Abstract
Background
The relation between para-aortic lymph nodes (PALN) involvement and pancreatic ductal adenocarcinoma (PDAC) survival, along with the optimal handling of this particular lymph node station remain unclear. A systematic review and meta-analysis was performed to assess this.
Methods
A search of Medline, Embase, Ovid and Cochrane databases was performed until July 2015 to identify studies reporting on the relation of PALN involvement and PDAC outcomes and a meta-analysis was performed following data extraction.
Results
Ten retrospective studies and two prospective non randomized studies (2467 patients) were included. Patients with positive PALN had worse one (p < 0.00001) and two year (p < 0.00001) survival when compared with patients with negative PALN. Even when comparing only patients with positive lymph nodes (N1), patients with PALN involvement presented with a significant lower one (p = 0.03) and two (p = 0.002) year survival. PALN involvement was associated with an increased possibility of positive margin (R1) resection (p < 0.00001), stations' 12, 14 and 17 malignant infiltration (p < 0.00001), but not with tumour stage (p = 0.78).
Discussion
Involvement of PALN is associated with decreased survival in pancreatic cancer patients. However, existence of long term survivors among this subgroup of patients should be further evaluated, in order to identify factors associated with their favourable prognosis.
Introduction
Despite recent advances in medical therapies, molecular biology and surgical techniques, pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in the United States.1 Only a small subset of patients are diagnosed with local disease and without distant metastases but, even with these favourable factors present, long term survival rarely exceeds 20%.2 Nodal status is considered as one of the most important prognostic factors for survival, while positive nodes are found in up to 90% of patients undergoing resection.3 Apart from the obvious discrimination between patients with positive (N1) and negative nodes (N0), many studies have tried to identify subgroups of patients, especially among N1 patients that may have different survival rates. Thus, different subsets of patients according to lymph node ratio (LNR) and node stations have been studied in an attempt to a more in-depth analysis of factors affecting survival.4, 5 One of the most controversial topics regarding these efforts remains the role and management of para-aortic nodes (PALN, station 16).
The necessity of para-aortic node excision during pancreatectomy either for oncological reasons or for accurate staging remains an area of debate. Resection of station 16 has been defined as part of an extended resection for pancreaticoduodenectomy although no specific consensus has been reached for station 16b1.6 Even more conflicting are the results regarding the effect on survival, with some studies reporting an adverse effect of positive PALN on survival, while others fail to reach a sound conclusion.7, 8 Consequently, resecting PALN for either frozen section or definite pathology, varies depending on the policy of individual surgeons or institutions.
The aim of this study was to define the optimal management of PALN for patients with pancreatic cancer by reviewing the current evidence regarding survival of patients with pancreatic cancer by PALN status and identifying any correlation between positive PALN and other clinicopathologic features.
Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.9 Study selection and data extraction were carried out independently by two reviewers.
Search strategy
A search of MEDLINE, EMBASE, OVID and COCHRANE databases was performed on all studies reporting on the impact of para-aortic nodes upon outcomes following resection for pancreatic cancer. The following Mesh terms were used and combined: pancreatic cancer, pancreatic neoplasms, lymph nodes, para-aortic lymph nodes, aortocaval lymph nodes, LN 16, LN 16b1, station 16. Last search was performed on July 2015.
Inclusion criteria
The inclusion criteria were: (i) report on the status of para-aortic or group 16 lymph nodes for pancreatic cancer, (ii) report of the number of patients included (minimum 10 patients), (iii) report of at least one outcome measures. Studies from the same institution or/and authors were included in the review provided there was no patients' overlap. In the event of patients' overlap, the study of higher quality or with the larger number of patients was analysed. The quality of the included studies was assessed with the tool adopted by Taylor et al. 10 Two independent reviewers (CA, NG) extracted the data. Discrepancies in the assessment of included studies and/or data were resolved by consensus among the authors.
Exclusion criteria
Studies were excluded in the event of: (i) unclear status of para-aortic or group 16 lymph nodes, (ii) mixed results for periampullary tumours (iii) considerable overlap between authors/centres or patient cohorts and (iv) inability to calculate necessary data from the published results.
Data extraction
The following data were extracted from each included study: (i) first author, (ii) year of publication, (iii) design of the study, (iv) patients' demographics, (v) tumour location, (vi) intraoperative outcomes, (vii) immediate postoperative outcomes (morbidity, mortality, hospital stay duration), (viii) total number and number of involved retrieved lymph nodes, (ix) lymph node mapping and status of each lymph node group, (x) grade of tumour, (xi) stage of the disease, (xii) loco-regional recurrence rate, (xiii) distant recurrence rate, and (xiv) overall and disease free survival.
Outcomes of interest
Outcomes of interest included number and status of retrieved lymph nodes, mapping of lymph nodes groups and overall survival.
