Abstract
Heart failure (HF) continues to be a highly prevalent syndrome, affecting millions of patients and costing billions of dollars in treatment per year in the United States alone. Studies in failing human heart and in transgenic HF models led to the recognition that enhanced neurohormonal signaling plays a causative role in HF progression, and the use of neurohormone receptor antagonists have proven to decrease hospitalization rates. It has also been long recognized that patients with HF have abnormal water retention, hypo-osmolality, and hyponatremia secondary to elevations in the levels of the neurohormone arginine vasopressin (AVP). AVP is released from the hypothalamus in response to changes in plasma osmolality and pressure, acting at three distinct G protein-coupled receptors: V1AR, V1BR and V2R. Persistent AVP release causes hyponatremia via renal V2R activation, a risk factor for death and hospitalization, and there is a correlation between plasma AVP levels and HF severity/survival of chronic HF patients. Because of the adverse clinical consequences associated with the development of hyponatremia, V2R antagonists were developed for the treatment of HF patients with hyponatremia, however in contrast to other neurohormone blockers they do not relay a survival benefit and may exacerbate decompensated HF requiring inotropic support. Renewed interest in the cardiac V1AR system during HF has arisen due to several recent findings: 1) mice with myocyte-selective transgenic overexpression of cardiac V1AR developed cardiomyopathy in the absence of any pathological insult, 2) cardiac V1AR expression was shown to be increased late-stage human HF, and 3) V1AR antagonism prevented cardiomyopathy development in a mouse model of HF. While cardiac V1AR expression is increased in HF, the role of V1AR signaling in various forms of cardiac injury and in distinct cardiac cell types has been controversial. Therefore this review will primarily focus on V1AR signaling as a potential therapeutic target for HF treatment.
1. HF Overview
Heart failure (HF) is a prevalent disease affecting over 5.1 million people in the United States and over 23 million people worldwide [1, 2]. Furthermore, cardiovascular disease causes more than 17.3 million deaths each year, making it the leading global cause of death [3]. The economic impact of cardiovascular disease is large, with the direct and indirect costs of cardiovascular disease in the United States alone totaling approximately $320.1 billion a year [3].
HF is the inability of the heart to pump sufficient outflow to meet the oxygen demands of the body and may be due to diastolic or systolic dysfunction. Cardiac output and systemic blood pressure are decreased, leading to the activation of neurohormonal systems such as the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system [4–6]. Chronic activation of these neurohormonal systems negatively impact the heart, independently of hemodynamic parameters [7]. Current treatment guidelines recommend using drugs that block β-adrenergic receptor (βAR) signaling (e.g. β-blockers) [8–10] or RAAS signaling (e.g. angiotensin receptor blockers or angiotensin converting enzyme inhibitors) [11–16]. In fact, clinical studies have shown that using drugs that block these neurohormones decreases hospitalizations, morbidity, and mortality in these HF patients [12–14, 17–19]; and these are the only classes of pharmacological therapies that have been shown to reduce patient mortality [20, 21].
Interestingly, the neurohormone arginine vasopressin (AVP), has been implicated in HF progression, and it may therefore be a potential target to reduce morbidity and mortality in HF patients. AVP is elevated in patients with heart failure and/or left ventricular (LV) dysfunction [22–25], and increases in AVP levels are associated with increasing severity of heart failure [26]. Additionally, persistent or inappropriate AVP in plasma impairs free water excretion and causes hyponatremia. Hyponatremia is often found in patients with HF [27, 28] and is associated with adverse prognosis and increased mortality of these patients [22, 29, 30]. Mechanisms contributing to hyponatremia in HF patients include enhanced neurohormone signaling leading to activation of the sympathetic nervous and RAAS, as well as diuretic use.
2. Physiologic and cellular consequences of AVP receptor signaling
2.1) AVP synthesis and receptor tissue distribution
AVP, or antidiuretic hormone (ADH), is a peptide hormone traditionally known for its role in the hypothalamic-pituitary-adrenal (HPA) axis. The AVP precursor peptide, pre-pro-AVP, is synthesized in magnocellular neurosecretory neurons in the paraventricular and supraoptic nuclei of the hypothalamus. Pre-pro-AVP is cleaved within the neurons into AVP, neurophysin II, and copeptin. Together, copeptin and AVP are released into the portal vessels, where they are transported to and stored in the posterior pituitary gland. AVP and copeptin are released in response to increases in plasma osmolarity or decreases in plasma volume in order to regulate body water content and excretion and blood pressure [31, 32]. Although AVP was originally believed to be solely synthesized in and released from the neurohypophysial system, studies on isolated rat hearts revealed that AVP mRNA and peptide synthesis was induced locally in hearts that were perfused to model pressure-overload [33]. These studies identified for the first time that AVP can be synthesized in the heart to act locally in a cardiac paracrine AVP system prior to release into effluent vessels, where it is predicted to act systemically.
