asia, Africa, and most countries in the American continent.22 The cost of Rowachol somewhat differs with various countries, but it usually costs less than a half a US dollar per capsule (100 mg dose).22 Medication was given to patients immediately after randomization, postoperative day (POD) 1 or 2 days. The use of other choleretic drugs, such as ursodeoxycholic acid (UDCA), during this period was prohibited. Follow-up evaluations were performed after cessation of study treatment for up to 3 months after surgery.
LC was performed by single or multiport methods at all institutes. All the operations were conducted by experienced laparoscopic surgeons. Technical difficulties were assessed as present (score of 1) or absent (score of 0) for each of the following 5 operative steps: (i) access into the peritoneal cavity, (ii) dissection of adhesions from the GB, (iii) dissection of the triangle formed by the common bile duct, cystic duct, and liver (Calot's triangle), (iv) dissection of the GB bed, and (v) extraction of the GB from the abdominal cavity (Table 1).23 All GB specimens were sent for histopathology.
Table 1.
Difficulty scores for operative steps in laparoscopic cholecystectomy23
| Category | Point: No (0)/Yes (1) |
|---|---|
| Difficult access into peritoneal cavity | 0/1 |
| Difficult dissection of adhesions from GB | 0/1 |
| Difficult dissection of Calot's triangle | 0/1 |
| Difficult dissection of GB bed | 0/1 |
| Difficult GB extraction from abdomen | 0/1 |
| Total score | (0–5) |
GB, gallbladder.
Efficacy measurements
As there are no validated or translated questionnaires in Korean suitable for evaluating PCP or other symptoms, PCP or other symptoms were measured using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire in Korean in the outpatient clinics 3 months postoperatively. Even though the EORTC QLQ C-30 was designed for cancer patients, it has widely been used in various non-cancer surgical diseases.24, 25 The Korean version of EORTC QLQ-C30 was developed by EORTC using a rigorous translation and back-translation process.26 The Patient comments led to appropriate modifications to the questions and scales, after which it was reviewed and approved by the EORTC QOL Study Group.26 The questionnaires were self-reported by patients, and a trained nurse was available for patients that required help in completing the surveys. Raw data underwent linear transformation to standardize the raw scores, ranging from 0 to 100, as recommended in the EORTC QLQ-C30 scoring manual.
Outcomes were analyzed according to the intention-to-treat principle. There have been several reports about PCP,1, 6, 7, 8, 9, 11 however, there have been no consensus on measuring and assessing the extent of the associated pain. After a thorough discussion among all the investigators, an EORTC QLQ C-30 score for abdominal pain exceeding 30 points at 3 months postoperatively defined the PCP as the primary endpoint. The Secondary endpoints in the study were for symptoms other than PCP as reported on the EORTC QLQ C-30, and for values of the liver function test (LFT).
Demographic information (age, sex, body mass index, and the American Society of Anesthesiologists class) and pertinent surgical information (presence of gallstone, type of surgery, conversion rate, operation time, and difficulty score) were all pre-defined. Routine hematology, biochemistry including LFT and abdominal ultrasonographic (USG) evaluations were performed at 3 months after LC. Outcomes were assessed by dedicated study nurses who submitted the data to a dedicated computerized database (MedicalDB, Seoul, KOR).
