Abstract
Ghost shrimps of Callianassidae and Ctenochelidae are soft-bodied, usually heterochelous decapods representing major bioturbators of muddy and sandy (sub)marine substrates. Ghost shrimps have a robust fossil record spanning from the Early Cretaceous (~ 133 Ma) to the Holocene and their remains are present in most assemblages of Cenozoic decapod crustaceans. Their taxonomic interpretation is in flux, mainly because the generic assignment is hindered by their insufficient preservation and disagreement in the biological classification. Furthermore, numerous taxa are incorrectly classified within the catch-all taxon Callianassa. To show the historical patterns in describing fossil ghost shrimps and to evaluate taphonomic aspects influencing the attribution of ghost shrimp remains to higher level taxa, a database of all fossil species treated at some time as belonging to the group has been compiled: 250 / 274 species are considered valid ghost shrimp taxa herein. More than half of these taxa (160 species, 58.4%) are known only from distal cheliped elements, i.e., dactylus and / or propodus, due to the more calcified cuticle locally. Rarely, ghost shrimps are preserved in situ in burrows or in direct association with them, and several previously unpublished occurrences are reported herein. For generic assignment, fossil material should be compared to living species because many of them have modern relatives. Heterochely, intraspecific variation, ontogenetic changes and sexual dimorphism are all factors that have to be taken into account when working with fossil ghost shrimps. Distal elements are usually more variable than proximal ones. Preliminary results suggest that the ghost shrimp clade emerged not before the Hauterivian (~ 133 Ma). The divergence of Ctenochelidae and Paracalliacinae is estimated to occur within the interval of Hauterivian to Albian (133–100 Ma). Callichirinae and Eucalliacinae likely diverged later during the Late Cretaceous (100–66 Ma), whereas Callianassinae did not appear before the Eocene (56 Ma).
Keywords: Crustacea, Decapoda, Callianassidae, Ctenochelidae, phylogeny, taxonomy, taphonomy, fossil record, burrow, heterochely
1. Introduction
The vernacular term “ghost shrimp” usually refers to taxa from the axiidean Callianassidae Dana, 1852 and its allies (Callianideidae Kossmann, 1880 and Ctenochelidae Manning & Felder, 1991). However, sometimes it is used also for Caprelloidea Leach, 1814 (Amphipoda) (e.g., Hirayama 1988) or, mostly in aquarium trading, for Palaemonidae Rafinesque, 1815 (Decapoda: Caridea). Here, the first usage is adopted.
Ghost shrimps of Callianassidae and Ctenochelidae (= Gourrettiidae Sakai, 1999) are soft-bodied, fossorial decapods with a pleon distinctly longer than the carapace (Fig. 1), inhabiting predominantly shallow intertidal and subtidal marine environments mainly in the tropics and subtropics (Dworschak 2000, 2005). Ghost shrimps represent major bioturbators of muddy and sandy substrates of fully marine environments as well as environments with a changing salinity (e.g., Dworschak 2000; Felder 2001).
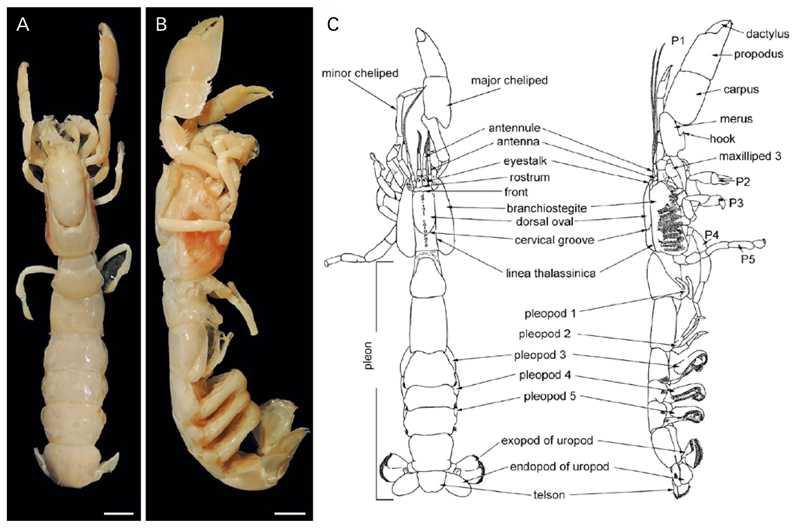
Fig. 1.
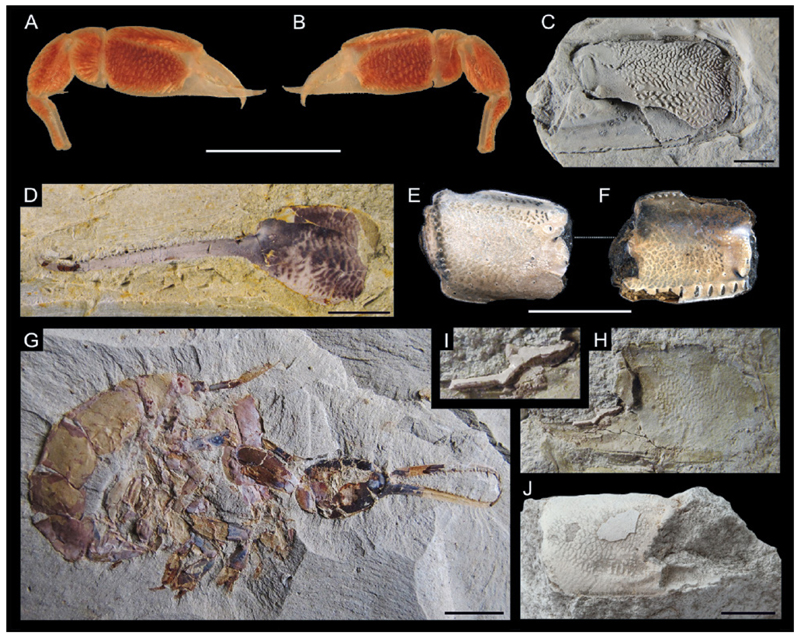
General morphology of a ghost shrimp. A,B: Dorsal and lateral view of Glypturus laurae (de Saint Laurent in de Vaugelas & de Saint Laurent, 1984) (NHMW 6973). C: Generalized ghost shrimp morphology of a different species with body parts indicated (modified after Biffar 1971). P1 – P5 = pereiopods 1 to 5. Scale bars: 10.0 mm.
While brachyuran crabs may be one of the best-preserved crustacean groups in the fossil record (Bishop 1986), ghost shrimps are one of the most ubiquitous. Their remains are present in most assemblages of Cenozoic decapod crustaceans described so far and, as Glaessner (1969: R435) noted, their “chelae are almost ubiquitous in Tertiary sediments”.
Interestingly, the number of fossil callianassid and ctenochelid species as recognized by De Grave et al. (2009) is comparable with extant species (230 fossil vs. 223 extant species), which is not the case for many arthropod groups. The fossil record is, thus, relatively rich, and, if interpreted correctly, questions regarding phylogeny and evolution of these animals can be answered. The interpretation of fossil material is difficult mainly because the generic assignment of ghost shrimp remains is often hindered by their insufficient preservation. Inconsistencies in the biological classification and taxonomy of the group are another issue (Dworschak et al. 2012; Poore et al. 2014). Furthermore, ghost shrimp are understudied as exemplified by their unresolved taxonomy, i.e., many taxa are still classified as “Callianassa” (see chapter 3.2.). Additionally, they are not always reported in the scientific literature. If present only as fragmentary elements, they were often neglected by scholars or mentioned only very briefly and treated in open nomenclature (e.g., Philippe & Secretan 1971; Vega et al. 1995; Schweitzer & Feldmann 2001; Schweitzer et al. 2006a; De Angeli et al. 2010).
Understanding their fossil record is crucial for correct interpretation of the role of ghost shrimps in their environments throughout geologic time. Recently, interpretations of the evolutionary history of fossorial shrimps (including ghost shrimps) have been proposed based on indirect (and partly dubious) evidence of trace fossils without taking into account the body fossil record (Baucon et al. 2014).
In comparison to brachyuran and anomuran decapods, only little attention has been paid to the systematics of fossil callianassid and ctenochelid ghost shrimps, which is a consequence of their puzzling fossil record. Inter- and intraspecific variations, heterochely, sexual dimorphism as well as ontogenetic changes have major impact on identifying isolated ghost shrimp elements. The review of thalassinidean taphonomy of Bishop & Williams (2005: p. 218) did not address taxonomy of the ghost shrimps claiming that, in the context of their study, “the classification of these burrowing shrimp is much less important than their functional role within ancient and modern ecosystems”. They (p. 233) noted, however, that it would be necessary to document the range of these variations in many extant taxa if the fossil record of these animals is ever to be understood. We take this conclusion as a stepping stone for our research. Moreover, several aspects of ghost shrimp taphonomy were not discussed by Bishop & Williams (2005).
Here, we address various major issues for understanding the fossil record of ghost shrimps. The aims of the present paper are (1) to discuss the taphonomy of ghost shrimps and its bearing on the identification of fossil material; (2) to address the taxonomic importance of the characters present on chelipeds; (3) and to evaluate the implications for systematics and phylogeny by using fossil material.
2. Material and methods
The database (see Electronic Supplement) consists of all fossil species-level taxa attributable to ghost shrimps or originally thought to belong among them (i.e., described as Callianassa). The census yielded 274 species, including 17 junior subjective synonyms. Extant species with a known fossil record were excluded from the database because their description is based on complete or near-complete individuals, which might skew the investigated taphonomic patterns. After removal of synonyms and taxa moved to families different from Callianassidae and Ctenochelidae, 250 valid fossil ghost shrimp species were retrieved.
The database was built using original published descriptions, gathering the following data: (1) original name, its authority and year of description; (2) current taxonomic placement; (3) stratigraphic age; (4) type of preservation; (5) and number of specimens on which the original description was based. Data from subsequent additions of known taxa and re-descriptions were not used.
For identifying stratigraphic ranges of genera, also extant species with a fossil record and taxa in open nomenclature were included in Fig. 3.
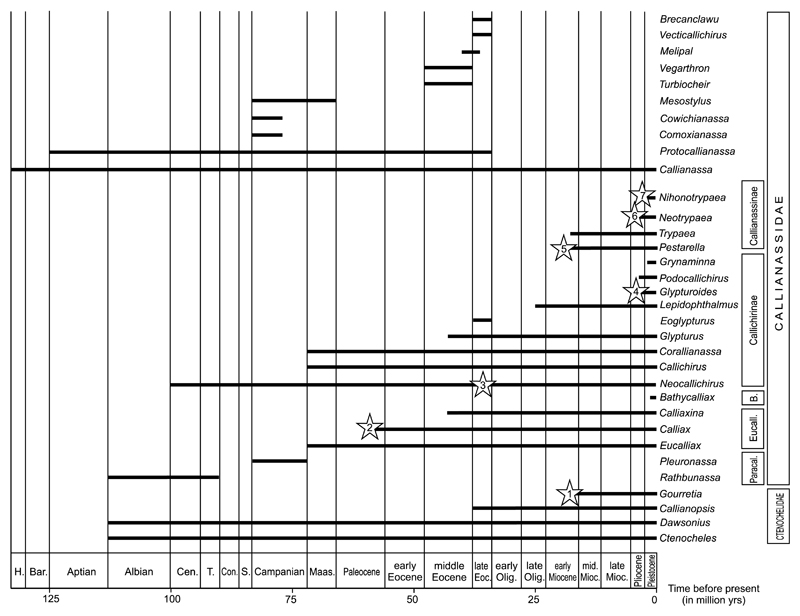
Fig. 3.
Stratigraphic ranges of ghost shrimp genera recognized in the fossil record based on the species database (Electronic Supplement). The oldest records of respective genera: Asterisk 1: Gourretia sp. from the early Miocene of Austria (Hyžný et al. 2015). Asterisk 2: Calliax sp. from the Paleocene (?Thanetian) of Pakistan (Charbonnier et al. 2013). Asterisk 3: Neocallichirus rhinos Schweitzer & Feldmann, 2002 from the late Eocene of Mexico (Schweitzer & Feldmann 2002); older occurrences were doubted by Hyžný & Karasawa (2012). Asterisk 4: Glypturoides trilobatus (Biffar, 1970) from the Plio-Pleistocene of Florida (Portell & Agnew 2004). Asterisk 5: Pestarella sp. from the early Miocene of Switzerland (Fraaije et al. 2010). Asterisk 6: Neotrypaea sp. from the Plio-Pleistocene of Florida (Portell & Agnew 2004). Asterisk 7: Nihonotrypaea sp. from the Pleistocene of Japan (Karasawa 2000a).
Issues concerning taphonomy and taxonomy are based on the study of both fossil and extant material. Numerous specimens deposited in various institutions were studied. The specimens directly mentioned in the text or figured are deposited in the following institutions or are part of private collections:
CBG/CD – Centro para la difusión e investigación de la Biodiversidad y geodiversidad, Ciudad de Lepe (Center for diffusion and research of biodiversity and geodiversity, City of Lepe), Spain; FI – Hungarian Geological and Geophysical Institute, Budapest, Hungary; GBA – Geological Survey, Vienna, Austria; HNHM – Department of Paleontology and Geology, Hungarian Natural History Museum, Budapest, Hungary; KGP-MH – Department of Geology and Palaeontology, Comenius University, Bratislava, Slovakia; MCZ – Museo Civico “G. Zannato”, Montecchio Maggiore (Vicenzia), Italy; MFM – Mizunami Fossil Museum, Mizunami, Japan; MNHN – Muséum National d’Histoire Naturelle, Paris, France; MSNM – Museo Civico di Storia Naturale, Milano, Italy; NHMW – Natural History Museum, Vienna, Austria; NM – National Museum, Prague, Czech Republic; RE – Ruhr Museum, Essen, Germany; RMM – Regional Museum, Most, Czech Republic; SMF – Senckenberg Museum, Frankfurt, Germany; SNM-Z – Natural History Museum of Slovak National Museum, Bratislava, Slovakia; SNSB-BSPG – Bayerische Staatssammlung für Paläontologie und historische Geologie, Munich, Germany; UF – Florida Museum of Natural History, University of Florida, Gainesville, Florida, USA; UMJGPA – Universalmuseum Joanneum, Graz, Austria; USNM – United States National Museum, Smithsonian Institution, Washington, D.C., USA; PCMH – Miroslav Hornáček private collection (Smolenice, Slovakia); PCGW – Gerhard Wanzenböck private collection (Bad Vöslau, Austria); PCYC – Yvonne Coole private collection (Stramproy, The Netherlands); PCZD – Zdeněk Dvořák private collection (Teplice, Czech Republic).
3. Ghost shrimps in the fossil record
3.1. (Palaeo)ecology of ghost shrimps
Ghost shrimps live in a variety of marine environments or environments under marine influence, e.g. estuaries, marshes, and mangroves (Dworschak & Ott 1993; Dworschak 2000, 2004, 2005; Felder 2001). Most extant species have been described from the intertidal environment (Dworschak 2000, 2005), which is at least partly a consequence of a collecting bias towards shallow-water settings (Rex et al. 2000). Many fossil ghost shrimps are known from shallow-water deposits. Although deepwater (i.e., bathyal: depths below 200 m) sediments are rarely preserved, such deposits can provide valuable data on deep-water faunas when exposed. For instance, the deep-water ghost shrimp Callianopsis De Saint Laurent, 1973 is only known from a limited number of specimens for extant species (Alcock & Anderson 1894; Rathbun 1902; Schweitzer Hopkins & Feldmann 1997; Lin et al. 2007), whereas numerous fossil individuals of fossil species attributed to the genus have been collected (Kato 1996; Schweitzer Hopkins & Feldmann 1997; East 2006; Hyžný & Schlögl 2011). From fossil occurrences, it is clear that representatives of the genus preferred soft siliciclastic muddy bottoms as is the case for extant representatives. The presence of individuals of Callianopsis marianae Hyžný & Schiögl, 2011 preserved in situ within their burrows from the early Miocene (~ 16 million years old; abbreviation “Ma [million years]” is used in all instances below) of Slovakia suggests that the animals fit tightly within their burrow (MH pers. obs.). In general, the sediments in which fossil ghost shrimps lived were quite variable, but usually siliciclastic (sandy to muddy; with or without volcanoclastic admixture) to carbonate mud (Dworschak et al. 2012: p. 163).
The latitudinal distribution of extant ghost shrimps is limited to 60° north and south (Dworschak 2005). It remains to be tested whether the dominant limiting factor is temperature and whether the latitudinal distribution changed in the course of the geologic history.
Ghost shrimps have a sophisticated behaviour involving digging complex permanent or semi-permanent burrow systems (Dworschak 1983; Griffis & Suchanek 1991; Dworschak & Ott 1993; Nickel & Atkinson 1995; Felder 2001), and they are important bioturbators. They can rework huge amounts of substrate (Rowden & Jones 1993; Kneer et al. 2013 and references therein) and are considered true ecosystem engineers (Berkenbusch & Rowden 2003; Siebert & Branch 2006; Berkenbusch et al. 2007; Kneer et al. 2013). This behaviour is often preserved in the fossil record as well as trace fossils representing burrows. Several ichnogenera have been attributed to decapod crustaceans by direct comparison to extant ghost shrimp burrows, i.e., Ophiomorpha Lundgren, 1891, Thalassinoides Ehrenberg, 1944 or Spongeliomorpha Saporta, 1887 (e.g., Shinn et al. 1968; Frey et al. 1978; Bishop & Bishop 1992; de Gibert & Ekdale 2010).
Ghost shrimps live in high densities. For instance, for Callianassa subterranea (Montagu, 1808) 40 individuals per m2 were reported by Stamhuis et al. (1997) and for Callianassa truncata Giard & Bonnier, 1890, Ziebis et al. (1996) mentioned up to 120 burrow openings per m2 (for more examples see Bishop & Williams 2005: p. 221). Great abundance of fossil ghost shrimp remains at some localities (e.g., Bishop 1983; East 2006; Schweitzer et al. 2006a; Hyžný & Schlögl 2011; Hyžný & Hudáčková 2012) suggests the same for the past.
3.2. History of describing fossil ghost shrimps
The fossil record of callianassid ghost shrimps has been characterized as being “essentially a series of major chelae” (Dworschak et al. 2012: p. 110). Although this is not always the case, numerous fossil taxa are based on a few isolated cheliped fragments. Thus far, 274 species were treated at some time as a ghost shrimp; 17 of them are currently recognized as junior subjective synonyms. As many as 99 species (36.1%) were described based only on the propodus. More than half of all described taxa (160 species, 58.4%) are known only from their distal cheliped elements, i.e., dactylus and / or propodus. An extreme case is a monograph of Rathbun (1935), who erected numerous taxa based on incomplete elements (e.g., Callianassa cretacea, C. valida, C. beta, C. gamma, C. delta), a few even based on isolated fingers alone (e.g. C. floridana, C. oktibbehana). Ironically, in that very work she (p. 29) noted that “the wide distribution of a species also promotes diversity of form. An extensive series of specimens is needed to determine the composition of a species in this genus [“Callianassa”]”. Recently, also some new taxa have been described based on isolated major propodi (e.g., Beschin et al. 2005, 2009; Breton 2011), although there is a trend to abandon this approach: from 54 ghost shrimp species described since 2000, only 16 (29.6%) were based on distal elements (i.e., propodus and / or dactylus).
Manning & Felder (1991) first considered that the cheliped characters were of great taxonomic importance in addition to the morphology of more weakly sclerotised parts of the exoskeleton (the latter is called „weak-part morphology“ in the following, as opposed to „hard-part morphology“ for strongly sclerotised body parts). Subsequently, several fossil callianassid and ctenochelid species were reassigned to extant genera, acknowledging the work of Manning & Felder (1991) (e.g., Schweitzer Hopkins & Feldmann 1997; Stilwell et al. 1997; Schweitzer & Feldmann 2002; Todd & Collins 2005). Since the 1980s, researchers on extant taxa have attempted to divide the genus Callianassa Leach, 1814 into several independent genera given the heterogeneous nature of this taxon and because they also assigned many species to “Callianassa” (see discussions in Biffar 1971; Ferrari 1981; Manning & Felder 1991). Numerous different genera have been erected since then, nearly always based on weak-part morphology (Poore 1994, 2008: table 1). Most recently, sakai and colleagues erected an array of new genera, largely based on male pleopods (Sakai 2011; Sakai et al. 2014), but their diagnoses are not supplemented with information on chelipeds so that their recognition in the fossil record becomes much more difficult.
A broadly defined concept of the genus has been used many times in the past for fossils: any ghost shrimp with mainstream cheliped morphology has been attributed to Callianassa, and, as a result, 190 species have been described under the collective taxon “Callianassa”. No attention has been paid to many of them since the first description.
Interestingly, since 2000 only 4 (out of 54) newly erected fossil ghost shrimps were attributed to Callianassa (see Karasawa 2000b, 2011; Breton 2011; Hyžný et al. 2013b), and, in most cases, it was clearly stated that the concept of Callianassa s.l. was adopted (e.g., Karasawa 2000b, 2011; Hyžný et al. 2013b). Given the common assignment of new ghost shrimp to other genera, the narrow definition of Callianassa as proposed by Manning & Felder (1991) has been adopted by palaeontologists. Prior to Manning & Felder (1991), 182 fossil Callianassa species were described (66.4% of all ghost shrimps), but only 8 fossil Callianassa species have been described (2.9%) since then.
Many taxa have also been ascribed to Protocallianassa. Beurlen (1930) erected Protocallianassa to accommodate Callianassa archiaci A. Milne-Edwards, 1860. This genus has subsequently been used for almost any callianassid remain from Cretaceous rocks or for those possessing a distal margin of the major propodus at an angle greater than 90° (see Schweitzer & Feldmann 2012). For example, Schweitzer et al. (2010) listed 21 species assigned to the genus. However, Schweitzer & Feldmann (2012) called attention to the type material of C. archiaci, showing that the distal margin of the propodus possesses an angle around 90°. Moreover, Hyžný (2012) opined that an angle of the carpus / propodus articulation is a subjective and variable character in some extant taxa (genus Eucalliax), suggesting that ascription of many taxa to Protocallianassa may not be justified. Therefore, all taxa previously assigned to the genus need to be revised. Thus, Callianassa and Protocallianassa, so well established in the palaeontological literature, appear to be “waste-basket taxa”, perhaps analogous to that of Hoploparia Mccoy, 1849 among nephropid lobsters as suggested by Tshudy & Sorhannus (2003; but see Feldmann et al. 2007).
In the last decade, more fossil ghost shrimp species were erected than during any comparable period since World War II (Fig. 2), suggesting that more taxa remain undiscovered and / or are undescribed. The most productive time for description of new fossil ghost shrimp taxa were 1920s and 1930s. At that time, Mary J. Rathbun erected numerous taxa (Rathbun 1918, 1919, 1926, 1930, 1935) based often on very fragmentary material. During her career, she erected 51 new fossil ghost shrimp species (nearly all of them as Callianassa), of which 25 were described on the basis of one or two specimens and as many as 40 were based on the description of the dactylus and /or propodus (i.e., the most variable elements). This calls for a revision of many of these taxa. Recently, Callianassa anguillensis Rathbun, 1919 and C. latidigita Rathbun, 1919 were reassigned to Glypturus by Klompmaker et al. (2015a). They, however, were treated in open nomenclature as Glypturus sp. given the incomplete preservation of the material.
Fig. 2.
Fossil ghost shrimp description curves since 1820s. One interval represents 5 years (main figure) and 10 years (inset). Blue curve = number of described species; red curve = number of species described as Callianassa; green curve = number of genera. The three main peaks result from the works of A. Milne-Edwards, M.J. Rathbun and scholars of the 21st century.