Once released into circulation, AVP acts on three distinct G-protein coupled receptors (GPCRs); V1AR, V1BR, and V2R. The V1AR are located in the heart, vascular smooth muscle cells, kidney, myometrium, central nervous system and liver, whereas V1BR is expressed predominantly in the pituitary. While the V2R is expressed substantially in the cardiovascular and renal systems, primarily located on vascular endothelial cells and collecting ducts of renal tubules, several studies have shown that V2R expression is not detectable in the heart or isolated cardiomyocytes [34–37]. V1AR has been shown to be involved in many cellular processes including vascular contraction as well as in the metabolism of glucose, lipids, and protein [38]. Additionally, studies using V1AR knockout mice and isolated cells showed that the V1AR regulates glucose homeostasis [39], mediates the release of aldosterone from adrenal gland cells [38, 40], controls social interactions [41], and platelet aggregation [42, 43]. V1BR mediates the release of adrenocorticotropic hormone and insulin from the pituitary gland and islet cells of the pancreas, respectively [38, 44]. V1BR knockout mice also show altered physiological behavior, including the ACTH response to stressors [45]. V2R is predominantly expressed on the collecting ducts of renal tubules, where AVP-stimulation leads to the formation and insertion of aquaporin (AQP) channels on the apical surface of collecting duct cells and allowing an increase in water permeability and water retention [46]. Interestingly, a recent study has shown that V1AR knockout mice have decreased expression of the AQP2 channels on collecting ducts [47]. V1AR in the kidney also regulates renal blow flow, and is expressed primarily in the vasculature and smooth muscle cells [48, 49]. Since the remainder of this review will focus on the impact of AVP-mediated signaling via V1AR and V2R in the cardiovascular system (Fig. 1), we will focus on these receptor classes in the subsequent sections.
Figure 1. Contributions of AVP signaling to heart failure (HF).
AVP stimulates vasopressin type 2 receptors (V2R) on renal tubules, leading to the formation and insertion of aquaporin channels on the apical surface of collecting duct cells, which increases and water retention and may cause or exacerbate hyponatremia. Stimulation of vasopressin type 1A receptors (V1AR) on vascular smooth muscle cells (VSMC) leads to vasoconstriction and increases afterload on the heart. Additionally, V1AR in the central nervous system (CNS) alter the baroreceptor response. Signaling via cardiac V1AR on cardiomyocytes (CM) and cardiac fibroblasts (CF) leads to increased cardiac hypertrophy and dilation and fibrosis, respectively.
2.2) V1AR signaling
Ligand binding to the V1AR induces Gq protein activation through the exchange of GDP for GTP. The activated Gq protein induces phospholipase C (PLC) activation, which triggers a downstream signaling cascade leading to the formation of inositol triphosphate, diacylglycerol and the activation of protein kinase C (PKC). In addition to Gq protein-dependent signaling, V1AR and other GPCRs can also signal via G protein-independent, G protein-coupled kinase (GRK)-dependent mechanisms [50]. Ligand binding results in GRK-mediated phosphorylation of the C-terminal of the GPCR. Then, β-arrestins bind to the phosphorylated C-terminal to facilitate internalization and desensitization of the receptor, and they can also act as scaffolding proteins for additional signaling pathways. V1AR internalization is dependent on β-arrestins, and studies suggest internalization is mediated by β-arrestin 2, but not β-arrestin 1 [51, 52].
The V1AR is transiently phosphosphorylated in the continued presence of agonists. In response to AVP, V1AR are rapidly phosphorylated and dephosphorylated, with a half-life of about 6 minutes [53]. Phosphorylation of the C-terminal of V1AR can be mediated by either PKC or GRKs. The C-terminus of V1AR contains a proximal GRK consensus motif upstream of phosphorylatable serine residues and three distal PKC consensus motifs [52, 54].
Activated PKC can phosphorylate V1AR in the absence of agonists, while GRK-mediated phosphorylation is constitutive and agonist-dependent [55]. Loss of function studies using a dominant negative GRK2 mutant showed reduction in ligand-induced phosphorylation, confirming role of GRKs in mediating PKC-independent phosphorylation [53]. In these studies, phosphorylation of the V1AR in the presence of the GRK2 mutant decreased by about 26%, suggesting that GRK-mediated phosphorylation is not solely due to GRK2 activity. In agreement with this hypothesis, immunoprecipitation studies in CHO cells overexpressing GFP-tagged V1AR, showed that GRK5 can associate with V1AR after stimulation with AVP [54]. Since many groups have shown differential signaling outcomes imparted via distinct GRKs acting at various GPCRs, including V1AR [50], further insight into AVP-mediated regulation of G protein-independent signaling and its impact on cellular processes is greatly needed.
