Abstract
Background
Hookworms (Necator americanus and Ancylostoma duodenale) remain a major public health problem worldwide. Infections with hookworms (e.g., A. caninum, A. ceylanicum and A. braziliense) are also prevalent in dogs, but the role of dogs as a reservoir for zoonotic hookworm infections in humans needs to be further explored.
Methodology/Principal Findings
As part of an open-label community based cluster-randomized trial in a tribal area in Tamil Nadu (India; 2013–2015), a total of 143 isolates of hookworm eggs from human stool were speciated based on a previously described PCR-RFLP methodology. The presence of hookworm DNA was confirmed in 119 of 143 human samples. N. americanus (100%) was the most prevalent species, followed by A. caninum (16.8%) and A. duodenale (8.4%). Because of the high prevalence of A. caninum in humans, dog samples were also collected to assess the prevalence of A. caninum in dogs. In 68 out of 77 canine stool samples the presence of hookworms was confirmed using PCR-RFLP. In dogs, both A. caninum (76.4%) and A. ceylanicum (27.9%) were identified. Additionally, to determine the contamination of soil with zoonotic hookworm larvae, topsoil was collected from defecating areas. Hookworm DNA was detected in 72 out of 78 soil samples that revealed presence of hookworm-like nematode larvae. In soil, different hookworm species were identified, with animal hookworms being more prevalent (A. ceylanicum: 60.2%, A. caninum: 29.4%, A. duodenale: 16.6%, N. americanus: 1.4%, A. braziliense: 1.4%).
Conclusions/Significance
In our study we regularly detected the presence of A. caninum DNA in the stool of humans. Whether this is the result of infection is currently unknown but it does warrant a closer look at dogs as a potential reservoir.
Author Summary
Hookworm infections remain a major public health problem in both tropical and subtropical parts of the world. To control the disease burden attributable to hookworms, large-scale deworming programs, in which drugs are administered to schoolchildren regardless of their infection status, are currently being implemented in endemic regions. However, these programs face some difficulties. One of them is the uncertainty about the role of animals in the transmission of hookworm infections. It is commonly believed that human-specific hookworms cause these infections, but there is growing evidence that the role of some animal-specific hookworms as cause of infection in humans should not be underestimated. We determined the different hookworms in humans, dogs and soil (eggs excreted by adult hookworms in stool are non-infectious, and need to develop and hatch on the soil before larvae can transmit disease by penetrating the skin) in a tribal area in India. In this area, the transmission of hookworms between humans and dogs is possible. Our results highlight the presence of DNA from animal-specific hookworms in both soil and human stool. Although these findings suggest that these animals could act as reservoir for zoonotic hookworm infections in humans, they should be interpreted with caution. This is because we lack the evidence to confirm A. caninum infections in our study population. Other potential reasons for the presence of DNA in stool are contamination of stool with environmental eggs or larvae during sample collection and passive passage in which eggs or larvae are ingested but did result in any infection.
Introduction
Infections with hookworms (Necator americanus and Ancylostoma duodenale) remain a major public health problem in several low and middle-income countries [1,2]. It is estimated that ~439 million people are infected, resulting in a global disease burden of ~3.5 million disability adjusted life years (DALYs; 62.3% of the DALYs attributable to soil-transmitted helminths; ~3% of all DALYs attributable to Neglected Tropical diseases (NTDs)) [3]. The major morbidity associated with hookworm infections is caused by intestinal blood loss, iron deficiency anaemia, and protein malnutrition [4], most of which occurs in children and pregnant women [5]. The current strategy to control the morbidity caused by these intestinal worms are embedded in large-scale school-based deworming programs, in which benzimidazole drugs (albendazole and mebendazole) are administered to schoolchildren regardless of their infection status [6,7]. However, it remains unclear whether these school-based deworming programs are the most efficient approach [8].
First, both prevalence and the intensity of hookworm infections increase as a function of age. Although most of the deworming programs target school-aged children, the major contributors of hookworm infection both in terms of prevalence and total egg excretion are adults, who are often not included in deworming programs [6,8]. Second, the eggs excreted in stool are non-infectious, and need to develop and hatch on the soil before larvae can transcutaneously enter the human host and cause disease [9]. Therefore it will be important to supplement deworming programs with improved water, sanitation and hygiene (WASH) to prevent re-infection [10]. Moreover, benzimidazole drugs have a moderate efficacy against hookworm and never reach 100% efficacy [11,12]. Third, it is traditionally assumed that hookworm infections are caused by the human hookworms N. americanus and A. duodenale, and hence hookworm infections in humans are solely due to the contamination of soil with human stool [13]. Infections with the hookworms (e.g. A. caninum, A. ceylanicum, A. braziliense and Uncinaria stenocephala) in dogs are also highly prevalent, and depending on the species these hookworms may also cause a variety of clinical symptoms in humans [14]. A. ceylanicum is the only known species to cause patent infection in humans [15] with symptoms ranging from gastrointestinal discomfort, epigastric pain, flatulence and diarrhoea, whereas the rest are mainly limited to lesions in the skin caused by migrating larvae (cutaneuos larva migrans) [16]. Migration to the intestine has been reported for A. caninum which may cause severe eosinophilic enteritis [14,17]. Recent studies also indicate that the role of animals as a source of hookworm infections in humans should not be ignored. For example, in a study done in a rural region of Cambodia [18] 64 out of 124 (51.6%) individuals were found to be infected with the animal hookworm A. ceylanicum, of which the majority were mono-infections (89%) [16]. Similarly, a study done in a tribal region in India [19], found that human hookworm infections (N. americanus 39/41; 95% and A. duodenale 6/41; 15%) accounted for majority of the infections, whereas the animal hookworm A. ceylanicum only accounted for a minority of the infections (2/41; 5%), and hence these findings suggest that the rate of zoonotic transmission might vary across different geographical areas.
