Abstract
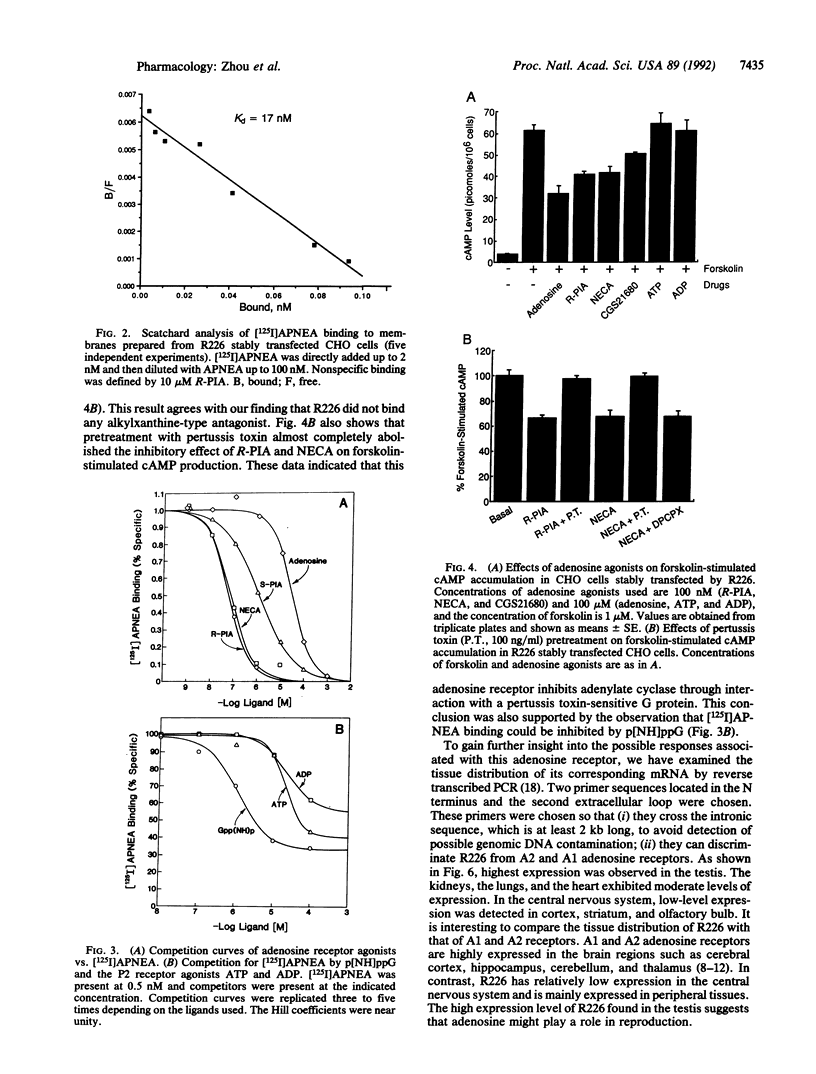
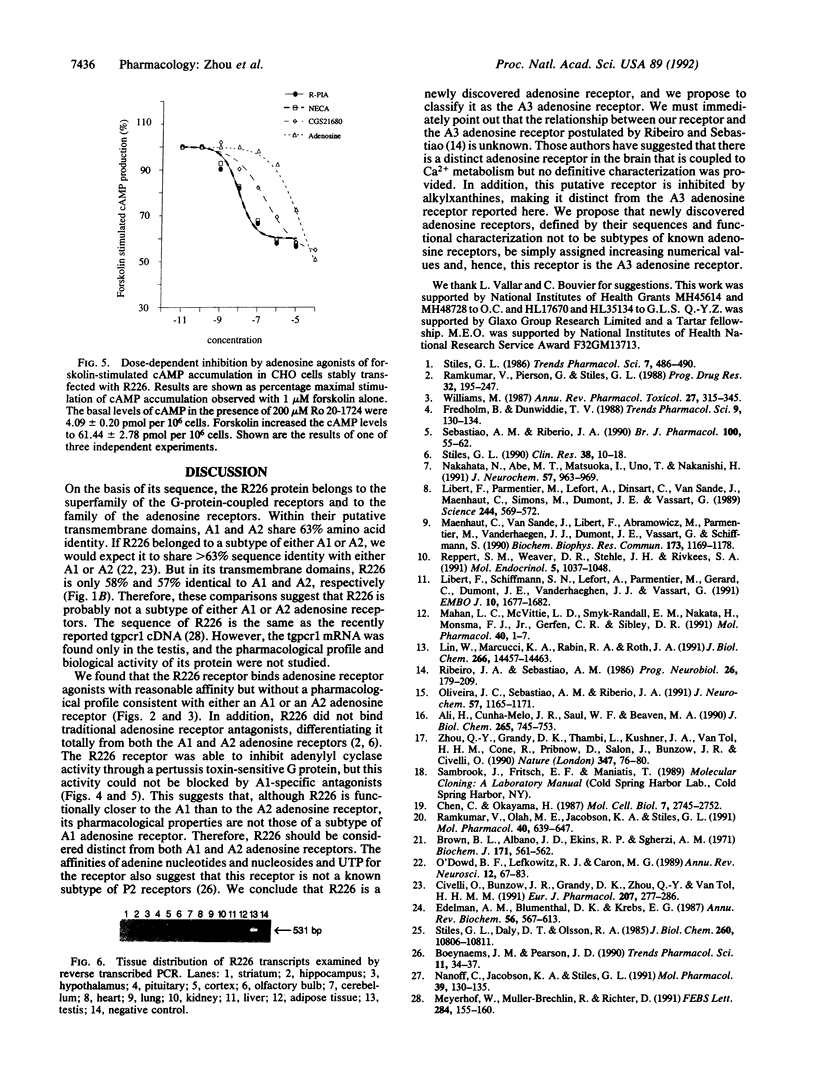
We have previously reported the selective amplification of several rat striatal cDNA sequences that encode guanine nucleotide-binding regulatory protein (G protein)-coupled receptors. One of these sequences (R226) exhibited high sequence identity (58%) with the two previously cloned adenosine receptors. A full-length cDNA clone for R226 has been isolated from a rat brain cDNA library. The cDNA clone encodes a protein of 320 amino acids that can be organized into seven transmembrane stretches. R226 has been expressed in COS-7 and CHO cells and membranes from the transfected cells were screened with adenosine receptor radioligands. R226 could bind the nonselective adenosine agonist tritiated N-ethyladenosine 5'-uronic acid ([3H]NECA) and A1-selective agonist radioiodinated N6-2-(4-amino-3-iodophenyl)-ethyladenosine ([125I]APNEA) but not A1-selective antagonists tritiated 1,3-dipropyl-8-cyclopentylxanthine ([3H]DPCPX) and 8-(4-[([[(2-aminoethyl)amino]carbonyl]methyl)oxy]-phenyl)-1, 3-dipropylxanthine ([3H]XAC) or the A2-selective agonist ligands tritiated 2-[4-(2-carboxyethyl)phenyl]ethyl-amino 5'-N-ethylcarboxamidoadenosine ([3H]CGS21680) and radioiodinated 2-[4-([2-[(4-aminophenyl)methylcarbonylamino] ethylaminocarbonyl]ethyl)phenyl]ethylamino 5'-N-ethylcarboxamidoadenosine. Extensive characterization with [125I]APNEA showed that R226 binds [125I]APNEA with high affinity (Kd = 15.5 +/- 2.4 nM) and the specific [125I]APNEA binding could be inhibited by adenosine ligands with a potency order of (R)-N6-phenyl-2-propyladenosine (R-PIA) = NECA greater than S-PIA greater than adenosine greater than ATP = ADP but not by antagonists XAC, isobutylmethylxanthine, and DPCPX. In R226 stably transfected CHO cells, adenosine agonists R-PIA, NECA, and CGS21680 inhibited by 40-50% the forskolin-stimulated cAMP accumulation through a pertussis toxin-sensitive G protein with an EC50 of 18 +/- 5.6 nM, 23 +/- 3.5 nM, and 144 +/- 34 nM, respectively. Based on these observations we conclude that R226 encodes an adenosine receptor with non-A1 and non-A2 specificity, and we thus name it the A3 adenosine receptor. mRNA analyses revealed that the highest expression of R226 was in the testis and low-level mRNAs were also found in the lung, kidneys, heart, and some parts of the central nervous system such as cortex, striatum, and olfactory bulb. The high-expression level of the A3 receptor in the testis suggests a possible role for adenosine in reproduction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Cunha-Melo J. R., Saul W. F., Beaven M. A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990 Jan 15;265(2):745–753. [PubMed] [Google Scholar]