2.3) V2R signaling
The V2R is a Gs-coupled GPCR, wherein AVP binding induces adenylate cyclase-mediated production of cAMP and activation of protein kinase A (PKA) [56], and can also lead to increased calcium mobilization [57]. The V2R also undergoes ligand-induced sequestration and desensitization, and although the last 14 amino acids of V2R are required for phoshophorylation of the AVP-bound V2R [58], Innamorati et. al. showed that desensitization and sequestration of V2R do not require receptor phosphorylation [58]. Unlike the rapid return of AVP-stimulated V1AR to the cell surface following removal of AVP from culture media [53], V2R remain at the cell surface 6h after AVP removal [59]. The recycling of V2R to the cell membrane is mediated by GRK-dependent phosphorylation of a cluster of serine residues (S362–S364) in the C-terminal of V2R [59]. Unlike β-arrestin-mediated desensitization for other GPCRs, β-arrestin 2 binding to V2R prolongs cAMP generation which is associated with V2R internalization to in endosomes, and receptor signaling is turned off by endosomal retromer complexes [60]. Additionally, three serine residues on the V2R C-terminal responsible for the formation of V2R/β-arrestin complexes and internalization to endosomes [61]. Physiologically, V2R stimulation on renal tubules leads to the formation and insertion of aquaporin channels on the apical surface of collecting duct cells and allowing an increase in water permeability and water retention. Additionally, persistent or inappropriate AVP in plasma causes decreased cardiac output and systemic blood pressure during HF, leading to increased water retention and hyponatremia [5], which is associated with adverse prognosis and increased mortality of HF patients [29, 30].
3. Rationale for and consequences of AVP receptor blocker usage in HF
Due to the adverse clinical consequences associated with the development of hyponatremia, especially during HF, V2R antagonists were developed for its treatment. Conivaptan (a V1AR/V2R mixed antagonist) and tolvaptan (a V2R-selective antagonist) are both FDA-approved for the treatment of HF with hyponatremia, and while they have shown favorable short-term outcome in terms of corrected hyponatremia, neither have been shown to improve survival or decrease hospitalizations [62]. Further, another V2R-selective antagonist, lixivaptan, was in phase III assessment for treatment of hyponatremic HF patients, but was shown in the Treatment of Hyponatremia Based on Lixivaptan in NYHA Class III/IV Cardiac Patient Evaluation (BALANCE) study to cause an early increase in patient mortality due to worsening HF, which led the Cardiovascular and Renal Advisory Panel of the FDA to vote against its use for HF treatment [63].
The phase II ACTIV in CHF (Acute and Chronic Therapeutic Impact of Vasopressin Antagonist in Congestive Heart Failure) trial showed that when added to standard of care therapy, tolvaptan improved net volume loss and increased serum sodium in patients hospitalized with CHF [64, 65]. However, tolvaptan-treated patients did not show decreased mortality compared to the placebo group. The phase III EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan) trial was a larger, multi-center trial that evaluated the long-term effects of tolvaptan. Although tolvaptan was shown to be safe in these patients, it did not alter LV remodeling, heart rate, or blood pressure, or decrease long-term mortality and HF-related morbidity [66–68]. However, improvements in body weight, or fluid retention, were detected [69], suggesting that although the acute effects of tolvaptan use seemed favorable, the short-term endpoint of body weight/fluid retention does not accurately reflect improved long-term mortality [70].
Interestingly, Lanfear et. al. noted that elevated baseline AVP levels were predictive of all-cause and cardiovascular mortality, independent of the tolvaptan response [26]. This observation is in agreement with additional studies showing that elevated AVP and/or copeptin increased risk of all-cause mortality in HF patients [26, 71–75]. It should be noted that exogenous administration of AVP to patients with HF leads to dose-dependent increases in systemic vascular resistance and pulmonary capillary wedge pressure (PCWP), and decreased stroke volume and cardiac output [76]. This hemodynamic deterioration in response to AVP and greater prognostic value of AVP compared to tolvaptan use suggests that increased AVP may contribute to HF deterioration. Additionally, studies in HF patients with hypervolemic hyponatremia revealed that V2R antagonists, including tolvaptan, lead to an increase in circulating AVP levels, potentially exacerbating its effects on other V2R-independent systems [26, 77, 78]. Morooka et. al. evaluated the long-term effects of tolvaptan on myocardial and kidney function in a hypertensive rat model for HF and found that circulating and myocardial levels of AVP, as well as myocardial levels of the V1AR and V1BR, were increased during the development of HF [79], while rats treated with tolvaptan had significantly suppressed levels of AVP and V1AR mRNA in LV tissue. Although this finding opposes the EVEREST findings that showed increased AVP levels following tolvaptan administration, the Dahl salt sensitive HF model used by Morooka et. al. assesses volume and pressure overload without hyponatremia, while the EVEREST trial focused on hyponatremic HF patients. This difference emphasizes the importance of conducting more studies to better understand not only how V2R antagonists alter plasma AVP levels, but also how a patient’s volume/natremic state impacts these changes, especially since plasma volume and osmolarity regulate AVP release from the pituitary.