Statistical analysis
Sample size computation was based on the reduction of pain score determined by the EORTC QLQ C-30. Adopting a power of 80%, a 2-sided type I error (α) of 0.05 and an anticipated dropout rate of 10%, the calculated sample size was 69 patients per group. The Chi-square test was used to determine whether prophylactic Rowachol therapy prevented the development of PCP in patients with LC. The remaining secondary endpoints for other symptoms on EORTC QLQ C-30, and for level of LFT were summarized with informal descriptive analyses. Multivariate analysis was performed using a proportional hazards regression model including 95% confidence interval (CI) and P value. Data were analyzed by use of the Statistical Package for the Social Sciences version 21.0 (SPSS, Chicago, IL, USA). The trial results were presented according to the CONSORT guidelines.27
Results
Patients
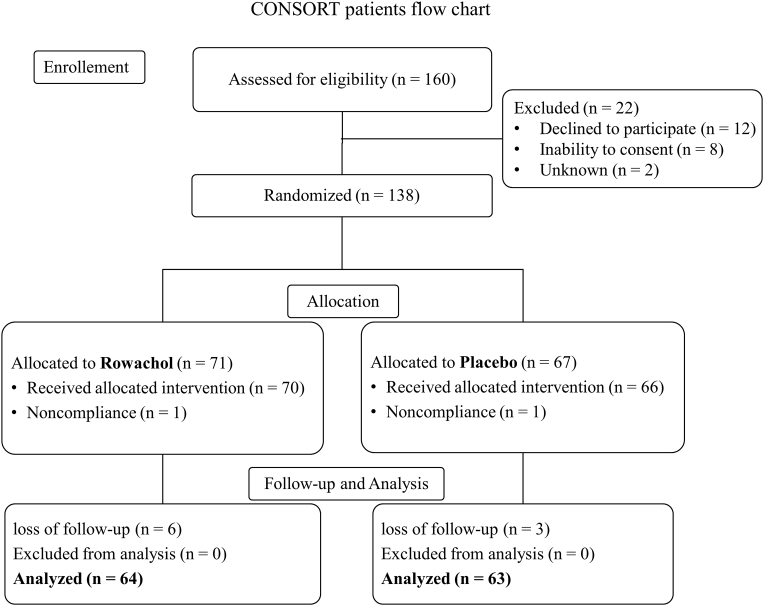
Of the 160 patients who were screened, 22 were excluded, including 8 who refused consent. In the intent-to-treat analysis, 138 patients with GB disease after LC were randomized to Rowachol (n = 71) or placebo (n = 67) (Fig. 1). Study enrollment and follow-up occurred between March 2013 and January 2014. Nine patients including 6 patients in the Rowachol group and 3 in the placebo group were non-evaluable due to loss to follow-up after postoperative 3 months (Fig. 1). No change to the methods or drug was made after the beginning of the trial. Also, no major side effects related to Rowachol occurred. None of the differences between groups including age, sex, difficulty score to perform LC, incidence of postoperative complication, or operative time was significant (Table 2).
Figure 1.
Screening, randomization, and follow-up of study participants
Table 2.
Patient characteristics in the intent-to-treat population
| Rowachol (n = 71) | Placebo (n = 67) | P | |
|---|---|---|---|
| Age in years | 51.5 ± 15.6 | 48.0 ± 13.8 | 0.21 |
| Gender (male: female) | 26: 45 | 33: 34 | 0.13 |
| BMI (kg/m2) | 24.6 ± 3.7 | 25.2 ± 3.5 | 0.90 |
| ASA class (minimal/moderate/severe) | 46/25/0 | 43/24/0 | 0.94 |
| Preoperative ERCP stone removal | 8 (11.3) | 6 (9.0) | 0.65 |
| GB stone | 66 (93.0) | 62 (92.5) | 0.81 |
| aCombined comorbid diseases | 22 (31.2) | 21 (31.3) | 0.96 |
| Single port cholecystectomy | 5 (7.0) | 7 (10.4) | 0.48 |
| Difficulty score in LC | 0.5 ± 1.0 | 0.8 ± 1.2 | 0.54 |
| bPostoperative complication | 0 | 1 (1.5) | 0.49 |
| Open conversions | 0 | 0 | 1.00 |
| cPathology (acute: chronic) | 6: 61 | 13: 53 | 0.17 |
| Operative time (min) | 51.9 ± 27.2 | 58.8 ± 30.0 | 0.14 |
Values denote mean values ± standard deviation or no. (%) of patients.
BMI indicates body mass index; ASA, American Society of Anesthesiologists; ERCP, Endoscopic Retrograde Cholangiopancreatography; LC, laparoscopic cholecystectomy.
Cardiovascular, Cerebrovascular, Diabetes mellitus, Chronic obstructive lung disease, Chronic renal failure, etc.