Despite these studies, the role of animals as a reservoir for hookworm infections in human remains poorly explored. This lack of understanding of disease transmission among both humans and animals, is largely due to the fact that diagnosis of hookworm infections are based on the microscopic demonstration of eggs in stool, but it is impossible to differentiate animal and human hookworm eggs based on morphology. For this, molecular tools are more appropriate [20]. Second, various studies have identified hookworm species separately across humans [19,21,22], dogs [23,24,25] or soil [26], but to our knowledge there are no studies which have identified hookworm species using molecular techniques within both hosts and environment in the same geographical region. The present study aims at molecular identification of hookworms isolates from humans, dogs and soil from a tribal area in Tamil Nadu, India. The selection of this study area was based on (i) a high prevalence of hookworm infection in humans (38%) [27] and (ii) the presence of factors that facilitates zoonotic hookworm transmission in humans.
Methods
Ethics Statement
This study was part of an open-label, community-based cluster randomized trial that was approved by the Institutional Review Board of Christian Medical College, Vellore, India. This trial is registered in the Clinical Trials Registry of India (CTRI/2013/05/003676). A description of the ethical considerations has been described in detail elsewhere (Sarkar et al., under review). In short, a written informed consent was obtained from parents/legal guardian for the collection of stool samples from children aged less than 18 years of age and an assent was obtained from 8–17 year old children. Participants older than 18 years of age signed their own informed consent form.
Study Setting, Sample Selection and Laboratory Procedure
Jawadhu hills are situated in Vellore and Thiruvannamalai district of Tamil Nadu (southern India). It covers an area of 150 km2 and a population of approximately 80,000 of which the majority is tribal. The population is organized in 11 ‘panchayat’ (a group of villages under one local administrative council) and 229 villages [28]. The area is known to have red loamy soil [28]. The temperatures of the region ranges between 12°C and 33°C [28]. There is excessive rainfall (>1000 mm) [28], with relative humidity varying from 40 to 85% [28]. The majority of the population is employed in the agricultural sector and lives in close proximity with animals, including dogs and cats. It is important to note that these animals are not confined, and although they belong to one household are found freely roaming through the village. Across the entire area there is a common practice of open-field defecation [27].
The study was part of an open-label, community-based cluster randomized trial conducted between 2013–2015. The aim of the trial was to compare the hookworm re-infection rates for one year in a population that was subjected to varying cycles of deworming using albendazole. Therefore, 15 clusters (villages) were randomized into one of three different treatment arms: (i) single cycle, (ii) two cycles and (iii) four cycles. The timing of deworming in each of the three groups have been described in detail elsewhere (Sarkar et al., 2016; under review). In short, in the single cycle, individuals received a single oral dose of albendazole once in the beginning of the study and stool samples were collected 3, 6, 9 and 12 months post-treatment. In the treatment arm of two cycles, individuals received two single oral doses of albendazole. The first dose was given at the start of the trial and second dose after one month. The stool samples were collected 3, 6, 9 and 12 month after the administration of the second dose. In the treatment arm of four cycles, the first two doses of albendazole were given at the start of the trial with one-month interval, and an additional two doses of albendazole were given at 6 months after the 2nd dose. Stool samples were also collected after 3 and 6 months post 2nd dose and 3, 6, 9 and 12 months post 4th dose of albendazole.
In the present study, stool (humans and dogs) and soil were collected from nine clusters included in the trial (3 per treatment arm), including Seramarthur, Jambudee, Alanjanur, Sinthalur, Koothatur, Villichanur, Keel Nadanur, Thimirimarathur and Pudhupattu. In 2013 the total population of the 9 villages was 2,906 habitants (1,492 males and 1,414 females) belonging to 683 families.
Human stool samples were collected as per trial protocol described above. Based on the treatment arm, stool samples were collected at an interval of three months until the end of one year after the last treatment. Field workers visited the house of the study a day before collection was scheduled and handed over a plastic stool container and wooden spatula. Containers were appropriately labelled. The stool samples were collected the following day and stored at the study area at 4°C before being transported to the laboratory using cold containers. In total 2,152 stool samples were collected from 711 individuals from the 9 selected villages. All samples were screened microscopically applying a saline wet mount. Stool in which hookworm eggs were found were subsequently screened using the McMaster egg counting method to estimate the intensity of infection (faecal egg counts (FECs) expressed in number of eggs per gram of stool (EPG)). Finally, one stool sample per infected subject was withheld for molecular analysis, and stored at -70°C. If hookworm eggs were found in multiple samples from the same individual, the sample with the highest FEC was selected. A total of 146 out of the 711 individuals were found to be excreting eggs in at least one time point. The median (25th quantile (Q25) - 75th quantile (Q75)) FEC among the infected individuals was 550 EPG (200–1,000). The number of infected humans and the corresponding median FEC across the different villages are summarized in Table 1.
Table 1. Molecular characterization of hookworm across 9 villages in human stool collected from Jawadhu hills.
| Village | N | Microscopy positive | Median FEC | PCR positive | Molecular characterization of hookworm | ||
|---|---|---|---|---|---|---|---|
| (Q25—Q75) | N. americanus | A. duodenale | A. caninum | ||||
| Alanjanur | 59 | 7 | 250 (150–1650) | 6 | 6 | 0 | 6 |
| Jambudee | 75 | 26 | 500 (200–950) | 20 | 20 | 1 | 2 |
| Keel Nadanur | 74 | 14 | 1,300 (500–1700) | 13 | 13 | 0 | 5 |
| Kootathur | 107 | 22 | 550 (250–750) | 21 | 21 | 0 | 4 |
| Pudhupattu | 120 | 11 | 300 (150–650) | 8 | 8 | 0 | 1 |
| Seramarathur | 63 | 23 | 1,100 (500–3450) | 23 | 23 | 7 | 1 |
| Sinthalur | 70 | 8 | 200 (125–450) | 5 | 5 | 0 | 1 |
| Thimirimarathur | 55 | 11 | 200 (100–450) | 6 | 6 | 1 | 0 |
| Villichanur | 88 | 21 | 650 (300–900) | 17 | 17 | 1 | 0 |
| Total | 711 | 143 (20.1%) | 550 (200–1000) | 119 (83.2%) | 119 (100%) | 10 (8.4%) | 20 (16.8%) |
FEC—Fecal egg count expressed as eggs per gram of stool
Q25:25th quantile; Q75: 75th quantile of an ordered range of data.