Since tolvaptan use in hyponatremic patients enhances AVP levels, and V1AR expression is increased 2-fold in patients with chronic HF compared to non-failing hearts [80], AVP elevation in combination with increased V1AR expression could lead to modified V1AR-mediated effects in the heart, as discussed further below. Interestingly, V1AR-deficient mice have significantly higher plasma concentrations of AVP than wild-type control mice during dehydration [47], thus it is possible that decreased expression or activity of one class of AVP receptor could cause a reciprocal increase in AVP stimulation of other classes of AVP receptors. However, there is currently no definitive proof that such a regulatory relationship exists between different AVP receptors. In light of this possibility, a combination of V1AR/V2R antagonists could offer increased benefit in the treatment of HF. Interestingly, Ikeda et. al. showed that myocardial V1AR and renal V1AR and V2R were significantly increased during the HF transition in a hypertensive rat model, and the combination of tolvaptan and a V1AR antagonist (OPC-21268) increased the median survival time of the rats more than either antagonist alone [81]. Furthermore, early clinical pilot studies using conivaptan are promising. Conivaptan increases urine output and decreases PCWP and right atrial pressure in HF [77, 82, 83], but no long-term clinical trials evaluating the effects of conivaptan of HF have been completed. Further, there have been no clinical trials testing selective V1AR antagonists in the context of HF, but based on the increased cardiac expression of V1AR and circulating levels of AVP in HF, further exploration of the role of V1AR in HF progression is warranted.
4. Exploration of V1AR effects on cardiac function and survival in HF
4.1) V1AR knockout (KO) mice
Studies using V1AR-KO mice have shown that the absence of V1AR causes hypotension and decreased plasma volume in mice [32, 84], despite plasma AVP levels being significantly higher than wild-type controls [47]. In addition, the V1AR-KO mice had a decreased pressor response to AVP, impaired baroreceptor reflexes, decreased sympathetic nerve activity, decreased levels of renin in plasma and in granule cells of the macula densa [85] accompanied by decreases in RAAS activity and decreased plasma aldosterone levels [32, 39, 84], all of which may contribute to the hypotensive phenotype observed in these mice. Additionally, V1AR are involved in the development of salt-induced hypertension, as V1AR-KO mice experience smaller increases in sympathetic activation and systolic blood pressure over time compared to wild-type mice in response to dietary salt loading [86].
4.2) V1AR contribution to chronic HF
Recent studies have shown that V1AR expression contributes to the development of HF. Stimulation of V1AR on vascular smooth muscle cells causes increased vascular resistance, increasing afterload. The increase in afterload and direct stimulation of V1AR on cardiomyocytes can lead to cardiac remodeling [87]. The effects of V1AR signaling on cardiomyocyte hypertrophy and cardiac fibrosis will be discussed in more detail in the cell-type specific sections below.
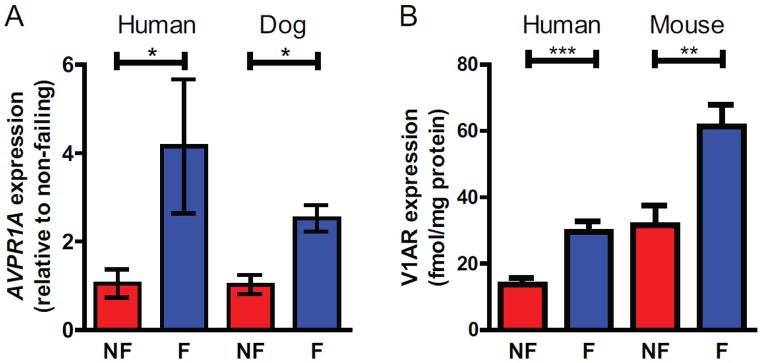
Cardiac V1AR expression is similarly increased in late stage human HF [80], the cardiac tachypacing dog model of HF as well as in the transverse aortic constriction (TAC) mouse model of chronic HF, highlighting a conserved enhancement of V1AR signaling in the heart across small and large animal HF models and human HF (Fig. 2). In the absence of any other pathological insult, mice with cardiac-specific overexpression of V1AR developed cardiac hypertrophy over several months eventually leading to dilation and left ventricular dysfunction, suggesting an essential role for V1AR in the development of chronic HF [88]. Furthermore, the V1AR-mediated cardiomyopathy was shown to be Gq protein-dependent, involving the activation of Akt and ERK1/2, as crossing the V1AR transgenic mice with mice expressing an inhibitory peptide against Gq protein completely ablated the HF phenotype [88]. Fittingly, V1AR-KO mice subjected to TAC had lower heart weight to body weight ratios compared to WT mice, thereby also demonstrating that AVP signaling through V1AR plays a role in the development of cardiac hypertrophy in response to pressure overload [89]. Furthermore, administration of the selective V1AR antagonist SR49059 to wild-type mice undergoing TAC not only preserved cardiac function compared to vehicle controlled-mice, but also blocked the HF-associated increase in V1AR expression and decrease in βAR density [90]. Conversely, in the tachypacing pig model of HF, V1AR antagonism was shown to improve LV loading but had limited effect on preservation of LV and isolated myocyte shortening, however V1AR expression was not measured, V1AR blockade was incomplete and V1AR blockade was not directly tested in the isolated cardiomyocyte contractility assays [91]. Thus, from a translational standpoint, assessing the impact of V1AR blockade in HF would greatly benefit from further exploration in large animal models.