It has to be noted that fossils were directly compared to extant material in only some cases (e.g., Hyžný & Müller 2010; Hyžný & Muñiz 2012; Baldanza et al. 2013; Klompmaker et al. 2015a). We argue that fossil material should always be compared to living species because many of them have modern relatives, to better understand the taxonomic placement of fossils, and to evaluate inter- and intraspecific variation properly (see chapter 5.).
3.3. Stratigraphic distribution of ghost shrimps
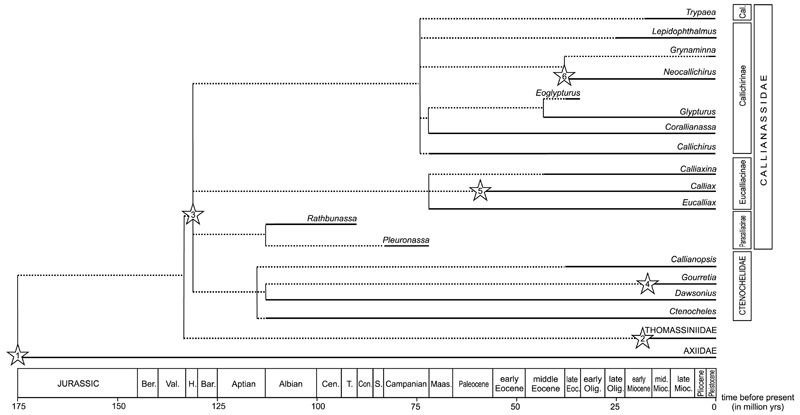
Ghost shrimps have a robust fossil record spanning from the Early Cretaceous to the Holocene. All Jurassic species previously referred to Callianassa are now interpreted to be representatives of Axiidae Huxley, 1879 (Förster 1977). Since the Early Cretaceous (~ 130 – 120 Ma), callianassid and ctenochelid ghost shrimps have become common macrofaunal elements in most studied fossil assemblages. Based on the compiled database (see Electronic Supplement), 250 valid fossil ghost shrimp species are known.
Nearly fifty extant callianassid and ctenochelid genera are currently recognized (De Grave et al. 2009; Anker 2010; see Sakai 2005, 2011 and Sakai et al. 2014 for a different view). However, as noted by Hyžný & Müller (2010: p. 37), less than a quarter of these have a fossil record that extends back before the Pliocene. This can be ascribed not only to collecting and reporting biases, but also to a preservational bias (see chapter 4.2.). As many extant genera are differentiated based on weak-part morphology, they will remain unrecognized in the fossil record if not re-diagnosed to include hard-part morphology. Discerning proxy characters present on chelipeds that are consistent throughout the genera will help to better classify their remains from older stratigraphic levels. This approach has led to relatively long stratigraphic ranges for genera previously unknown from the fossil record, specifically Calliaxina Ngoc-Ho, 2003 (Hyžný 2012: middle Miocene, 13 Ma); Calliax de Saint Laurent, 1973 (Charbonnier et al. 2013: Paleocene [?Thanetian, ~ 58 Ma]; Hyžný & Gašparič 2014: Oligocene [Rupelian], 28 Ma); and Lepidophthalmus Holmes, 1904 (Hyžný & Dulai 2014: Oligocene [Rupelian], 28 Ma).
Most fossil ghost shrimp taxa (78 species) have been described from Eocene strata (56 – 34 Ma). More than forty species are described from Upper Cretaceous strata (100 – 66 Ma) and more than thirty and fifty species from the Oligocene (34 – 23 Ma) and Miocene strata (23 – 5 Ma), respectively. No more than fifteen fossil species are known from the Paleocene (66 – 56 Ma). From the Pliocene (5 – 2.5 Ma) and Pleistocene (2.5 – 0.01 Ma), less than ten exclusively fossil species are known (Electronic Supplement), but several extant species are also known from Pliocene and Pleistocene strata (e.g., Abrard 1947; Portell & Agnew 2004). It is difficult to interpret these raw data as there still are numerous species classified within Callianassa s.l. that need to be revised, which may alter diversity patterns. Pending thorough species revisions, the oldest fossil occurrences of genera indicated in Fig. 3 should be taken as preliminary.
4. Taphonomy of ghost shrimps
4.1. Decay
Ghost shrimp decay has not been investigated in detail. Therefore, observations on other shrimps are used herein. For example, Plotnick (1986) argued that the physical disturbance of a buried decaying shrimp by bioturbation and scavenging are important processes in addition to bacterial decay based on field experiments using the caridean shrimp Pandalus danae Stimpson, 1857. Jar experiments (where bacterial decomposition dominated) resulted in the disassociation of legs, pleon, and carapace; major disintegration of soft tissue; and softening of the cuticle after two weeks. Doubling that time resulted in tiny fragments for open jars. Likewise, Allison (1988) found that only a few fragments of the carapace were left when another caridean shrimp, Palaemon adspersus Rathke, 1837, was put in a jar for 25 weeks. For the same caridean shrimp, Allison (1986) found that turbulent movement by tumbling resulted in faster disintegration after the shrimp had been decaying for a while prior to the experiment. For freshly killed specimens, tumbling for five hours resulted only in the carapace being separated from the pleon. Several stages of decay were postulated by Briggs & Kear (1994) based on jar experiments with the caridean shrimps Crangon crangon (Linnaeus, 1758) and Palaemon elegans Rathke, 1837: swollen, rupturing, hollow (muscle degeneration), disarticulation, and fragmentation. Complete decay was on the order of tens of weeks.
The availability of oxygen does not seem to play a major role in the decay of shrimps (Allison 1988; Briggs & Kear 1995), but the open or closed nature of the system does by influencing the pH and early mineralization enhancing their preservation potential (Briggs & Kear 1995). Closed systems may be promoted by deep burial, the formation of microbial films on the carcass’s surface, or by a large size promoting an internal closed system (Briggs & Kear 1995). The amount of calcium present in the cuticle may influence caridean shrimp decay as Briggs & Kear (1995) noted that Crangon crangon (< 0.1% Ca in cuticle) decayed faster than did Palaemon elegans (8% Ca). It is important to note that these shrimps exhibit no major, well-calcified claw, as opposed to nearly all ghost shrimps. Therefore, complete decay of the major claw may take longer in the absence of other physical disturbances, which is confirmed by the fossil record as elements of the major chelae, namely the propodus and to a lesser extent the carpus and dactylus, are more often preserved than other parts of these shrimps. This is further supported by their ubiquitous presence in the fossil record (e.g., Glaessner 1969; Bishop & Williams 2005), also relative to modern representatives (De Grave et al. 2009).
4.2. Preservation
Due to the delicate nature of most of the cuticle of ghost shrimps, only the hardened parts are usually preserved, i.e., the chelae, the antero-dorsal portion of the carapace, and sometimes the posterior pleonal segments and the telson. Heavily calcified chelipeds are preserved most frequently (Schäfer 1972: p. 314; Bishop & Williams 2005), although other parts are sometimes preserved as well. Altogether, 38 fossil ghost shrimp species (13.9%) were described based on material including at least a partial pleon and / or carapace. In some other species, however, weakly sclerotised parts were found after their first description.
4.2.1. Types of preservation
Three main types of preservation in terms of completeness of the material can be observed for fossil ghost shrimps (modified after Bishop & Williams 2005), discussed in decreasing order of completeness:
-
(1)
(Near) complete body fossil – completely preserved decapod crustacean, although some parts may be missing, but the majority of all three main parts of the shrimp should be present (i.e., carapace, legs, and pleon). Whole-body fossils of ghost shrimps are rare; from the 250 valid fossil species of Calianassidae and Ctenochelidae described so far, only 19 species (6.9%) were originally described from whole-body fossils. They usually represent moults with the chelipeds positioned anteriorly to the rest of the body. The central part of the carapace together with the branchiostegites is flipped over, whereas the pleon is bent inward so that the telson points anteriorly. Examples include Protocallianassa archiaci (A. Milne-Edwards, 1860) from the “Cretaceous” of France (Schweitzer & Feldmann 2012: fig. 1); Callianassa jahringensis Glaessner, 1928 from the middle Miocene (~ 16 Ma) of Slovenia (Glaessner 1928: pl. 4); Rathbunassa aquilae (Rathbun, 1935) from the mid-Cretaceous (Albian, ~ 110 Ma) of Mexico (Vega et al. 2007: fig. 6.9) and Colombia (Bermúdez et al. 2013: fig. 5D).
-
(2)
Disassociation unit – a natural aggregation of exoskeleton elements commonly preserved together. As ghost shrimps decompose, they disintegrate into disassociation units comprised of the more heavily calcified parts of the exoskeleton. Disassociated chelipeds are more common than disassociated pleonal units: 90 species (32.9% of all species) were described using chelae consisting of at least three elements, usually preserved as a disassociation unit. Examples of disassociated chelipeds include: Lepidophthalmus crateriferus (Lőrenthey in Lőrenthey & Beurlen, 1929) from the Oligocene (~ 28 Ma) of Hungary (Hyžný & Dulai 2014: figs. 2A,C,F); Callianassa oregonensis Dana, 1849 from the Oligocene (~ 23 Ma) of Oregon, USA (Rathbun 1926: pl. 28.9); C. parinasensis Woods, 1922 from the middle Eocene (~ 40 Ma) of Peru (Woods 1922: pl. 17.4); Callianopsis marianae Hyžný & Schlögl, 2011 from the early Miocene (~ 16 Ma) of Slovakia (Hyžný & Schlögl 2011: text-fig. 2). More examples are shown in Bishop & Williams (2005: fig. 4a – j,l).
-
(3)
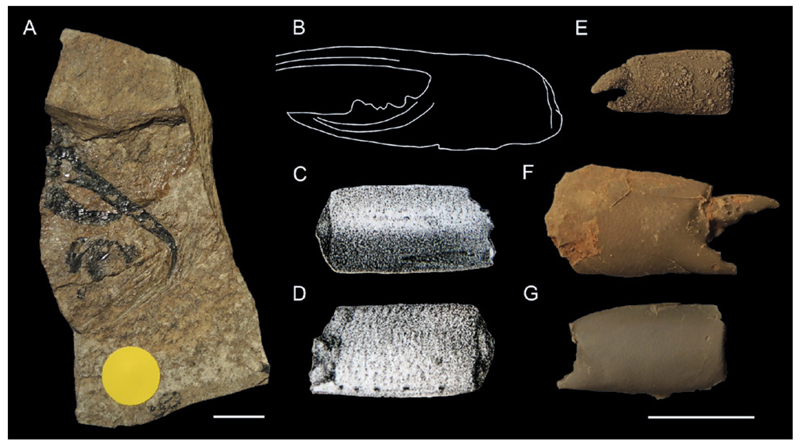
Isolated elements – single part of the exoskeleton found without any associated parts from the same specimen. If the cheliped disassociation unit disintegrates further, only often fragmentary, isolated cheliped elements remain (that is, an isolated propodus or dactylus not attached to one another and not in the immediate proximity of another). This mode of preservation constitutes the most abundant portion of the ghost shrimp fossil record: 160 species (58.4% of all species) were originally described based on the distal cheliped elements, i.e., dactylus and / or propodus. A cephalic disc disassociation unit sensu Bishop & Williams (2005) is here considered an example of an isolated part because it usually does not break into recognizable parts unlike a complete cheliped. Examples of such preservation are shown in Fig. 4B – C. Some of the many examples of species known from the isolated cheliped elements include: Calliaxina chalmasii (Brocchi, 1883) from the middle Miocene (~ 12.5 Ma) of Hungary (Hyžný 2012: fig. 4); Neocallichirus brocchii (Lőrenthey, 1897) from the middle Miocene of Slovakia (Hyžný & Hudáčková 2012: figs. 3 – 5); Podocallichirus laepaensis Hyžný & Muñiz, 2012 from the late Miocene (~ 5 Ma) of Spain (Hyžný & Muñiz 2012: figs. 5 – 6); Glypturus spp. from the Oligocene– Pleistocene of the Western Atlantic (Rathbun 1935; Klompmaker et al. 2015a); and callianassids from the Early Cretaceous of Europe (Klompmaker et al. 2012).
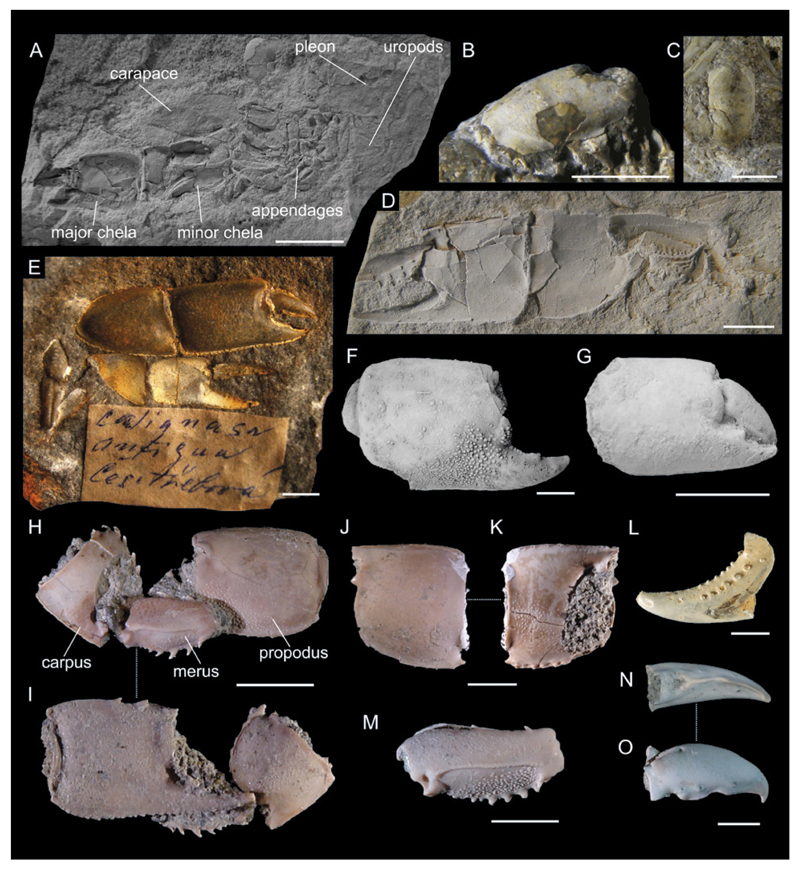
Fig. 4.
Types of fossil ghost shrimp preservation as discussed in chapter 4.2.1. A: Type 1: near-complete animal, Callianopsis marianae Hyžný & Schlögl, 2011 (early Miocene of Slovakia), KGP-MH/CL010. B,C: Type 3: isolated dorsal carapace, Callianassa oroszyi (Bachmayer, 1954) (middle Miocene of Austria), holotype NHMW 2009/0095/0001 (B) and paratype NHMW 2009/0095/0002 (C). D: Type 2: cheliped disassociation unit (isolated major chela), Callianassa floriana Glaessner, 1928 (early Miocene of Slovenia), holotype NHMW 1846/0049/0009. E: Type 2: cheliped disassociation unit (both chelae), Protocallianassa antiqua (Roemer, 1841) (Late Cretaceous of the Czech Republic), NM-O7577. F: Type 3: isolated major propodus, Glypturus munieri (Brocchi, 1883) (middle Miocene of Hungary), FI M.2355. G: Type 2: major chela (propodus + dactylus), Eucalliax pseudorakosensis (Lőrenthey in Lőrenthey & Beurlen, 1929) (middle Miocene of Romania), lectotype FI M.21. H,I: Type 2: cheliped disassociation unit (major propodus + carpus + merus), Glypturus sikesi Klompmaker et al., 2015a (late Miocene of Florida, USA), paratype UF 248042. J,K: Type 3: isolated major carpus, Glypturus sikesi (late Miocene of Florida, USA), paratype UF 248029. L: Type 3: isolated major propodus (fixed finger only), Podocallichirus laepaensis Hyžný& Muñiz, 2012 (late Miocene of Spain), CBG/CD/066. M: Type 3: isolated major merus, Glypturus sikesi (late Miocene of Florida, USA), paratype UF 248038. N,O: Type 3: isolated major dactylus, Glypturus sikesi (late Miocene of Florida, USA), paratype UF 235166. H–K and M – O from Klompmaker et al. (2015a). Specimens in A, D, F and G were coated with ammonium chloride prior to photography. Scale bars equal 5.0 mm.
Based on the completeness of the original material and reflecting the classification given above, fossil ghost shrimp species can be divided in taxa described using the near-complete body fossils (Type 1), chelae including proximal elements (Type 2) and using only distal cheliped elements (Type 3) (Fig. 5). Five species were described either as isolated carapaces [Callianassa oroszyi (Bachmayer, 1954), Callianassa taiwanica Hu & Tao, 1996 (no ghost shrimp, see Karasawa 2000a: p. 192), and Dawsonius tigris Franţescu, 2014] or the information of the type of preservation is not available (Callianassa persica A. Milne-Edwards, 1860, and C. primaeva Philippi, 1887).
Fig. 5.
Proportions of fossil ghost shrimp descriptions (n = 274) based on different types of elements. Only original species descriptions are included in the diagram. Near-complete body fossils correspond to Type 1 preservation in chapter 4.2.1.; chelae including proximal elements largely correspond to Type 2 in chapter 4.2.1.; and only distal elements largely correspond to Type 3 in chapter 4.2.1.
4.2.2. Preservation in burrows
Ghost shrimp burrows function as structural components of the animals’ skeletal support (as the animals themselves are largely soft-bodied) and act as their shelter. As a result, much of the shrimp’s integument is reduced because the burrow walls replace many of the cuticle’s functions such as protection from predation.
Ghost shrimps may be preserved in burrows as a disk, button (sensu Bishop & Williams 2005), or a tube-like portion of sediment that formed around the ghost shrimp remains. Burrow buttons represent body fossils preserved as parts of burrows themselves (e.g., Hyžný 2011; Hyžný & Hudáčková 2012). Death or moulting within burrows followed by rapid burial may lead to this mode of preservation. Other examples of ghost shrimps preserved within their burrows or in close association with them are known (e.g., Mertin 1941; Shinn 1968; Beikirch & Feldmann 1980; Schweitzer & Feldmann 2000; Crawford et al. 2006; Hyžný 2011: table 1; Hyžný & Muñiz 2012: fig. 7; Table 1). Bishop & Williams (2005) argued that the preservation of the major and minor chelipeds in close proximity may be taken as evidence for burrows, because the chelipeds would almost certainly have been separated otherwise.
Table 1.
Occurrences of fossil ghost shrimp remains preserved in burrows or associated with burrows, arranged by family and age.
| Taxon | Age | Country | Remarks | Major reference |
|---|---|---|---|---|
| Callianassidae | ||||
| “callianassid claws” | Early Cretaceous (Berriasian / Valanginian, ~ 140 Ma) | Argentina | associated with burrows | Mángano & Buatois (1991) |
| Mesostylus faujasi | Late Cretaceous (Cenomanian, ~ 95 Ma) | Czech Republic | in a burrow | Veselská (2009) |
| Mesostylus faujasi | Late Cretaceous (Cenomanian, ~ 95 Ma) | Czech Republic | associated with burrows | Veselská (2009) |
| Protocallianassa antiqua | Late Cretaceous (Cenomanian, ~ 95 Ma) | Germany | associated with burrows | Müller (1970) |
| Protocallianassa antiqua | Late Cretaceous (Turonian, ~ 90 Ma) | Czech Republic | associated with burrows | Kříž & Čech (1974) |
| Rathbunassa aquilae (as “a shrimp”) | Late Cretaceous (Turonian, ~ 90 Ma) | USA (Texas) | in a burrow | Shinn (1968) |
| Mesostylus faujasi | Late Cretaceous (Santonian, ~ 85 Ma) | Germany | associated with burrows | Förster (1973) |
| Protocallianassa ex aff. antiqua | Late Cretaceous (Santonian, ~ 85 Ma) | Germany | in a burrow | Mertin (1941) |
| “Callianassa” sp. | Late Cretaceous (Campanian, ~ 80 Ma) | USA (Texas) | in a burrow | Beikirch & Feldmann (1980) |
| Mesostylus faujasi | Late Cretaceous (Campanian, ~ 80 Ma) | Germany | in a burrow | Mourik et al. (2005) |
| Mesostylus mortoni | Late Cretaceous (Campanian, ~ 80 Ma) | USA (Delaware) | associated with burrows | Picket et al. (1971) |
| Mesostylus mortoni | Late Cretaceous (Campanian, ~ 80 Ma) | USA (Delaware) | in a burrow | Feldmann et al. (2013) |
| Mesostylus faujasi | Late Cretaceous (Maastrichtian, ~ 70 Ma) | The Netherlands | in a burrow | Swen et al. (2001) |
| Callichirus waagei | Late Cretaceous (Maastrichtian, ~ 70 Ma) | USA (South Dakota) | associated with burrows | Waage (1968), Crawford et al. (2006) |
| Eucalliax burckhardti | early Paleocene (Danian, ~ 65 Ma) | Argentina | associated with burrows | Feldmann et al. (1995) |
| Callichirus symmetricus | middle Eocene (Lutetian, ~ 45 Ma) | Antarctica | in a burrow | Stilwell et al. (1997), Schweitzer & Feldmann (2000) |
| Neocallichirus rhinos | middle Eocene (~ 40 Ma) | Mexico | in a burrow | Schweitzer & Feldmann (2002) |
| Vegarthron santiago | middle Eocene (~ 40 Ma) | Mexico | in a presumed burrow | Schweitzer & Feldmann (2002) |
| Melipal chilensis | late middle-late Eocene (~ 38 Ma) | Chile | associated with burrows | Schweitzer et al. (2006) |
| Neocallichirus borensis | late Eocene (Priabonian, ~ 35 Ma) | Italy | in a burrow | pers. obs. (MH, Nov. 2014) |
| “Callianassa” sp. | late Eocene (Priabonian, ~ 35 Ma) | USA (Georgia) | in a burrow | Bishop & Whitmore (1986) |
| Calliax michelottii | late Oligocene (Chattian, ~ 25 Ma) | Germany | in a burrow | Polkowsky (2004) |
| Neocallichirus okamotoi | late Oligocene (Chattian, ~ 25 Ma) | Japan | associated with burrows | Karasawa (1993) |
| “Callianassa” cf. C. awakina | early Miocene (Burdigalian, ~ 17 Ma) | New Zealand | in burrows | Hayward (1976) |
| “Callianassa” sp. | early Miocene (Burdigalian, ~ 17 Ma) | Austria | in a burrow | Ehrenberg (1938) |
| “Callianassa” almerai | middle Miocene (Langhian, ~ 15 Ma) | Austria | in a burrow | Hyžný (2011) |
| Eucalliax pseudorakosensis | middle Miocene (Serravallian, ~ 13 Ma) | Slovakia | in a presumed burrow | Hyžný & Hudáčková (2012) |
| “Callianassa” ?pseudorakosensis | middle Miocene (Serravallian, ~ 13 Ma) | Poland | associated with burrows | Radwański & Wysocka (2004) |
| Glypturus munieri | middle Miocene (Serravallian, ~ 13 Ma | Hungary | in a burrow | Kókay & Müller (1993) |
| Neocallichirus brocchii | middle Miocene (Serravallian, ~ 13 Ma) | Slovakia | in a presumed burrow | Hyžný & Hudáčková (2012) |
| “Callianassa” sp. 1 | middle Miocene (Serravallian, ~ 13 Ma) | Hungary | in a burrow | Hyžný (2011) |
| “Callianassa” sp. 2 | middle Miocene (Serravallian, ~ 13 Ma) | Hungary | in a burrow | Hyžný (2011) |
| Grynaminna grandis | Pleistocene (~ 2 Ma) | Japan | in burrows | Karasawa et al. (2006) |
| Callianassa kraussi | mid-Holocene (0 Ma) | South Africa | associated with burrows | Compton (2001) |
| Ctenocheles inaequidens | Late Cretaceous (Maastrichtian, ~ 70 Ma) | The Netherlands | associated with burrows | Pelseneer (1886) |
| Ctenocheles madagascariensis | Late Cretaceous (?Maastrichtian, ~ 70 Ma) | Madagascar | in a burrow | pers. obs. (MH, Nov. 2014) |
| Ctenocheles bakeri | middle Paleocene (Selandian, ~ 60 Ma) | Australia | associated with burrows | Glaessner (1947) |
| Ctenocheles sp. | middle Eocene (Lutetian, ~ 45 Ma) | Italy | in a burrow | herein |
| Ctenocheles sp. | late Eocene (Priabonian, ~ 35 Ma) | Italy | associated with burrows | pers. comm. (A. De Angeli, Sept. 2011) |
| Ctenocheles fragilis | late Oligocene / early Miocene (~ 23 Ma) | Australia | in burrows | Jenkins (1972) |
| Callianopsis marianae | early Miocene (Burdigalian, ~ 17 Ma) | Slovakia | in a burrow | pers. obs. (MH, Nov. 2014) |
| Callianopsis spp. | middle Miocene (Langhian, ~ 15 Ma) | Japan | associated with burrows | Kato (1996) |
Schäfer (1972) noted that moulting of Callianassa takes place in the burrow, but the moult is taken outside the burrow subsequently (see Murray & Hanley 1986 for a different strategy for the mud lobster Thalassina anomala (Herbst, 1804)). Importantly, the heavy (= major) claw of Callianassa separates from the exuvia and is left in the burrow, apparently too large and rounded to be moved outside the burrow as well. He further noted that these animals leave the burrow prior to death, whereas Bishop & Williams (2005) mention that “thalassinideans” are thought to die in their burrow (p. 223), not citing Schäfer (1972) in their paper. Bishop & Williams (2005: fig. 1b), however, showed a dead specimen of Callichirus major (Say, 1818) outside its burrow. This was also supported by Frey et al. (1978), who observed moribund specimens of C. major outside their burrow after they destroyed the burrow’s narrow apertural neck. This species may leave the burrow due to noxious chemical stimuli (Schäfer 1972) or at night for foraging (Frey et al. 1978). Wienberg Rasmussen (1971) explained that when the Callianassa animal is near death or sick, it leaves the burrow because it cannot maintain the water current (for breathing purposes, see Schäfer 1972: p. 314).