1 case of minor bile leak only, no other complication occurred.
3 cases xanthogranulomatous cholecystitis, 2 cases T1 GB cancer.
Laboratory findings after postoperative 3 months
There were no significant differences between the Rowachol and placebo groups in laboratory findings, such as, bilirubin levels and white blood cell counts (Table 3).
Table 3.
Laboratory findings between Rowachol and placebo groups at 3 months postoperatively
| Rowachol (n = 64) | Placebo (n = 63) | P | |
|---|---|---|---|
| Total bilirubin (mg/dL) | 0.7 ± 0.5 | 0.6 ± 0.2 | 0.43 |
| Direct bilirubin (mg/dL) | 0.3 ± 0.3 | 0.2 ± 0.2 | 0.40 |
| Alkaline phosphatase (IU/L) | 242.8 ± 449.8 | 209.6 ± 145.5 | 0.670 |
| Aspartate aminotransferase (IU/L) | 105.1 ± 60.5 | 50.8 ± 41.2 | 0.33 |
| Alanine aminotransferase (IU/L) | 44.3 ± 43.8 | 56.1 ± 49.1 | 0.35 |
| White blood cells (× 106/μl) | 6.8 ± 2.1 | 7.2 ± 2.4 | 0.38 |
Values denote mean ± standard deviation.
Incidence of abdominal pain after postoperative 3 months
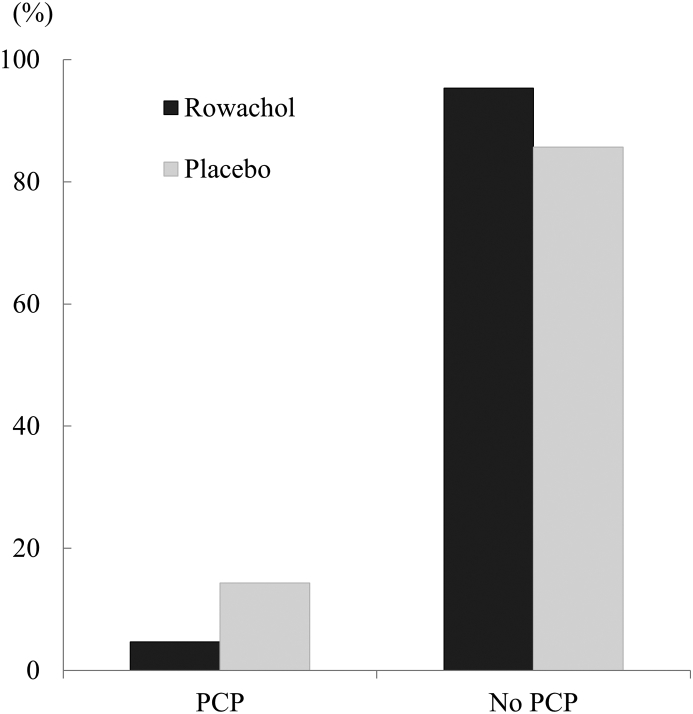
The incidence of PCP after LC as determined using the EORTC QLQ C-30 questionnaire was 4.7% (3 of 64) in the Rowachol group and 14.3% (9 of 63) in the placebo group with a statistically marginal significance (P = 0.08; Fig. 2). Patients with PCP were more likely to have more difficulty in performing LC (1.8 ± 1.5 vs. 0.6 ± 1.0, P < 0.01), and had a higher frequency of acute cholecystitis (41.7%, 5 of 12 vs. 10.4%, 12 of 115, P = 0.03) compared to those without PCP on univariate analysis (Table 4). Twelve patients (3 patients with PCP in the Rowachol group and 9 patients with PCP in placebo group) scored ≥30 points in the questionnaire completed 3 months after LC. The characteristics of these patients are summarized in Table 5. Multivariate analysis revealed that, a more difficult LC (HR = 5.78, 95% CI 1.36–24.40, P = 0.02), and absence of postoperative Rowachol treatment (HR = 2.54, 95% CI 1.10–10.39, P < 0.05) were independent risk factors for the development of PCP (Table 6).