Figure 2. Increased V1AR expression in human HF as well as large and small animal models of HF.
A) Realtime PCR reveals a significant increase in AVPR1A expression in failing (F) versus non-failing (NF) human and dog left ventricular samples, normalized to RPS18 and presented as RQ with RQmin and RQmax as error bars. Human heart tissue was obtained with institutional review board approval and patient consent as described in [80]. Failing dog heart samples were generated by pacing with IACUC approved as described in [148]. B) Saturation radioligand binding of 125I-AVP in membrane preparations of non-failing (NF) and failing (F) human heart and mouse left ventricular samples as previously described in [80 and 90]. Failing mouse heart samples were generated via transaortic constriction (TAC)-induced pressure overload with IACUC approval as described in [90]. *P<0.05, **P<0.01, ***P<0.001 unpaired t-test with Welch’s correction for unequal variance. Adapted from [80] with permission from Elsevier.
As with HF patients, rat models of chronic HF and LV dysfunction show increased plasma levels of AVP [92, 93]. Additionally, AVP was increased in regions of the brain stem known to be involved in regulating cardiovascular and circulatory function [93], suggesting that the central activity of AVP may be a potential target for HF or hypertension (HTN). Since HTN is the most prevalent and preventable risk factor for HF development and HTN contributions to a large proportion of HF cases [1, 94, 95], a better understanding the role of V1AR in HTN may provide insights about the management of HTN-induced HF. Recent studies suggest that V1AR are also involved in the development of genetic hypertension. Selective V1AR antagonists attenuated blood pressure increases in spontaneously hypertensive rats (SHR) when given short-term during HTN development [96]. However, chronic V1AR antagonism did not reduce blood pressure, suggesting that the role of AVP during HTN development is not due to its pressor effects [97].
4.3) V1AR contribution to acute HF
Although V1AR signaling during chronic HF appears to be largely detrimental, the effects of V1AR on acute cardiac injury are less clear. Some studies have shown that V1AR are functionally and numerically downregulated in rat cardiomyocytes four weeks after MI, and it was suggested that V1AR blockade after MI may not be beneficial [34]. However, this study evaluated the chronic use of orally administered V1AR antagonists starting one day after MI surgery, though significant increases in AVP following MI occurs within 6 hours of MI [98] and decrease to baseline levels within 24 hours of initial presentation [18]. Conversely, it was shown that cardiac V1AR mRNA was increased 14 weeks after MI, again suggesting a role for V1AR signaling in the long-term post-injury remodeling phase [99]. Interestingly, exogenous administration of AVP to rats provides cardioprotection against reperfusion injury [100], presumably via preconditioning, while V1AR inhibition reduced these effects. V1AR antagonism alone did not decrease infarct size, suggesting that V1AR preconditioning only occurred when “superphysiological” levels of AVP are present. Therefore, the acute impact versus chronic outcome of AVP signaling following cardiac injury may vary temporally and V1AR-directed therapeutics for myocardial ischemia may need to be considered within this context.
Following MI, changes in V1AR expression in non-cardiac tissue may also contribute to HF. Significantly lower expression of V1AR mRNA and protein in were detected in the cerebellum of rats after MI compared to control rats and rats after chronic stressing [101]. The cerebellum is involved with autonomic regulation in the cardiovascular system, including the baroreceptor reflex. Interestingly, V1AR knockout mice show decreased baroreceptor reflexes [102], which may partially be due to the lack of V1AR in the cerebellum. Although there was no difference between the cerebellar V1AR expression of rats undergoing chronic stressing after MI compared to MI alone, responses to stress following MI can be altered by cerebral and cerebellar V1AR signaling [103, 104]. Furthermore, V1AR mRNA and protein were significantly lower in the mesencephalopontine region of infarcted rats compared to control rats [105]. Along with the cardiac and central effects, V1AR also alter blood pressure following MI via coronary artery constriction [106]. Additionally, V1AR expression in the renal medulla is increased after MI [105], and has been shown to contribute to hypertension [107]. However, others have suggested that changes in vasoconstriction and fluid retention after chronic MI may be the result of a shift in vascular receptor expression from V1AR to V2R [108, 109].