Assuming that Schäfer’s (1972) observations apply to ancient ghost shrimps as well, callianassid remains preserved in burrows should primarily be moulted major claws. Furthermore, it should be rare to find wellpreserved shrimp bodies within, unless a sudden event (storm, anoxia) caused the animal to die in its burrow. In general, carcasses would typically be found only outside burrows, implying a limited preservation potential. However, various fossil ghost shrimps preserved in situ or in direct association with burrows are known (Table 1, Figs. 6 – 7). Interestingly, individuals of presumable representatives of Rathbunassa aquilae (Rathbun, 1935) from the Late Cretaceous (Turonian, ~ 90 Ma) of Texas (Shinn 1968: pl. 11, fig. 3), Mesostylus faujasi Desmarest, 1822 from the Late Cretaceous (Campanian, ~ 80 Ma) of Germany (Mourik et al. 2005: pl. 2), and “Callianassa” almerai Müller, 1993 from the middle Miocene (~ 15 Ma) of Austria (Hyžný 2011: figs. 2 – 3), are preserved lying on their side and retaining both chelae and / or pleon within the burrows. Hyžný (2011: p. 43) hypothesized that supposedly dead animals or moults sank down with the heaviest exoskeleton part (major chela) to the bottom of the burrow tunnel. A crucial question is in which part of the burrow ghost shrimps moult and in which orientation they do so. If they moult in the deepest part of the burrow (for example to avoid any disturbance) then not much transport is possible. Alternatively, the animal itself may move the moult to the deepest part of the burrow to avoid potential inaccessibility of part of the burrow (see also below). The water current within the burrow may not be strong enough to transport the relatively heavy claw.
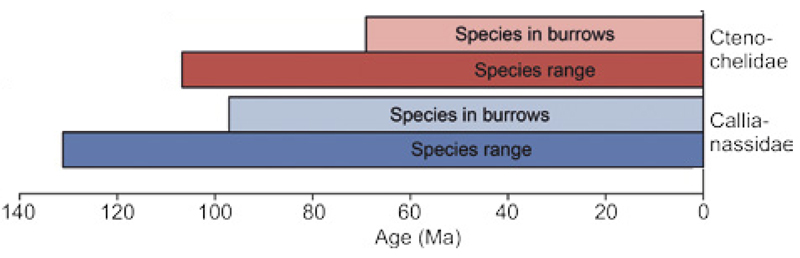
Fig. 6.
The confirmed stratigraphic ranges of named species of Ctenochelidae and Callianassidae as well the stratigraphic ranges of their species found in burrows (see Table 1 in situ for full data). Given that both groups are known to burrow, a large overlap is to be expected.
Fig. 7.
Ghost shrimp body fossils preserved in burrows. A: Grynaminna grandis (Karasawa et al., 2006) from the Pleistocene of Japan in a hollow burrow (cross-sectional view) (MFM 142500). B: Eucalliax pseudorakosensis (Lőrenthey in Lőrenthey & Beurlen, 1929) from the middle Miocene of Slovakia, three individuals (moults?) in a single burrow (SNM-Z 21373). C – E: Mesostylus faujasi (Desmarest, 1822), from the Late Cretaceous (Campanian) of Germany. Three individuals (numbered) in a single burrow (C) (RE A 6075). Single individual (moult?) at the end of a burrow tunnel (D, E) (RE A 5009/03). F: Mesostylus faujasi from the Late Cretaceous of the Czech Republic, major chela (circled) in a burrow (NM-O7576). G: “Callianassa” almerai Müller, 1993 from the middle Miocene of Austria (GW RET93-021). H – K: Ctenocheles sp. from the middle Eocene of Italy (see also Beschin et al. 1998: fig. 6.1) in a large burrow (MCZ 1484) in three different views (I – K) and a closeup of the specimen (H). Specimens in A and B were coated with ammonium chloride prior to photography. A is from Hyžný & Karasawa (2012). Scale bars equal 10.0 mm.
The preservation of several individuals preserved in situ within the same burrow as reported for Mesostylus faujasi (Mourik et al. 2005; Fig. 7C – E), “Callianassa” almerai (Hyžný 2011), and Eucalliax pseudorakosensis (Lőrenthey in Lőrenthey & Beurlen, 1929) (Hyžný 2011; Hyžný & Hudáčková 2012; Fig. 7B) is difficult to explain. Mourik et al. (2005) hypothesized that the dead individuals were carried away and stored in dead-end tunnels by living ones. Bishop & williams (2005) mentioned such systematic removal of exoskeletal fragments (moults or corpses) into disposal chambers that subsequently may be closed off. This behaviour, however, has never been directly observed in extant species, although this may be due to the limited number of studies on behavioural aspects of ghost shrimps in general. We can only note that the preservation of successive moults of the same individual seems unlikely, because the moults/corpses reported by Mourik et al. (2005), Hyžný (2011), and Hyžný & Hudáčková 2012 are of similar size. Moreover, specimens preserved within a single tunnel do not always have the major chela preserved on the same side of the body (Mourik et al. 2005: pl. 1D).
Ghost shrimps may vary markedly in exhibiting different behaviours dealing with stressful situations as well as indifferent ways of handling the moults. The possibility that at least some species live or lived gregariously should not be excluded based on observations of antagonistic behaviour under laboratory conditions (Macginitie 1934; Felder & Lovett 1989; Rodrigues & Hödl 1990; Shimoda et al. 2005).
4.2.3. Soft tissue preservation
Only rarely soft tissue is preserved in ghost or mud shrimps, requiring special taphonomic conditions including rapid burial. Haug et al. (2013) reported on muscles in Upogebia aronae Haug, Nyborg & Vega, 2013 from the Eocene (~ 46 Ma) of California (USA). No example of similarly preserved callianassid or ctenochelid ghost shrimp has been reported yet.
Scars from muscle attachments are often preserved in fossil ghost shrimps. This reticulate pattern has been reported or depicted for isolated callianassid propodi (e.g., Glaessner 1928; Karasawa 1997; Hyžný & Müller 2010; Breton 2011; Klompmaker et al. 2012; Hyžný & Gašparič 2014), but it has barely been discussed in detail. The scars show direct places where muscle bands were attached during the animal’s life (Fig. 8A– B). During the fossilisation process, these scars can be expressed in different ways depending on the type of preservation. Usually, they are preserved on the specimens without the upper layers of cuticle preserved (Hyžný & Müller 2010) or without any cuticular surfaces (Fig. 8E– F). The scars can even attain positive relief, which can happen when an internal mould is preserved without any remains of cuticule (Hyžný & Gašparič 2014: fig. 8A– B; Fig. 8C), suggesting that the rock around the location where the muscles were present is more prone to erosion.
Fig. 8.
Preservation of the muscle scars and cuticle in fossil ghost shrimps. A,B: Right major chela of a modern specimen of Paragourretia phuketensis Sakai, 2002 (SMF 29522) in outer (A) and inner lateral view (B); transparent cuticle allows observation of the cheliped musculature. C: Internal mould of the major chela of Calliax michelottii (A. Milne-Edwards, 1860) (UMJGPA 77874) from the early Miocene of Slovenia showing muscle scars preserved as positive relief. D: Major propodus of Ctenocheles fritschi Hyžný, Kočová Veselská & Dvořák, 2014 (RMM G-Pa 1031) from the Late Cretaceous (Coniacian) of the Czech Republic showing muscle scars as stains preserved on the cuticular surface. E,F: Right propodus of Callianassa hulli Rathbun, 1935 (holotype USNM MO371576) from the Paleocene of the USA (Arkansas) in two views showing muscle scars. G: Near-complete individual of C. fritschi with preserved cuticle on all parts including abdominal somites (PCZD). H,I: Major chela of Callianopsis marianae Hyžný & Schlögl, 2011 (SNM-Z 24826) from the early Miocene of Slovakia exhibiting preservation of the cuticle on the occlusal surface of the fixed finger. It also shows a reticulate pattern of propodal muscle scars. J: Right major propodus of C. michelottii (GBA 2009/014/0027) from the middle Miocene of Slovenia with preserved muscle scars. Note that the muscle scars can be seen on the internal mould, but not on the cuticle. Specimens in C and J were coated with ammonium chloride prior the photography. Scale bars equal 5.0 mm.
4.2.4. Taphonomy and preservation of the cuticle
Decapod cuticle in general tends to disintegrate within weeks to months in experimental settings (see above). Another major factor impacting the preservation of the cuticle is the type of substrate. As noted by Förster (1966), the uppermost layer of the decapod cuticle, the epicuticle, is often absent in carbonate rich deposits, whereas it is preserved in lithographic limestones and in clays. When preserved in concretions, the decapod fossils usually break along the exo-endocuticle boundary (Waugh et al. 2009). All these aspects have to be taken into account when interpreting characters supposed to be present on the cuticular surfaces, such as tubercles or spines. These features often are eroded away and may hinder identification of the specimens if such characters are considered of taxonomic importance. Recently, Klompmaker et al. (2015b) discussed the effect of preservation of cuticle on the taxonomy of decapod crustaceans. Ghost shrimps preserved in fine siliciclastics may exhibit preservation of the cuticle (Fig. 8D,G– I).
The types of cuticle preservation in ghost shrimps can be divided in three main types:
-
(1)
No cuticle – cheliped elements are preserved as internal moulds, often three-dimensionally. The specimens are smooth and shiny and muscle scars may be visible as coloured patches (Fig. 8E– F).
-
(2)
Altered cuticle – no or few structural details of the cuticle are observable due to obvious recrystallization or, rarely, replacement. Some cuticle layers may be absent as well. This preservation is typical in carbonates (Hyžný 2012: fig. 5).
-
(3)
Cuticle preserved completely – the specimens have the complete cuticle including all layers preserved, seemingly unaltered. They can be flattened if preserved in lithographic limestones (Garassino 2001: fig. 5), marls (Fig. 8G), or clays (Fig. 8H – I). In clays, however, the elements can be preserved also three-dimensionally (Hyžný & Gašparič 2014: fig. 8D – H).
4.3. Major vs. minor chelae
A strong preservational bias is observed in the chelipeds of heterochelous ghost shrimps. Schäfer (1972: p.314) noted that “mainly the large palmae of the claws” of Callianassa were found as fossils. Major chelae constitute one of the most common fossil decapod remains; on the other hand, minor chelae are rare (Klompmaker et al. 2015a, for Glypturus). Because of their less calcified and / or thinner cuticle and their relatively small size, they preserve less commonly than major chelipeds do. Moreover, a collecting bias may also exists because they are usually much smaller, and, therefore, are easily overlooked. Hyžný & Hudáčková (2012) reported numerous disassociated chelipeds and isolated elements of Eucalliax pseudorakosensis and Neocallichirus brocchii from a single site. Major claws were extremely abundant and only a handful of minor chelae have been identified (Hyžný & Hudáčková 2012: tables 1 – 2). In the studied sample of E. pseudorakosensis, 57 majors and 7 (10.9%) minors were identified, whereas for N. brocchii, 46 majors and 6 (11.5%) minors were present. Conversely, Calliaxina chalmasii (Brocchi, 1883) has subequal chelae, and, indeed, minors (originally described as Callianassa rakosensis Lőrenthey, 1897) were nearly as abundant as majors (Müller 1984; Hyžný 2012: table 2, fig. 4), constituting 38.7% of the sample. In this particular case, however, the calculation is somewhat biased because material from three localities with a different number of specimens was included.
In a study on Glypturus Stimpson, 1866, Klompmaker et al. (2015a) mentioned that fossil minors should be smaller than the majors (by definition), may have no tubercles, and may have a relatively long fixed finger compared to the major by analogy with modern Glypturus acanthochirus Stimpson, 1866. Despite their large samples of fossil Glypturus, no unequivocal minors were found, possibly due to their small size making them more fragile, although the thickness of equal-sized majors and minors is not known. Their observation that minors are rare is in line with Schäfer (1972), who found that the heavy claw of Callianassa separates from the exuvia and is left in the burrow, increasing the preservation potential of majors.
Thus far, no study explored possible differences in the thickness of equal-sized majors and minors from the same taxon (P.C. Dworschak pers. comm., February 2015). The assumption that the cuticle of minor chelae is thinner than that of the majors of equal size, as tentatively suggested by Klompmaker et al. (2015a), requires further testing.
4.4. Isopod induced swellings
A part of modern ghost shrimp specimens exhibit swellings in the branchial region, usually attributed to parasitic bopyroid isopods (e.g., Markham 2001; An et al. 2009). Thus far, not a single fossil ghost shrimp with such a swelling in the branchial region is known (e.g., Wienberg Rasmussen et al. 2008; Klompmaker et al. 2014). However, one fossil (Early Cretaceous, Albian, ~ 110 Ma) representative of Axiidae showed such a swelling (Franțescu 2014: fig. 2C). It is not surprising that such swellings, ichnotaxonomically named Kanthyloma crusta Klompmaker, Artal, Van Bakel, Fraaije & Jagt, 2014 (see also Klompmaker & Boxshall 2015), have not been found in fossil ghost shrimps because carapaces are only rarely preserved (see 4.2.1.). A swelling in a propodus of Glypturus panamacanalensis Klompmaker, Hyžný, Portell & Kowalewski, 2015a, has another origin most likely (Klompmaker et al. 2015a).
4.5. Fecal pellets
Ghost shrimps produce rod-shaped striated feces, which are compact and greatly resistant to disintegration (Pryor 1975; Bromley 1990), but Bishop & Williams (2005) stated that, upon settling, fecal pellets slowly lose their coherent shape and become indistinguishable as pellets. Nevertheless, decapod pellets have been preserved in the sedimentological record (e.g., Shinn 1968; Brönnimann 1972; Pryor 1975; Kuss & Senowbari-Daryan 1992; Schweigert et al. 1997; Mehling 2004; Senowbari-Daryan et al. 2009), but ascribing them to taxa of lower taxonomic ranks is difficult because no detailed comparative studies on fecal pellets of extant axiidean and gebiidean taxa have been performed, to our knowledge. Kuss & Senowbari-Daryan (1992) reported on fecal pellets associated with Thalassinoides burrows from the Upper Cretaceous (Cenomanian and Turonian, ~ 100–90 Ma) limestones of Egypt and Jordan, but no body fossils were found. Mehling (2004) reported on fecal pellets from the Late Cretaceous (Campanian, ~ 80 Ma) of Monmouth County, New Jersey, USA, interpreted as products of callianassids based on the fact that numerous remains of Mesostylus mortoni (Pilsbry, 1901) were known previously from the same formation (Rathbun 1935), although not directly associated with fecal pellets. Later, this interpretation has been confirmed, as Feldmann et al. (2013) reported and figured the only occurrence of the ghost shrimp fecal pellets preserved together with a supposed producer Mesostylus mortoni from the Late Cretaceous (Campanian, ~ 80 Ma) of Delaware, USA.
Although Pryor (1975: p. 1246) claimed that the morphology of decapod fecal pellets are probably species-specific, Schweigert et al. (1997: p. 67) concluded that microcoprolites alone “only allow distinguishing different families of producing crustaceans, but a separation on the biospecies-level seems rather impossible”. Identification on the family-level, however, would be sufficient to identify ghost shrimp fecal pellets in the fossil record. More comparative studies on this topic are needed to demonstrate whether differences in pellet morphology and structure can be used for genus-level identifications in ghost shrimps.
5. Taxonomy of the fossil ghost shrimps based on chelipeds
Since Callianassa sensu Manning & Felder (1991) or Ngoc-Ho (2003) is currently diagnosed on the basis weakly sclerotised parts, most of the fossil species of “Callianassa” do not fit the diagnosis. Rather, fossil “Callianassa” represents a heterogeneous mixture of several independent genera. Thus, a major issue in deciphering the fossil record of ghost shrimp is the ascription of fossil specimens to extant genera. If the material is complete enough, i.e., exceptionally preserved including some weak-part morphology, direct comparison with extant taxa is possible. However, as mentioned above (chapter 4.2.) palaeontology is often necessarily focused on isolated cheliped remains. Identification of proxy characters of taxonomic importance of these elements is crucial to assign the fossil material to higher taxa.
5.1. Taxonomically important characters in neontological studies
Several views exist regarding the evaluation of taxonomically important characters (see Biffar 1971; Manning & Felder 1991; Poore 1994, 2008; Sakai 1999, 2005, 2011). The major revisions by Sakai (1999, 2005, 2011) remain questionable at the subfamily- and genus-levels (cf. Dworschak 2007; Poore 2008; Dworschak et al. 2012).
The chelipeds of Callianassidae and many Ctenochelidae are subject to intraspecific variation as well as sexual dimorphism (e.g., Manning & Felder 1986; Felder & Lovett 1989). This is the main reason as to why neontological studies do not consider them to be taxonomically significant. Instead, features of the carapace, maxillipeds, eyes, pleopods, uropods, and telson are used to assign extant species to a genus. These characters are very rarely preserved in the fossil record. As a consequence, more recent literature dealing with fossil ghost shrimps usually emphasizes the contribution of Manning & Felder (1991), who opined that certain characters on the ischium and merus of the major cheliped (serration, presence / absence of proximal hook) are taxonomically important for genus-level assignment (see also chapter 5.3.). The taxonomic importance of the chelipeds for systematics of extant callianassid genera was also emphasized by Ngoc-Ho (2003: table 1) when comparing genera within Eucalliacinae (specifically Calliax, Calliaxina, and Paraglypturus Türkay & Sakai 1995). In addition to weak-part morphology, she also considered the degree of heterochely (unequal vs. subequal chelae), morphological similarity of chelae (similar vs. dissimilar), and whether the chelipeds were laterally compressed (Calliax, Calliaxina) or massive (Paraglypturus).
Both studies (Manning & Felder 1991; Ngoc-Ho 2003) discussed characters present on chelipeds in addition to other characters from weak-part morphology. Careful examination of chelipeds in all congeneric species is needed to identify taxonomically important characters at the genus-level, as was done for Glypturus. For instance, the presence of spines on the upper margin of the propodus is diagnostic for all representatives of Glypturus (Manning 1987; Hyžný & Müller 2012; Klompmaker et al. 2015a). This does not imply that spination is generically important for all ghost shrimp taxa. For example, all Glypturus species possess major carpi with spines on their lower margin, but the same character is also present in Corallianassa intesi (de Saint Laurent & Le Loeuff, 1979) (de Saint Laurent & Le Loeuff 1979: fig. 21a,c), whereas other Corallianassa species do not have a spinulose carpus.
5.2. Variation in chelipeds within species
Heterochely, intraspecific variation, ontogenetic changes and sexual dimorphism are all factors that have to be taken into account when working with fossil ghost shrimps. As pointed out by Bishop & Williams (2005: p. 233), “it will be necessary to document the range of these variations in many extant ghost shrimp taxa if the fossil record of these structures is ever to be understood”. To document variations in fossil taxa statistically, many specimens need to be evaluated.
5.2.1. Heterochely
Heterochely is a condition in decapods where the two claws of an individual differ in size, shape, and often function, which usually occurs in both sexes of a species (Hartnoll 2012). In many decapod taxa heterochely becomes more evident in larger specimens, and is generally more obvious in males (Przibram 1905; Schäfer 1954; Hartnoll 2012).
Ghost shrimps are usually strongly heterochelous (conspicuous asymmetry sensu Babcock 2005) as only a few taxa (Calliaxina and Eucalliax Manning & Felder, 1991) exhibit subequally-sized chelipeds (subtle asymmetry sensu Babcock 2005). The extent of handedness in heterochelous decapod species varies from rigid handedness (left vs. right with the preference for one; directional asymmetry sensu Babcock 2005) to a lack of preference (random asymmetry sensu Babcock 2005). The only major decapod group in which rigid handedness is universal are the hermit crabs (Hartnoll 1982, 2012), whereas there is a complete lack of preference in ghost shrimps (Labadie & Palmer 1996; Felder & Lovett 1989; Nates & Felder 1999). In the fossil record, a lack of preference can be observed using large samples, in which the same propodus morphotype usually occurs as right and left-handed (dextral and sinistral forms) in about equal numbers. For example, for the middle Miocene (~ 13 Ma) Calliaxina chalmasii 8 right vs. 11 left major chelae were reported (Hyžný 2012), for the middle Miocene (~ 12 Ma) Neocallichirus brocchii 23 right vs. 24 left major chelae were reported (Hyžný & Hudáčková 2012), for the middle Miocene (~ 12 Ma) Eucalliax pseudorakosensis 27 right vs. 28 left major chelae were reported (Hyžný & Hudáčková 2012), for the late Miocene (~ 8 Ma) Glypturus sikesi 36 right vs. 34 left major chelae were reported (Klompmaker et al. 2015a), and for the Holo-Pleistocene (~ 2.5 – 0 Ma) G. panamacanalensis 193 right vs. 159 left major chelae were reported (Klompmaker et al. 2015a).