Statistical analysis
Qualitative outcomes were expressed as percentages over the total number of patients. Quantitative outcomes were expressed as overall mean. Meta-analytical techniques were used to compare outcomes between 16+ and 16− patients. The meta-analysis was in accordance with the recommendations from the Cochrane Collaboration and the Quality of Reporting of Meta-analyses guidelines. Odds ratio (OR) was used as the summary statistic to perform statistical analysis of dichotomous variables and was reported with 95% confidence intervals (CI). Odds ratios represent the odds of an event occurring in the 16+ group compared with the 16− group. OR < 1 favoured the 16− group, and the point estimate of the OR was considered to be statistically significant at the p < 0.05 level if the 95% CI did not include the value one. Two strategies were used to quantitatively assess heterogeneity. A fixed (weighted with inverse variance) or a random effects model was used for this meta-analysis. Heterogeneity between studies was assessed by the chi-square and I2 statistic. Higher chi-square and I2 statistic indicates greater heterogeneity between studies. The assumption of homogeneity between the groups was deemed invalid if the p-value was less than 0.1 and the random effects model was reported after exploring the causes of heterogeneity. Otherwise, the fixed-effects model was reported. All meta-analyses were performed with Review Manager Version 5.3.3 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. The analysis of the association between T-stage and PALN status was done using the chi-square test and was performed with the use of SPSS software package for Windows (IBM SPSS Statistics version 21, Chicago, Illinois, USA).
Results
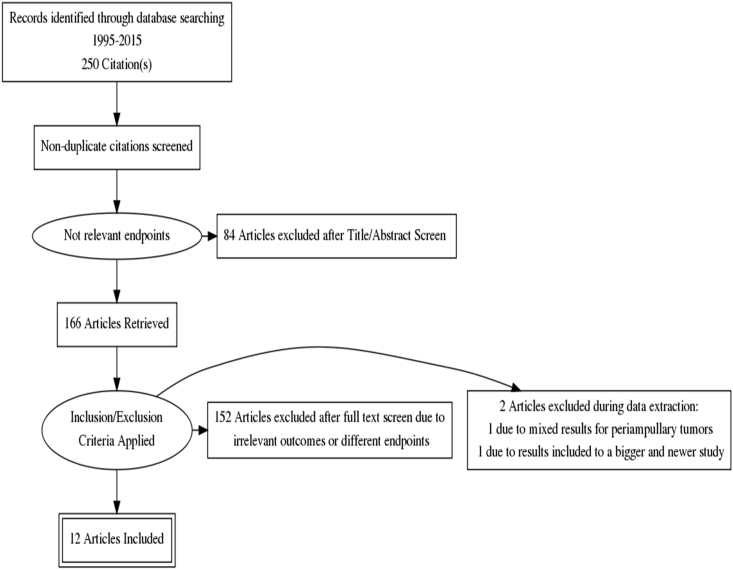
Literature search retrieved 250 studies without any duplicates of which 12 were included for final analysis. A PRISMA flow chart showing the reasons for exclusion at each stage of the study process is presented in Fig. 1.
Figure 1.
PRISMA flow chart of the study selection process
Characteristics of the studies
Ten studies were retrospective7, 8, 11, 12, 13, 14, 15, 16, 17, 18 and two prospective,19, 20 with a total of 2467 patients and a mean age of 63 years. Two studies presented results regarding only pancreaticoduodenectomies, whereas the remaining studies used data also on total and/or distal pancreatectomies. Five studies included results for the number of PALN retrieved. Characteristics of the included studies are shown in Table 1.
Table 1.
Studies' characteristics
| Study | Year | Type | Patients | PD (%) | Total (%) | Distal (%) | Patients with PALNs+ (%) | PALN/patient (mean/sd) |
|---|---|---|---|---|---|---|---|---|
| Kayahara8 | 1999 | Retrospective | 99 | 62 (63) | 16 (16) | 21 (21) | 18 (18) | N/S |
| Yoshida18 | 2004 | Retrospective | 34 | N/S | N/S | 0 | 9 (26) | N/S |
| Sakai14 | 2005 | Retrospective | 178 | 124 (69) | 0 | 54 (31) | 34 (19) | 7 (8.25) |
| Shimada15 | 2006 | Retrospective | 133 | 87 (65) | 41 (31) | 5 (4) | 27 (20) | N/S |
| Doi7 | 2007 | Retrospective | 133 | 133 (100) | 0 | 0 | 19 (14) | N/S |
| Yamada17 | 2009 | Retrospective | 335 | 206 (61) | 59 (18) | 70 (21) | 45 (13) | 7.4 (8) |
| Kurosaki19 | 2009 | Prospective | 27 | 23 (85) | 0 | 4 (15) | 7 (26) | N/S |
| Kanda12 | 2011 | Retrospective | 429 | 278 (65) | 73 (17) | 78 (18) | 49 (11) | N/S |
| Choi11 | 2013 | Retrospective | 99 | 85 (86) | 13 (13) | 1 (1) | 10 (10)a | 4.9 (4.5) |
| Schwarz20 | 2014 | Prospective | 111 | 111 (100) | 0 | 0 | 32 (29)b | N/S |
| Sho16 | 2015 | Retrospective | 822 | 617 (75) | 161 (20) | 44 (5) | 102 (12) | 4.3 (4.2) |
| Paiella13 | 2015 | Retrospective | 67 | 63 (94) | 0 | 4 (6) | 14 (21) | 5 (5.49) |
| 2467 | 1789 (72.5) | 363 (14.5) | 315 (13) | 366 (14.8) |
PD: pancreaticoduodenectomy.