4.4) AVP tested as a potential therapeutic in animal models of cardiovascular disease
Targeting of the V1AR, alone and in conjunction with other GPCR including α1AR, has been explored in human and large animal models of cardiac arrest and shock, wherein AVP with or without epinephrine (Epi) has been administered to assess the outcome on cardiac function and survival in these contexts. The concurrent use of AVP and Epi during resuscitation has been studied in both humans and large animal models of cardiac arrest over the last several years. However, studies in human cardiac arrest have failed to demonstrate a consistent benefit of AVP. In a recent study, combined AVP+Epi and methylprednisolone during CPR and stress-dose hydrocortisone in post-resuscitation shock, compared with Epi/saline placebo, resulted in improved survival to hospital discharge with favorable neurological status [110]. However, this study enrolled only 130 patients in each treatment group and it was impossible to ascertain whether the benefits seen in the comparator group was due to AVP or methylprednisolone and hydrocortisone. The definitive study in humans compared AVP+Epi versus Epi alone in approximately 4000 patients and failed to show a benefit of AVP in out-of-hospital arrests [111]. In a study of patients with cardiac arrest presenting to or in the emergency department, the combination of AVP+Epi also did not improve long term survival, although there was a trend towards improved survival in patients with prolonged cardiac arrest – however only eight patients in the Epi group and eleven patients in the AVP group survived to discharge [112]. Furthermore, meta-analysis of studies that included nearly 4500 patients found no overall benefit or harm of AVP use in the resuscitation of cardiac arrest patients [113]. In aggregate, the studies assessing the efficacy of AVP in cardiogenic shock have failed to show benefit – leading to practice guidelines not recommending its use in shock.
A recent study demonstrated that in pigs with shock secondary to electrically induced ventricular fibrillation, the treatment of AVP+Epi increased the likelihood of successful defibrillation [114]. However, this large animal model lacked additional study arms with which to compare this effect (i.e. AVP given alone or prior to Epi infusion, or Epi given again instead of AVP), thus the comparative impact of Epi+AVP versus Epi in this context is unclear. A study which induced cardiac arrest by clamping the endotracheal tube found that while the administration of Epi or Epi+AVP resulted in similar restoration of spontaneous circulation and survival rates, there were improved neurological and cerebral histopathological outcomes [115]. However, these findings were not seen consistently. In a study of bupivacaine-induced cardiac arrest, first-line rescue with Epi and Epi+intralipid was more effective with regard to survival than intralipid alone or AVP+intralipid [116].
Following earlier studies of cardiac arrest in pigs it was proposed that AVP enhances myocardial blood flow via strong peripheral vasoconstriction [117], but these studies showed varying success of AVP in increasing coronary blood flow alone or in conjunction with Epi, depending on the intervention (asphyxiation versus ventricular fibrillation-induced cardiac arrest). When cardiac arrest was induced by asphyxiation in a pediatric porcine model, return of spontaneous circulation was significantly more likely in Epi-treated pigs than in animals resuscitated with AVP alone [118], though in an adult model of asphyxiation the combination of AVP+Epi was better than either agent alone at restoring myocardial blood flow [119], while in a ventricular fibrillation-induced pediatric pig model of cardiac arrest, AVP+Epi was shown to increase coronary blood flow better than either AVP or Epi alone [120]. The AVP analog terlipressin had no statistically significant effect on outcomes in an infant pig model of cardiac failure when used alone or with Epi [121].
In addition, others have demonstrated that AVP failed to have a positive effect on outcomes in a murine model of ischemia-reperfusion injury. In fact, AVP infusion significantly increased myocardial dysfunction and mortality in comparison with both saline and dobutamine [122]. Thus, the potential protective versus detrimental roles of AVP may depend on the type of cardiovascular injury and requirement for systemic vasoconstriction to enhance myocardial blood flow.
5. Cardiac cell type-dependent V1AR signaling
5.1) V1AR in cardiomyocytes
A majority of studies focusing on the impact of AVP signaling in myocytes have been performed in neonatal cardiomyocytes and H9c2 rat myoblasts, which each express high levels of V1AR in the absence of V1BR or V2R [89, 123]. (The major roles of AVP signaling in myocytes are summarized in Fig. 3) AVP signaling via V1AR promotes hypertrophy in both cell types [89, 124] via enhanced protein synthesis [35, 89, 125, 126] and cell surface area [89], with no impact on cell proliferation [125]. V1AR-mediated protein synthesis occurs in a PLC- and PKC-dependent manner involving elevated Ca2+ responses and c-fos expression [35, 89, 125], and it has been shown that AVP strongly regulates the GATA-4 transcription factor in hypertrophied cardiomyocytes [127]. Interestingly, V1AR regulation of GATA-4 may also aid in ventricular precursor differentiation and maturation as V1AR mediates GATA-4 during D3 embryonic stem cell differentiation via the NOS pathway [128]. Studies examining the expression of vasopressin receptors on rat hearts have shown that although V2R expression is barely detectable in adult rat hearts, its expression increases until post-natal day 5 and then declines [129]. The V2R expression and presence of AVP in maturing hearts suggests that V2R may play a role in cardiac development and maturation; indeed, while V1AR is mainly responsible for ventricular differentiation of embryonic stem cells, V2R were shown to modulate atrial precursor cell differentiation [128].