- Boeynaems J. M., Pearson J. D. P2 purinoceptors on vascular endothelial cells: physiological significance and transduction mechanisms. Trends Pharmacol Sci. 1990 Jan;11(1):34–37. doi: 10.1016/0165-6147(90)90039-b. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Bunzow J. R., Grandy D. K., Zhou Q. Y., Van Tol H. H. Molecular biology of the dopamine receptors. Eur J Pharmacol. 1991 Aug 14;207(4):277–286. doi: 10.1016/0922-4106(91)90001-x. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Dunwiddie T. V. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988 Apr;9(4):130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Libert F., Schiffmann S. N., Lefort A., Parmentier M., Gérard C., Dumont J. E., Vanderhaeghen J. J., Vassart G. The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J. 1991 Jul;10(7):1677–1682. doi: 10.1002/j.1460-2075.1991.tb07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. H., Marcucci K. A., Rabin R. A., Roth J. A. 2-Chloroadenosine decreases tyrosylprotein sulfotransferase activity in the Golgi apparatus in PC12 cells. Evidence for a novel receptor. J Biol Chem. 1991 Aug 5;266(22):14457–14463. [PubMed] [Google Scholar]
- Maenhaut C., Van Sande J., Libert F., Abramowicz M., Parmentier M., Vanderhaegen J. J., Dumont J. E., Vassart G., Schiffmann S. RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1169–1178. doi: 10.1016/s0006-291x(05)80909-x. [DOI] [PubMed] [Google Scholar]
- Mahan L. C., McVittie L. D., Smyk-Randall E. M., Nakata H., Monsma F. J., Jr, Gerfen C. R., Sibley D. R. Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol. 1991 Jul;40(1):1–7. [PubMed] [Google Scholar]
- Meyerhof W., Müller-Brechlin R., Richter D. Molecular cloning of a novel putative G-protein coupled receptor expressed during rat spermiogenesis. FEBS Lett. 1991 Jun 24;284(2):155–160. doi: 10.1016/0014-5793(91)80674-r. [DOI] [PubMed] [Google Scholar]
- Nakahata N., Abe M. T., Matsuoka I., Ono T., Nakanishi H. Adenosine inhibits histamine-induced phosphoinositide hydrolysis mediated via pertussis toxin-sensitive G protein in human astrocytoma cells. J Neurochem. 1991 Sep;57(3):963–969. doi: 10.1111/j.1471-4159.1991.tb08244.x. [DOI] [PubMed] [Google Scholar]