N/S: not stated.
One patient had only micrometastasis.
15 patients had micrometastasis.
Overall survival
Eleven studies reported data on median survival in patients undergoing pancreatectomy. Data of the included studies on survival rates are shown in Table 2.
Table 2.
Overall survival (months)
| Study | All patients |
p-Value | N1 patients |
p-Value | ||
|---|---|---|---|---|---|---|
| PALN− | PALN+ | PALN− | PALN+ | |||
| Doi7 | 12.4 (1.175) | 5.1 (1.325) | <0.001 | |||
| Kayahara8 | 12 | 8.4 | 0.050 | |||
| Choi11 | 31 (3.55) | 17 (2.2) | 0.008 | |||
| Kanda12 | 11.5 | 8.3 | 0.006 | |||
| Sakai14 | 9 | 8.1 | 0.117 | |||
| Schwarz20 | 27.2 | 15.7 | 0.050 | 21 | 15.1 | 0.110 |
| Shimada15 | 30 | 13 | <0.001 | |||
| Yamada17 | 11 | 8 | 0.002 | |||
| Yoshida18 | 24.8 | 8 | 0.003 | |||
| Sho16 | 22.6 | 16.9 | <0.001 | |||
| Paiella13 | 30 | 17 | <0.001 | |||
Numbers express mean values (SD) in months, (SD) where it could be extracted.
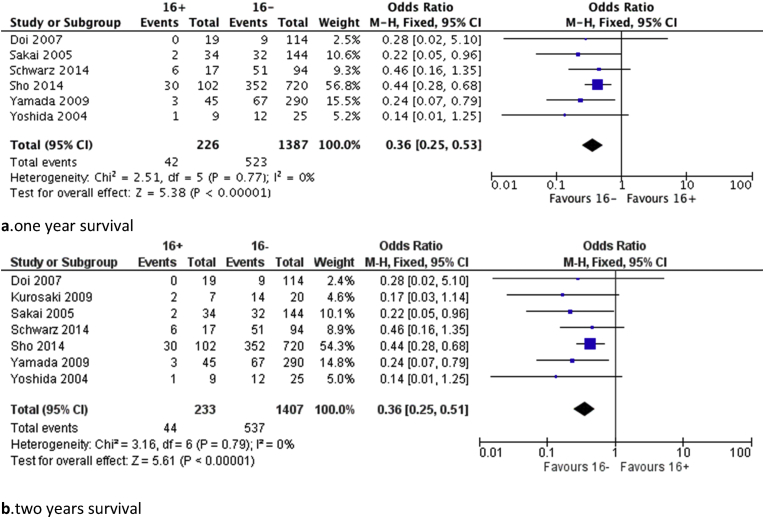
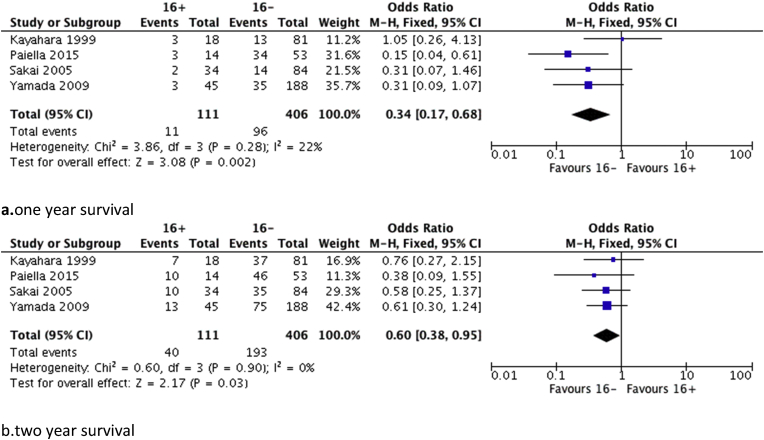
Further analysis based on available data from the studies was performed regarding one and two-year survival. Seven studies were included in the analysis comparing all patients and those with positive para-aortic lymph nodes. Results regarding either 1 or 2-year survival demonstrated a significant benefit in favour of the PALN negative group (p < 0.001) and are shown in Fig. 2. Data on survival rates for N1 patients were available from four studies. N1 patients with negative PALN showed a significantly better 1 (p = 0.030) and 2-year (p = 0.002) survival compared with those with positive PALN as it is shown in Fig. 3.