Field workers identified 10 houses per village based on structured questionnaire that had previously claimed dog ownership, and volunteered to help collect stool samples. As the dogs in these villages were not usually confined, the owners chained their dogs for a day and stool sample was collected into the plastic stool container using a spatula after the dog defecated. In each village, a single stool sample from 10 dogs was collected (n = 90). The stool samples were collected and stored at 4°C at the site of collection. The samples were transported to the laboratory in cold containers. As with human stool samples, dog stool were first screened with saline wet mount, after which stool of infected animals were re-examined using the McMaster egg counting method. All samples containing hookworm eggs were withheld for molecular analysis, and stored at -70°C. In total, 77 out of 90 dogs excreted hookworm eggs in stool. The median (Q25—Q75) FEC across infected dogs was 350 EPG (100–650). The number of infected dogs and the corresponding median FEC across the different villages are summarized in Table 2.
Table 2. Molecular characterization of hookworm across 9 villages in dog stool collected from Jawadhu hills.
| Village | N | Microscopy positive | Median FEC | PCR positive | Molecular characterization of hookworm | |
|---|---|---|---|---|---|---|
| (Q25—Q75) | ||||||
| A. caninum | A. ceylanicum | |||||
| Alanjanur | 10 | 10 | 875 (450–1350) | 9 | 5 | 6 |
| Jambudee | 10 | 7 | 100 (50–850) | 5 | 2 | 3 |
| Keel Nadanur | 10 | 8 | 350 (75–475) | 8 | 8 | 0 |
| Kootathur | 10 | 8 | 150 (50–200) | 8 | 8 | 1 |
| Pudhupattu | 10 | 10 | 300 (150–2200) | 10 | 9 | 1 |
| Seramarathur | 10 | 8 | 525 (250–1875) | 7 | 5 | 2 |
| Sinthalur | 10 | 8 | 450 (300–775) | 7 | 6 | 1 |
| Thimirimarathur | 10 | 10 | 500 (250–550) | 7 | 3 | 4 |
| Villichanur | 10 | 8 | 325 (175–350) | 7 | 6 | 1 |
| Total | 90 | 77 (85.5%) | 350 (100–650) | 68 (88.3%) | 52 (76.4%) | 19 (27.9%) |
FEC: Fecal egg count expressed as eggs per gram of stool; Q25: 25th quantile; Q75: 75th quantile
Soil samples were collected from common open defecation areas for each of these villages. Field workers opportunistically collected soil samples from hot spots of the defecation site chosen for the study. The soil samples that were collected were found to be loamy and wet. The collection of soil samples was in conjunction with human stool samples. Hookworm larvae are known to be present in the top soil early in the morning [29,30] and therefore sample collection was done between 8 a.m. and 10 a.m. Depending on the number of open defecation areas in a village and the number of cycles of deworming, 20 to 40 soil samples were collected per village. Approximately 250–300 grams of topsoil was collected and transported in plastic bags at room temperature to the laboratory on the same day. All samples were screened for the presence of hookworm-like nematode larvae applying a modified saline wet mount. In total, 271 samples were collected from 22 open defecation sites. In 78 samples out of 271 samples hookworm-like nematode larvae were identified. The total number of soil samples collected and the number of soil samples containing hookworm-like nematode larvae across the different villages are summarized in Table 3. All samples were stored at 4°C until the molecular identification of the larvae.
Table 3. Molecular characterization of hookworm across 9 villages in soil samples collected from Jawadhu hills.
| Village | N | Microscopy positive | PCR positive | Molecular characterization of hookworm | ||||
|---|---|---|---|---|---|---|---|---|
| A. caninum | A. ceylanicum | A. duodenale | A. braziliense | N. americanus | ||||
| Alanjanur | 40 | 11 | 10 | 5 | 4 | 1 | 0 | 0 |
| Jambudee | 30 | 11 | 10 | 2 | 7 | 1 | 0 | 0 |
| Keel Nadanur | 30 | 5 | 5 | 1 | 4 | 0 | 0 | 0 |
| Kootathur | 40 | 21 | 20 | 6 | 12 | 2 | 1 | 0 |
| Pudhupattu | 31 | 5 | 4 | 1 | 3 | 0 | 0 | 0 |
| Seramarathur | 20 | 5 | 4 | 1 | 2 | 1 | 0 | 0 |
| Sinthalur | 20 | 2 | 2 | 1 | 0 | 1 | 0 | 0 |
| Thimirimarathur | 40 | 10 | 9 | 5 | 3 | 2 | 0 | 1 |
| Villichanur | 20 | 8 | 8 | 1 | 6 | 1 | 0 | 0 |
| Total | 271 | 78 (28.8%) | 72 (92.3%) | 20 (27.7%) | 41 (56.9%) | 8 (11.1%) | 1 (1.4%) | 1 (1.4%) |
Microscopic examination
The saline wet mount was performed on stool as described in laboratory manual by WHO [31]. For soil samples, two grams of soil sample was suspended in 10 ml buffered saline (0.85% NaCl). The suspension was subsequently filtered twice using a tea strainer to withhold any large debris. The obtained filtrate was transferred into a 15 ml falcon tube, and saline was added up to a volume of 10 ml. The suspension was centrifugation at 3,150 g for 10 minutes. The supernatant was discarded and a saline wet mount was performed.
McMaster egg counting method was performed as described by Levecke and colleagues, 2011 [32]. In short, two grams of stool were suspended in 30 ml of saturated NaCl solution (specific gravity ∼1.2). The fecal suspension was run through filter (250 μm) three times to remove any large debris. Then, 500 μl of the remaining suspension was added to each of the two chambers of a McMaster slide (http://www.mcmaster.co.za). Both chambers were examined under a light microscope using a 100x magnification and the FEC, expressed as EPG for hookworms, were obtained by multiplying the total number of hookworm eggs by 50. A visual tutorial on how to perform a McMaster can be found at https://www.youtube.com/watch?v=bwIFyZ7NrFw.