Figure 3. AVP signaling in cardiomyocytes (CM).
AVP stimulation of V1AR on CM leads to enhanced protein synthesis and cellular hypertrophy in phospholipase C (PLC)-protein kinase C (PKC)-dependent increases in calcium and c-fos. AVP-induced hypertrophy also increases atrial myosin light chain-1 (ALC-1) activation via a Ca2+-calmodulin-calcineurin-NFAT pathway. G protein-coupled receptor kinase (GRK)-dependent V1AR signaling decreases beta-adrenergic receptor (βAR) responsiveness and increases cell survival during hypoxia. V1AR signaling also increases ventricular differentiation and maturation via GATA-4 regulation. AVP also alters cellular metabolism, increasing glycolysis and decreasing fatty acid oxidation.
PKC-dependent V1AR signaling modulates many signaling pathways including regulation of IL-1β-induced iNOS expression [130] and potentiation of L-type Ca2+ channel currents [37]. Moreover, AVP-mediated increases in protein synthesis are partially regulated by the rapid release of sequestered SR Ca2+ [131]. While the effects of AVP on protein synthesis are additive with those of PDGF, the chronic depletion of Ca2+ caused by AVP decrease mitogenic effects of PDGF in favor of the hypertrophic effects [131]. In addition to mediating cardiomyocyte hypertrophy, V1AR signaling also alters cellular metabolism during hypertrophy. AVP acutely increases glycolysis in a Ca2+-dependent, AMPK independent manner in hypertrophied H9c2 cells and simultaneously decreased fatty acid oxidation [132]. Furthermore, AVP-induced hypertrophy also increases atrial myosin light chain-1 activation via a Ca2+-calmodulin-calcineurin-NFAT pathway, which may improve cardiac contractility [133].
The potential for AVP signaling to regulate contractility is also supported by the V1AR-dependent increases in free cytosolic Ca2+ observed in rat neonatal cardiomyocytes [36]. However, the ability of V1AR to mobilize Ca2+ in cardiomyocytes has been shown to be variable, ranging from a small increase (~10-fold less than Ang II) to no response or even a small decrease depending on the developmental stage (adult, neonatal) and organism (rat, mouse, guinea pig, chick) [34–37, 134, 135]. Our group has observed no response up to 1μM AVP in adult mouse cardiomyocytes [90], and it was shown that adult mouse cardiomyocytes with V1AR overexpression had diminished Ca2+ transients [88]. Interestingly, we showed that although V1AR stimulation alone did not impact cardiomyocyte Ca2+ transients and slightly reduced cardiac contractility, pretreatment with AVP prior to catecholamine stimulation greatly reduced βAR responsiveness [90]. Thus, the V1AR may play a more important role in fine-tuning cardiomyocyte contractility in response to sympathetic drive. Interestingly, the ability of V1AR to modulate βAR responsiveness was shown to be mediated in a Gq protein-independent, but GRK-dependent manner. While the particular GRK isoform responsible for this effect has not been established, we also showed in H9c2 cells that GRK2-dependent V1AR signaling enhanced ERK1/2 activity and promoted survival under hypoxic conditions in vitro [50]. Although the role this pathway could play in vivo has not been determined, it highlights the potential dichotomy between V1AR signaling pathways; even if using the same initial effectors such as GRKs, the physiologic results at the cardiomyocyte level could be divergent with the simultaneous promotion of survival and inhibition of contractility, both essential to maintain under stress conditions such as HF. Further exploration of V1AR effects on cardiomyocyte signaling and function, alone or in conjunction with other neurohormone systems, is required to address this issue.