Figure 2.
Forest plots regarding survival rates between 16+ patients and 16− patients (N0 and N1), a. one year, b. two years
Figure 3.
Forest plots regarding survival rates between 16+ patients and 16− patients (N1), a. one year, b. two years
Association of PALN with clinicopathologic features
One study identified a significant correlation between positive PALN and perineural invasion8 whereas in the study of Paiella et al., perineural invasion was not associated with PALN positive status.13 Six studies presented data on T-stage and 4 of them concluded that infiltration of station 16 lymph nodes was strongly associated with increased T-stage and especially T3 and T4 tumours.8, 12, 13, 14, 15, 16, 18, 20 In this study an analysis was performed to identify possible relation of T status with PALN invasion, grouping patients either as T1/T2 or T3/T4. No significant association between T-stage and PALN invasion could be revealed (p = 0.78) (Table 3).
Table 3.
Association of T-stage with station 16 infiltration
| Study | 16+ patients |
16− patients |
p | ||
|---|---|---|---|---|---|
| T1/T2 | T3/T4 | T1/T2 | T3/T4 | ||
| Kayahara8 | 11/18 (61) | 7/18 (39) | 51/81 (63) | 30/81 (37) | |
| Choi11 | 0/10 (0) | 10/10 (100) | 9/89 (10) | 80/89 (90) | |
| Kanda12 | 0/49 (0) | 49/49 (100) | 23/380 (6) | 357/380 (94) | |
| Sho16 | 2/102 (1) | 100/102 (99) | 61/720 (8) | 659/720 (92) | |
| Paiella13 | 0/14 (0) | 14/14 (100) | 3/53 (5) | 50/53 (95) | |
| Overall | 13/193 (7) | 180/193 (93) | 147/1323 (11) | 1176/1323 (89) | 0.78 |
Numbers in parentheses express percentages.
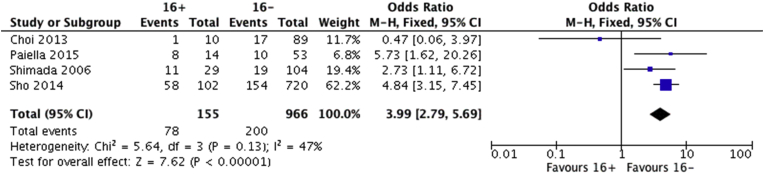
Only four studies included analysis regarding R status at the final pathology and all apart from the latest study from Italy reached the conclusion that PALN metastasis was correlated with R1 status.11, 13, 15, 16 After pooling the aforementioned data, a significant association was identified between R1 status and PALN invasion as displayed in Fig. 4.
Figure 4.
Forest plot comparing R1 resections between 16+ and 16− patients
Association with different lymph node stations
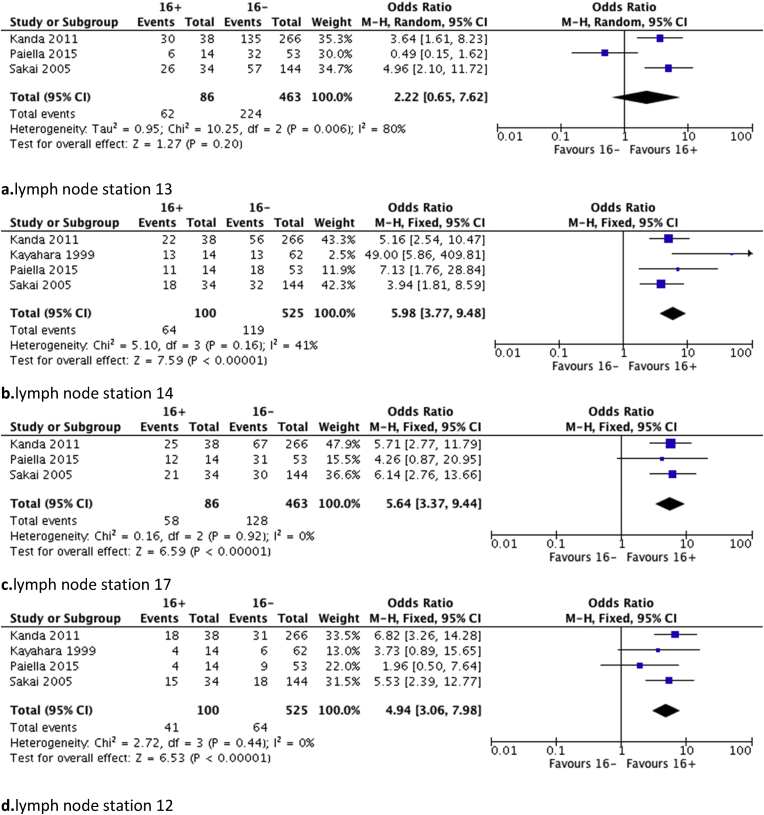
Only five out of the 12 included studies reported data on other lymph node stations' status. In the study by Kanda et al., the only lymph node station strongly associated with positive PALN was station 12, also reported in the study by Sakai et al.12, 14 In three studies, a significant association was found between lymph node stations 14 and 16,8, 13, 14 whereas also in three studies such an association could be identified between stations 13 and 16.8, 14, 18 Moreover, in one study station 17 was also found to be strongly associated with station 16 metastasis.14 Finally, only in the study by Kayahara et al. presented the results regarding distal pancreatectomies but without reaching any significant association.8
Pooled analysis showed that positive lymph nodes of stations 12, 14 and 17 are significantly associated with the presence of malignant invasion of station 16 (p < 0.001). On the contrary, station 13 invasion is not associated with the possibility of having 16+ patients (Fig. 5).