Figure 2.
Incidence of abdominal pain 3 months postoperatively. Pain was assessed by the EORTC QLQ C-30 questionnaire 3 months postoperatively. The Rowachol group (n = 3, 4.7%) showed lower incidence of PCP compared with that of placebo group (n = 9, 14.3%) with statistically marginal significance (P = 0.076). *PCP was defined as pain score ≥30 on the EORTC QLQ C-30 questionnaire 3 months postoperatively. PCP, postcholecystectomy pain; EORTC QLQ C-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30
Table 4.
Risk factor analysis for postcholecystectomy pain at 3 months postoperatively
| PCP (n = 12) | No PCP (n = 115) | P | |
|---|---|---|---|
| Age in years | 51.3 ± 12.1 | 49.3 ± 14.7 | 0.61 |
| Sex (male: female) | 5:7 | 48: 67 | 1.00 |
| BMI (kg/m2) | 26.4 ± 4.0 | 24.9 ± 3.5 | 0.24 |
| ASA class (minimal/moderate/severe) | 7: 5 | 75: 40: 0 | 0.64 |
| Preoperative ERCP stone removal | 1 | 11 (9.6) | 1.00 |
| GB stone | 12 | 105 (91.3) | 0.60 |
| aCombined comorbid diseases | 4 | 36 (31.3) | 1.00 |
| Single port cholecystectomy | 0 | 11 (9.6) | 0.60 |
| Difficulty score to perform LC | 1.8 ± 1.5 | 0.6 ± 1.0 | <0.01 |
| bPostoperative complication | 1 | 0 | 0.81 |
| Open conversions | 0 | 0 | |
| cPathology (acute: chronic) | 5 | 12 (10.4) | 0.03 |
| Operative time (min) | 52.1 ± 20.4 | 56.0 ± 29.5 | 0.56 |
| Postoperative Rowachol treatment | 3 | 61 (53.0) | 0.08 |
Values denote mean values ± standard deviation or no. (%) of patients.
BMI indicates body mass index; ASA, American Society of Anesthesiologists; ERCP, Endoscopic Retrograde Cholangiopancreatography; LC, laparoscopic cholecystectomy.
Cardiovascular, Cerebrovascular, Diabetes mellitus, Chronic obstructive lung disease, Chronic renal failure, etc.
1 case of minor bile leak only, no other complication occurred.
3 cases xanthogranulomatous cholecystitis, 2 cases T1 GB cancer.
Table 5.
Detailed characteristics in patients with postcholecystectomy pain
| Age/Gender | Group | Difficulty score | Pathology | Combined CBD stone | Postoperative complication | LFT abnormality at follow-up | Radiologic diagnosis using USG at follow-up | Management for pain |
|---|---|---|---|---|---|---|---|---|
| 70/F | Rowachol | 0 | Chronic | – | – | – | Nonspecific | Conservative |
| 41/M | Rowachol | 0 | Chronic | – | – | – | Nonspecific | Conservative |
| 36/F | Rowachol | 0 | Chronic | – | – | – | Nonspecific | Conservative |
| 66/F | Placebo | 4 | Acute | Yes | – | – | Nonspecific | Conservative |
| 35/M | Placebo | 4 | Chronic | – | – | – | Nonspecific | Conservative |
| 61/F | Placebo | 3 | Acute | – | – | – | Nonspecific | Conservative |
| 44/M | Placebo | 3 | Chronic | – | – | – | Nonspecific | Conservative |
| 64/M | Placebo | 2 | Acute | – | Bile leak | – | Mild bile duct dilatation | Conservative |
| 44/F | Placebo | 2 | Acute | – | – | – | Nonspecific | Conservative |
| 46/F | Placebo | 2 | Acute | – | – | – | Nonspecific | Conservative |
| 58/M | Placebo | 1 | Chronic | – | – | – | Nonspecific | Conservative |
| 51/F | Placebo | 0 | Chronic | – | – | – | Nonspecific | Conservative |
CBD, common bile duct; LFT, liver function test.