Molecular identification of hookworm isolates in stool samples
DNA was extracted from stool using the Qiagen stool DNA-mini kit (Qiagen, Hilden, Germany). One gram of stool sample was mixed with 1 ml of lysis buffer and thoroughly mixed using a vortex for 10 minutes. Subsequently, 0.1 gram of 425–600 μm acid-washed glass beads was added to the suspension and was continuously beaded at variable shaking speeds of 2,000–3,450 strokes/min for 9 minutes. To further enhance the recovery of DNA, the samples were then subjected to 5 freeze-thaw cycles; first putting the samples in water bath of 95°C for 5 minutes followed by a freeze step in liquid nitrogen for 2 minutes. With the exception of the elution step, which was repeated twice, the remaining of the DNA extraction was performed according to the manufacturer’s protocol.
Molecular identification of hookworm isolates in soil samples
Prior to DNA extraction, larvae were isolated from the soil. To this end, 20 grams of the soil samples was suspended in 10 ml of distilled water. The choice of the amount of soil was based on larval recovery rates across 2, 4, 5, 10 and 20 grams of soil. Subsequently, the sample was filtered twice through a tea strainer to retain any larger debris. The obtained filtrate was subjected once more to 2 consecutive filtration steps, using a sieve filter of pore size 0.4 mm and 0.2 mm respectively. The remaining material was then scrapped off the 0.2 mm sieve filter where the larvae retained and transferred into a 15 ml falcon tube, and 3 ml of MgSO4 solution (specific gravity = 1.2). The suspension was centrifuged at 2,000 g for 2 minutes, the supernatant was transferred into a new 15 ml falcon tube, and 3 ml of distilled water was added. Finally, the end solution was concentrated to 1 ml, which was then processed using the Qiagen Blood and Tissue kit (Qiagen, Hilden, Germany).
Identification of hookworm species
The ITS 1,2 and 5.8s region of the hookworm genome was amplified using a semi-nested PCR protocol described by George and colleagues, 2015 [19]. The first-round PCR resulted in a product of 597 bp (N. americanus) and in a product of 449 bp (Ancylostoma spp. and U. stenocephala), while the second PCR resulted in a product of 552 bp (N. americanus), 404–408 bp (Ancylostoma spp. and U. stenocephala). Both a negative (water) and positive (hookworm DNA) control was included in each run. The amplification reactions and conditions have been described in detail elsewhere [19]. The amplified product was detected using 1.5% agarose gel electrophoresis using ethidium bromide. To determine the species of Ancylostoma, the second-round PCR products were subsequently digested using the restriction enzymes as mentioned by George et al., 2015 [19]. To differentiate between A. braziliense, A. ceylanicum, A. caninum and A. duodenale, DNA from these species were subjected to two different restriction enzymes (MvaI and Psp1406I) at 37°C for 13 hours. MvaI digest A. braziliense into three fragments of 64, 122 and 222 bp using and A. ceylanicum into two fragments of 255 and 149 bp; but is not able to digest A. duodenale, A. caninum and U. stenocephala. Psp1406I digests both A. braziliense and A. duodenale into two fragments (A. braziliense: 259 and 149 bp; A. duodenale: 255 and 149 bp), but not A. caninum. The restricted products were detected using 2% agarose gel electrophoresis and ethidium bromide. Based on previous findings, the likelihood of observing the canine U. stenocephala and the feline hookworm A. tubaeforme was expected to be low in our study setting [33], and hence these hookworm species were not prioritized while selecting RFLP enzymes. Our analysis on the reference sequence from GenBank for both A. tubaeforme and U. stenocepahala revealed that both MvaI and Psp1406I do not have restriction sites in their genome. To confirm the specificity of the PCR-RFLP method and to exclude any U. stenocephala and A. tubaeforme infections, DNA sequencing was performed on a subset of the isolates. DNA sequencing was done using a dye terminator cycle sequencing kit and a four-capillary array genetic analyser (Applied Biosystems 3130) directly from the purified amplicons, which were sequenced in both directions using the same oligonucleotide primers used by George et al., 2015 [19]. In the presence of mixed infections, the amplified products were run on 1.5% agarose gel and the desired product cut from the gel and purified using fast bind-wash-elute method using QIAquick gel extraction kit. Sequences were aligned by pairwise alignment using the ClustalW method with MegAlign DNASTAR software. The pairwise alignment was done to draw inference on the relationship between hookworms from humans, dogs and soil. A bootstrap consensus tree inferred from 100 replicates was used to generate the tree. For each of the different hookworm species reference samples were included (A. braziliense: GenBank accession no DQ359149; A. caninum: GenBank accession no DQ438070; A. ceylanicum: GenBank accession no DQ381541; A. duodenale: GenBank accession no EU344797 and N. americanus: GenBank accession no AB793527).
Analytic sensitivity of detecting N. americanus L3-larvae in soil
In order to verify the analytic limit of detecting N. americanus L3-larvae recovered from soil, a spiking experiment was set up. To this end, a series of known number of N. americanus L3-larvae were added to a fixed aliquot of sterile soil. In this experiment 70, 140, 280 and 700 larvae were added to 20 gram of soil, resulting in a concentration of 3.5, 7, 14 and 35 larvae per gram of soil. Each of the number of larvae was added to 5 different aliquots of soil, resulting in 20 seeded aliquots of soil. These seeded aliquots were processed as described above in the section Molecular identification of hookworm isolates. Prior to DNA extraction, the number of larvae isolated from 20 gram of soil was determined by microscopically screening 4x 10 μl of the final 1 ml elute.
The L3 larvae were obtained by applying the Harada-Mori method to human stool submitted to the laboratory for routine microscopic examination. The larvae were kept at a concentration of 14,000 larvae / ml, and hence 70, 140, 280 and 700 larvae were represented by a larval stock volume 5 μl, 10 μl, 20 μl and 50 μl, respectively. To fix the total volume added to the soil, sterile water was added up to a volume of 1,000 μl for all experiments. One kilo of soil, which was negative on the saline wet mount, was dry heat sterilized (160°C for 2 hours). The sample was subsequently processed as described in Molecular Identification of Hookworm Isolates and found to be negative for N. americanus-DNA.