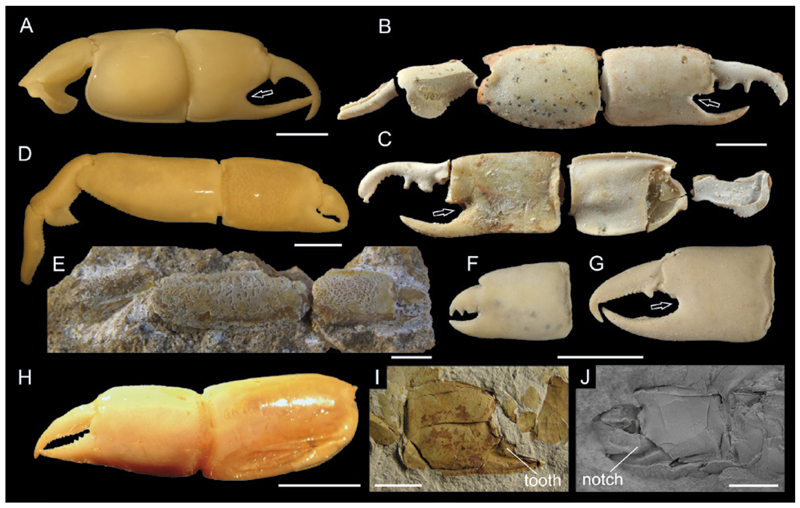
Minor chelipeds are often distinctly different from major ones other than size. This may lead to the recognition of two separate species in the fossil record, especially when dealing with isolated cheliped elements. For instance, Callianassa chalmasii (Fig. 9C) and Callianassa rakosiensis (Fig. 9B), both from the middle Miocene (~ 13 Ma) of Austria and Hungary are now interpreted to represent major and minor chelae of a single species re-assigned to Calliaxina (Hyžný 2012). Similarly, Callianassa nuda Beurlen, 1939 (Fig. 9H) from the Oligocene (~ 28 Ma) of Hungary was recently identified as the minor chela of Ctenocheles rupeliensis (Beurlen, 1939) (Fig. 9G), known from the same locality (Hyžný & Dulai 2014). The latter example is particularly interesting because C. rupeliensis was originally described as the lobster Thaumastocheles Wood-Mason, 1874. Thus, major and minor chelae of a single animal were originally misinterpreted to represent not only two distinct taxa within a single monophyletic group, but as representatives of two different unrelated groups, i.e., ghost shrimps and nephropid lobsters.
Fig. 9.
Heterochely in ghost shrimps. A: Holotype of Callianopsis marianae Hyžný & Schlögl, 2011 (SNM-Z 24.810) from the early Miocene of Slovakia showing both chelae. B: Minor propodus of Calliaxina chalmasii Brocchi, 1883 from the middle Miocene of Hungary (holotype FI M.29 of Callianassa rakosiensis Lőrenthey, 1897). C: Major propodus of C. chalmasii from the middle Miocene of Hungary (HNHM PAL 2011.33). D: Major propodus and dactylus of Neocallichirus brocchii (Lőrenthey, 1897) (PCRB-DH008) from the middle Miocene of Slovakia. E: Minor propodus of N. brocchii (PCRB-DH013). F: Both chelae of Corallianassa acucurvata Swen et al., 2001 from the Late Cretaceous (Maastrichtian) of the Netherlands (PCYC). G: Major propodus and dactylus of Ctenocheles rupeliensis (Beurlen, 1939) (HNHM M.66.961) from the Oligocene of Hungary. H: Minor chela of C. rupeliensis (HNHM M.59.4691), originally described as Callianassa nuda Beurlen, 1939. I,J: Major and minor chela from a modern specimen of Calliax cf. C. lobata (NHMW 25511). K: Major chela of Eucalliax pseudorakosensis (LőrentHey in Lőrenthey & Beurlen, 1929) (PCMH-005) from the middle Miocene of Slovakia. L: Minor chelae of E. pseudorakosensis (KGP-MH/DH070). Note that the couples in B–C, D – E, G – H, and K – L are not from the same individuals. All specimens except F, I, and J were coated with ammonium chloride prior to photography. Scale bars equal 5.0 mm.
Minor chelipeds usually do not bear the pronounced features as observed in major chelipeds. Consequently, there are plenty of species whose minor chelipeds are very similar within a single genus (e.g., for Callichirus see Kensley 1974: fig. 2B; de Saint Laurent & Le Loeuff 1979: fig. 16f – g; Manning & Felder 1986: figs. 1d, 2d). In the fossil record, the attribution of minor chelae to certain species is difficult unless they co-occur with major chelae in the same assemblage (Hyžný & Hudáčková 2012; Hyžný & Dulai 2014; Fig. 10) or based on morphological evidence from extant species. Collecting all material by hand picking or even better by bulk sampling of unlithified sediments from one locality or horizon is crucial. This may help to statistically attribute morphologically distinct specimens to the same species.
Fig. 10.
Sexual dimorphism in the chelipeds of ghost shrimps. A: Male of a modern specimen of Trypaea australiensis Dana, 1852 (NHMW 24988). B,C: Males of Callianassa macrodactyla A. Milne-Edwards, 1860 from the middle Eocene of France (MNHN.F.B71835). D: Sexually mature male of a modern specimen of Callichirus major Say, 1818 (NHMW 19354). E: Sexually mature male of Callichirus bertalani Hyžný & Müller, 2010 from the middle Miocene of Hungary (holotype HNHM M.2009.2334.1). F: Presumed female propodus and dactylus of Callianassa heberti A. Milne-Edwards, 1860 from the middle Eocene of France (NHMW 2011/0169/0009). G: Male propodus and dactylus of C. heberti from the middle Eocene of France (NHMW 2011/0169/0011). H: Female chela from a modern specimen of Callichirus seilacheri (Bott, 1955) (SMF 2184). I: Female chela of Callianopsis marianae Hyžný & Schlögl, 2011 from the early Miocene of Slovakia (SNM-Z 24815). J: Male chela of C. marianae (KGP-MH uncatalogued). Arrows show deep notch typical of sexually mature males. Specimen in J was coated with ammonium chloride prior to photography. B and C were photographed by Ch. Lemzaouda (MNHN). Scale bars equal 5.0 mm.
5.2.2. Sexual dimorphism
Males and females differ morphologically for most ghost shrimp species. There is only one exception known within Callianassidae: in all studied individuals of Callianassa aqabaensis Dworschak, 2003 with a carapace longer than 3 mm both male and female gonopores were reported (Dworschak 2003).
The knowledge on the reproductive morphology of fossorial shrimps is very poor or lacking completely (Dworschak et al. 2012: p. 155). Sexual dimorphism is mainly observed in the first and second pleopods. All females possess these appendages, but they are lacking in many male callianassids (Dworschak et al. 2012), i.e., males of some genera within Callianassinae do not have them at all (Sakai 2011: table 9).
The chelipeds are sexually dimorphic in some callianassid species because the major cheliped becomes larger and more massive in mature males (Shimoda et al. 2005 and references therein). Sexual dimorphism in decapods is usually a consequence of the widespread use of chelae by males in combat, display or courtship (Hartnoll 1974, for crabs). Sexually dimorphic chelipeds, accompanied by allometric growth enhancing the differences between male and female chelae, have been convincingly demonstrated for several ghost shrimp taxa, including representatives of Callichirinae such as Callichirus (Botelho De Souza et al. 1998) or Lepidophthalmus (Felder & Lovett 1989; Nates & Felder 1999), and representatives of Callianassinae such as Neotrypaea Manning & Felder, 1991 (Labadie & Palmer 1996), Nihonotrypaea Manning & Tamaki, 1998 (Shimoda et al. 2005), Paratrypaea Komai & Tachikawa, 2007 (Dworschak 2012), Pestarella Ngoc-Ho, 2003 (Dworschak 1998), and Trypaea Dana, 1852 (Hailstone & Stephenson 1961).
Sexual dimorphs can express not only different growth rates of chelipeds but also different morphologies of the major chela. This has been demonstrated in representatives of the ctenochelid Callianopsis in which the major propodus differs distinctly between sexes (Schweitzer Hopkins & Feldmann 1997; Fig. 10I – J). Two distinct morphotypes of major chelae can also be recognized for Lepidophthalmus spp., which have been tentatively attributed to sexual dimorphism (Hyžný & Dulai 2014). Two morphs of majors, possibly sexual dimorphs, have also been identified for Neocallichirus brocchii and Eucalliax pseudorakosensis from the middle Miocene (~ 13 Ma) of Hungary and Slovakia (Hyžný & Hudáčková 2012). A special case is Callichirus; sexually mature males of Callichirus spp. usually possess greatly elongated elements of the major chela (Manning & Felder 1986; Fig. 10D). This characteristic can be identified also in the fossil record (Hyžný & Müller 2010; Fig. 10E).
In the fossil record, differences in the sexual morphs can lead to the incorrect recognition of two separate taxa, as was the case of Callianopsis clallamensis (Withers, 1924) from the Oligocene and Miocene (~ 25 – 20 Ma) of Washington state, USA. Its female morph was originally described as Callianassa twinensis Rathbun, 1926 (for details see Schweitzer Hopkins & Feldmann 1997 and East 2006).
For ghost shrimps, usually only a single animal occupies a burrow (Dworschak et al. 2012; but see Pryor 1975: p. 1246) and under laboratory conditions, antagonistic behavior has been reported. Documented examples include species of Neotrypaea (MacGinitie 1934), Nihonotrypaea (Tamaki et al. 1997; Shimoda et al. 2005), Callichirus (Rodrigues 1983), Sergio (Rodrigues & Hödl 1990), Lepidophthalmus (Felder & Lovett 1989), and Callianassa subterranea (Witbaard & Duineveld 1989; Rowden & Jones 1994). This behaviour seems more frequently employed by males. Shimoda et al.(2005) documented attacks in several species of Nihonotrypaea, usually between males, in which major cheliped acts as a tool to grip the rival’s one. This is in agreement with the observation of Labadie & Palmer (1996), who analyzed the morphology of chelipeds of its close relative Neotrypaea and argued that the presence of propodal notch, distally hooked dactylus, and serration on fingers seem to be functional in gripping / grappling of the rival. They came to conclusion that sexual dimorphism in the nature of major chelae probably reflects the antagonistic behaviour occurring more often among males than in females. In all the above mentioned genera, of which representatives show antagonistic behaviour (Neotrypaea, Nihonotrypaea, Callichirus, Sergio, Lepidophthalmus, and Callianassa), the presence of notch is observed (Fig. 10). There are ghost shrimps lacking a notch or any sexual dimorphism in the morphology of chelipeds. A good example is Glypturus with males and females having the same cheliped morphology (Biffar 1971; Poore & Suchanek 1988). It may be speculated that representatives of this genus may not express antagonistic behaviour frequently.
5.2.3. Intraspecific variation and polymorphism
Ghost shrimps can express definite intraspecific variation in the major cheliped morphology causing major difficulties for the taxonomic interpretation of the isolated elements in the fossil record. The morphology of merus and carpus are usually quite consistent within the genus, but the nature of the propodus and dactylus, especially the fixed fingers, can be variable. For instance, in extant Sergio mericeae Manning & Felder, 1995 the fixed finger can be armed with numerous teeth or completely unarmed (Manning & Felder 1995: fig. 1b,d,h). Another example is the fossil species Neocallichirus brocchii exhibiting a variable armature on the major dactylus (Hyžný & Hudáčková 2012: fig. 2F). The same can be postulated for Podocallichirus laepaensis Hyžný & Muñiz, 2012 (Hyžný & Muñiz 2012: fig. 6, see also Fig. 11). Klompmaker et al. (2015a) reported intraspecific variation in Glypturus panamacanalensis and G. sikesi in the strength of the teeth on the dactylus and the tooth on the fixed finger.
Fig. 11.
Intraspecific variation in the major chelae of ghost shrimps. Examples include Podocallichirus laepaensis Hyžný & Muñiz, 2012 from the late Miocene of Spain (A– M) and Callianassa heberti A. Milne-Edwards, 1860 from the middle Eocene of France (N – T). Note variation in the teeth formula of the dactylus and the curvature of the fixed finger in P. laepaensis. Note different propodus length / height ratio, variation in the development of propodal notch and the tooth on the fixed finger (arrows) in C. heberti. A: CBG/CD/029. B: CBG/CD/076a. C: Paratype CBG/CD/010. D: CBG/CD/015. E: Paratype CBG/CD/037. F: CBG/CD/018. G: CBG/CD/076b. H: CBG/CD/025. I: Paratype CBG/CD/003. J: CBG/CD/071. K: Paratype CBG/CD/064. L: CBG/CD/066. M: CBG/CD/062. N: NHMW 2011/0169/0014. O: HMW 2011/0168/0006. P: NHMW 2011/0169/0015. Q: NHMW 2011/0168/0007. R: NHMW 2011/0169/0013. S: NHMW 2011/0169/0010. T: NHMW 2011/0169/0012. Scale bars equal 5.0 mm.
Polymorphism in relative growth refers to differences in form not related to sex or maturity, whereby two or more morphs co-occur within the same growth phase (Hartnoll 2012). Definitive polymorphism appears to be a purely male phenomenon for crustaceans in general (Hartnoll 2012). For ghost shrimps, polymorphism is known to occur only in male populations of Callichirus species, e.g., Callichirus major (Botelho De Souza et al. 1998). Regarding the fossil record, it is questionable whether polymorphism can be detected and distinguished from intraspecific variation. Swen et al. (2001) reported polymorphism in Mesostylus faujasi from the Late Cretaceous (Maastrichtian, ~ 70 Ma) of the Netherlands. Their results, however, do not seem to be a case of definitive polymorphism as defined above. Within the studied sample, Swen et al. (2001) recognized three morphotypes interpreted as mature females, mature males and immature males; however, within the same growth phase only one morphotype for each sex was determined.
5.2.4. Size
The size (total length from rostrum to telson) of adult ghost shrimp ranges from about 1.5 cm to ~ 16 cm (Dworschak 2015). It is, however, difficult to estimate their maximum size because they supposedly have indeterminate growth (i.e., without terminal anecdysis). For decapods in general, the percentage of moult increment (i.e., the percentage of size increase at each moult) declines and the intermoult period increases with size, thereby limiting growth (Hartnoll 1983). Growth rate decrease has not been determined for any ghost shrimp known to the authors, in part due to difficulty with catching ghost shrimps (Garcia et al. 2003; Dworschak 2015), especially the large-sized tropical species (Shinn 1968; de Vaugelas 1985; Kneer et al. 2013; Dworschak 2015). Assuming that large specimens have a greater fossilization potential, the fossil record can provide insight into this issue, i.e., maximum size.
Size on its own should never be considered of taxonomic importance because size can be influenced by environmental conditions (ecophenotypic variation), but in some cases it may supplement other characteristic differences among taxa. For instance, representatives of extant Paratrypaea usually does not exceed a total length of 2 cm (Dworschak 2012), and, thus, it may be speculated that its size would be similar for fossil species of this genus. On the other hand, representatives of Callichirinae can reach a total length of 10 – 16 cm (De Vaugelas 1985; Dworschak 2008, 2015), including the largest ghost shrimps known to date (Glypturus, Neocallichirus). As a result, one would not expect to identify typically smallsized ghost shrimp genera with a total body length usually not exceeding 2 cm (e.g., Biffarius Manning & Felder, 1991, Paratrypaea, Pseudobiffarius Heard & Manning, 2000) in a sample of exclusively large-sized isolated propodi. Size may influence the composition of fossil assemblages as poorly calcified, small-sized callianassid genera have a lower fossilisation potential compared to larger-sized specimens. For instance, Thomassiniidae, consisting of small-sized taxa (total body length ~ 1 – 2 cm; Dworschak 2015: p. 5), is represented in the fossil record by only one known species, Crosniera schweitzerae Hyžný & Schlögl, 2011 from the early Miocene (~ 16 Ma) of Slovakia. Not surprisingly, this occurrence is known from very fine clays and the largest propodus found is not longer than 3.5 mm (Hyžný & Schlögl 2011: table 3).
Growth can influence the morphology of cheliped elements. Especially length / height ratios of the propodus and merus are prone to change with growth. This has been reported for both extant (for Neocallichirus see Dworschak 2008) and fossil taxa (for Glypturus see Klompmaker et al. 2015a). Based on three Glypturus spp. assemblages from different stratigraphic levels (Recent, Holo-Pleistocene and late Miocene), Klompmaker et al. (2015a) observed that the propodal length / height ratios increased faster throughout growth in geologically older assemblages, suggesting possible heterochrony.
5.3. Evaluation of characters present on chelipeds
Of a great importance is the selection of systematically important characters to be used for the identification of taxa in the fossil record. Ghost shrimp chelae offer numerous characters for evaluation. Some of them can be subject to intraspecific variation. Therefore, a thorough study of extant material is needed prior to any assignment of fossil material to an extant genus. Distal elements (i.e., dactylus and propodus) are usually more variable than proximal ones (i.e., carpus, merus, and ischium).
5.3.1. Suprageneric-level
In cases when only the distal parts of the chelipeds (dactylus, propodus, carpus) are available, ctenochelids and callianassids occasionally cannot be distinguished, with the exception of the genus Ctenocheles Kishinouye, 1926. As a result, numerous authors have used Callianassa as a collective name resulting in its waste-basket nature of the genus (i.e., mixture of several genera).
Without having diagnostic characters of the maxillipeds and pleopods (e.g., Manning & Felder 1991) on which neontologists base their subfamily assignment, the assignment of fossil species to a subfamily is virtually impossible. For fossils, the subfamilial assignment is dependent on identifying fossil specimens as a representative of an extant genus. Consequently, several exclusively fossil callianassid genera have not been assigned to any subfamily (see De Grave et al. 2009: p. 22). Re-diagnosing the subfamilies with the implementation of characters with higher fossilization potential may resolve this problem.
Beurlen (1930: p. 332) proposed Protocallianassa to be placed within its own monotypic subfamily Protocallianassinae based on well-developed pleurae on pleonal segments 2 – 6. Since species attributed once to Protocallianassa are in a need of revision (Schweitzer & Feldmann 2012), the usage of Protocallianassinae has been abandoned for the time being (De grave et al. 2009; Schweitzer et al. 2010).
5.3.2. Genus-level
Since the work of Manning & Felder (1991), who called attention to the taxonomic importance of the nature of the chelipeds in generic assignments, there has been a strong tendency to classify fossil ghost shrimps within neontologically diagnosed genera for Cenozoic ghost shrimp. In the case this is not possible, Collins et al. (1996) recommended classifying fossil specimens within Callianassidae (or Callianassa) s.l. without reference to a subfamily or genus. This is not a solution to the problem. Without a thorough comparison to extant counterparts, almost any fossil ghost shrimp would be classified as Callianassa s.l.. Unfortunately, there are several different neontological concepts of the genus Callianassa (e.g., Manning & Felder 1991; Ngoc-Ho 2003; Sakai 1999, 2005, 2011), hampering progress with fossil representatives. The practice of using Callianassa s.l. for problematic ghost shrimp remains creates a difficult situation when distinguishing between Callianassa s.str. and Callianassa as a waste-basket taxon. For instance Müller (1984: p. 48) used ‘Callianassa’ as a collective taxon, whereas Karasawa (2000b) and Hyžný et al. (2013b) used Callianassa (s.l.) to distinguish a waste-basket genus from the one diagnosed by Manning & Felder (1991) or Ngoc-Ho (2003).
When evaluating the characters on the major cheliped, much attention was paid to the morphology of the merus of the major cheliped, notably the nature of its lower margin and the presence or absence of the meral hook (Manning & Felder 1991). The discussion on this topic between Raymond Manning and Rodney Feldmann (for details see Schweitzer & Feldmann 2002: p. 940) resulted in two critical points: for confident generic assignment, the merus and carpus have to be preserved and the angle of the propodus / carpus joint is significant. It has been argued (Schweitzer & Feldmann 2002) that living forms typically exhibit the same type of articulation (at right angle along the axis of the articles). This assumption has recently been doubted (Hyžný 2012; Hyžný et al. 2013b) and the presence of the propodus / carpus joint with an angle more than 100° in extinct Protocallianassa has also been re-evaluated (Schweitzer & Feldmann 2012). Instead, a unique suite of more than one character present on chelipeds is needed to diagnose ghost shrimp genera.
Although the work of Manning & Felder (1991) was a very important step forward for classifying fossil ghost shrimps, more progress is called for. Diagnoses presented in their contribution appear insufficient for recognizing genera in the fossil record: whether or not the taxon exhibits a proximal meral hook has to be supplemented with other characters. For assignment to Callianopsis, the presence of a small proximal meral hook has been considered taxonomically important, but Hyžný & Dulai (2014 ; see also Gašparič & Hyžný 2015) demonstrated that a very similar meral hook can also be present in Lepidophthalmus. They suggested the usage of an additional character, the distal meral lobe, to be present for generic assignment to Lepidophthalmus. Additionally, the shape of the meral hook can be subjected to changes during growth (e.g., Dworschak 2012: fig. 6); therefore the developmental stage should be taken into account.
For some genera, the morphology of the major propodus can also be of taxonomic importance: Calliaxina (Hyžný 2012), Glypturus (Hyžný & MüIler 2012), and Calliax (Hyžný & Gašparič 2014). The preservation of minor chela, at least for representatives of Eucalliacinae (Calliax, Eucalliax, and Calliaxina), helps in distinguishing respective genera (Hyžný 2012: fig. 2; Hyžný & Gašparič 2014).
Many extant genera are based on rather minor differences in weak-part morphology or even on the basis of a single character. If not rediagnosed with respect to most durable body parts (i.e., chelipeds), it is difficult to recognise these taxa in the fossil record. For instance, Sergio is differentiated from the morphologically similar Neocallichirus on the basis of differences in the telson and uropods (Manning & Lemaitre 1994). A molecular study by Felder & Robles (2009) cast doubt on the monophyletic nature of the genus Sergio in its present arrangement. The problem dates back to 1988, when Sakai erected Neocallichirus. Its original diagnosis (Sakai 1988: p. 61) is vague resulting in the erection of several additional extant genera (including Sergio) from the “common stock” of Neocallichirus as well as erecting numerous fossil species of Neocallichirus based on a limited number of characters of minor taxonomic significance (Hyžný & Karasawa 2012: table 1). Sometimes, the assignment was even based on a single character (Neocallichirus manningi Schweitzer, Feldmann, Fam, Hessin, Hetrick, Nyborg & Ross, 2003 = N. rodfeldmanni Hyžný, 2010). Given the many different morphologies considered to be typical for the genus, Neocallichirus may be heterogeneous (Hyžný & Karasawa 2012: p. 61).