Table 6.
Multivariate analysis for postcholecystectomy pain at 3 months postoperatively
| Hazard ratio | 95% Confidence intervals | P | |
|---|---|---|---|
| Higher difficulty score in LC (≥3) | 5.78 | 1.36–24.40 | 0.02 |
| Pathology (acute cholecystitis) | 2.03 | 0.42–9.78 | 0.38 |
| Absence of postoperative Rowachol treatment | 2.54 | 1.10–10.39 | <0.05 |
LC, laparoscopic cholecystectomy.
Comparison of the other symptoms at postoperative 3 months
Other symptom scales, excepting PCP on EORTC QLQ C-30 questionnaire and functional scales, were not statistically significantly different between groups (Table 6).
Discussion
LC is a very effective treatment for GB disease, but PCP is not uncommon.3, 8, 9, 10, 11, 28 Approximately 30–50% of patients who undergo cholecystectomy remain symptomatic, where the management of PCP can be quite challenging.1, 6, 7, 8, 9, 28, 29 The efficacy of choleretic drugs has been questioned,16, 20, 21 as those studies did not focus directly on preventing or minimizing PCP. This study demonstrates that Rowachol can be beneficial for prevention of PCP after LC; absence of postoperative Rowachol treatment was an independent risk factor to develop PCP after the multivariate analysis (Table 5). Also, no major side effects related to Rowachol occurred. As over 50,000 cholecystectomies are performed annually in Korea,30 a relatively large number of patients might benefit from Rowachol treatment for their PCP.
Bile crystals or microlithiasis have been previously identified in patients with PCP.6, 12 Rowachol, as a mixture of terpene, inhibits hepatic hydroxymethyl glutamylcoenzyme A reductase, alters biliary cholesterol saturation, and can dissolve bile crystals. It enhances the solubility of cholesterol, calcium carbonate, and calcium phosphate, which makes it a potent choleretic agent.17, 18, 31 Terpene preparations are known to be effective in resolving biliary stones.19 Thus, Rowachol treatment for patients with postcholecystectomy pain might be beneficial.
It has been reported that SOD may develop in some patients after a cholecystectomy and may cause similar pain.1, 12, 13 Also, remnant GB after partial cholecystectomy or cystic duct calculi can be a source of recurrent biliary pain.1, 15 Patients in this study did not meet the criteria for type I or type II SOD, and were not found retained GB and cystic duct calculi because there was no evident bile-duct dilatation or retained stone on USG imaging, and serum LFT abnormalities (Table 5).
Another possible explanation for PCP is that intraoperative damage to nerves innervating visceral structures during the operation causes postoperative pain.8 In this study, after multivariate analysis, score indicative of higher difficulty in performing LC, in the absence of other definite visceral organ damage, was an independent risk factor in developing PCP (Table 6). This may be explained that the difficulty in dissection of the triangle formed by the common bile duct, cystic duct, and liver (Calot's triangle) might cause intraoperative nerve damage innervating the visceral structures. To our knowledge, this is the first report that the difficulty in surgical procedure without other visceral organ damage affected PCP.
Cholecystectomy is associated with several physiological changes in the upper gastrointestinal tract, which may account for persistence of symptoms or the development of new symptoms after GB removal besides abdominal pain.1, 7, 9, 10 Cholecystectomy can give rise to gastrointestinal symptoms like indigestion, nausea, vomiting, and food intolerance.7, 9, 32 In this study, the other symptom scales, except PCP on EORTC QLQ C-30 and functional scales, did not differ significantly between groups (Table 7). However, this study did not include a relationship analysis for pain with other symptoms. Further planned studies will address the so-called postcholecystectomy syndrome among various symptoms after LC, with the aim of identifying risk factors affecting postcholecystectomy syndrome for patient management.
Table 7.