Results
Molecular Characterization of Hookworm in Humans, Dogs and Soil
From the 711 individuals that participated in the study, 146 individuals were found to be infected with hookworm using saline wet mount microscopy. Out of the 146 infected individuals, 143 individuals provided adequate quantity of stool samples to perform molecular characterization, and hookworm-DNA was detected in 119 individuals (83.2%). All had N. americanus, while A. caninum was found in 20 individuals and A. duodenale in 10 individuals. The distribution of the different hookworm species in human stool across the 9 villages is summarized in Table 1. Based on a structured questionnaire, the odds of being infected with A. caninum when claiming dog ownership (11/299) was 1.71 (95% confidence interval = 0.69–4.18) times higher when no dog ownership was claimed (9/412), but this was not statistically significant.
On account of high prevalence of A. caninum DNA in human stool samples, dog stool samples were collected from the 9 villages. A total of 90 dog stool samples were collected, of which hookworm was detected in 77 samples using saline wet mount microscopy. Hookworm was detected in 68 of the 77 dog samples (88.3%) selected for molecular characterization. A. caninum was the predominant species, being found in 52 dogs. A. ceylanicum was found in 19 (27.9%) dogs. Among them three dogs had mixed A. caninum and A. ceylanicum infections. The distribution of the different hookworm species across the 9 villages is summarized in Table 2.
To assess the role of soil as a source of zoonotic hookworm infection, a total of 271 soil samples were collected from defecating areas across 9 villages. Hookworm-like nematode larvae were found in 78 out of the 271 samples that were collected. Of the 78 soil samples identified positive for hookworm-like nematode larvae, 72 (92.3%) were found positive by PCR for hookworms. Molecular characterization of these 78 soil samples revealed the presence of a variety of hookworm species, including A. caninum, A. ceylanicum, A. duodenale, A. brazilense and N. americanus. The majority of these soil samples were contaminated with A. ceylanicum (n = 41; 56.9%), followed by A. caninum (n = 20; 27.7%) and A. braziliense (n = 1; 1.4%). The human hookworms were only found in the minority of the samples (A. duodenale: n = 8; 11.1%; N. americanus: n = 1; 1.4%). The distribution of the hookworm species across the different villages is summarized in Table 3.
Sequence Analysis
In total 69 hookworm isolates from different sources (29 human, 26 Dog and 14 soil) were sequenced in the present study. The phylogenetic tree is provided in Fig 1, and highlights that each species identified by the PCR-RFLP cluster nicely together with their respective reference sequences. The human-derived A. caninum sequences (Study ID nos. Human20493, Human16162, Human11351, Human11017, Human9949, Human9931, Human9851, Human9843, Human9661, Human9659, Human6365, Human6303, Human6253, Human1882, Human1649 and Human1596) clustered with the A. caninum sequences from dogs and soil, forming a cluster with A. caninum reference sequences (GenBank accession no. DQ438070). The human-derived sequences of A. duodenale (Study ID nos. Human2545, Human2880, Human2552 and Human2544) centred within a clade with the reference A. duodenale sequence (GenBank accession no. EU344797). The N. americanus sequences from humans clustered as a separate larger cluster. All 69 sequences were submitted to GenBank and assigned the accession numbers from KU996361 to KU996390, and from KX155777 to KX155815
Fig 1. Phylogenetic tree constructed using Pairwise Alignment to draw inferences on relationship between different hookworm species.
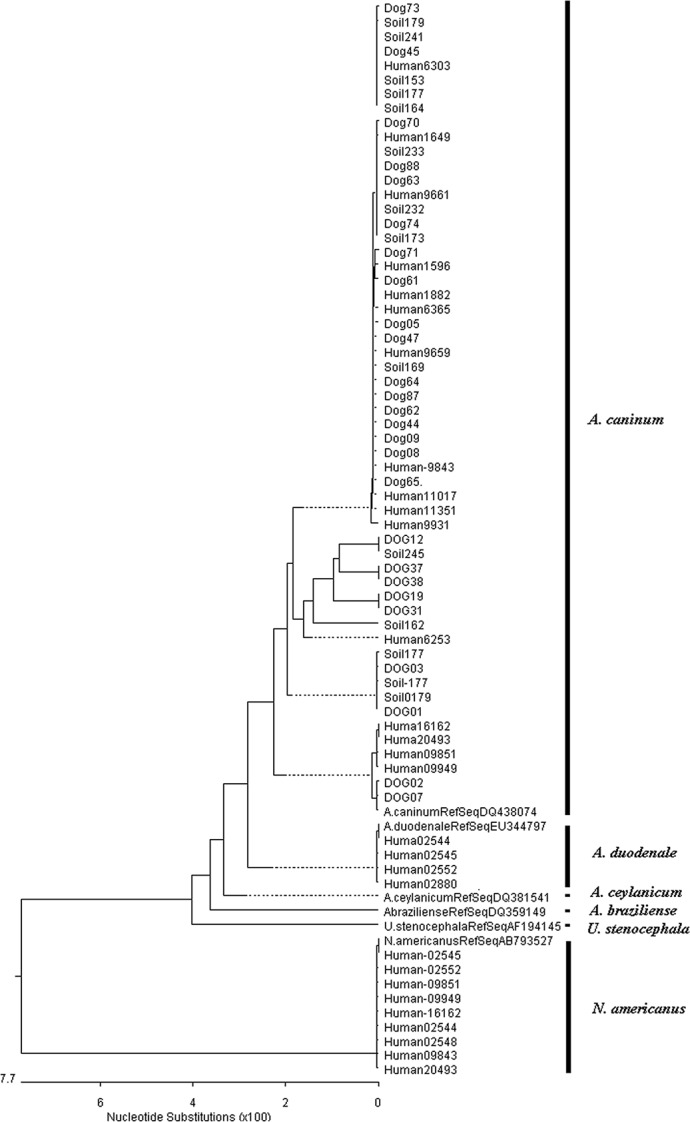
Sequences were aligned by pairwise alignment using the ClustalW method with MegAlign DNASTAR software. The pairwise alignment was done to draw inference on the relationship between hookworms from humans, dogs and soil. A bootstrap consensus tree inferred from 100 replicates was used to generate the tree.