For assignment on the genus-level, a certain suite of characters can be used. For instance, Glypturus can be readily distinguished from all other genera by spines on the upper margin of the propodus and merus and on the lower margin of the carpus (Hyžný & Müller 2012). This does not mean that a different configuration of spines calls for erection of a new genus, but rather that prominent spines consistently present at the same place allow distinguishing one genus from a different one. To not confuse an intraspecifically variable feature with a taxonomically important character on the genus-level, many specimens need to be examined typically. In this respect, some of the extinct genera are based on only a limited number of specimens. Twenty-six ghost shrimp genera are known from the fossil record, of which 11 are exclusively fossil. Eight of them are monotypic genera. Some of them (Brecanclawu Schweitzer & Feldmann, 2001; Comoxianassa Schweitzer, Feldmann, Ćosović, Ross & Waugh, 2009; Cowichianassa Schweitzer, Feldmann, Ćosović, Ross & Waugh, 2009; Eoglypturus Beschin, De Angeli, Checchi & Zarantonello, 2005, and Turbiocheir Schweitzer, Feldmann, Casadío & Raising, 2012) are based on less than five specimens, implying that the intraspecific variability is difficult to evaluate. Moreover, their justification often is insufficient. For instance, chelipeds of Turbiocheir were compared only to Neocallichirus (i.e., a vaguely defined genus) without referring to any published or studied comparative material. The authors (Schweitzer et al. 2012: p. 176) also stated: “To avoid needless confusion and additional biogeographic chaos, it seems most prudent to erect a new genus for the new material from Río Turbio, instead of referring the material to an extant genus.” It is questionable whether such approach can lead to a better understanding of the ghost shrimp fossil record. Careful comparison to extant taxa may resolve the affinities of fossil taxa better than erecting new genera without sound reasoning as has been shown by Hyžný and others (e.g. Schweitzer-Hopkins& Feldmann 1997; Hyžný 2012; Hyžný & Hudáčková 2012; Hyžný & Dulai 2014; Hyžný & Gašparič 2014). Additionally, the fact that there are 11 exclusively fossil and largely monotypic genera tell us more about the taxonomic practice within the group than about real diversity / disparity pattern of the ghost shrimps in the geological past.
5.3.3. Species-level
Unfortunately, most fossil species are based on fragmentary material comprising propodi and dactyli only (160 species, 58.4%). These elements can be subject to intraspecific and possible size-related variation (see chapters 5.2.3. and 5.2.4.). Therefore it is important to gather a large number of specimens containing a growth series of the (supposedly) same taxon to recognize variable features. Congruence in the presence of otherwise variable features (tuberculation, armature of fingers) may point to recognition of the diagnostic characters on the species-level.
Prominent tuberculation is often considered a taxonomically important character in decapod taxonomy including ghost shrimps. One should, however, be aware of character changes during ontogeny because the tuberculation on ghost shrimp chelipeds may appear in large specimens in some cases, whereas much smaller individuals may be completely smooth. Dworschak (2008) reported this phenomenon for extant Neocallichirus karumba Poore & Griffin, 1979, in which individuals with a total length not exceeding 40 mm exhibit smooth major chelipeds (except for a few tubercles on the lateral surface of the propodus below the articulation with the dactylus), whereas larger specimens can be heavily tuberculated (Dworschak 2008: figs. 2e – f, 4b – c). Thus, tuberculation can be successfully used for taxonomy on the species-level in cases where its development is not linked to growth. In Glypturus, it has been demonstrated that already very small specimens (propodus length less than 5 mm) possess tubercles on the lateral surfaces of major propodus (Klompmaker et al. 2015a). The extent and presence of tuberculation differs among species (Klompmaker et al. 2015a) so that this character can be used for species identification in almost any case.
Setal pores are present in all ghost shrimps, but their shape, number, and arrangement vary markedly. It has to be stressed, however, that the mere presence or absence of setal pores cannot be taken as taxonomically important because it represents often only a preservational artefact. All ghost shrimps possess sensitive setae on the chelipeds, and, if preserved without cuticle as fossils, the setal pores may not be discernible. Polkowsky (2004) erected Ctenocheles chattiensis as a new species largely based on the presence of setal pores on isolated fingers. He stated that the pores are not present in its relative C. rupeliensis, which appears incorrect. Moreover, the new species was based on fragmentary material, namely an isolated dactylus and a doubtful minor propodus (Polkowsky 2004: figs. 22 – 24). Therefore, Hyžný & Dulai (2014) considered Ctenocheles chattiensis to be a junior subjective synonym of the well-documented C. rupeliensis.
6. Origins and evolution of the ghost shrimps
The evolutionary history of ghost shrimps is very much understudied. Without proper classification of the often fragmentary fossils of fossorial shrimps, no reliable calibration points based on fossils can be identified. Re-evaluation of the ghost shrimp fossil record has the potential to provide answers about the origin and phylogeny of this decapod group.
6.1. Phylogenetics of Axiidea
As inferred from molecular data (Robles et al. 2009; Fig. 12F), two distinct lineages seem to be present within Axiidea: one embracing Axiidae (= Calocarididae ortmann, 1891 = Eiconaxiidae Sakai & Ohta, 2005), and the second consisting of Callianassidae and Ctenochelidae (both sensu Manning & Felder 1991) together with less diverse Strahlaxiidae Poore, 1994, Callianideidae (= Thomassiniidae De Saint Laurent, 1979b), and Micheleidae Sakai, 1992. These two lineages are sometimes ascribed to Axioidea and Callianassoidea (e.g., Sakai 2011; Ahyong et al. 2011) in which Axiidae and Callianassidae apparently represent the most derived states within their lineages. The position of less diverse clades, however, is contentious (cf. Poore 1994; Tudge et al. 2000; Robles et al. 2009; Fig. 12). It should be noted that the superfamily Callianassoidea as in Poore (1994, 2004; Fig. 12A), Martin & Davis (2001), Sakai (2011) and Ahyong et al. (2011) is not supported by molecular analyses (e.g., Robles et al. 2009; see also Dworschak et al. 2012: p. 186). Within Axiidea, Callianassidae and Ctenochelidae seem to constitute a monophyletic group (cf. Tudge et al. 2000; Felder & Robles 2009; Robles et al. 2009; but see Tsang et al. 2008; Fig. 12), but its internal relationships are not clear. Ctenochelidae appear to be paraphyletic in several phylogenetic analyses (e.g., Poore 1994), whereas it is considered monophyletic in others (Tudge et al. 2000; Felder & Robles 2009). Callianassidae in the present arrangement (sensu De Grave et al. 2009) appears to be a non-natural grouping in the latest molecular analysis (Felder & Robles 2009; Fig. 12E). Specifically, Eucalliacinae Manning & Felder, 1991 appears to be paraphyletic, suggesting that Calliaxina may share a common lineage with Ctenochelidae. Both Eucalliacinae and Ctenochelidae appear to be ancestral with respect to the more advanced Callichirinae and Callianassinae. This seems supported by fossils from the Cretaceous. For Ctenochelidae, the fossil record of Ctenocheles goes back to the Cenomanian (~ 95 Ma) (Hyžný & Dulai 2014; Hyžný et al. 2014) and the oldest and only fossil representative of Dawsonius Manning & Felder, 1991 is known from the Albian (~ 105 Ma) (Franīescu 2014), if the generic assignment is correct. The oldest known representative of Eucalliacinae appears to be Eucalliax burckhardti (Böhm, 1911) from the Late Cretaceous – Paleocene (Maastrichtian–Dania, ~ 70 – 65 Ma) of Argentina and Mexico (Hyžný et al. 2013b). From coeval strata, also Callianassa (s.l.) ocozocoautlaensis Hyžný, Vega & Coutiño, 2013 is known, interpreted as a representative of Callianassinae. The Cretaceous occurrences of Neocallichirus, a representative of Callichirinae, have been recently doubted by Hyžný & Karasawa (2012).
Fig. 12.
An overview of published phylogenies involving ghost shrimps. The schemes are simplified to include only generic and familial taxa. Outgroup taxa or taxa different from axiidean and gebiidean shrimps are indicated with shorter branches. An indication of Axiidea and Gebiidea does not necessarily mean that the respective study recognized these taxa as separate infraorders. A: Phylogeny based on morphology, after Poore (1994: fig. 9). B: A composite of the consensus trees from the Bayesian analysis and the single maximum-likelihood (ML) tree for the combined mitochondrial 16S rDNA and nuclear 18S rDNA data, after Tudge & Cunningham (2002: fig. 3). C: ML tree from combined 16S, 18S and 28S rDNA analysis, after Tsang et al. (2008: fig. 2). D: ML tree from combined 16S and 18S rDNA analysis, after Tsang et al. (2008: fig. 1). E: Cladogram inferred from a maximum parsimony (MP) analysis of 16S and 12S rDNA, after Felder & Robles (2009: fig. 1). F: Cladogram inferred from a Bayesian analysis of 16S and 18S rDNA, after Robles et al. (2009: fig. 1). Abbreviations: GEB. = Gebiidea; TH. = Thalassinoidea. For commentary see the text (chapter 6.1.).
6.2. Origins of Axiidea
Fossils provide important calibration points for the divergence times of major clades. Because the ghost shrimp fossil record is understudied, the selection of fossil taxa as calibration points is rather difficult.
Porter et al. (2005), using a limited dataset for molecular clock estimates, argued for a possible divergence time of “thalassinideans” (to which ghost shrimps belong) from other reptantian decapods around the mid-Carboniferous (~ 325 Ma). No fossil representatives of Axiidea or Gebiidea were chosen as calibration points, and, as pointed out by Dworschak et al. (2012: pp. 110 – 111), this estimate must apply only to axiideans because the five extant representatives used in the analysis of Porter et al. (2005) were all callianassids (no gebiidean was analysed). An alternate view has been presented by Bracken et al. (2010), who suggested an independent radiation of Gebiidea to have occurred within the Carboniferous (~ 309 Ma) and a radiation of Axiidea within the Permian (~ 255 Ma). Although these results are based on a more robust molecular phylogenetic analysis, no calibration point for Axiidea or Gebiidea based on fossils was used. Calibration points based on the oldest known axiidean and gebiidean taxa were used for the first time by Bracken-Grissom et al. (2013), namely Callianassa bonjouri Étallon, 1861 (now treated as Magila Oppel, 1861; see Förster 1977 and Schweitzer et al. 2010) from the Early Jurassic (Toarcian, ~ 180 Ma) of France and ?Gebia obscura von Meyer, 1834 (now treated as Upogebia Leach, 1814; see Schweitzer et al. 2010) from the Early Triassic (~ 248 Ma) of France. Both taxa, however, need to be revised. Förster (1977: p. 145) noted that the holotype of Magila bonjouri could not be found and the original figure does not allow detailed comparison to congeneric species. Regarding Upogebia obscura Von Meyer, 1834, even Von Meyer (1834) classified the poorly preserved specimen as ?Gebia. The analysis of Bracken-Grissom et al. (2013) was primarily focused on anomurans with other higher taxa of decapods serving as outgroup taxa. Thus, it is difficult to make further conclusions about the estimated divergence times of Axiidea and Gebiidea during the Devonian (~ 370 Ma) and Permian (~ 265 Ma), respectively.
Most recently, Baucon et al. (2014) studied the fluvial succession of the Permian – Triassic (270 Ma) boundary in Sardinia, Italy. Based on the presence of Ophiomorpha ichnofossils in freshwater sediments, they suggested a sister-group relationship between Thalassinidea and Astacidea (see Porter et al. 2005; Tsang et al. 2008). Baucon et al. (2014: p. 99) concluded that both lineages derived from the same biological population and interpreted that “astacid / thalassinid diversification to have taken place in fluvial environment between the Carboniferous (~ 310 Ma) and early Permian (~ 295 Ma) times, while ghost shrimps had invaded marine environments at the Permian – Triassic boundary”. Their usage of the term “ghost shrimp” is, however, rather vague because they use it instead of “thalassinideans”, i.e., in a rather broad sense. Most importantly, identifying the tracemaker of freshwater Ophiomorpha to be of “thalassinidean” origin does not take into account the possibility of other decapod taxa that may be able to produce these burrows. As Frey et al. (1978: p. 214) noted, Thalassinideans are “by no means the only animals to employ sediment pellets in the construction of at least a part of a burrow”. Crayfishes can produce burrows with knobby walls (Chamberlain 1975; Hasiotis & Bourke 2006 and references therein) and this morphology was linked to crayfish in the fossil record (Babcock et al. 1998; Bedatou et al. 2008), although fossil burrows referred to crayfish were not ascribed to Ophiomorpha. Baucon et al. (2014) ascribed some of the investigated trace fossils to crayfishes. Indeed, these animals may have been around at that time because Babcock et al. (1998) already reported on Early Permian (~ 295 Ma) crayfish. Could it be possible that crayfishes exhibited different burrowing behaviours to produce burrow similar to Ophiomorpha? If so, then this may be more reasonable than to postulate a freshwater origin of otherwise fully marine axiideans and gebiideans. In this respect, it has to be stressed that “thalassinidean” taxa tolerating salinity fluctuations are positioned relatively high in the proposed phylogenies (Tsang et al. 2008; Robles et al. 2009), suggesting that resistance to freshwater may be derived.
6.3. What is the oldest ghost shrimp?
The oldest known representative of Axiidea (to which ghost shrimps belong) is the axiid Magila bonjouri (Étallon, 1861) from the Early Jurassic (Toarcian, ~ 180 Ma) of France. Garassino & Teruzzi (2001) questionably assigned a fragmentary chela (Fig. 13A) from the Toarcian of Italy to ?Etallonia. Later, Schweigert (2003) suggested a possible relationship to his newly described axiid Megachela frickhingeri Schweigert, 2003 from the Late Jurassic (early Tithonian, 150 Ma) of Germany and re-assigned the Italian ?Etallonia to ?Megachela sp. Reexamination (MH, pers. obs. Nov. 2014) of the original material of Garassino & Teruzzi (2001) cast doubt on the axiidean affinity of the specimen. We suggest that it may represent a polychelid chela because the dactylus is curved in a manner resembling polychelids (e.g., Audo et al. 2014). Moreover, polychelid lobsters are known from the same strata as the discussed specimen (Garassino & Teruzzi 2001. No material attributable to Callianassidae or Ctenochelidae is known from the Jurassic strata.
Fig. 13.
Some of the supposedly oldest ghost shrimps. A: ?Etallonia sensu Garassino & Teruzzi (2001) from the Early Jurassic (Toarcian) of Italy, MSNM i 10855. B: Protocallianassa antarctica Taylor, 1979 from the Early Cretaceous (early Aptian) of Antarctica (redrawn after Taylor 1979: fig. 9a). C,D: Callianassa infracretacea de Tribolet, 1874 from the Early Cretaceous (Hauterivian) of France; digital image from de Tribolet (1874: pl. 15.1). E – G: Callianassa uncifera Harbort, 1905 from the Early Cretaceous (late Hauterivian) of Germany, SNSB-BSPG 1988 III 373 (E), 1988 III 372 (F), and 1988 III 374 (G). Scale bars equal 5.0 mm. Specimens in A and B are not considered ghost shrimps herein, whereas C. infracretacea and C. uncifera await revision. For commentary see the text (chapter 6.3.).
There are several candidates for the oldest representative of Callianassidae, all of Cretaceous age: Callianassa uncifera Harbort, 1905 is known from the Hauterivian – Barremian (125 – 133 Ma) of Germany (Fig. 13E – G), C. sakakuraorum Karasawa, 2000b has been reported from the Barremian (~ 127 Ma) of Japan, and Protocallianassa patagonica Aguirre Urreta, 1982 is known from the late Barremian (~ 126 Ma) of Argentina (Aguirre Urreta 1982, 1989). Callianassa infracretacea de Tribolet, 1874, from the Hauterivian (~ 130 Ma) of France (Fig. 13C – D), most probably is a representative of Axiidae because Förster (1977) suggested assignment to Etallonia Münster, 1839. Glyphea carinata de Tribolet, 1875 from the same formation was recently questionably re-assigned to ?Callianassa by Charbonnier et al. (2013), to which we concur.
Karasawa (2000b: p. 237) noted that “the earliest representatives of Callianassa (s.l.) have been recorded from the Neocomian [Berriasian – Hauterivian, 145 – 130 Ma] of Europe (Glaessner 1929) and the Valanginian (~ 135 Ma) of Argentina (Aguirre Urreta 1989)”. No European pre-Hauterivian callianassid occurrences are known to the authors. Aguirre Urreta (1989: text-fig.6) provided a table summarizing Cretaceous decapod crustacean occurrences of Argentina and Antarctica. Decapod taxa of the Fossil Bluff Formation of the Western Atlantic Basin originally described by Taylor (1979) are in the aforementioned table listed in the column spanning the range of Berriasian to Aptian (145 – 115 Ma). Protocallianassa antarctica Taylor, 1979 appears to occur in the Berriasian (~ 140 Ma), but this age seems incorrect because Aguirre Urreta (1989) filled the stratigraphic column of the Fossil Bluff Formation, known to span from the Berriasian to Aptian, with the names of taxa. Taylor (1979) mentioned the probable age of P. antarctica to be early Aptian (~ 120 Ma). The species is based on several chelae with long and robust fingers with prominent longitudinal ridges (Fig. 13B) reminiscent of the axiid Schlueteria Fritsch in Fritsch & Kafka, 1887. Interestingly, the Berriasian Schlueteria carinata Taylor, 1979 is known from the same formation. Based on the comparison with chelipeds of Schlueteria (Fritsch & Kafka 1887: pl. 6; Charbonnier et al. 2012: fig. 24), Protocallianassa antarctica may be a representative of this genus or its close relative. In the table presented by Aguirre Urreta (1989: text-fig. 6), Callianassa aff. C. peruviana is reported from the Lower Cretaceous of the Neuquen Basin. It appears to represent a Valanginian (~ 135 Ma) occurrence, but given the nature of the table (taxa listed across the entire stratigraphic span of the formation in which they occur), one cannot be certain about this. Callianassa peruviana Rathbun, 1947 was originally described from the Albian (~ 110 Ma) of Peru.
Mángano & Buatois (1991) reported “callianassid claws” associated with burrows from the Berriasian / Valanginian (~ 140 Ma) of Argentina. Since no additional information or detailed photo-documentation was included, it is difficult to accept this as the oldest ghost shrimp occurrence.
To conclude, no unequivocal ghost shrimp (i.e., callianassid or ctenochelid) older than the Hauterivian (133 Ma) is known to date. Thus, based on fossils, the emergence of axiideans can be expected to occur in the Jurassic followed by a major radiation during the Cretaceous. This hypothesis contradicts the Paleozoic origin of axiideans proposed by others (see chapter 6.2.).
6.4. Evolution of ghost shrimps
The evolutionary scheme plotted against the geological time scale in Fig. 14 is based largely on phylogenies proposed by Felder & Robles (2009) and Robles et al. (2009: fig.1); see also Dworschak et al. 2012: fig. 69.32). The scheme considers only taxa with a known fossil record. Since numerous fossil ghost shrimp taxa await generic or familial re-assignment, it necessarily represents only a preliminary outline of suggested relationships.
Fig. 14.
Evolutionary scheme of ghost shrimps based on the fossil record and phylogenies of modern ghost shrimps. For commentary see the text (chapter 6.4.).
The oldest axiidean fossil Magila bonjouri is also the oldest record for Axiidae (asterisk 1 in Fig. 14). In his morphological analysis Poore (1994) resolved Thomassiniidae / Callianideidae as a sister taxon to ghost shrimps (Ctenochelidae + Callianassidae). Based on Robles et al. (2009), the sister group to ghost shrimps is a clade containing representatives of Strahlaxiidae and Thomassiniidae / Callianideidae. Since only one fossil occurrence is known from this grouping, Crosniera schweitzerae Hyžný & Schlögl, 2011, from the early Miocene (~ 16 Ma) of Slovakia (asterisk 2 in Fig. 14), Thomassiniidae (considered synonymous to Callianideidae by Dworschak et al. 2012) is shown as a sister taxon to ghost shrimps in Fig. 14.
The relationship between the ingroups (subfamilies in the Linnean classification) of ghost shrimps is largely unresolved and, therefore, all lineages are shown here as a polytomy. This is because of contradictory results from the phylogenetic analyses conducted so far (Tudge et al. 2000; Tudge & Cunningham 2002; Tsang et al. 2008; Felder & Robles 2009; Robles et al. 2009). The oldest ghost shrimp as recognized herein and discussed in chapter 6.3. is considered the “calibration point” of this polytomy (asterisk 3 in Fig. 14).
Felder & Robles (2009) resolved Eucalliacinae and Callichirinae as paraphyletic groupings, with Eucalliacinae sharing the lineage with Ctenochelidae. Dawsonius has been recognized as a sister taxon to Gourretia by Felder & Robles (2009) and Robles et al. (2009), although it must be noted that no other representatives of Ctenochelidae were included in their analyses. Thus, the relationships among Ctenocheles, Callianopsis, and Gourretia / Dawsonius are largely unexplored, although the monophyly of Ctenochelidae is presumed here. As far as the status of Ctenochelidae as a sister taxon to Callianassidae, the results of Tsang et al. (2008) and Robles et al. (2009) offer only modest support. The oldest fossil record of Gourretia is the occurrence of Gourretia sp. from the early Miocene (18 Ma) of Austria (Hyžný et al. 2015; asterisk 4 in Fig. 14). Observations (MH pers. obs.), however, suggest a much older history of the genus, going back at least into the middle Eocene (~ 42 Ma).
Paracalliacinae includes two extinct genera, Rathbunassa Hyžný in Bermúdez et al., 2013, and Pleuronassa Ossó-Morales, Garassino, Vega & Artal, 2011. Both were included in the subfamily by Bermúdez et al. (2013). Rathbunassa, known from the Early Cretaceous (Albian, ~ 110 Ma) (Bermúdez et al. 2013), is consistent with the results in Poore (1994), who recognized its extant relative Paracalliax to be positioned even more basally than Ctenocheles. The fossil record cannot resolve this issue yet because the oldest records of Rathbunassa and Ctenocheles are roughly coeval.
In the ghost shrimp phylogeny, pronounced heterochely appears to be a derived state, as representatives of Thomassiniidae are only slightly heterochelous. Interestingly, fossil members of Paracalliacinae show slight heterochely as well. From the viewpoint of the character evolution, Paracalliacinae appears more plesiomorphic than Ctenochelidae (suggested also by Poore 1994: fig. 9), as Ctenocheles clearly is already a specialized, strongly heterochelous form.
Eucalliacinae was resolved as a paraphyletic grouping by Felder & Robles (2009). Interestingly, it appears to represent early branching off the lineage towards Callichirinae and Callianassinae, but the fossil record is not sufficiently known to support this. The oldest record is Eucalliax burckhardti from the Late Cretaceous (Maastrichtian, ~ 70 Ma) of Argentina and Mexico (Hyžný et al. 2013b), but numerous older taxa (treated as Callianassa) from the Late Cretaceous possess characters typical for Eucalliacinae (see Hyžný 2012). The oldest record of Calliax is Calliax sp. from Paleocene (?Thanetian, ~ 58 Ma) of Pakistan (Charbonnier et al. 2013; asterisk 5 in Fig. 14). Eucalliacinae include also nearly isochelous taxa (Calliaxina and Eucalliax); thus, the combination of their suggested early branching off the lineage towards Callichirinae and Callianassinae (Felder & Robles 2009), and the near-isochely of Jurassic axiids (Beurlen 1930) would imply character evolution from ancestral isochelous to derived heterochelous chelipeds. If correct, heterochely would have arisen at least two times within Axiidea, once in Axiidae and once in ghost shrimp clade. However, convergence within the ghost shrimp lineage cannot be ruled out.
The oldest fossil occurrence of a representative of Callichirinae is Callichirus waagei and Corallianassa acucurvata from the Late Cretaceous (Maastrichtian, ~ 70 Ma) of the USA (Crawford et al. 2006) and the Netherlands (Swen et al. 2001), respectively. Corallianassa rigoi De angeli & Garassino, 2006 from the Early to Late Cretaceous (Aptian – Campanian) of Italy is herein considered to be closer to Protocallianassa and Mesostylus. Analyses of Felder & Robles (2009) and Robles et al. (2009) resolved Callichirus as the sister group to the rest of Callichirinae, in disagreement with Tsang et al. (2008). Moreover, due to the unstable position of Lepidophthalmus within Callichirinae / Callianassinae clade, the relationships among derived callianassids are unresolved in Fig. 14. Lepidophthalmus was considered a representative of Callichirinae by Manning & Felder (1991), and, as such, appeared also in major revisions by Sakai (2005, 2011). The analysis of Felder & Robles (2009) contested this view because it resolved Lepidophthalmus positioned basally within the clade together with Callianassinae. The fossil record of the genus is largely unknown: only recently fossils have been identified (Hyžný & Dulai 2014; Gašparič & Hyžný 2015). It is likely that after applying proxy characters as discussed by Hyžný & Dulai (2014), more ghost shrimp taxa will be re-assigned to this genus. So far, its oldest record is L. crateriferus from the late Oligocene (~ 28 Ma) of Hungary (Hyžný & Dulai 2014).