Comparison of other symptoms on EORTC QLQ C-30 at 3 months postoperatively
| Rowachol (n = 64) | Placebo (n = 63) | P | |
|---|---|---|---|
| aFrequently present symptom scales | |||
| Fatigue | 42 (65.6) | 37 (58.7) | 0.42 |
| Nausea and vomiting | 21 (32.8) | 20 (31.7) | 0.90 |
| Dyspnea | 12 (18.8) | 13 (20.6) | 0.79 |
| Insomnia | 39 (60.9) | 35 (55.6) | 0.59 |
| Appetite loss | 39 (60.9) | 38 (60.3) | 0.94 |
| Constipation | 28 (43.8) | 22 (34.9) | 0.31 |
| Diarrhea | 44 (68.8) | 35 (55.6) | 0.13 |
| Financial difficulties | 4 (6.3) | 5 (7.9) | 0.71 |
| bImpaired functional scale | |||
| Physical function | 1 (1.6) | 1 (1.6) | 1.00 |
| Role function | 1 (1.6) | 2 (3.2) | 0.62 |
| Emotional function | 2 (3.1) | 2 (3.2) | 1.00 |
| Cognitive function | 5 (7.8) | 2 (3.2) | 0.44 |
| Social function | 0 | 1 (1.6) | 0.50 |
| Global health status/Quality of Life | 17 (26.6) | 15 (23.8) | 0.72 |
Values denote no. (%) of patients.
EORTC QLQ C-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30.
Symptom scales ≥30 points at postoperative 3 months.
Functional scales ≤50 points at postoperative 3 months.
There are some potential limitations of the study. Ideally, a bile examination after Rowachol treatment should be performed to correlate clinical outcomes with resolution or persistence of microlithiasis. However, in this study, ERCP was not indicated in all patients with PCP because all patients showed normal LFT level and no significant abnormality at postoperative USG examination (Table 5). Also, the types and proportions of persistent symptoms are reported to differ from those that rise de novo, suggesting that these two entities may have different causes.9 In this study, the symptomatic evaluation using the EORTC QLQ C-30 questionnaire was done once at 3 months postoperatively, and we could not differentiate between persistent and de novo postoperative symptoms. Future research should explore the preventive effect of Rowachol on PCP with these distinctions particularly with longer follow-up.
In this study, we revealed absence of postoperative Rowachol treatment was an independent risk factor to develop PCP after multivariate analysis (Table 6), the incidence of PCP after LC in the Rowachol group was lower than that in the placebo group with statistically marginal significance after univariate analysis (Fig. 2). It might be caused by relatively small sample size. As a result, future research should take an expanded sample size into account.
In conclusion, this prospective, multicenter, randomized, single-blind, placebo-controlled study compared the efficacy of Rowachol versus placebo as prophylaxis for the prevention of PCP in patients with LC. Multivariate analysis revealed that Rowachol might be beneficial for prevention of postcholecystectomy pain after LC. Most prior studies for symptomatic evaluation after LC had not focused on prevention or treatment of symptoms, but rather on the etiology or natural course of symptoms.8, 9, 10, 11, 15
To our knowledge, this is the first report that the difficulty to perform LC could contribute to onset of PCP. It is noteworthy that difficulty in performing LC, without any injury to the bile duct or other visceral organs, can produce postoperative pain.
Funding
In Woong Han and Seung Eun Lee were currently receiving a grant from the Phambio Korea Co., Ltd.
Conflicts of interest
None declared.
Acknowledgments
In Woong Han and Seung Eun Lee were currently receiving a grant from the Phambio Korea Co., Ltd. Also, the supply of drug was provided by the Phambio Korea Co., Ltd.
Footnotes
This manuscript was presented at A-PHPBA Singapore 2015, taking place on March 18–21, 2015.