Analytic Sensitivity of Detecting N. Americanus L3-Larvae in Soil
Due to low detection of N. americanus larvae in the soil samples but the high prevalence of hookworm (N. americanus) in humans, an experiment was carried out to assess the analytic sensitivity of detecting N. americanus larvae in soil samples using the standardized assay as described in material and methods section:. For the various concentrations (70, 140, 280 and 700 larvae) of the larvae in the stock that was used to spike, there was a large variation in mean recovery rate (%) of the N. americanus L3-larvae, ranging from 51.1% when 70 larvae were added to 92.9% when 700 larvae were added. The results of the larvae recovery rate are presented in Table 4. The larvae that were isolated from these experiments were characterized using PCR and were confirmed to be N. americanus.
Table 4. The recovery rates (%) with different larval concentrations in five batches of soil aliquots.
| N | Mean (SD*) | Recovery rate (%) |
|---|---|---|
| Larvae added to the soil | Recovered larvae | |
| 70 | 40 (13.7) | 57.1 |
| 140 | 125 (0) | 89.3 |
| 280 | 255 (11.2) | 91.1 |
| 700 | 650 (30.6) | 92.9 |
*SD: standard deviation
Discussion
There has been a worldwide upscale of drug donations to control the morbidity caused by hookworms, and to even attempts to eliminate these worms in confined geographical areas [6]. It is traditionally assumed that infections in humans are solely due to the human hookworms (N. americanus and A. duodenale) [6,34], hence ignoring the possible role of animals as a reservoir for hookworm infections. This study determined the hookworm species in humans, dogs and soil from a tribal area in Tamil Nadu, India. The findings from our present work confirm that N. americanus are responsible for the majority of the hookworm infections in humans in these tribal communities [19]. In addition, we also found an unexpectedly high prevalence of animal hookworm DNA in humans, while our previous study [19] in Jawadhu hills had a low prevalence of animal hookworms (5%). These differences in occurrence might be explained by variation in prevalence across villages. Both studies covered different villages, and as illustrated in Table 1, there was a large variation in animal hookworm infections in humans across villages (A. caninum was found in 6 out 6 cases in Alanjanur, but was absent in Thimirimarathur and Villichanur). There was no significant evidence of an increased risk of hookworm infections with dog ownership, which is in contrast with the studies reported by Traub et al., and Ugbomoiko US et al., [24,35], who did observe a significant increased risk. The lack of this evidence maybe due to the fact that animals are not confined, but are found freely roaming through the village. As a consequence of this they are able to randomly defecate within the village, which subsequently will increase the likelihood of infecting other habitants beyond their owner.
In the present study a large proportion of human stools were found to contain A. caninum DNA. These observations can be explained by (i) A. caninum infections, (ii) passive passage of A. caninum eggs or larvae that are accidently ingested, but do not result in any infection and (iii) contamination of stool during sample collection with environmental A. caninum eggs or larvae. Although it is unlikely that passive passage explains the high proportion of stool samples containing A. caninum DNA, we have no conclusive evidence for any of the remaining potential causes either. Traditionally it is assumed that parasite DNA in stool is due the presence of eggs shed by adult worms. However, up to today there is no evidence yet that egg-laying adult A. caninum worms can develop in humans [14,36,37], and hence one would not expect any amplification of DNA from A. caninum extracted eggs in human stool. In our study we were not able to provide evidence for the presence of A. caninum eggs in stool, as the human hookworm N. americanus was also detected in all cases of A. caninum. As a consequence of this, it is possible that the eggs in stool were shed by adult N. americanus worms only. A single-egg based speciation would have been ideal. Another potential source of parasite DNA is DNA that is directly released by immature or mature non-egg producing worms. To demonstrate the presence of both immature and non-egg producing mature A. caninum worms expulsion studies are required [36]. In these studies stool is collected over consecutive days following treatment to recover worms, which are then individually speciated. Both a single-egg based speciation and an expulsion study were out of scope of the present study. Another aspect that needs to be considered during the interpretation of our findings is the way the human samples were collected. Although all the study participants were informed about the importance of the study and need to collect fecal samples devoid of any soil, stool samples could have been contaminated with soil during collection because people in the study area defecate in the open and samples might have been scooped from the ground which could result in the presence of A. caninum DNA in human stool. In either case, these results emphasize the need for additional epidemiological surveys across various geographical settings to further explore the role of animals as a reservoir for zoonotic transmission. It is important to note that this does not only apply for hookworms, but is also of concern for other soil-transmitted helminths (Trichuris trichiura and Ascaris lumbricoides). This is because dogs are known to harbor Trichuris spp. (T. vulpis), which, similarly to canine hookworm species, is known to infect humans causing symptoms ranging from an asymptomatic infection to diarrhea or even dysentery [38]. T. vulpis has also been reported as a causative agent of visceral larva migrans [39,40,41].
In the dog stool samples, both known zoonotic hookworm (A. caninum and A. ceylanicum) species were found. Although A. ceylanicum is the only canine hookworm species that is known to cause patent infections in humans [42], our present study did not identify any human A. ceylanicum infections, in spite of detecting it in both soil and canine stool samples. In contrast, a study from a rural village in Cambodia reported more than half of the hookworm infected individuals to be positive for A. ceylanicum (51.6%) [18] A possible explanation for the presence of A. ceylanicum in dogs and soil, but not in humans, is the existence of two sub species (haplotypes) of A. ceylanicum, one with an animal origin and one with a human origin [42]. To differentiate these subspecies the cytochrome c oxidase (COX) subunit 1 gene of the hookworm is recommended [42]. In addition, the applied PCR-RFLP method may lack some sensitivity; this is in particular for mixed infections. As previously illustrated for other gastro-intestinal parasites (e.g. Giardia; Geurden et al., 2008 and Levecke et al., 2009), genus specific PCRs will preferentially amplify the most abundant species, and hence presence of the least abundant species may be underestimated [43,44]. This could be one possible explanation for missing out zoonotic A. ceylanicum infections in humans. For the soil samples that were collected from defecating areas, it was interesting to observe variety of different species of hookworm larvae (A. braziliense, A. caninum, A. ceylanicum, A. duodenale and N. americanus). This can be attributed to open defecation practised in the study area and indiscriminate defecation by freely roaming stray dogs.