- Nanoff C., Jacobson K. A., Stiles G. L. The A2 adenosine receptor: guanine nucleotide modulation of agonist binding is enhanced by proteolysis. Mol Pharmacol. 1991 Feb;39(2):130–135. [PMC free article] [PubMed] [Google Scholar]
- O'Dowd B. F., Lefkowitz R. J., Caron M. G. Structure of the adrenergic and related receptors. Annu Rev Neurosci. 1989;12:67–83. doi: 10.1146/annurev.ne.12.030189.000435. [DOI] [PubMed] [Google Scholar]
- Oliveira J. C., Sebastião A. M., Ribeiro J. A. Solubilized rat brain adenosine receptors have two high-affinity binding sites for 1,3-dipropyl-8-cyclopentylxanthine. J Neurochem. 1991 Oct;57(4):1165–1171. doi: 10.1111/j.1471-4159.1991.tb08275.x. [DOI] [PubMed] [Google Scholar]
- Ramkumar V., Olah M. E., Jacobson K. A., Stiles G. L. Distinct pathways of desensitization of A1- and A2-adenosine receptors in DDT1 MF-2 cells. Mol Pharmacol. 1991 Nov;40(5):639–647. [PMC free article] [PubMed] [Google Scholar]
- Ramkumar V., Pierson G., Stiles G. L. Adenosine receptors: clinical implications and biochemical mechanisms. Prog Drug Res. 1988;32:195–247. doi: 10.1007/978-3-0348-9154-7_7. [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Weaver D. R., Stehle J. H., Rivkees S. A. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol. 1991 Aug;5(8):1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. Adenosine receptors and calcium: basis for proposing a third (A3) adenosine receptor. Prog Neurobiol. 1986;26(3):179–209. doi: 10.1016/0301-0082(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Sebastião A. M., Ribeiro J. A. Interactions between adenosine and phorbol esters or lithium at the frog neuromuscular junction. Br J Pharmacol. 1990 May;100(1):55–62. doi: 10.1111/j.1476-5381.1990.tb12051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles G. L. Adenosine receptors and beyond: molecular mechanisms of physiological regulation. Clin Res. 1990 Jan;38(1):10–18. [PubMed] [Google Scholar]
- Stiles G. L., Daly D. T., Olsson R. A. The A1 adenosine receptor. Identification of the binding subunit by photoaffinity cross-linking. J Biol Chem. 1985 Sep 5;260(19):10806–10811. [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- Zhou Q. Y., Grandy D. K., Thambi L., Kushner J. A., Van Tol H. H., Cone R., Pribnow D., Salon J., Bunzow J. R., Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990 Sep 6;347(6288):76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]