References
- 1.Jaunoo S.S., Mohandas S., Almond L.M. Postcholecystectomy syndrome (PCS) Int J Surg. 2010;8:15–17. doi: 10.1016/j.ijsu.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 2.McPherson K., Wennberg J.E., Hovind O.B., Clifford P. Small-area variations in the use of common surgical procedures: an international comparison of New England, England, and Norway. N Engl J Med. 1982;307:1310–1314. doi: 10.1056/NEJM198211183072104. [DOI] [PubMed] [Google Scholar]
- 3.Lirici M.M., Califano A.D., Angelini P., Corcione F. Laparo-endoscopic single site cholecystectomy versus standard laparoscopic cholecystectomy: results of a pilot randomized trial. Am J Surg. 2011;202:45–52. doi: 10.1016/j.amjsurg.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds W., Jr. The first laparoscopic cholecystectomy. JSLS. 2001;5:89–94. [PMC free article] [PubMed] [Google Scholar]
- 5.Tiong L., Oh J. Safety and efficacy of a laparoscopic cholecystectomy in the morbid and super obese patients. HPB. 2015;17:600–604. doi: 10.1111/hpb.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okoro N., Patel A., Goldstein M., Narahari N., Cai Q. Ursodeoxycholic acid treatment for patients with postcholecystectomy pain and bile microlithiasis. Gastrointest Endosc. 2008;68:69–74. doi: 10.1016/j.gie.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Filip M., Saftoiu A., Popescu C., Gheonea D.I., Iordache S., Sandulescu L. Postcholecystectomy syndrome – an algorithmic approach. J Gastrointestin Liver Dis. 2009;18:67–71. [PubMed] [Google Scholar]
- 8.Blichfeldt-Eckhardt M.R., Ording H., Andersen C., Licht P.B., Toft P. Early visceral pain predicts chronic pain after laparoscopic cholecystectomy. Pain. 2014;155:2400–2407. doi: 10.1016/j.pain.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Lamberts M.P., Lugtenberg M., Rovers M.M., Roukema A.J., Drenth J.P., Westert G.P. Persistent and de novo symptoms after cholecystectomy: a systematic review of cholecystectomy effectiveness. Surg Endosc. 2013;27:709–718. doi: 10.1007/s00464-012-2516-9. [DOI] [PubMed] [Google Scholar]
- 10.Berger M.Y., Olde Hartman T.C., Bohnen A.M. Abdominal symptoms: do they disappear after cholecystectomy? Surg Endosc. 2003;17:1723–1728. doi: 10.1007/s00464-002-9154-6. [DOI] [PubMed] [Google Scholar]
- 11.Finan K.R., Leeth R.R., Whitley B.M., Klapow J.C., Hawn M.T. Improvement in gastrointestinal symptoms and quality of life after cholecystectomy. Am J Surg. 2006;192:196–202. doi: 10.1016/j.amjsurg.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Quallich L.G., Stern M.A., Rich M., Chey W.D., Barnett J.L., Elta G.H. Bile duct crystals do not contribute to sphincter of Oddi dysfunction. Gastrointest Endosc. 2002;55:163–166. doi: 10.1067/mge.2002.121340. [DOI] [PubMed] [Google Scholar]
- 13.Cheon Y.K., Cho Y.D., Moon J.H., Im H.H., Jung Y., Lee J.S. Effects of vardenafil, a phosphodiesterase type-5 inhibitor, on sphincter of Oddi motility in patients with suspected biliary sphincter of Oddi dysfunction. Gastrointest Endosc. 2009;69:1111–1116. doi: 10.1016/j.gie.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Topazian M., Salem R.R., Robert M.E. Painful cystic duct remnant diagnosed by endoscopic ultrasound. Am J Gastroenterol. 2005;100:491–495. doi: 10.1111/j.1572-0241.2005.41153.x. [DOI] [PubMed] [Google Scholar]