There are a few limitations to our present study. First, N. americanus was rarely found in soil samples, whereas this hookworm species was found in all human stool samples. For this reason, we determined the analytical sensitivity of the isolation procedure using N. americanus L3-larvae to confirm efficiency of the assay to isolate and identify the species. The results of this seeding experiment suggest that the procedure was able to detect 3.5 larvae per gram of soil, and hence ruling out false negative test results due to loss of larvae. Another potential cause of absence of N. americanus could be inappropriate storage of the soil samples prior to the molecular analysis. In this study, the soil samples were first processed for hookworm-like larvae using modified saline wet mount microscopy, subsequently they were stored at 4°C (up to 14 months) until further processed for molecular identification. Unlike Ancylostoma spp., N. americanus stored in cold temperature do not survive long [29]. It is therefore important to mention that the only case of N. americanus was observed in one out of four samples containing hookworm-like larvae that were processed almost immediately after collection for molecular speciation.
Second, for the collection of soil samples, the samples were collected from area around the site of defecation/presence of stool (human and dog), which makes the selection biased, and hence it increased the probability of finding hookworm larvae.
In conclusion, in our study we regularly detected the presence of A. caninum DNA in the stool of humans. Whether this is the result of an infection is currently unknown but it does warrant a closer look at dogs as a potential reservoir. Nevertheless, there is a need for additional epidemiological surveys across different geographical settings to further unravel the role of animals as a reservoir for zoonotic transmission, and ultimately inform the health policy makers to adapt or improve measures to control soil-transmitted helminths as a public health problem.
Supporting Information
(DOCX)
(DOC)
Acknowledgments
The authors are grateful to the study subjects and the parents of the children, who allowed their children to participate. We whole-heartedly thank the field workers who put a considerable amount of time and effort in the collection of human and dog stool samples and soil samples. We acknowledge the support extended by R. Raju for his help in conveying to the study participants the importance and need for the study. In addition, we would like to thank the staff of Division of Gastrointestinal sciences, Christian Medical College, Vellore for processing and examining the stool and soil samples.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study is supported by the Wellcome Trust/DBT India Alliance trough an Early Career Fellowship to RS. SG is supported by the Special Research Fund of Ghent University (scholarship code - 01W01511) (http://www.ugent.be/nl/onderzoek/financiering/bof). BL is a postdoctoral fellow of FWO (Grant number - 1285316N) (www.fwo.be). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.de Silva NR (2003) Impact of mass chemotherapy on the morbidity due to soil-transmitted nematodes. Acta Trop 86: 197–214. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Diemert D, Bacon KM, Beaumier C, Bethony JM, et al. (2013) The Human Hookworm Vaccine. Vaccine 31 Suppl 2: B227–232. 10.1016/j.vaccine.2012.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 4.Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A (2010) Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol 8: 814–826. 10.1038/nrmicro2438 [DOI] [PubMed] [Google Scholar]
- 5.Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, et al. (2011) Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis 5: e948 10.1371/journal.pntd.0000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooker S, Bethony J, Hotez PJ (2004) Human hookworm infection in the 21st century. Adv Parasitol 58: 197–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savioli L, Stansfield S, Bundy DA, Mitchell A, Bhatia R, et al. (2002) Schistosomiasis and soil-transmitted helminth infections: forging control efforts. Trans R Soc Trop Med Hyg 96: 577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson RM, Truscott JE, Pullan RL, Brooker SJ, Hollingsworth TD (2013) How effective is school-based deworming for the community-wide control of soil-transmitted helminths? PLoS Negl Trop Dis 7: e2027 10.1371/journal.pntd.0002027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plotkin S, Diemert DJ, Bethony JM, Hotez PJ (2008) Hookworm Vaccines. Clinical Infectious Diseases 46: 282–288. 10.1086/524070 [DOI] [PubMed] [Google Scholar]
- 10.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, et al. (2014) Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med 11: e1001620 10.1371/journal.pmed.1001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keiser J, Utzinger J (2008) Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA 299: 1937–1948. 10.1001/jama.299.16.1937 [DOI] [PubMed] [Google Scholar]
- 12.Levecke B, Montresor A, Albonico M, Ame SM, Behnke JM, et al. (2014) Assessment of anthelmintic efficacy of mebendazole in school children in six countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis 8: e3204 10.1371/journal.pntd.0003204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.N. Cary Engleberg MD (Author) TDPA, Victor DiRita PhD (Author) (2013) Schaechter's Mechanisms of Microbial Disease.