Eoglypturus, although originally not ascribed to a subfamily (Beschin et al. 2005; see also De Grave et al. 2009), was considered a representative of Callichirinae by Hyžný & Müller (2012). The presence of spines on the upper margin of the propodus is considered a taxonomically important character on the supraspecific level (Hyžný & Müller 2012; Hyžný et al. 2013a). Eoglypturus possesses similar spines and is, therefore, considered a sister genus to Glypturus. Corallianassa was resolved as a sister taxon to Glypturus by Felder & Robles (2009).
Based on Hyžný & Karasawa (2012: table 1), Neocallichirus rhinos from the middle Eocene (~ 45 Ma) of Mexico (Schweitzer & Feldmann 2002) is considered the oldest confirmed record of the genus (asterisk 6 in Fig. 14), although there are several older occurrences described as Neocallichirus. The oldest one, Neocallichirus agadirensis from the Late Cretaceous (Cenomanian, ~ 95 Ma) of Morocco (Garassino et al. 2011), is currently being redescribed by one of us (MH).
The sister group relationship between Grynaminna and Neocallichirus was resolved by Felder & Robles (2009). Interestingly, although numerous fossil species of Neocallichirus were described, only one species was assigned to Grynaminna (Hyžný & karasawa 2012).
The fossil record of Callianassinae is poorly known. Most species attributed to Callianassa do not conform to Callianassa sensu Manning & Felder (1991) or Ngoc-Ho (2003). The oldest recorded representative of the subfamily is Trypaea mizunamiensis Karasawa, 1993 from the early Miocene (~ 20 Ma) of Japan. However, older taxa may be accommodated within Callianassinae as well, e.g., Callianassa heberti Milne-Edwards, 1860 or C. macrodactyla Milne-Edwards, 1860 from the Eocene (Bartonian, 40 Ma) of France. Formal reassignment of these taxa, however, awaits a more detailed study.
7. Conclusions
The ghost shrimp fossil record is rich, but largely understudied. This is mainly because the generic assignment of ghost shrimp remains is often hindered by their insufficient preservation and inconsistencies in the biological classification and taxonomy of the group. Furthermore, a broadly defined concept of the genus Callianassa has been used many times in the past: almost any ghost shrimp with mainstream cheliped morphology has been attributed to that genus. Altogether, 190 fossil species have been described under the collective taxon “Callianassa”, which represents a heterogeneous mixture of several genera.
So far, 274 fossil species once attributed to ghost shrimps were recognized, and 250 of them are considered valid taxa. Interestingly, in the last decade, more fossil ghost shrimp species were erected than during any comparable period since World War II, suggesting that more taxa remain undiscovered and/ or undescribed.
Major conclusions from this fossil ghost shrimp review involve (1) taphonomical aspects, (2) generic assignment of the fossil taxa, and (3) remarks on the ghost shrimp phylogeny.
-
(1)
Due to the delicate nature of most ghost shrimp cuticle, only the hardened parts are usually preserved, i.e., the chelae, the antero-dorsal portion of the carapace, and sometimes the posterior pleonal segments and the telson. Heavily calcified chelipeds are preserved most frequently. Whole-body fossils of ghost shrimps are rare; only 19 species were described as such. Disassociated chelipeds are more common than disassociated pleonal units and cephalothoracic region units. When a cheliped disassociation unit disintegrates further, only often fragmentary, isolated cheliped elements remain. This mode of preservation constitutes the most abundant portion of the ghost shrimp fossil record. Altogether 160 species, which is more than half of all described species, are known only from their distal cheliped elements, i.e., dactylus and/ or propodus.
The majors of ghost shrimp chelae constitute one of the most common fossil decapod remains, but minor chelae are rare. Scars from muscle attachments are often preserved in the fossil ghost shrimps. Only rarely, ghost shrimps are preserved in situ in burrows or in direct association with them.
-
(2)
For successful generic assignment, fossil material should always be compared to modern genera because many of them have modern relatives. In the neontological literature, the morphology of the major cheliped is rarely closely examined for features that can be used for identification on the genus-level. Instead the telson, uropods, and maxillipeds are used typically. It is suggested that neontologists should provide detailed descriptions of cheliped morphology more frequently and that paleontologists should look at modern taxa more often.
Heterochely, intraspecific variation, ontogenetic changes, and sexual dimorphism are all factors that have to be taken into account when working with fossil ghost shrimps. In the fossil record, differences in the sexual morphs can lead to the incorrect recognition of two separate taxa. Despite being used frequently necessarily to erect fossil ghost shrimp species, distal elements (i.e., dactylus and propodus) are usually more variable than proximal ones (i.e., carpus, merus, and ischium).
-
(3)
Based on fossils, the emergence of axiideans can be expected to occur within Jurassic and a major radiation is suggested for the Cretaceous. No ghost shrimp (i.e., callianassid or ctenochelid) older than the Early Cretaceous (Hauterivian, ~ 133 Ma) is known to date. The divergence of Ctenochelidae and Paracalliacinae is estimated to occur at least before the Albian (~ 113 Ma), but most probably not before the Hauterivian (~ 133 Ma), as the oldest ghost shrimp is known from around that time. Callichirinae and Eucalliacinae likely diverged during the Late Cretaceous, whereas Callianassinae did not appear before the Eocene (56 Ma).
Electronic Supplement Files
at http://www.senckenberg.de/arthropod-systematics (“Contents”)
File 1:hyzny&klompmaker-ghostshrimps-asp2015-electronicsupplement-1.xlsx. – Database of fossil ghost shrimp species.
File 2:hyzny&klompmaker-ghostshrimps-asp2015-electronicsupplement-2.docx. – References used in the database of fossil ghost shrimp species.
8. Acknowledgements
We are grateful to P.C. Dworschak (NHMW) for invaluable discussions on all aspects of ghost shrimp systematics and biology. Numerous individuals are thanked for access to respective collections (in alphabetic order): S. Charbonnier (MNHN), A. Dulai (HNHM), P.C. Dworschak (NHMW), E. Bodor and K. Palotás (FI), V. Frisone (MCZ), A. Garassino (MSNM), M. Gross (UMJGPA), M. Nose (SNSB-BSPG), U. Scheer (RE), J. Sklenář (NM), M. Türkay (SMF), B. Zahradníková (SNM-Z), and I. Zorn (GBA). Private collectors M. Hornáček, and G. Wanzenböck were generous in providing access to their collections. H. Karasawa (MZM) is thanked for permission to use image of Grynaminna grandis (Fig. 7A), T.E. Jorstadt (USNM) is thanked for permission to use images of Callianassa hulli (Fig. 8E,F), Journal of Systematic Palaeontology is thanked for permission to reuse images of Glypturus sikesi (Figs. 4H – K,M – O). Ch. Lemzaouda (MNHN) took photographs of Callianassa macrodactyla (Fig. 10B– C). Private collectors Y. Coole and Z. Dvořák are thanked for providing photos of Corallianassa acucurvata (Fig. 9F) and Ctenocheles fritschi (Fig. 8D), respectively. Constructive comments of Joachim T. Haug (LMU Munich, Germany) and an anonymous reviewer improved the manuscript considerably. M.H. has been supported by Austrian Science Fund (FWF; Lise Meitner Program M 1544-B25) and by the Slovak Research and Development Agency under contracts no. APVV-0644-10 and APVV-0436-12. This work was supported by the Jon L. and Beverly A. Thompson Endowment Fund to A.A.K. This is University of Florida Contribution to Paleobiology 688.
References
- Abrard R. Fossiles néogènes et quaternaires des Nouvelles-Hébrides (Missions E. Aubert de la Rüe 1934–1936) Annales de Paléontologie. 1947;32:3–113. [Google Scholar]
- Aguirre Urreta MB. Crustaceos deacpodos Barremianos de la region del Tucu-Tucu, provincial de Santa Cruz. Ameghiniana. 1982;19:303–317. [Google Scholar]
- Aguirre Urreta MB. The Cretaceous decapod Crustacea of Argentina and the Antarctic Peninsula. Palaeontology. 1989;32:499–552. [Google Scholar]
- Ahyong ST, Lowry JK, Alonso M, Bamber R, Boxshall GA, Castro P, Gerken S, Karaman GS, Goy JW, Jones DS, Meland K, et al. Subphylum Crustacea Brünnich, 1772. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. In: Zhang Z-Q, editor. Zootaxa. Vol. 3148. 2011. pp. 165–191. [Google Scholar]
- Anker A. Ctenocheloides attenboroughi n. gen., n. sp. (Crustacea: Decapoda: Axiidea: Ctenochelidae), a new ghost shrimp with pectinate claw fingers from Madagascar. Journal of Natural History. 2010;44:1789–1805. [Google Scholar]
- Alcock A, Anderson ARS. An account of a recent collection of deep-sea Crustacea from the Bay of Bengal and Laccadive Sea. Natural history notes from H.M. Royal Indian Marine Survey Steamer “Investigator”, commander C.F. Oldham, R.N., commanding. (Series II No. 14).Journal of the Asiatic Society of Bengal. 1894;63(3):141–185. pl. 9. [Google Scholar]
- Allison PA. Soft-bodied animals in the fossil record: The role of decay in fragmentation during transport. Geology. 1986;14(12):979–981. [Google Scholar]
- Allison PA. The role of anoxia in the decay and mineralization of proteinaceous macro-fossils. Paleobiology. 1988;14:139–154. [Google Scholar]
- An J, Williams JD, Yu H. The Bopyridae (Crustacea: Isopoda) parasitic on thalassinideans (Crustacea: Decapoda) from China. Proceedings of the Biological Society of Washington. 2009;122:225–246. [Google Scholar]
- Audo D, Charbonnier S, Schweigert G, Saint Martin J-P. New eryonid crustaceans from the Late Jurassic Lagerstätten of Cerin (France), Canjuers (France), Wattendorf (Germany) and Zandt (Germany) Journal of Systematic Palaeontology. 2014;12:459–479. [Google Scholar]
- Baldanza A, Bizzarri R, Famiani F, Garassino A, Hyžný M, Pasini G. The bathyal decapod crustacean community from the Poggio i Sodi quarries (Siena Basin, Tuscany, Italy) Boletín de la Sociedad Geológica Mexicana. 2013;65:335–353. [Google Scholar]
- Babcock LE. Asymmetry in the fossil record. European Review. 2005;13:135–143. [Google Scholar]
- Babcock LE, Miller MF, Isbell JL, Collinson JW, Hasiotis ST. Paleozoic-Mesozoic crayfish from Antarctica: Earliest evidence of freshwater decapod crustaceans. Geology. 1998;26:539–542. [Google Scholar]
- Bachmayer F. Zwei bemerkenswerte Crustaceen-Funde aus dem Jungtertiär des Wiener Beckens. Sitzungsberichte der Mathematisch-Naturwissenschaftlichen Klasse der Kaiserlichen Akademie der Wissenschaften, Wien. 1954;163:63–70. [Google Scholar]
- Baucon A, Ronchi A, Felletti F, Neto De Carvalho C. Evolution of crustaceans at the edge of the end-Permian crisis: Ichnonetwork analysis of the fluvial succession of Nurra (Permian – Triassic, Sardinia, Italy) Palaeogeography, Palaeoclimatology, Palaeoecology. 2014;410:74–103. [Google Scholar]
- Bedatou E, Melchor RN, Bellosi E, Genise JF. Crayfish burrows from Late Jurassic-Late Cretaceous continental deposits of Patagonia: Argentina. Their palaeoecological, palaeoclimatic and palaeobiogeographical significance. Palaeogeography, Palaeoclimatology, Palaeoecology. 2008;257:169–184. [Google Scholar]
- Beikirch DW, Feldmann RM. Decapod crustaceans from the Pflugerville Member, Austin Formation (Late Cretaceous Campanian) of Texas. Journal of Paleontology. 1980;54:309–324. [Google Scholar]
- Berkenbusch K, Rowden AA. Ecosystem engineering – moving away from ‘just-so’ stories. New Zealand Journal of Ecology. 2003;27:67–73. [Google Scholar]
- Berkenbusch K, Rowden AA, Myers TE. Interactions between seagrasses and burrowing ghost shrimps and their influence on infaunal assemblages. Journal of Experimental Marine Biology and Ecology. 2007;341:70–84. [Google Scholar]
- Bermúdez HD, Gómez-Cruz A, De J, Hyžný M, Moreno-BedMar JA, Barragán R, Moreno Sánchez M, Vega FJ. Decapod Crustacea from the Cretaceous San Gil Group (Aptian-Albian) at Villa de Leyva section, central Colombia. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen. 2013;267:255–272. [Google Scholar]
- Beschin C, Busulini A, De Angeli A, Tessier G, Ungaro S. Crostacei eocenici di “Cava Rossi” presso Monte di Malo (Vicenza – Italia settentrionale) Studi Trentini di Scienze Naturali – Acta Geologica. 1998;73:7–34. [Google Scholar]
- Beschin C, De Angeli A, Checchi A, Zarantonello G. Studi e Ricerche – Associazione Amici del Museo – Museo Civico. Vol. 12. G. Zannato; Montecchio Maggiore (Vicenza): 2005. Crostacei eocenici di Grola presso spagnago (Vicenza, Italia Settentrionale) pp. 5–35. [Google Scholar]
- Beschin C, De Angeli A, Zorzin R. Crostacei fossili del Veneto: una inedita fauna eocenica die Lessini orientali (Monte Serea di San Giovanni Ilarione, Verona), con descrizione di tre nuove specie. Bolletino del Museo Civico di Storia Naturale di Verona, Geologia Paleontologia Preistoria. 2009;33:59–83. [Google Scholar]
- Beurlen K. Vergleichende Stammesgeschichte Grundlagen, Methoden, Probleme unter besonderer Berücksichtigung der höheren Krebse. Fortschritte in der Geologie und Paläontologie. 1930;8:317–586. [Google Scholar]
- Beurlen K. Neue Dekapoden-Krebse aus dem ungarischen Tertiär. Paläontologische Zeitschrift. 1939;21:135–161. [Google Scholar]
- Biffar TA. Three new species of callianassid shrimp (Decapoda, Thalassinidea) from the western Atlantic. Proceedings of the Biological Society of Washington. 1970;83(3):35–50. [Google Scholar]
- Biffar TA. The genus Callianassa (Crustacea, Decapoda, Thalassinidea) in South Florida, with keys to the western Atlantic species. Bulletin of Marine Science. 1971;21:637–715. [Google Scholar]
- Bishop GA. Fossil decapod crustaceans from the Lower Cretaceous Glen Rose Limestone of Central Texas. Transactions of the San Diego Society of Natural History. 1983;20:27–55. [Google Scholar]
- Bishop GA. Taphonomy of the North American decapods. Journal of Crustacean Biology. 1986;6:326–355. [Google Scholar]
- Bishop GA, Bishop EC. Distribution of ghost shrimp; North Beach, St. Catherines Island, Georgia. American Museum Novitates. 1992;3042:1–17. [Google Scholar]
- Bishop GA, Williams AB. Taphonomy and preservation of burrowing thalassinidean shrimps. Proceedings of the Biological Society of Washington. 2005;118:218–236. [Google Scholar]
- Böhm J. Callianassa burckhardti n. sp. nebst einer Zusammenstellung der fossilen Arten der Gattung Callianassa. Zeitschrift der Deutschen Geologischen Gesellschaft, Monatsberichte. 1911;63:37–46. [Google Scholar]
- Botelho de Souza JR, Borzone CA, Brey T. Population dynamics and secondary production of Callichirus major (Crustacea: Thalassinidea) on a southern Brazilian sandy beach. Archives of Fisheries and Marine Research. 1998;46:151–164. [Google Scholar]
- Bott R. Litorale Dekapoden, außer Uca. Dekapoden (Crustacea) aus El Salvador. Seckenbergiana Biologica. 1955;36:45–72. [Google Scholar]
- Bracken HD, De Grave S, Toon A, Felder DL, Crandall KA. Phylogenetic position, systematic status, and divergence time of the Procarididea (Crustacea: Decapoda) Zoologica Scripta. 2010;39:198–212. [Google Scholar]
- Bracken-Grissom HD, Cannon ME, Cabezas P, Feldmann RM, Schweitzer CE, Ahyong ST, Felder DL, Lemaitre R, Crandall KA. A comprehensive and integrative reconstruction of evolutionary history for Anomura. BMC Evolutionary Biology. 2013;13:128–28. doi: 10.1186/1471-2148-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton G. Deux nouvelles espèces de crustacés décapodes de l’Albien du Bassin de Paris. Geodiversitas. 2011;33:279–284. [Google Scholar]
- Brocchi P. Notes sur les Crustacés fossiles des terres tertiaires de la Hongrie. Annales des Sciences Géologiques. 1883;14:1–8. [Google Scholar]
- Bromley RG. Trace Fossils: Biology and Taphonomy. London: Unwin Hyman; 1990. p. 280. [Google Scholar]
- Brönnimann P. Remarks on the classification of fossil anomuran coprolites. Paläontologische Zeitschrift. 1972;46:99–103. [Google Scholar]
- Casadío S, De Angeli A, Feldmann RM, Garassino A, Hetler JL, Parras A, Schweitzer CE. New decapod crustaceans (Thalassinidea, Galatheoidea, Brachyura) from the middle Oligocene of Patagonia, Argentina. Annals of Carnegie Museum. 2004;73:85–107. [Google Scholar]
- Chamberlain CK. Recent lebensspuren in nonmarine aquatic environments. In: Frey RW, editor. The study of Trace Fossils. Springer-Verlag; Berlin-Heidelberg-New York: 1975. pp. 431–458. [Google Scholar]
- Charbonnier S, Garassino A, Pasini G. Revision of Mesozoic decapod crustaceans from Madagascar. Geodiversitas. 2012;34:313–357. [Google Scholar]
- Charbonnier S, Garassino A, Pasini G, Métais G, Merle D, Bartolini A, Brohi IA, Solangi SH, Lashari RA, Wel-comme J-L, Marivaux L. Early Paleogene decapod crustaceans from the Sulaiman and Kirthar Ranges, Pakistan. Annales de Paléontologie. 2013;99:101–117. [Google Scholar]
- Collins JSH, Donovan SK, Dixon HL. Crabs and barnacles (Crustacea: Decapoda & Cirripedia) from the late Pleistocene Port Morant Formation of southeast Jamaica. Bulletin of the Mizunami Fossil Museum. 1996;23:51–63. [Google Scholar]
- Compton JS. Holocene sea-level fluctuations inferred from the evolution of depositional environments of the southern Langebaan Lagoon salt marsh, South Africa. The Holocene. 2001;11:395–405. [Google Scholar]
- Crawford RS, Feldmann RM, Waugh DA, Kelley BM, Allen JG. Decapod crustaceans from the Maastrichtian Fox Hills Formation. Bulletin of the Peabody Museum of Natural History. 2006;47:3–28. [Google Scholar]
- Dana JD. United States Exploring Expedition during the years 1838 – 1842, under the command of Charles Wilkes, U.S.N., 10, C. Sherman; Philadelphia: 1849. Geology; p. 756. [Google Scholar]
- Dana JD. Conspectus Crustaceorum, &c. Conspectus of the Crustacea of the Exploring Expedition under Capt. Wilkes, U.S.N. Macroura. Proceedings of the Academy of Natural Sciences of Philadelphia. 1852;6:10–28. [Google Scholar]
- De Angeli A, Garassino A, Ceccon L. New report of coral-associated decapods from the “Formazione di Castelgomberto” (early Oligocene) (Vicenza, NE Italy) Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. 2010;151:145–177. [Google Scholar]
- De Grave S, Pentcheff ND, Ahyong ST, Chan T-Y, Crandall KA, Dworschak PC, Felder DL, Feldmann RM, Fransen CHJM, Goulding LYD, Lemaitre R, et al. A classification of living and fossil genera of decapod crustaceans. The Raffles Bulletin of Zoology. 2009;Supplements 21:1–109. [Google Scholar]
- Desmarest AG. Les Crustacés Proprement Dits. plates V– XI. In: Brongniart A, Desmarest A-G, editors. Histoire Naturelle des Crustacés Fossiles sous les Rapports Zoologiques et Géologiques. Paris: F-G Levrault; 1822. pp. 67–154. [Google Scholar]
- Dworschak PC. The biology of Upogebia pusilla (Petagna) (Decapoda, Thalassinidea). I. The burrows. Pubblicazioni della Stazione Zoologica di Napoli I Marine Ecology. 1983;4:19–43. [Google Scholar]
- Dworschak PC. Observations on the biology of Callianassa tyrrhena and C. candida (Crustacea, Thalassinidea) Journal of Natural History. 1998;32:1535–1548. [Google Scholar]
- Dworschak PC. Global diversity in the Thalassinidea (Decapoda) Journal of Crustacean Biology. 2000;20(Special Number 2):238–245. [Google Scholar]
- Dworschak PC. A new species of ghost shrimp from the Gulf of Aqaba, Red Sea (Crustacea: Decapoda: Callianassidae) Annalen des Naturhistorischen Museums in Wien. 2003;104B:415–428. [Google Scholar]
- Dworschak PC. Biology of Mediterranean and Caribbean Thalassinidea (Decapoda) In: Tamaki A, editor. Proceedings of the Symposium on “Ecology of large bioturbators in tidal flats and shallow sublittoral sediments – from individual behavior to their role as ecosystem engineers”. Nagasaki: Nagasaki University; 2004. pp. 15–22. November: 1 – 2, 2003. [Google Scholar]
- Dworschak PC. Global diversity in the Thalassinidea (Decapoda): an update (1998 – 2004) Nauplius. 2005;13:57–63. [Google Scholar]
- Dworschak PC. Book review: Sakai, K. 2005. Callianassoidea of the world (Decapoda: Thalassinidea). Crustaceana Monographs 4, i – vi 285 pp., 44 textfigs. Koninklijke Brill, NV Leiden, The Netherlands, ISBN 90 04 14211 8, hardcover, € 89 / U.S.$ 120. Journal of Crustacean Biology. 2007;27:158–169. [Google Scholar]
- Dworschak PC. Neocallichirus kempi Sakai, 1999, a junior synonym of Callianassa karumba Poore & Griffin, 1979 (Decapoda: Callianassidae) Raffles Bulletin of Zoology. 2008;56(1):75–84. [Google Scholar]
- Dworschak PC. On the identities of Callianassa bouvieri Nobili, 1904, C. maldivensis Borradaile, 1904, and C. gravieri Nobili, 1905 (Crustacea: Decapoda: Callianassidae): a morphometric approach. Zootaxa. 2012;3149:5–21. [Google Scholar]
