To The Editor
Familial hypocalciuric hypercalcemia is a genetically heterogeneous disorder with three variants: types 1, 2, and 3. Type 1 is caused by calcium-sensing receptor (CASR) mutations,1 and type 2 is caused by guanine nucleotide–binding protein (G-protein) subunit α11 (GNA11) mutations.2 Type 3, which is the most severe variant clinically, is caused by adaptor-related protein complex 2 (AP2), sigma 1 subunit (AP2S1) heterozygous mutations. AP2S1 mutations cause Arg15Cys, Arg15His, and Arg15Leu substitutions, and the mutant AP2-sigma proteins result in impaired calcium-sensing receptor–mediated signal transduction.3,4
There is currently no effective therapy for familial hypocalciuric hypercalcemia type 3. We therefore evaluated the usefulness of cinacalcet, a licensed calcium-sensing receptor allosteric activator,5 in correcting signaling defects due to AP2S1 mutations and in ameliorating symptomatic hypercalcemia.
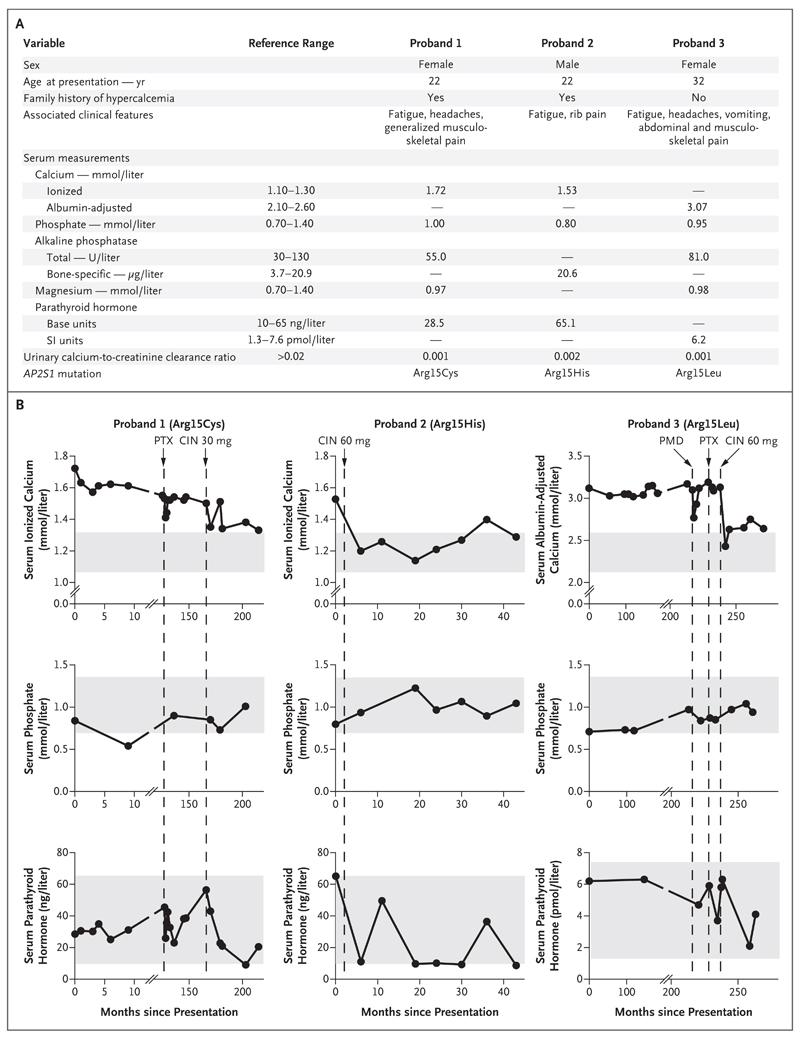
We evaluated three previously unreported cases of familial hypocalciuric hypercalcemia type 3. Each of the three probands had an AP2S1 mutation causing an Arg15Cys, Arg15His, or Arg15Leu substitution (Fig. 1A). The probands included a 34-year-old woman who had a 12-year history of hypercalcemia. She presented with fatigue, headaches, and persistent generalized aches that did not resolve after parathyroidectomy. In addition, a 22-year-old man presented with hypercalcemia, fatigue, and generalized rib pain, and a 52-year-old woman who had an approximately 20-year history of hypercalcemia presented with headaches, abdominal pain, vomiting, fatigue, and musculoskeletal pain that did not resolve after pamidronate infusion or parathyroidectomy (Fig. 1A and 1B).