Figure 5.
Forest plots of association between 16+ patients and other infiltrated lymph node stations, a. station 13, b. station 14, c. station 17, d. station 12
Factors associated with positive PALN or survival in patients with positive PALN
Some studies have reported other associations of positive PALN with clinicopathologic or laboratory factors. Moreover two studies have analysed factors associated with survival in patients with positive PALN. These results are presented in Table 4.
Table 4.
Factors associated with survival and positive PALN
| Study | Factors influencing survival | Factors associated with positive PALN |
|---|---|---|
| Yamada17 | Age <59 years (p = 0.001), tumours >4 cm (p = 0.007), pPV(+) (p = 0.036), perineural invasion (p = 0.111) | |
| Kanda12 | Arterial infiltration (p = 0.006), perineural invasion (p < 0.001) | |
| Sakai14 | Distal bile duct invasion (p < 0.050) | |
| Shimada15 | Postoperative elevated CA 19-9 (p = 0.030) | |
| Sho16 | Number of positive PALN (p < 0.001), postoperative chemotherapy (p < 0.001) | Pre- and post-operative elevated CA 19-9 (p < 0.001) |
| Paiella13 | G3 tumours (p = 0.025), >8 involved lymph nodes (p = 0.002) |
pPV equals for pathologic Portal Vein invasion.
Discussion
The present review and meta-analysis clearly demonstrates a decreased 1 and 2-year survival rate in patients operated for pancreatic adenocarcinoma with positive para-aortic lymph node involvement, when compared with those having no infiltration of this particular lymph node station. These results are of course expected when comparing populations with and without positive PALNs, because in the latter group are also included patients with N0 status, which are known of bearing better survival rates.21 On the other hand, the adverse effect of station 16 infiltration on survival is becoming clearer when comparing N1 patients. One and two-year survival rates are significantly higher in patients with N1 16− status as it is demonstrated in this meta-analysis. Analysis for more extended survival was not performed, mainly because in the included studies five year survival was not reached especially in N1 patients.
The critical issue is how the information derived from this meta-analysis could be of clinical value for patients. There are studies proposing that the presence of positive PALN during frozen section analysis, should be regarded as a contraindication for surgery and that these patients should be offered other palliative treatment and not surgery.7, 15, 20 On the other hand, other investigators propose that intraoperative positive findings in station 16 should not prevent surgeons from performing a resection, based on presence of long term survivors in this group of patients and the beneficial effect of aggressive adjuvant therapy.11, 16, 17 Moreover, although in some studies PALN infiltration is regarded as M1 disease, extended survival of this subgroup of patients compared with those with liver or peritoneal metastasis, should justify pancreatic resection with interaortocaval clearance.22 The results of the present meta-analysis, highlight the survival superiority of patients with negative PALN, but certainly cannot propose abandoning of resection in those with positive PALN. However, in certain patients an intraoperative decision has to be made based on the characteristics of the tumour, like vein invasion and local extension, along with patient characteristics like the presence of severe co morbidities.