- 15.Walsh R.M., Ponsky J.L., Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc. 2002;16:981–984. doi: 10.1007/s00464-001-8236-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee T.H., Han J.H., Kim H.J., Park S.M., Park S.H., Kim S.J. Is the addition of choleretic agents in multiple double-pigtail biliary stents effective for difficult common bile duct stones in elderly patients? A prospective, multicenter study. Gastrointest Endosc. 2011;74:96–102. doi: 10.1016/j.gie.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Leiss O., von Bergmann K. Effect of Rowachol on biliary lipid secretion and serum lipids in normal volunteers. Gut. 1985;26:32–37. doi: 10.1136/gut.26.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis W.R., Somerville K.W., Whitten B.H., Bell G.D. Pilot study of combination treatment for gall stones with medium dose chenodeoxycholic acid and a terpene preparation. Br Med J Clin Res Ed. 1984;289:153–156. doi: 10.1136/bmj.289.6438.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han J., Moon J.H., Koo H.C., Kang J.H., Choi J.H., Jeong S. Effect of biliary stenting combined with ursodeoxycholic acid and terpene treatment on retained common bile duct stones in elderly patients: a multicenter study. Am J Gastroenterol. 2009;104:2418–2421. doi: 10.1038/ajg.2009.303. [DOI] [PubMed] [Google Scholar]
- 20.Galandi D., Schwarzer G., Bassler D., Allgaier H.P. Ursodeoxycholic acid and/or antibiotics for prevention of biliary stent occlusion. Cochrane Database Syst Rev. 2002:CD003043. doi: 10.1002/14651858.CD003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luman W., Ghosh S., Palmer K.R. A combination of ciprofloxacin and Rowachol does not prevent biliary stent occlusion. Gastrointest Endosc. 1999;49:316–321. doi: 10.1016/s0016-5107(99)70007-6. [DOI] [PubMed] [Google Scholar]
- 22.Gaby A.R. Nutritional approaches to prevention and treatment of gallstones. Altern Med Rev. 2009;14:258–267. [PubMed] [Google Scholar]
- 23.Cho K.S., Baek S.Y., Kang B.C., Choi H.Y., Han H.S. Evaluation of preoperative sonography in acute cholecystitis to predict technical difficulties during laparoscopic cholecystectomy. J Clin Ultrasound. 2004;32:115–122. doi: 10.1002/jcu.20001. [DOI] [PubMed] [Google Scholar]
- 24.Heits N., Meer G., Bernsmeier A., Guenther R., Malchow B., Kuechler T. Mode of allocation and social demographic factors correlate with impaired quality of life after liver transplantation. Health Qual Life Outcomes. 2015;13:162. doi: 10.1186/s12955-015-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olesen S.S., Juel J., Nielsen A.K., Frokjaer J.B., Wilder-Smith O.H., Drewes A.M. Pain severity reduces life quality in chronic pancreatitis: implications for design of future outcome trials. Pancreatology. 2014;14:497–502. doi: 10.1016/j.pan.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Yun Y.H., Park Y.S., Lee E.S., Bang S.M., Heo D.S., Park S.Y. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004;13:863–868. doi: 10.1023/B:QURE.0000021692.81214.70. [DOI] [PubMed] [Google Scholar]
- 27.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:834–840. doi: 10.1016/j.jclinepi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Thistle J.L., Longstreth G.F., Romero Y., Arora A.S., Simonson J.A., Diehl N.N. Factors that predict relief from upper abdominal pain after cholecystectomy. Clin Gastroenterol Hepatol. 2011;9:891–896. doi: 10.1016/j.cgh.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Girometti R., Brondani G., Cereser L., Como G., Del Pin M., Bazzocchi M. Post-cholecystectomy syndrome: spectrum of biliary findings at magnetic resonance cholangiopancreatography. Br J Radiol. 2010;83:351–361. doi: 10.1259/bjr/99865290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Statistics Korea [Internet]. Daejeon2014.
- 31.Clegg R.J., Middleton B., Bell G.D., White D.A. Inhibition of hepatic cholesterol synthesis and S-3-hydroxy-3-methylglutaryl-CoA reductase by mono and bicyclic monoterpenes administered in vivo. Biochem Pharmacol. 1980;29:2125–2127. doi: 10.1016/0006-2952(80)90183-5. [DOI] [PubMed] [Google Scholar]
- 32.Kim G.H., Lee H.D., Kim M., Kim K., Jeong Y., Hong Y.J. Fate of dyspeptic or colonic symptoms after laparoscopic cholecystectomy. J Neurogastroenterol Motil. 2014;20:253–260. doi: 10.5056/jnm.2014.20.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]