- Dworschak PC. Methods collecting Axiidea and Gebiidea (Decapoda): a review. Annalen des Naturhistorischen Museums in Wien. 2015;117B:415–428. [Google Scholar]
- Dworschak PC, Felder DL, Tudge CC. Infraorders Axiidea de Saint Laurent, 1979 and Gebiidea de Saint Laurent, 1979 (formerly known collectively as Thalassinidea) In: Schram FR, Von Vaupel Klein JC, Charmantier-Daures M, Forest J, editors. Treatise on Zoology – Anatomy, Taxonomy, Biology – The Crustacea, Decapoda, Volume 9 Part B Decapoda: Astacidea P.P. (Enoplometopoidea, Nephropoidea), Glypheidea, Axiidea, Gebiidea, and Anomura. 9B. 2012. pp. 109–219. [Google Scholar]
- Dworschak PC, Ott JA. Decapod burrows in mangrove-channel and back-reef environments at the Atlantic Barries Reef, Belize. Ichnos. 1993;2:277–290. [Google Scholar]
- East EH. Reconstruction of the fossil mud shrimp Callianopsis clallamensis. Journal of Crustacean Biology. 2006;26:168–175. [Google Scholar]
- Ehrenberg K. Ergänzende Bemerkungen zu den seinerzeit aus dem Miozän von Burgschleinitz beschriebenen Gangkernen und Bauten dekapoder Krebse. Paläontologische Zeitschrift. 1944;23:354–359. [Google Scholar]
- Felder DL. Diversity and ecological significance of deep-burrowing macrocrustaceans in coastal tropical waters of the Americas (Decapoda: Thalassinidea) Interciência. 2001;26:440–449. [Google Scholar]
- Felder DL, Lovett DL. Relative growth and sexual maturation in the estuarine ghost shrimp Callianassa louisianensis Schmitt, 1935. Journal of Crustacean Biology. 1989;9:540–553. [Google Scholar]
- Felder DL, Robles R. Molecular phylogeny of the family Callianassidae based on preliminary analysis of two mitochondrial genes. In: Martin JW, Crandall KA, Felder DL, editors. Decapod Crustacean Phylogenetics. Taylor & Francis; CRC Boca Raton, Fl: 2009. pp. 327–342. [Google Scholar]
- Feldmann RM, Schweitzer CE, Redman CM, Morris NJ, Ward DJ. New Late Cretaceous lobsters from the Kyzylkum desert of Uzbekistan. Journal of Paleontology. 2007;81:701–713. [Google Scholar]
- Feldmann RM, Schweitzer CE, Baltzly LM, Bennett OA, Jones AR, Mathias FF, Weaver KL, Yost SL. New and previously known decapod crustaceans from the Late Cretaceous of New Jersey and Delaware, USA. Bulletin of the Mizunami Fossil Museum. 2013;39:7–37. [Google Scholar]
- Ferrari L. Aportes para el conocimiento de la familia Callianassidae (Decapoda, Macrura) en el Oceano Atlantico sudoccidental. Physis (Buenos Aires), Seccion A. 1981;39(97):11–21. [Google Scholar]
- Förster R. Über die Erymiden, eine alte konservative Familie der mesozoischen Dekapoden. Palaeontographica. 1966;125:61–175. [Google Scholar]
- Förster R. Die Krebse und ihre Bauten aus dem Santon der Gehrdener Berge. Bericht der Naturhistorischen Gesellschaft Hannover. 1973;117:149–162. [Google Scholar]
- Förster R. Untersuchungen an jurassischen Thalassinoidea (Crustacea, Decapoda) Mitteilungen der Bayerischen Staatssammlung für Paläontologie und historische Geologie. 1977;17:137–156. pl. 14. [Google Scholar]
- Fraaije RHB, Menkveld-Gfeller UE, Van Bakel BWM, Jagt JWM. Decapod crustaceans from the type area of the Helvetian Stage (lower Miocene) in the Bern area, Switzerland. Bulletin of the Mizunami Fossil Museum. 2010;36:1–11. [Google Scholar]
- Franțescu OD. Fossil mudshrimps (Decapoda: Axiidea) from the Pawpaw Formation (Cretaceous: Albian), northeast Texas, USA. Bulletin of the Mizunami Fossil Museum. 2014;40:13–22. [Google Scholar]
- Frey RW, Howard JD, Wayne AP. Ophiomorpha: Its morphologic, taxonomic, and environmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology. 1978;23:199–229. [Google Scholar]
- Fritsch A, Kafka J. Die Crustaceen der böhmischen Kreideformation. F. Řivnáč; Prague: 1887. p. 53. [Google Scholar]
- Garassino A, De Angeli A, Pasini G. A new species of ghost shrimp (Decapoda, Thalassinidea, Callianassidae) from the Late Cretaceous (Cenomanian) of Agadir (W Morocco) Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. 2011;152:45–55. [Google Scholar]
- Garcia KE, Embry SJ, Grossblat D, Holbrook A-M, Mclaren WM, Reed SK, Wildey HC, Shuster SM. A comparison of two methods for sampling the Gulf of California mud shrimp, Neotrypaea uncinata (Crustacea: Thalassinidea) Journal of Natural History. 2003;37:1847–1854. [Google Scholar]
- Gašparič R, Hyžný M. An early Miocene deep-water decapod crustacean faunule from the Slovenian part of the Styrian Basin, and its palaeoenvironmental and palaeobiogeographical significance. Papers in Palaeontology. 2015;1:141–166. doi: 10.1002/spp2.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard A, Bonnier J. Sur une espèce nouvelle de Callianasse du Golfe de Naples (Callianassa truncata) Bulletin Scienti fique de la France et de la Belgique. 1890;22:362–366. [Google Scholar]
- de Gibert JM, Ekdale AA. Paleobiology of the crustacean trace fossil Spongeliomorpha iberica in the Miocene of south-eastern Spain. Acta Palaeontologica Polonica. 2010;55:733–740. [Google Scholar]
- Glaessner MF. Crustacea Decapoda. In: Pompeckj FJ, editor. Fossilium Catalogus, 1 Animalium, 41. W. Junk; Berlin: 1929. pp. 1–464. [Google Scholar]
- Glaessner MF. In: Decapoda. Treatise on Invertebrate Paleontology, Part R. Arthropoda 4 (2) Moore RC, editor. Geological Society of America, Boulder, and University of Kansas Press: Lawrence; 1969. pp. R399–R533. [Google Scholar]
- Griffis RB, Suchanek TH. Ecological significance of species-specific burrow architecture in thalassinid shrimp (Decapoda: Thalassinidea) (Series).Marine Ecology Progress. 1991;79:171–183. [Google Scholar]
- Hailstone TS, Stephenson W. The biology of Callianassa (Trypaea) australiensis Dana 1852 (Crustacea, Thalassinidea) University of Queensland Papers, Department of Zoology. 1961;1(12):259–282. pls. 1 – 3. [Google Scholar]
- Harbort E. Über die stratigraphischen Ergebnisse von zwei Tiefbohrungen durch die Untere Kreide bei Stederdorf und Horst im Kreise Peine. Jahrbuch der Königlich Preussischen Geologischen Landesanstalt und Bergakademie zu Berlin. 1905;26:20–42. [Google Scholar]
- Hartnoll RG. Variation in growth pattern between some secondary sexual characters in crabs (Decapoda, Brachyura) Crustaceana. 1974;27:131–136. [Google Scholar]
- Hartnoll RG. Strategies of crustacean growth. In: Lowry JK, editor. Papers from the conference on the biology and evolution of Crustacea, held at the Australian Museum Sydney, 1980. Vol. 18. Australian Museum Memoir; 1983. pp. 121–131. [Google Scholar]
- Hartnoll RG. Relative growth: description and analysis. Treatise on Zoology – Anatomy, Taxonomy, Biology – The Crustacea, Decapoda. In: Forest J, Von vaupel Klein JC, Charmantier-Daures M, Schram FR, editors. Vol. 3. 2012. pp. 365–401. [Google Scholar]
- Hasiotis ST, Bourke MC. Continental trace fossils and museum exhibits: displaying organism behaviour frozen in time. The Geological Curator. 2006;8:211–226. [Google Scholar]
- Haug C, Nyborg T, Vega FJ. An exceptionally preserved upogebiid (Decapoda: Reptantia) from the Eocene of California. Boletín de la Sociedad Geológica Mexicana. 2013;65:235–248. [Google Scholar]
- Hayward BW. Lower Miocene bathyal and submarine canyon ichnocoenosis from Northland, New Zealand. Lethaia. 1976;9:149–162. [Google Scholar]
- Heard RW, Manning RB. A new genus and species of ghost shrimp from Tobago, West Indies (Crustacea: Decapoda: Callianassidae) Proceedings of the Biological Society of Washington. 2000;113:70–76. [Google Scholar]
- Herbst JFW. Versuch einer Naturgeschichte der Krabben und Krebse nebst einer systematischen Beschreibung ihrer verschiedenen Arten 1[1782 – 1790]: 1 – 274, pls. 1 – 21; 2[1791 – 1796]: i – viii, iii, iv 1 – 225, pls. 22 – 46; 3[1799 – 1804]: 1 – 66, pls. 47 – 50. 1782 – 1804 [Google Scholar]
- Hirayama A. A ghost-shrimp with four-articulate fifth pereopods (Crustacea: Caprellidea: Phtisicidae) from Northwest Australia. Zoological Science. 1988;5:1089–1093. [Google Scholar]
- Holmes SJ. On some new or imperfectly known species of West American Crustacea. (series 3).Proceedings of the California Academy of Sciences. 1904;3:307–328. [Google Scholar]
- Hu C-H, Tao H-J. Crustacean Fossils of Taiwan. Taipei, Taiwan. 1996 [Google Scholar]
- Huxley TH. On the classification and the distribution of the crayfishes. Proceedings of the Scientific Meetings of the Zoological Society of London. 1879;46:752–788. [for 1878] [Google Scholar]
- Hyžný M. Neocallichirus rodfeldmanni, a proposed replacement name for Neocallichirus manningi Schweitzer, Feldmann, Fam, Hessin, Hetrick, Nyborg & Ross, 2003 (Crustacea: Decapoda: Axiidea: Callianassidae) Bulletin of the Mizuna mi Fossil Museum. 2010;36:129. [Google Scholar]
- Hyžný M. In situ mud shrimps (Decapoda: Axiidea: Callianassidae) preserved within their burrows from the middle Miocene of the Central Paratethys. Bulletin of the Mizunami Fossil Museum. 2011;37:37–46. [Google Scholar]
- Hyžný M. Calliaxina chalmasii (Brocchi, 1883) comb. nov. (Decapoda: Axiidea: Callianassidae: Eucalliacinae), a ghost shrimp from the Middle Miocene of Europe, with reappraisal of the fossil record of Eucalliacinae. Zootaxa. 2012;3492:49–64. [Google Scholar]
- Hyžný M, Bahrami A, Klompmaker AA, Yazdi M, Portell RW, Neumann C. The fossil record of Glypturus (Decapoda: Axiidea: Callianassidae) revisited with additional observations and description of a new species. Swiss Journal of Palaeontology. 2013a.;132:129–139. [Google Scholar]
- Hyžný M, Dulai A. Deep-water fossorial shrimps from the Oligocene Kiscell Clay of Hungary: Taxonomy and palaeoecology. Acta Palaeontologica Polonica. 2014;59:947–965. doi: 10.4202/app.2012.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyžný M, Gašparič R. Ghost shrimp Calliax de Saint Laurent, 1973 (Decapoda: Axiidea: Callianassidae) in the fossil record: systematics, palaeoecology and palaeobiogeography. Zootaxa. 2014;3821:37–57. doi: 10.11646/zootaxa.3821.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyžný M, Harzhauser M, Danninger W. Decapod Crustacea of the Central Paratethyan stage Ottnangian (middle Burdigalian): implications for systematics and biogeography. Geologica Carpathica. 2015;66:217–233. doi: 10.1515/geoca-2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyžný M, Hudáčková N. Redescription of two ghost shrimps (Decapoda: Axiidea: Callianassidae) from the Middle Miocene of the Central Paratethys: systematics, intraspecific variation, and in situ preservation. Zootaxa. 2012;3210:1–25. [Google Scholar]
- Hyžný M, Karasawa H. How to distinguish Neocallichirus, Sergio, Podocallichirus and Grynaminna (Decapoda: Callianassidae: Callichirinae) from each other in the fossil record? Bulletin of the Mizunami Fossil Museum. 2012;38:59–68. [Google Scholar]
- Hyžný M, Kočová Veselská M, Dvořák P. On the occurrence of Ctenocheles (Decapoda, Axiidea, Callianassidae) in the Bohemian Cretaceous Basin. Bulletin of Geosciences; 2014;89:245–256. doi: 10.3140/bull.geosci.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyžný M, Müller PM. The first fossil record of the genus Callichirus (Decapoda, Axiidea, Callianassidae) from the middle Miocene of Hungary, with description of a new species. Bulletin of the Mizunami Fossil Museum. 2010;36:37–43. [Google Scholar]
- Hyžný M, Müller PM. The fossil record of Glypturus Stimpson, 1866 (Crustacea, Decapoda, Axiidea, Callianassidae) revisited, with notes on palaeoecology and palaeobiogeography. Palaeontology. 2012;55:967–993. [Google Scholar]
- Hyžný M, Muñiz F. Podocallichirus laepaensis, a new ghost shrimp (Crustacea, Decapoda, Callianassidae) from the Late Miocene of southwest Spain. Journal of Paleontology. 2012;86:616–625. [Google Scholar]
- Hyžný M, Schlögl J. An early Miocene deep-water decapod crustacean faunule from the Vienna Basin (Western Carpathians, Slovakia) Palaeontology. 2011;54:323–349. [Google Scholar]
- Hyžný M, Vega FJ, Coutiño MA. Ghost shrimps (Decapoda: Axiidea: Callianassidae) of the Maastrichtian (Late Cretaceous) Ocozocoautla Formation, Chiapas (Mexico) Boletín de la Sociedad Geológica Mexicana. 2013b;65:255–264. [Google Scholar]
- Jenkins RJF. Australian fossil decapod Crustacea: faunal and environmental changes. 1972. p. 392. Unpublished Ph.D. dissertation, University of Adelaide, Australia, 9 tables, 63 figs., 23 pls. [Google Scholar]
- Karasawa H. Cenozoic decapod Crustacea from southwest Japan. Bulletin of the Mizunami Fossil Museum. 1993;20:1–92. 24 pls. [Google Scholar]
- Karasawa H. A monograph of Cenozoic stomatopod, decapod, isopod and amphipod Crustacea from West Japan. Monograph of the Mizunami Fossil Museum. 1997;8:1–81. [Google Scholar]
- Karasawa H. Coral-associated decapod Crustacea from the Pliocene Daito Limestone Formation and Pleistocene Ryukyu Group, Ryukyu Islands, Japan. Bulletin of the Mizunami Fossil Museum. 2000a;27:167–189. [Google Scholar]
- Karasawa H. Discovery of Early Cretaceous (Barremian) decapod Crustacea from the Arida Formation of Wakayama Prefecture, Japan. Paleontological Research. 2000b;4:235–238. [Google Scholar]
- Karasawa H. New axiidean Decapoda from the Albian (Lower Cretaceous) chemosynthetic community of Hokkaido, Japan. Bulletin of the Mizunami Fossil Museum. 2011;37:27–29. [Google Scholar]
- Kato H. Miocene decapod Crustacea from the Chichibu Basin, Central Japan. (new series 183).Transactions and Proceedings of the Palaeontological Society of Japan. 1996:500–521. [Google Scholar]
- Kensley B. The genus Callianassa (Crustacea, Decapoda, Thalassinidea) from the west coast of South Africa with a key to the South African species. Annals of the South African Museum. 1974;62(8):265–278. [Google Scholar]
- Kishinouye K. Two rare and remarkable forms of macrurous Crustacea from Japan. Annotationes Zoologicae Japonenses. 1926;11:63–70. [Google Scholar]
- Klompmaker AA, Artal P, Van Bakel BWM, Fraaije RHB, Jagt JWM. Parasites in the fossil record: a Cretaceous fauna with isopod-infested decapod crustaceans, infestation patterns through time, and a new ichnotaxon. PLOS ONE. 2014;9(3):e92551. doi: 10.1371/journal.pone.0092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompmaker AA, Boxshall GA. Fossil crustaceans as parasites and hosts. Advances in Parasitology. 2015;90:233–289. doi: 10.1016/bs.apar.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Klompmaker AA, Feldmann RM, Schweitzer CE. New European localities for coral-associated, Cretaceous decapod crustaceans. Bulletin of the Mizunami Fossil Museum. 2012;38:69–74. [Google Scholar]
- Klompmaker AA, Hyžný M, Portell RW, Kowalewski M. Growth, inter- and intraspecific variation, palaeobiogeography, taphonomy, and systematics of the Cenozoic ghost shrimpGlypturus . Journal of Systematic Palaeontology. 2015a doi: 10.1080/14772019.2015.1009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompmaker AA, Hyžný M, Jakobsen SL. Taphonomy of decapod crustacean cuticle and its effect on the appearance as exemplified by new and known taxa from the Cretaceous – Danian crab Caloxanthus . Cretaceous Research. 2015b;55:141–151. [Google Scholar]
- Kneer D, Asmus H, Jompa J. Do burrowing callianassid shrimp control the lower boundary of tropical seagrass beds? Journal of Experimental Marine Biology and Ecology. 2013;446:262–272. [Google Scholar]
- Komai T, Tachikawa H. New genus and species of axiid shrimp (Crustacea, Decapoda, Thalassinidea) from Japan. (series A (Zoology)).Bulletin of the National Museum of Nature and Science. 2007;33:113–126. [Google Scholar]
- Kossmann R. III. Malacostraca, (2. Theil: Anomura) Zoologische Ergebnisse einer im Auftrage der Königlichen Academie der Wissenschaften zu Berlin ausgeführten Reise in die Küstengebiete des Roten Meeres. In: Kossmann R, editor. Vol. 2. Leipzig: Wilhelm Engelmann; 1880. pp. 67–140. pls. 4 – 15 in: [Google Scholar]
- Kuss J, Senowbari-Daryan B. Anomuran coprolites from Cretaceous shallow water limestones of NE Africa. Cretaceous Research. 1992;13:147–156. [Google Scholar]
- Labadie LV, Palmer AR. Pronounced heterochely in the ghost shrimp, Neotrypaea californiensis (Decapoda: Thalassinidea: Callianassidae): allometry, inferred function and development. Journal of Zoology. 1996;240:659–675. [Google Scholar]
- Leach WE. Crustaceology. In: Brewster D, editor. The Edinburgh Encyclopedia. Blackwood; Edinburgh: 1814. pp. 383–437. [Google Scholar]
- Lin F-J, Komai T, Chan T-Y. First record of the thalassinidean genus Callianopsis de Saint Laurent, 1973 (Decapoda, Ctenochelidae) in the West Pacific, with the description of a new species from Taiwan. Crustaceana. 2007;80:1193–1203. [Google Scholar]
- Linnaeus C. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata. Laurentius Salvius: Holmiae. 1758;ii:824. [Google Scholar]
- Lőrenthey E. Adatok Magyarország harmadkorú rák faunájához. Mathematikai és Természettudományi Értesito. 1897;15:149–169. [Google Scholar]
- Lőrenthey I, Beurlen K. Die fossilen Dekapoden der Länder der Ungarischen Krone. (Series Palaeontologica).Geologica Hungarica. 1929;3:1–421. [Google Scholar]
- Lundgren B. Studier öfver fossilförande lösa block. Geologiska Föreningens I Stockholm Förhandlingar. 1891;13:111–121. [Google Scholar]
- Macginitie GE. The natural history of Callianassa californiensis Dana. American Midland Naturalist. 1934;15:166–177. [Google Scholar]
- Mangano MG, Buatois LA. Discontinuity surfaces in the Lower Cretaceous of the High Andes (Mendoza, Argentina): Trace fossils and environmental implications. Journal of South American Earth Sciences. 1991;4:215–229. [Google Scholar]
- Manning RB. Notes on western Atlantic Callianassidae (Crustacea: Decapoda: Thalassinidea) Proceedings of the Biological Society of Washington. 1987;100:386–401. [Google Scholar]
- Manning RB, Felder DL. The status of the callianassid genus Callichirus Stimpson, 1866 (Crustacea: Decapoda: Thalassinidea) Proceedings of the Biological Society of Washington. 1986;99:437–443. [Google Scholar]
- Manning RB, Felder DL. Revision of the American Callianassidae (Crustacea: Decapoda: Thalassinidea) Proceedings of the Biological Society of Washington. 1991;104:764–792. [Google Scholar]
- Manning RB, Felder DL. Description of the ghost shrimp Sergio merceae a new species from south Florida, with reexamination of S. guassutinga (Crustacea: Decapoda: Callianassidae) Proceedings of the Biological Society of Washington. 1995;108:266–280. [Google Scholar]
- Manning RB, Lemaitre R. Sergio a new genus of ghost shrimp from the Americas (Crustacea: Decapoda: Callianassidae) Nauplius. 1994;1:39–43. [Google Scholar]
- Manning RB, Tamaki A. A new genus of ghost shrimp from Japan (Crustacea: Decapoda: Callianassidae) Proceedings of the Biological Society of Washington. 1998;111:889–892. [Google Scholar]
- Markham JC. A review of the bopyrid isopods parasitic on thalassinidean decapods. Crustacean Issues. 2001;13:195–204. [Google Scholar]
- Martin JW, Davis GE. An updated classification of the Recent Crustacea. (Science Series).Natural History Museum of Los Angeles County. 2001;39:1–124. [Google Scholar]
- Mccoy F. On the classification of some British fossil Crustacea with notices of new forms in the University Collection at Cambridge. (series 2).Annals and Magazine of Natural History. 1849;4:161–179. 330 – 335. [Google Scholar]
- Mehling CM. Occurrence of callianassid coprolites in the Cretaceous of New Jersey. The Mosasaur. 2004;7:59–63. [Google Scholar]
- Mertin H. Decapode Krebse aus dem subhercynen und Braunschweiger Emscher und Untersenon sowie Bemerkungen über einige verwandte Formen in der Oberkreide. Nova Acta Leopoldina, Neue Folge 10. 1941;68:149–264. [Google Scholar]
- Meyer von H. Krebse im bunten Sandstein. Museum Senckenbergianum. 1834;1:293–295. [Google Scholar]
- Milne-Edwards A. Histoire des Crustacés podophthalmaires fossils et monographie des Décapodes macroures de la famille des Thalassiens fossiles. Annales des Sciences Naturelles, (Zoologie) (4) 1860;14:129–293. pls. 1 – 10. [Google Scholar]
- Montagu G. Description of several marine animals found on the south coast of Devonshire. Transactions of the Linnean Society of London. 1808;9:81–114. [Google Scholar]
- Mourik AA, Fraaije RHB, Van Der Zwaan GJ, Scheer U. The burrowing shrimp, Protocallianassa faujasi (Crustacea, Decapoda, Thalassinoidea), from the Lower Campanian at Dülmen, Germany. Bulletin of the Muzinami Fossil Museum. 2005;32:1–12. [Google Scholar]
- Murray PF, Hanley JR. Unmuddling the mud lobster; observations on the age and taphonomy of fossil Thalassina. The Beagle, Occasional papers of the Northern Territory Museum of Arts and Sciences. 1986;3(1):59–70. [Google Scholar]
- Müller AH. Über Ichnia vom Typ Ophiomorpha und Thalassinoides (Vestigia invertebratorum, Crustacea) Monatsberichte der Deutschen Akademie der Wissenschaften zu Berlin. 1970;12:775–787. [Google Scholar]