- 14.Prociv P, Croese J (1990) Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum. Lancet 335: 1299–1302. [DOI] [PubMed] [Google Scholar]
- 15.Ngui R, Lim YA, Ismail WH, Lim KN, Mahmud R (2014) Zoonotic Ancylostoma ceylanicum infection detected by endoscopy. Am J Trop Med Hyg 91: 86–88. 10.4269/ajtmh.13-0756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR (2010) Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol 26: 162–167. 10.1016/j.pt.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 17.Walker NI, Croese J, Clouston AD, Parry M, Loukas A, et al. (1995) Eosinophilic enteritis in northeastern Australia. Pathology, association with Ancylostoma caninum, and implications. Am J Surg Pathol 19: 328–337. [DOI] [PubMed] [Google Scholar]
- 18.Inpankaew T, Schar F, Dalsgaard A, Khieu V, Chimnoi W, et al. (2014) High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg Infect Dis 20: 976–982. 10.3201/eid2006.131770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George S, Kaliappan SP, Kattula D, Roy S, Geldhof P, et al. (2015) Identification of Ancylostoma ceylanicum in children from a tribal community in Tamil Nadu, India using a semi-nested PCR-RFLP tool. Trans R Soc Trop Med Hyg 109: 283–285. 10.1093/trstmh/trv001 [DOI] [PubMed] [Google Scholar]
- 20.Gasser RB (2006) Molecular tools—advances, opportunities and prospects. Vet Parasitol 136: 69–89. [DOI] [PubMed] [Google Scholar]
- 21.Ngui R, Ching LS, Kai TT, Roslan MA, Lim YA (2012) Molecular identification of human hookworm infections in economically disadvantaged communities in Peninsular Malaysia. Am J Trop Med Hyg 86: 837–842. 10.4269/ajtmh.2012.11-0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traub RJ, Robertson ID, Irwin P, Mencke N, Andrew Thompson RC (2004) The prevalence, intensities and risk factors associated with geohelminth infection in tea-growing communities of Assam, India. Trop Med Int Health 9: 688–701. [DOI] [PubMed] [Google Scholar]
- 23.Traub RJ, Robertson ID, Irwin PJ, Mencke N, Thompson RC (2005) Canine gastrointestinal parasitic zoonoses in India. Trends Parasitol 21: 42–48. [DOI] [PubMed] [Google Scholar]
- 24.Traub RJ, Robertson ID, Irwin P, Mencke N, Thompson RC (2002) The role of dogs in transmission of gastrointestinal parasites in a remote tea-growing community in northeastern India. Am J Trop Med Hyg 67: 539–545. [DOI] [PubMed] [Google Scholar]
- 25.Ng-Nguyen D, Hii SF, Nguyen VA, Van Nguyen T, Van Nguyen D, et al. (2015) Re-evaluation of the species of hookworms infecting dogs in Central Vietnam. Parasit Vectors 8: 401 10.1186/s13071-015-1015-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tun S, Ithoi I, Mahmud R, Samsudin NI, Kek Heng C, et al. (2015) Detection of Helminth Eggs and Identification of Hookworm Species in Stray Cats, Dogs and Soil from Klang Valley, Malaysia. PLoS One 10: e0142231 10.1371/journal.pone.0142231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaliappan SP, George S, Francis MR, Kattula D, Sarkar R, et al. (2013) Prevalence and clustering of soil-transmitted helminth infections in a tribal area in southern India. Trop Med Int Health 18: 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranganathan RV R., Parameswari P. (2012) Ethnomedicinal Survey Of Jawadhu Hills In Tamil Nadu. Asian Journal of Pharmaceutical and Clinical Research 5. [Google Scholar]
- 29.Udonsi JK, Atata G (1987) Necator americanus: temperature, pH, light, and larval development, longevity, and desiccation tolerance. Exp Parasitol 63: 136–142. [DOI] [PubMed] [Google Scholar]
- 30.Liu D (2012) Molecular Detection of Human Parasitic Pathogens: CRC Press; 1 edition. [Google Scholar]
- 31.WHO (1991) Basic laboratory methods in medical parasitology: World health Organisation, Geneva. [Google Scholar]
- 32.Levecke B, Behnke JM, Ajjampur SS, Albonico M, Ame SM, et al. (2011) A comparison of the sensitivity and fecal egg counts of the McMaster egg counting and Kato-Katz thick smear methods for soil-transmitted helminths. PLoS Negl Trop Dis 5: e1201 10.1371/journal.pntd.0001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traub RJ, Pednekar RP, Cuttell L, Porter RB, Abd Megat Rani PA, et al. (2014) The prevalence and distribution of gastrointestinal parasites of stray and refuge dogs in four locations in India. Vet Parasitol 205: 233–238. 10.1016/j.vetpar.2014.06.037 [DOI] [PubMed] [Google Scholar]
- 34.PJ. H (1995) Human hookworm infection; Farthing MJG KG, Wakelin D, editor: London: Chapman and Hall. [Google Scholar]
- 35.Ugbomoiko US, Ariza L, Heukelbach J (2008) Parasites of importance for human health in Nigerian dogs: high prevalence and limited knowledge of pet owners. BMC Vet Res 4: 49 10.1186/1746-6148-4-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landmann JK, Prociv P (2003) Experimental human infection with the dog hookworm, Ancylostoma caninum. Med J Aust 178: 69–71. [DOI] [PubMed] [Google Scholar]
- 37.Croese J, Loukas A, Opdebeeck J, Fairley S, Prociv P (1994) Human enteric infection with canine hookworms. Ann Intern Med 120: 369–374. [DOI] [PubMed] [Google Scholar]
- 38.Dunn JJ, Columbus ST, Aldeen WE, Davis M, Carroll KC (2002) Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J Clin Microbiol 40: 2703–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masuda Y, Kishimoto T., Ito H., and Tsuji M. (1987) Visceral larva migrans caused by Trichuris vulpis presenting as a pulmonary mass. Thorax 42: 990–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakano T, Hamamoto K., Kobayashi Y., Sakata Y., Tsuji M., and Usui T. (1980) Visceral larva migrans caused by Trichuris vulpis. Arch Dis Child 55: 631–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Areekul Pannatat CP, Pattanawong Urassaya, Sitthicharoenchai Prasert, Jongwutiwes Somchai (2010) Trichuris vulpis and T. trichiura infections among school children of a rural community in northwestern Thailand: the possible role of dogs in disease transmission. Asian Biomedicine 4: 49–60. [Google Scholar]
- 42.Ngui R, Mahdy MA, Chua KH, Traub R, Lim YA (2013) Genetic characterization of the partial mitochondrial cytochrome oxidase c subunit I (cox 1) gene of the zoonotic parasitic nematode, Ancylostoma ceylanicum from humans, dogs and cats. Acta Trop 128: 154–157. 10.1016/j.actatropica.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 43.Geurden T, Geldhof P, Levecke B, Martens C, Berkvens D, et al. (2008) Mixed Giardia duodenalis assemblage A and E infections in calves. Int J Parasitol 38: 259–264. [DOI] [PubMed] [Google Scholar]
- 44.Levecke B, Geldhof P, Claerebout E, Dorny P, Vercammen F, et al. (2009) Molecular characterisation of Giardiaduodenalis in captive non-human primates reveals mixed assemblage A and B infections and novel polymorphisms. Int J Parasitol 39: 1595–1601. 10.1016/j.ijpara.2009.05.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