Furthermore, a significant correlation of PALN involvement and positive surgical margins is demonstrated by this meta-analysis. Taken into account that PALN involvement is considered as the next step following peripancreatic and superior mesenteric lymphatic spread in patients with pancreatic cancer,23 association between mesopancreas clearance and PALN dissection seems anatomically inevitable. The term “mesopancreas” refers to an area without boundaries including areolar and adipose tissue, with blood and lymphatic vessels without a true fascia or a sheath surrounding them.24, 25 Mesopancreas' extension in the retroperitoneal area, the anatomic site of embryologic fusion of peritoneal layers is also important to understand the connection with the PALN.26 Connor et al. suggested that station 16 involvement maybe a reflection of local invasion through the fascia of Treitz and not a real second line nodal involvement,27 whereas Sho et al. recognized the increased rate of posterior positive margins in these patients. Identification of this association should lead to an extensive mesopancreas dissection which by definition will include the para-aortic area.16 This by definition is leading to an «extended lymphadenectomy», highlighting not only the problems regarding the optimal handling of this particular lymph node station, but also the definition of extended resection because due to the aforementioned reasons, this is the correct resection that follows the embryological planes of pancreas.
Another emerging issue is the lymph routes that lead in station 16 invasion, along with the presence and role of micrometatastases. In two of the pivotal studies regarding the lymph node spreading in pancreatic cancer, the main proven routes towards the para-aortic region were through the posterior part of the pancreatic head and around the superior mesenteric artery, considered as stations 13 and 14 respectively.28, 29 Sakai et al., found that only 3% of the patients had para-aortic metastasis without involvement of either stations 13, 14 or 17.14 Similar results, especially regarding station 14 are presented in the studies of Paiella and Kayahara, leading investigators in using the term «junctional lymph nodes» for the aforementioned lymph node stations, highlighting their role in the pancreatic cancer lymph spread.8, 13 This important role is partly proven by this meta-analysis. Stations 12, 14 and 17 are significantly associated with the presence of station 16 metastasis, whereas this is not the case with station 13, which can only be explained by the presence of skip metastases or the presence of micrometastases. Especially the latter seems to play a significant role in other solid organs cancers like bile duct and colon,30, 31 but their presence in pancreatic cancer patients has not been until now proven of being associated with worse survival.11, 20
Consequently, one of the most attractive fields of research should be the preoperative recognition of metastatic PALN. Unfortunately, until now no imaging modality has been able to clarify the nature of these nodes in the staging of pancreatic cancer. In the study of Maemura et al. positron emission tomography (PET) had a sensitivity of 50%,32 whereas another study using computed tomography (CT), magnetic resonance imaging (MRI) and 18F-flurodeoxyglucose positron emission tomography (FDG-PET) reached a disappointing sensitivity of 0% in identifying metastasis in para-aortic nodes.33 Newer, more evolved imaging tools, like the promising nano-particle enhanced MRI,34 are needed in order to preoperatively identify this subset of patients with increased probability of para-aortic lymph node metastasis. Moreover, as demonstrated by the studies of Shimada and Sho preoperative elevated CA 19-9 is strongly associated with PALN infiltration and could be combined with preoperative imaging in the effort of identifying these patients.15, 16, 25 This combination could be used as a useful tool in individualizing patient treatment, like selecting those that will probably benefit from the use of neoadjuvant regimens.
Pooled analysis performed in this study clearly shows that patients with positive para-aortic lymph nodes present with decreased rates of survival. On the other hand, long term survivors can be found even in this group of patients. Adjuvant chemotherapy is one of the factors affecting survival of these patients. In the near future tailored therapy based on biological markers and genetic alterations could further improve survival rates.16 Furthermore, number of positive PALN, age and tumour size are associated with survival in PALN positive patients and may also be used as a useful differentiation tool.16, 17, 35 Individualizing pre- and post-operative treatment based on the aforementioned prognostic factors, can increase survival rates in this subset of patients.
The relatively small number of included studies and their heterogeneity, especially in terms of R status definition, is the first limitation of this meta-analysis. Moreover, a possible source of bias could be the design of the included studies, which are all but one retrospective. Finally, lack of a standard consensus in which para-aortic clearance is defined as extended or standard lymphadenectomy and especially the fact this particular lymph node station was not resected in a routine fashion in most of the studies, has possibly led to severe biases regarding survival rates. Large prospective or randomized trials, with intraoperative routine sampling of para-aortic space and lymph nodes, should be conducted in order to provide safe results regarding the role of PALN in pancreatic cancer.
Conclusions
Infiltration of para-aortic lymph nodes is associated with decreased survival in pancreatic cancer patients. Station 16 involvement is associated with positive resection margins but is independent of the tumour size, whereas lymph node stations 12, 14 and 17 can be regarded as junctional to station 16. Although, PALN+ patients have a worse prognosis, recognition of the factors affecting their survival and identification of the characteristics of the long term survivors should be the tool which will guide the selection of the optimal treatment modality for these pancreatic cancer patients.
Funding sources
None.
Conflicts of interest
Authors declare there are no conflicts of interest.