- Müller P. Decapod Crustacea of the Badenian. (Series Palaeontologica).Geologica Hungarica. 1984;42:3–317. [Google Scholar]
- Müller P. Neogene decapod crustaceans from Catalonia. Scripta Musei Geologici Seminarii Barcinonensis. 1993;225:1–39. [Google Scholar]
- Münster G Graf Zu. Decapoda Macroura. Abbildung und Beschreibung der fossilen langschwänzigen Krebse in den Kalkschiefern von Bayern. Münster G Graf Zu., editor. Beiträge zur Petrefacten-Kunde. 1839;2:1–88. [Google Scholar]
- Nates SF, Felder DL. Growth and maturation of the ghost shrimp Lepidophthalmus sinuensis Lemaitre and Rodrigues 1991 (Crustacea, Decapoda, Callianassidae), a burrowing pest in penaeid shrimp culture ponds. Fishery Bulletin. 1999;97:526–541. [Google Scholar]
- Ngoc-Ho N. European and Mediterranean Thalassinidea (Crustacea, Decapoda) Zoosystema. 2003;25:439–555. [Google Scholar]
- Nickel LA, Atkinson RJA. Functional morphology of burrows and trophic modes of three thalassinidean shrimp species, and a new approach to the classification of thalassinidean burrow morphology. Marine Ecology Progress Series. 1995;128:181–197. [Google Scholar]
- Oppel A. Die Arten der Gattungen Eryma Pseudastacus Magila und Etallonia. Jahreshefte des Vereins für Vaterländische Naturkunde in Württemberg. 1861;17:355–361. [Google Scholar]
- Ortmann A. Die Decapoden-Krebse des Strassburger Museums. 3. Die Abtheilungen der Reptantia Boas: Homaridae, Loricata und Thalassinidea. Zoologische Jahrbücher (Systematik, Ökologie, und Geographie der Tiere) 1891;6:1–58. [Google Scholar]
- Ossó-Morales À, Garassino A, Vega FJ, Artal P. Pleuronassa timerchidouensis n. gen., n. sp. (Axiidea, Callianassidae) from the Calcaires à slumps de Taghit Fm., Late Campanian of the Moyenne Moulouya, NE Morocco. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. 2011;152:165–175. [Google Scholar]
- Pelseneer P. Notice sur les Crustacés décapodes du Maastrichtien du Limbourg. Bulletin du Musée royal d’Histoire Naturelle de Belgique. 1886;4(3):161–175. [Google Scholar]
- Philippe M, Secretan S. Crustacés decapods du Burdigalien des Courennes (Vaucluse) Annales de Paléontologie (Invertébrés) 1971;57:117–134. [Google Scholar]
- Philippi RA. Los fósiles terciarios I cuartarios de Chile. Brockhaus, Leipzig [German version] and Santiago de Chile [Spanish version] 1887:256. 56 pls. [Google Scholar]
- Pilsbry HA. Crustacea of the Cretaceous Formation of New Jersey. Proceedings of the Academy of Natural Sciences of Philadelphia. 1901;53:111–118. [Google Scholar]
- Plotnick RE. Taphonomy of a modern shrimp: Implications for the arthropod fossil record. Palaios. 1986;1:286–293. [Google Scholar]
- Polkowsky S. Decapode Krebse aus dem oberoligozänen Sternberger Gestein von Kobrow (Mecklenburg) Tassados. 2004;1:1–126. [Google Scholar]
- Poore GCB. A phylogeny of the families of Thalassinidea (Crustacea: Decapoda) with keys to families and genera. Memoirs of the Museum of Victoria. 1994;54:79–120. [Google Scholar]
- Poore GCB. CSIRO Publishing; Melbourne: 2004. Marine decapod Crustacea of southern Australia. A guide to identification. With chapter on Stomatopoda by Shane Ahyong; 574 pp. [Google Scholar]
- Poore GCB. Thalassinidean shrimps (Crustacea: Decapoda) from north-western Australia, including five new species. Records of the Western Australian Museum, Supplement. 2008;73:161–179. [Google Scholar]
- Poore GCB, Ahyong ST, Bracken-Grissom HD, Chan T-Y, Chu KH, Crandall KA, Dworschak PC, Felder DL, FeldMann RM, Hyžný M, Karasawa H, et al. On stabilising the names of the infraorders of thalassinidean shrimps, Axiidea de Saint Laurent, 1979 and Gebiidea de Saint Laurent, 1979 (Decapoda) Crustaceana. 2014;87:1258–1272. [Google Scholar]
- Poore GCB, Griffin DJG. The Thalassinidea (Crustacea: Decapoda) of Australia. Records of the Australian Museum. 1979;32:217–321. [Google Scholar]
- Poore GCB, Suchanek TH. Glypturus motupore a new callianassid shrimp (Crustacea: Decapoda) from Papua New Guinea with notes on its ecology. Records of the Australian Museum. 1988;40:197–204. [Google Scholar]
- Portell RW, Agnew JG. Pliocene and Pleistocene decapod crustaceans. Florida Fossil Invertebrates. 2004;4:1–29. [Google Scholar]
- Pryor WA. Biogenic sedimentation and alteration of argillaceous sediments in shallow marine environments. Geological Society of America Bulletin. 1975;86:1244–1254. [Google Scholar]
- Przibram H. Die „Heterochelie“ bei decapoden Crustaceen (zugleich: Experimentelle Studien über Regeneration. Dritte Mitteilung) Archiv für Entwicklungsmechanik der Organismen. 1905;19(2):181–247. [Google Scholar]
- Rafinesque CS. Analyse de la Nature, ou Tableau de l’Univers et des Corps Organisés. Palermo, L’Imprimerie de Jean Barravecchia, Palermo pp. 1815;224 [Google Scholar]
- Rathbun MJ. Descriptions of new decapod crustaceans from the west coast of North America. Proceedings of the United States National Museum. 1902;24:885–905. [Google Scholar]
- Rathbun MJ. Decapod crustaceans from Panama. Vaughan TW, editor. Contributions to the geology and paleontology of the Canal Zone, Panama and geologically related areas in Central America and the West Indies. United States National Museum Bulletin. 1918;103:123–184. pls. 54 – 66. [Google Scholar]
- Rathbun MJ. West Indian Tertiary decapod crustaceans. Vaughan TW, editor. Contributions to the geology and paleontology of the West Indies. Carnegie Institution of Washington Publication pls. 1919;291:159–184. pls 1 – 9. [Google Scholar]
- Rathbun MJ. The fossil stalk-eyed Crustacea of the Pacific Slope of North America. Bulletin of the United States National Museum. 1926;138:1–155. [Google Scholar]
- Rathbun MJ. A new Callianassafrom the Cretaceous of South Dakota. Journal of the Washington Academy of Sciences. 1930;20:1–3. [Google Scholar]
- Rathbun MJ. Fossil Crustacea of the Atlantic and Gulf Coastal Plain. Geological Society of America, Special Papers. 1935;2:1–160. [Google Scholar]
- Rathbun MJ. Phylum Arthropoda. Knechtel MM, Richards EF, Rathbun MJ, editors. Mesozoic fossils of the Peruvian Andes. Johns Hopkins University Studies in Geology. 1947;15:1–150. [Google Scholar]
- Rathke H. Zur Fauna der Krym. Mémoires de l’Académie Impériale des Sciences de St. Pétersbourg. 1837;3:291–454. pls. 1–10. [Google Scholar]
- Rex MA, Stuart CT, Coyne G. Latitudinal gradient of species richness in the deep-sea benthos of the North Atlantic. Proceedings of the National Academy of Sciences. 2000;97:4082–4085. doi: 10.1073/pnas.050589497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles R, Tudge CC, Dworschak PC, Poore GCB, Felder DL. Molecular phylogeny of the Thalassinidea based on nuclear and mitochondrial genes. In: Martin JW, Crandall KA, Felder DL, editors. Decapod Crustacean Phylogenetics. Taylor & Francis / CRC Press; Boca Raton: 2009. pp. 309–326. [Google Scholar]
- De Rodrigues SA. Aspectos da biologia de Thalassinidea do Atlântico tropical Americano. Thesis presented to the Instituto de Biociencias da Universidada de Sao Paulo in partial fulfillment of obtaining the title of Livre Docente, Univ. de São Paulo, São Paulo. 1983:174. [Google Scholar]
- De Rodrigues SA, Hödl W. Burrowing behaviour of Callichirus major and C. mirim. Begleitveröffentlichung zum wissenschaftlichen Film C 2199 des ÖWF. (Accompanying guide with the scientific film C 2199 of the Austrian Scientific Film Organization.) Wissenschaftl. Film. 1990 Apr;41:48–58. [Google Scholar]
- Roemer FA. Die Versteinerungen des Norddeutschen Kreidegebirges. Hahnsche Hofbuchhandlung, Hannover. 1841:136. [Google Scholar]
- Rowden AA, Jones MB. Critical evaluation of sediment turnover estimates for Callianassidae (Decapoda: Thalassinidea) Journal of Experimental Marine Biology and Ecology. 1993;446:262–272. [Google Scholar]
- Rowden AA, Jones MB. A contribution to the biology of the burrowing mud shrimp, Callianassa subterranea (Decapoda: Thalassinidea) Journal of the Marine Biological Association of the United Kingdom. 1994;74:623–635. [Google Scholar]
- De Saint Laurent M. Sur la systématique et la phylogénie des Thalassinidea: définition des familles des Callianassidae et des Upogebiidae et diagnose de cinq genres nouveaux (Crustacea Decapoda) Comptes Rendus Hebdomadaires de Séances de l’Académie des Sciences, série D. 1973;277:513–516. [Google Scholar]
- De Saint Laurent M. Sur la classification et la phylogénie des Thalassinides: définitions de la superfamille des Axioidea, de la sous-famille des Thomassiniinae et de deux genres nouveaux (Crustacea Decapoda) Comptes Rendus Hebdomadaires de Séances de l’Académie des Sciences, Paris. 1979;288:1395–1397. [Google Scholar]
- De Saint Laurent M, Le Loeuff P. Upogebiidae et Callianassidae. Crustacés Décapodes Thalassinidea, 1. Campagnes de la Calypso au large des cotes Atlantiques africaines (1956 et 1959) (suite), 22. Résultats scientifiques des campagnes de la Calypso. 1979;11:29–101. [Google Scholar]
- Sakai K. The families Callianideidae and Thalassinidae, with the description of two new subfamilies, one new genus and two new species (Decapoda: Thalassinidea) Naturalists, Tokushima Biological Laboratory, Shikoku Women’s University. 1992;4:1–33. [Google Scholar]
- Sakai K. Synopsis of the family Callianassidae, with keys to subfamilies, genera and species, and the description of new taxa (Crustacea: Decapoda: Thalassinidea) Zoologische Verhandelingen, Leiden. 1999;326:1–152. [Google Scholar]
- Sakai K. Callianassidae (Decapoda, Thalassinidea) in the Andaman Sea Thailand. Phuket Marine Biological Center Special Publication. 2002;23:461–532. [Google Scholar]
- Sakai K. Callianassoidea of the world (Decapoda: Thalassinidea) Crustaceana Monographs. 2005;4:1–285. [Google Scholar]
- Sakai K. Axioidea of the world and a reconsideration of the Callianassoidea (Decapoda, Thalassinidea, Callianassida) Crustaceana Monographs. 2011;13:1–520. [Google Scholar]
- Sakai K, Ohta S. Some thalassinid collections by R/V “Hakuhou-Maru” and R/V “Tansei-Maru”, University of Tokyo, in the Sulu Sea Philippines, and in Sagami Bay and Suruga Bay Japan, including two new species, one new genus, and one new family (Decapoda, Thalassinidea) Crustaceana. 2005;78:67–93. [Google Scholar]
- Sakai K, Türkay M, Beuck L, Freiwald A. A collection of the Infraorder Callianassidea (Decapoda, Pleocyemata) with one new genus and five new species from the Eastern Atlantic off Mauritania (R/V Maria S. Merian cruise MSm 16/3 “PHAETON”) Marine Biodiversity. 2014;45:113–133. [Google Scholar]
- De Saporta G. Nouveaux documents relatifs aux organismes problématiques des anciennes mers. Société Géologique de France, Bulletin. 1887;Série 3(15):286–302. [Google Scholar]
- Say T. An account of the Crustacea of the United States. Journal of the Academy of Natural Sciences, Philadelphia. 1817 – 1818;1:57–63. 65 – 80 (pl. 4), 97 – 101, 155 – 160, 161 – 169, 235 – 253, 313 – 319, 374 – 380, 381 – 401, 423 – 441. [Google Scholar]
- Schäfer W. Form und Funktion der Brachyuren-Schere. Abhandlungen der Senckenbergischen Naturforschenden Gesselschaft. 1954;489:1–65. [Google Scholar]
- Schäfer W. Ecology and Paleoecology of Marine Environments. In: Oertel I, translator; Craig GY, editor. Chicago: Univ. Chicago Press; 1972. 568 pp. [Google Scholar]
- Schweigert G, Seegis DB, Fels A, Leinfelder RR. New internally structured decapod microcoprolites from Germany (Late Triassic/Early Miocene), Southern Spain (Early/Middle Jurassic) and Portugal (Late Jurassic): Taxonomy, palaeoecology and evolutionary implications. Paläontologische Zeitschrift. 1997;71(1/2):51–69. [Google Scholar]
- Schweitzer CE, Feldmann RM. Callichirus? symmetricus (Decapoda: Thalassinoidea) and associated burrows, Eocene, Antarctica. Stilwell JD, Feldmann RM, editors. Paleobiology and Paleoenvironments of Eocene Rocks, McMurdo Sound, East Antarctica. (Antarctic Research Series).American Geophysical Union. 2000;76:348. [Google Scholar]
- Schweitzer CE, Feldmann RM. New Cretaceous and Tertiary decapod crustaceans from western North America. Bulletin of the Mizunami Fossil Museum. 2001;28:173–210. [Google Scholar]
- Schweitzer CE, Feldmann RM. New Eocene decapods (Thalassinidea and Brachyura) from Southern California. Journal of Crustacean Biology. 2002;22:938–967. [Google Scholar]
- Schweitzer CE, Feldmann RM. Revision of Decapoda deposited in the Muséum national d’Histoire naturelle, Paris. Bulletin of the Mizunami Fossil Museum. 2012;38:15–27. [Google Scholar]
- Schweitzer CE, Feldmann RM, Encinas A, Suárez M. New Cretaceous and Eocene Callianassoidea (Thalassinidea, Decapoda) from Algarrobo, Chile. Journal of Crustacean Biology. 2006b;26:73–81. [Google Scholar]
- Schweitzer CE, González-Barba G, Feldmann RM, Waugh DA. Decapoda (Thalassinidea and Paguroidea) from the Eocene Bateque and Tepetate Formations, Baja California Sur México: systematics, cuticle microstructure, and paleoecology. Annals of Carnegie Museum. 2006a;74:275–293. [Google Scholar]
- Schweitzer CE, Feldmann RM, Casadío S, Raising MR. Eocene decapod Crustacea (Thalassinidea and Brachyura) from Patagonia, Argentina. Annals of Carnegie Museum. 2012;80:173–186. [Google Scholar]
- Schweitzer CE, Feldmann RM, Ćosović V, Ross RLM, Waugh D. New Cretaceous and Eocene Decapoda (Astacidea: Thalassinidea: Brachyura) from British Columbia, Canada. Annals of Carnegie Museum. 2009;77:403–423. [Google Scholar]
- Schweitzer CE, Feldmann RM, Fam J, Hessin WA, Hetrick SW, Nyborg TG, Ross RLM. Cretaceous and Eocene decapod crustaceans from Southern Vancouver Island, British Columbia, Canada. NRC Research Press; Ottawa, Ontario: 2003. p. 66. [Google Scholar]
- Schweitzer CE, Feldmann RM, Garassino A, Karasawa H, Schweigert G. Systematic list of fossil decapod crustacean species. Crustaceana Monographs. 2010;10:1–222. [Google Scholar]
- Schweitzer-Hopkins C, Feldmann RM. Sexual dimorphism in fossil and extant species of Callianopsis de Saint Laurent. Journal of Crustacean Biology. 1997;17:236–252. [Google Scholar]
- Senowbari-Daryan B, Nagm E, Blau J, Wilmsen M. Crustacean microcoprolites from the Upper Cretaceous of Egypt. Revue de Paléobiologie. 2009;28:511–518. [Google Scholar]
- Shimoda K, Wardiatno Y, Kubo K, Tamaki A. Intraspecific behaviors and major cheliped sexual dimorphism in three congeneric callianassid shrimps. Marine Biology. 2005;146:543–557. [Google Scholar]
- Shinn EA. Burrowing in Recent lime sediments of Florida and the Bahamas. Journal of Paleontology. 1968;42:879–894. [Google Scholar]
- Siebert T, Branch GM. Ecosystem engineers: interactions between eelgrass Zostera capensis and the sandprawn Callianassa kraussi and their indirect effects on the mudprawn Upogebia africana. Journal of Experimental Marine Biology and Ecology. 2006;338:253–270. [Google Scholar]
- Stamhuis EJ, Schreurs CE, Videler JJ. Burrow architecture and turbative activity of the thalassinid shrimp Callianassa subterranea from the central North Sea. (Series).Marine Ecology Progress. 1997;151:155–163. [Google Scholar]
- Stilwell JD, Levy RH, Feldmann RM, Harwood DM. On the rare occurrence of Eocene callianassid decapods (Arthropoda) preserved in their burrows, Mount Discovery, East Antarctica. Journal of Paleontology. 1997;71:284–287. [Google Scholar]
- Stimpson W. On the Crustacea and Echinodermata of the Pacific shores of North America. Boston Journal of Natural History. 1857;6:444–532. pls. 18 – 23. [Google Scholar]
- Stimpson W. Descriptions of new genera and species of macrurous Crustacea from the coasts of North America. Proceedings of the Chicago Academy of Sciences. 1866;1:46–48. [Google Scholar]
- Swen K, Fraaije RHB, Van Der Zwaan GJ. Polymorphy and extinction of the Late Cretaceous burrowing shrimp Protocallianassa faujasi and first record of the genera Corallianassa and Calliax (Crustacea, Decapoda, Thalassinoidea) from the Cretaceous. Contributions to Zoology. 2001;70:85–98. [Google Scholar]
- Tamaki A, Ingole B, Ikebe K, Muramatsu K, Taka M, Tanaka M. Life history of the ghost shrimp, Callianassa japonicaOrtmann (Decapoda: Thalassinidea), on an intertidal sandflat in western Kyushu, Japan. Journal of Experimental Marine Biology and Ecology. 1997;210:223–250. [Google Scholar]
- Taylor BJ. Macrurous Decapoda from the Lower Cretaceous of south-eastern Alexander Island. British Antarctic Survey Scientific Reports. 1979;81:1–39. pls. 1 – 5. [Google Scholar]
- Todd JA, Collins JSH. Neogene and Quaternary crabs (Crustacea, Decapoda) collected from Costa Rica and Panama by members of the Panama Paleontology Project. Bulletin of the Mizunami Fossil Museum. 2005;32:53–85. [Google Scholar]
- de Tribolet M. Description des Crustacés du terrain néocomien du Jura neuchâtelois et vaudois. Bulletin de la Societé Géologique de France, série. 1874;3(2):350–365. pl. 12. [Google Scholar]
- de Tribolet M. Description des Crustacés Décapodes des étages néocomien et urgonien de la Haute-Marne du terrain néocomien du Jura neuchâtelois et vaudois. Bulletin de la Societé Géologique de France, série 3. 1875;3:451–459. pl. 16. [Google Scholar]
- Tsang LM, Lin F-J, Chu KH, Chan T-Y. Phylogeny of Thalassinidea (Crustacea, Decapoda) inferred from three rDNA sequences: implications for morphological evolution and superfamily classification. Journal of Zoological Systematics and Evolutionary Research. 2008;46:216–223. [Google Scholar]
- Tshudy D, Sorhannus U. Hoploparia, the best-known fossil clawed lobster (family Nephropidae), is a “wastebasket” genus. Journal of Crustacean Biology. 2003;23:700–711. [Google Scholar]
- Tudge CC, Cunningham CW. Molecular phylogeny of the mud lobsters and mud shrimps (Crustacea: Decapoda: Thalassinidea) using nuclear 18S rDNA and mitochondrial 16S rDNA. Invertebrate Systematics. 2002;16:839–847. [Google Scholar]
- Tudge CC, Poore GCB, Lemaitre R. Preliminary phylogenetic analysis of generic relationships within the Callianassidae and Ctenochelidae (Decapoda: Thalassinidea: Callianassoidea) Journal of Crustacean Biology. 2000;20(Special issue 2):129–149. [Google Scholar]
- Türkay M, Sakai K. Decapod crustaceans from a volcanic hot spring in the Marianas. Senckenbergiana Maritima. 2005;26:25–35. [Google Scholar]
- de Vaugelas J. A new technique for collecting large-sized callianassid mud-shrimp (Decapoda, Thalassinidea) Crustaceana. 1985;49:105–109. [Google Scholar]
- de Vaugelas J, De Saint Laurent M. Premières données sur l’écologie de Callichirus laurae de Saint Laurent sp. nov. (Crustaceé Décapode Callianassidae): son action bioturbatrice sur les formations sédimentaires du golfe d’Aqaba (Mer Rouge) Comptes Rendus Hebdomadaires de Séances de l’Académie des Sciences, Paris. 1984;298(6):147–152. [Google Scholar]
- Vega FJ, Feldmann RM, Villalobos-Hiriart JL. Additions to the crustacean (Decapoda) fauna from the Potrerillos Formation (Late Cretaceous) in northeastern Mexico. Annals of Carnegie Museum. 1995;64(3):39–49. [Google Scholar]
- Vega FJ, Nyborg T, Rojas-Briceño A, Patarroyo P, Luque J, Porras-Múzquiz H, Stinnesbeck W. Upper Cretaceous Crustacea from Mexico and Colombia: similar faunas and environments during Turonian times. Revista Mexicana de Ciencias Geológicas. 2007;24:403–422. [Google Scholar]
- Waage KM. The type Fox Hills Formation, Cretaceous (Maestrichtian), South Dakota, part 1. Stratigraphy and paleoenvironments. Bulletin Peabody Museum of Natural History. 1968;27:1–175. [Google Scholar]
- Waugh DA, Feldmann RM, Schweitzer CE. Systematic evaluation of raninid cuticle microstructure. Bulletin of the Mizunami Fossil Museum. 2009;35:15–41. [Google Scholar]
- Wienberg Rasmussen H. Echinoid and crustacean burrows and their diagenetic significance in the Maastrichtian-Danian of Stevns Klint, Denmark. Lethaia. 1971;4:191–216. [Google Scholar]
- Wienberg Rasmussen H, Jakobsen SL, Collins JSH. Raninidae infested by parasitic Isopoda (Epicaridea) Bulletin of the Mizunami Fossil Museum. 2008;34:31–49. [Google Scholar]
- Witbaard R, Duineveld GCA. Some aspects of the biology and ecology of the burrowing shrimp Callianassa subterranea (Montagu) (Thalassinidea) from the southern North Sea. Sarsia. 1989;74:209–219. [Google Scholar]
- Withers TH. Some decapod crustaceans (Callianassaand Ranina) from the Oligocene of Washington State, U.S.A. (series 9).Annals and Magazine of Natural History. 1924;14:121–127. [Google Scholar]
- Wood-Mason J. Blind Crustacea. Proceedings of the Asiatic Society of Bengal. 1874;1874:180–181. [Google Scholar]
- Woods H. Crustacea from the Eocene deposits of Peru. In: Bosworth TO, editor. Geology of the Tertiary and Quaternary Periods in the Northwest Part of Peru. MacMillan and Co.; London: 1922. pp. 114–118. [Google Scholar]
- Ziebis W, Forster S, Huettel M, Jørgensen BB. Complex burrows of the mud shrimp Callianassa truncata and their geochemical impact in the sea bed. Nature. 1996;382:619–622. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.