Figure 1. Cinacalcet for Hypercalcemia Associated with AP2S1 Mutations.
Panel A shows the clinical and biochemical findings at presentation in three unrelated probands with familial hypocalciuric hypercalcemia who had heterozygous adaptor-related protein complex 2 (AP2), sigma 1 subunit (AP2S1) mutations resulting in Arg15Cys, Arg15His, or Arg15Leu substitutions in the AP2-sigma mutant proteins. Reference ranges are from Nesbit et al.2 Panel B shows the effect of cinacalcet on serum concentrations of calcium, phosphate, and parathyroid hormone in the three probands. Vertical dashed lines indicate initiation of cinacalcet (CIN) therapy, pamidronate (PMD) infusion, or parathyroidectomy (PTX). Shaded gray areas indicate reference ranges.
The in vitro effects of cinacalcet on the signaling responses of cells expressing the calciumsensing receptor and familial hypocalciuric hypercalcemia type 3–associated AP2-sigma mutants were assessed by measurement of intracellular calcium concentrations and of the activity of the mitogen-activated protein kinase (MAPK)–serum response element reporter in response to alterations in extracellular calcium (see the Methods section in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Cinacalcet (at a concentration of 10 nM) corrected the abnormal intracellular calcium signaling and MAPK responses to extracellular calcium in cells expressing the Cys15, His15, or Leu15 AP2-sigma mutants (Fig. S1 and S2 in the Supplementary Appendix).
Oral cinacalcet (at a dose of 30 to 60 mg daily) was administered to the three probands with familial hypocalciuric hypercalcemia type 3 over a 33-to-45-month period. This treatment led to more than 20% reductions in serum calcium concentrations (Fig. 1B) and abatement of symptoms. Cinacalcet also increased serum phosphate concentrations and reduced serum parathyroid hormone concentrations, although these values remained within the normal range (Fig. 1B).
Adverse effects such as nausea, vomiting, and hypocalcemia did not develop in any of the probands. However, long-term surveillance will be required to assess safety and monitor for hypocalcemia.
Our results show that cinacalcet-mediated allosteric modulation of the calcium-sensing receptor can correct the loss of function of AP2S1 mutations. In addition, in the short term, cinacalcet can reduce the symptoms of familial hypocalciuric hypercalcemia type 3 associated with all three AP2S1 mutations.
Supplementary Material
Acknowledgments
Supported by program grants (G9825289 and G1000467, to Drs. Hannan, Gorvin, Nesbit, and Thakker) from the United Kingdom Medical Research Council, a grant (to Drs. Nesbit and Thakker) from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme, a grant (FP7-264663, to Dr. Babinsky) from the European Commission Seventh Framework Program, Wellcome Trust clinical training fellowships (to Drs. Howles and Rogers), a Wellcome Trust Investigator Award (to Dr. Thakker), and an NIHR Senior Investigator Award (to Dr. Thakker).
Footnotes
References
- 1.Hannan FM, Nesbit MA, Zhang C, et al. Identification of 70 calcium-sensing receptor mutations in hyper- and hypo-calcaemic patients: evidence for clustering of extracellular domain mutations at calcium-binding sites. Hum Mol Genet. 2012;21:2768–78. doi: 10.1093/hmg/dds105. [DOI] [PubMed] [Google Scholar]
- 2.Nesbit MA, Hannan FM, Howles SA, et al. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368:2476–86. doi: 10.1056/NEJMoa1300253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesbit MA, Hannan FM, Howles SA, et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat Genet. 2013;45:93–7. doi: 10.1038/ng.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannan FM, Howles SA, Rogers A, et al. Adaptor protein-2 sigma subunit mutations causing familial hypocalciuric hypercalcaemia type 3 (FHH3) demonstrate genotype-phenotype correlations, codon bias and dominant-negative effects. Hum Mol Genet. 2015;24:5079–92. doi: 10.1093/hmg/ddv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeth EF, Shoback D. Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract Res Clin Endocrinol Metab. 2013;27:373–84. doi: 10.1016/j.beem.2013.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.