References
- 1.Society AC . American Cancer Society; Atlanta: 2014. Cancer Facts & Figures. [Google Scholar]
- 2.Hartwig W., Werner J., Jager D., Debus J., Buchler M.W. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14:e476–e485. doi: 10.1016/S1470-2045(13)70172-4. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz R.E., Smith D.D. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 4.Malleo G., Maggino L., Capelli P., Gulino F., Segattini S., Scarpa A. Reappraisal of nodal staging and study of lymph node station involvement in pancreaticoduodenectomy with the standard international study group of pancreatic surgery definition of lymphadenectomy for cancer. J Am Coll Surg. 2015;221 doi: 10.1016/j.jamcollsurg.2015.02.019. 367–379 e364. [DOI] [PubMed] [Google Scholar]
- 5.Slidell M.B., Chang D.C., Cameron J.L., Wolfgang C., Herman J.M., Schulick R.D. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 6.Tol J.A., Gouma D.J., Bassi C., Dervenis C., Montorsi M., Adham M., International Study Group on Pancreatic S Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156:591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doi R., Kami K., Ito D., Fujimoto K., Kawaguchi Y., Wada M. Prognostic implication of para-aortic lymph node metastasis in resectable pancreatic cancer. World J Surg. 2007;31:147–154. doi: 10.1007/s00268-005-0730-5. [DOI] [PubMed] [Google Scholar]
- 8.Kayahara M., Nagakawa T., Ohta T., Kitagawa H., Ueno K., Tajima H. Analysis of paraaortic lymph node involvement in pancreatic carcinoma: a significant indication for surgery? Cancer. 1999;85:583–590. doi: 10.1002/(sici)1097-0142(19990201)85:3<583::aid-cncr8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor R.S., Van Buyten J.P., Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91–101. doi: 10.1016/j.ejpain.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Choi S.H., Kim S.H., Choi J.J., Kang C.M., Hwang H.K., Lee W.J. Clinical necessity of the immunohistochemical reassessment of para-aortic lymph nodes in resected pancreatic ductal adenocarcinoma. Oncol Lett. 2013;6:1189–1194. doi: 10.3892/ol.2013.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanda M., Fujii T., Nagai S., Kodera Y., Kanzaki A., Sahin T.T. Pattern of lymph node metastasis spread in pancreatic cancer. Pancreas. 2011;40:951–955. doi: 10.1097/MPA.0b013e3182148342. [DOI] [PubMed] [Google Scholar]
- 13.Paiella S., Malleo G., Maggino L., Bassi C., Salvia R., Butturini G. Pancreatectomy with para-aortic lymph node dissection for pancreatic head adenocarcinoma: pattern of nodal metastasis spread and analysis of prognostic factors. J Gastrointest Surg – Off J Soc Surg Aliment Tract. 2015;19:1610–1620. doi: 10.1007/s11605-015-2882-4. [DOI] [PubMed] [Google Scholar]
- 14.Sakai M., Nakao A., Kaneko T., Takeda S., Inoue S., Kodera Y. Para-aortic lymph node metastasis in carcinoma of the head of the pancreas. Surgery. 2005;137:606–611. doi: 10.1016/j.surg.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Shimada K., Sakamoto Y., Sano T., Kosuge T. The role of paraaortic lymph node involvement on early recurrence and survival after macroscopic curative resection with extended lymphadenectomy for pancreatic carcinoma. J Am Coll Surg. 2006;203:345–352. doi: 10.1016/j.jamcollsurg.2006.05.289. [DOI] [PubMed] [Google Scholar]
- 16.Sho M., Murakami Y., Motoi F., Satoi S., Matsumoto I., Kawai M. Postoperative prognosis of pancreatic cancer with para-aortic lymph node metastasis: a multicenter study on 822 patients. J Gastroenterol. 2015;50:694–702. doi: 10.1007/s00535-014-1005-4. [DOI] [PubMed] [Google Scholar]
- 17.Yamada S., Nakao A., Fujii T., Sugimoto H., Kanazumi N., Nomoto S. Pancreatic cancer with paraaortic lymph node metastasis: a contraindication for radical surgery? Pancreas. 2009;38:e13–e17. doi: 10.1097/MPA.0b013e3181889e2d. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida T., Matsumoto T., Sasaki A., Shibata K., Aramaki M., Kitano S. Outcome of paraaortic node-positive pancreatic head and bile duct adenocarcinoma. Am J Surg. 2004;187:736–740. doi: 10.1016/j.amjsurg.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Kurosaki I., Kawachi Y., Nihei K., Tsuchiya Y., Aono T., Yokoyama N. Liver perfusion chemotherapy with 5-Fluorouracil followed by systemic gemcitabine administration for resected pancreatic cancer: preliminary results of a prospective phase 2 study. Pancreas. 2009;38:161–167. doi: 10.1097/MPA.0b013e31818815f7. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz L., Lupinacci R.M., Svrcek M., Lesurtel M., Bubenheim M., Vuarnesson H. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br J Surg. 2014;101:530–538. doi: 10.1002/bjs.9444. [DOI] [PubMed] [Google Scholar]
- 21.Bassi C., Stocken D.D., Olah A., Friess H., Buckels J., Hickey H., European Study Group for Pancreatic C Influence of surgical resection and post-operative complications on survival following adjuvant treatment for pancreatic cancer in the ESPAC-1 randomized controlled trial. Dig Surg. 2005;22:353–363. doi: 10.1159/000089771. [DOI] [PubMed] [Google Scholar]
- 22.Shrikhande S.V., Kleeff J., Reiser C., Weitz J., Hinz U., Esposito I. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2007;14:118–127. doi: 10.1245/s10434-006-9131-8. [DOI] [PubMed] [Google Scholar]
- 23.Peparini N., Chirletti P. Mesopancreas: a boundless structure, namely R1 risk in pancreaticoduodenectomy for pancreatic head carcinoma. Eur J Surg Oncol – J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2013;39:1303–1308. doi: 10.1016/j.ejso.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Bouassida M., Mighri M.M., Chtourou M.F., Sassi S., Touinsi H., Hajji H. Retroportal lamina or mesopancreas? Lessons learned by anatomical and histological study of thirty three cadaveric dissections. Int J Surg. 2013;11:834–836. doi: 10.1016/j.ijsu.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Gaedcke J., Gunawan B., Grade M., Szoke R., Liersch T., Becker H. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: relevance for clinical trials. Langenbeck's Arch Surg/Deutsche Ges Chir. 2010;395:451–458. doi: 10.1007/s00423-009-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agrawal M.K., Thakur D.S., Somashekar U., Chandrakar S.K., Sharma D. Mesopancreas: myth or reality? JOP – J Pancreas. 2010;11:230–233. [PubMed] [Google Scholar]
- 27.Connor S., Bosonnet L., Ghaneh P., Alexakis N., Hartley M., Campbell F. Survival of patients with periampullary carcinoma is predicted by lymph node 8a but not by lymph node 16b1 status. Br J Surg. 2004;91:1592–1599. doi: 10.1002/bjs.4761. [DOI] [PubMed] [Google Scholar]
- 28.Nagai H., Kuroda A., Morioka Y. Lymphatic and local spread of T1 and T2 pancreatic cancer. A study of autopsy material. Ann Surg. 1986;204:65–71. doi: 10.1097/00000658-198607000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagakawa T., Kobayashi H., Ueno K., Ohta T., Kayahara M., Miyazaki I. Clinical study of lymphatic flow to the paraaortic lymph nodes in carcinoma of the head of the pancreas. Cancer. 1994;73:1155–1162. doi: 10.1002/1097-0142(19940215)73:4<1155::aid-cncr2820730406>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Rahbari N.N., Bork U., Motschall E., Thorlund K., Buchler M.W., Koch M. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol – Off J Am Soc Clin Oncol. 2012;30:60–70. doi: 10.1200/JCO.2011.36.9504. [DOI] [PubMed] [Google Scholar]
- 31.Yonemori A., Kondo S., Matsuno Y., Ito T., Tanaka E., Hirano S. Prognostic impact of para-aortic lymph node micrometastasis in patients with regional node-positive biliary cancer. Br J Surg. 2009;96:509–516. doi: 10.1002/bjs.6585. [DOI] [PubMed] [Google Scholar]
- 32.Maemura K., Takao S., Shinchi H., Noma H., Mataki Y., Kurahara H. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepato-biliary-pancreatic Surg. 2006;13:435–441. doi: 10.1007/s00534-006-1102-8. [DOI] [PubMed] [Google Scholar]
- 33.Imai H., Doi R., Kanazawa H., Kamo N., Koizumi M., Masui T. Preoperative assessment of para-aortic lymph node metastasis in patients with pancreatic cancer. Int J Clin Oncol. 2010;15:294–300. doi: 10.1007/s10147-010-0066-5. [DOI] [PubMed] [Google Scholar]
- 34.McDermott S., Thayer S.P., Fernandez-Del Castillo C., Mino-Kenudson M., Weissleder R., Harisinghani M.G. Accurate prediction of nodal status in preoperative patients with pancreatic ductal adenocarcinoma using next-gen nanoparticle. Transl Oncol. 2013;6:670–675. doi: 10.1593/tlo.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami Y., Uemura K., Sudo T., Hashimoto Y., Yuasa Y., Sueda T. Prognostic impact of para-aortic lymph node metastasis in pancreatic ductal adenocarcinoma. World J Surg. 2010;34:1900–1907. doi: 10.1007/s00268-010-0577-2. [DOI] [PubMed] [Google Scholar]