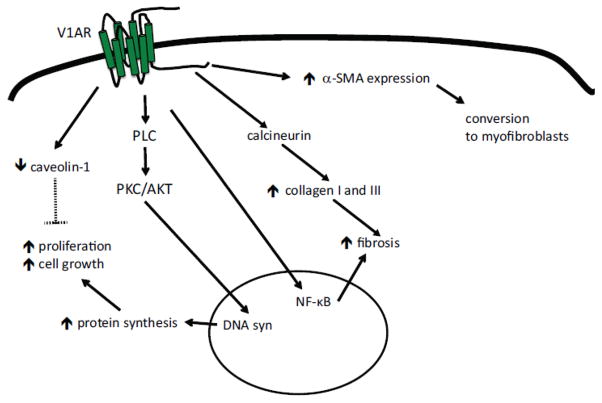
5.2) V1AR in Fibroblasts
AVP-induced DNA synthesis in cardiac fibroblasts promotes both cell growth and proliferation in a PKC- and AKT-dependent manner [136]. Interestingly, the HMG-CoA reductase inhibitor simvastatin inhibits AVP-induced PKC, ERK1/2, and AKT activation and prevents AVP-induced cell growth in fibroblasts and other cell types [136, 137]. Studies on cultured 3T3 fibroblasts support that activation of the V1AR promotes cell growth [125], and AVP promotes DNA and RNA synthesis in and the proliferation of fibroblasts [138–140]. Additionally, AVP reduces caveolin-1 expression, which aids in the regulation of cell proliferation [141].
Increased fibroblast proliferation is accompanied by increased collagen I and III synthesis [142], suggesting that AVP can alter myocardial fibrosis, and the AVP-dependent fibroblast growth and collagen synthesis can be inhibited by blocking AVP-induced calcineurin activity [143]. In agreement with this, studies have shown that AVP signaling alters fibrosis via NF-κB and iNOS-NO signaling [144]. AVP-induced collagen synthesis is accompanied by nuclear translocation of NF-κB, and IL-1β acts synergistically with AVP to increase iNOS-NO system in cardiac fibroblasts [144, 145]. V1AR signaling also increases 3H-proline incorporation and α-SMA expression in fibroblasts and aids in the conversion of fibroblasts to myofibroblasts [146]. In addition to the profibrotic effects of direct V1AR signaling on cardiac fibroblasts (summarized in Fig. 4), V1AR signaling also contributes to the angiotensin II- mediated increase in fibrosis during acute pressure overload [147]. In this context, V1AR deficiency was shown to prevent the onset of HF, independently of myocyte hypertrophy, suggesting that distinct V1AR-mediated effects in other cardiac cell populations, such as fibroblasts, also contributes to the development of HF.
Figure 4. AVP signaling in cardiac fibroblasts (CF).
AVP signaling in CF leads to increased cell growth and proliferation in a protein kinase C (PKC)- and AKT-dependent manner. Fibrosis in increased via NF-kB signaling and through calcineurin-dependent increases in collagen I and III. Additionally, V1AR signaling increases α-smooth muscle actin (α-SMA) expression, contributing to the conversion of CF into myofibroblasts.
6. Conclusion
HF remains a prevalent and burdensome disease, both in western countries and increasingly in developing nations. Therapeutics targeting neurohormone pathways continue to be the standard for reducing cardiac remodeling and mortality associated with progressive cardiac dysfunction. Because of the adverse clinical consequences associated with the development of hyponatremia, V2R antagonists were developed for the treatment of patients with hyponatremia. However, in contrast with the βAR and AT1R pathways, therapeutic interventions of V2R to date have proven of no benefit in patients with HF. Conversely, the cardiac V1AR system may have significant clinical importance since: 1) circulating AVP is elevated in patients with HF, which is associated with increased morbidity and mortality; 2) the use of FDA-approved V2R-selective antagonists increase levels of circulating AVP, which is also associated with increased mortality in patients with acutely decompensated HF; 3) V1AR expression is increased two-fold in hearts from patients with end-stage HF, results replicated in both small and large animal models of HF; and 4) increased cardiac V1AR expression is associated with cardiac remodeling, decreased βAR expression and contractility, effects reversed by V1AR blockade. While the molecular, cellular and physiological outcomes of V1AR-dependent signaling varies between cell types, animal models and pathological insults, the potential for V1AR antagonism in the treatment of HF is high. Further exploration of the relationship between AVP signaling and cardiac function during various HF etiologies, and within distinct cardiac cell populations, will provide rationale for targeting this system as a therapeutic approach for HF patients with elevated AVP.
Highlights.
Circulating arginine vasopressin (AVP) is elevated in patients with heart failure (HF), which is associated with increased morbidity and mortality.
In the cardiovascular system, chronic AVP signaling via V1AR (heart, blood vessels) and V2R (kidney) leads to increased hypertrophy and water reabsorption, respectively.
Use of FDA-approved V2R-selective antagonists symptomatically reduce water reabsorption, but increase levels of circulating AVP, which is also associated with increased mortality in patients with acutely decompensated HF.
V1AR expression is increased two-fold in hearts from patients with end-stage HF, results replicated in both small and large animal models of HF.
Increased V1AR signaling is associated with cardiac remodeling, decreased βAR expression and cardiac contractility, effects reversed by V1AR blockade, providing rationale for targeting this system as a therapeutic approach for HF patients with elevated AVP.
Footnotes
Disclosures
M.A.W., A.M.F and D.G.T. wrote the article; V.D.M and F.A.R. generated and analyzed the dog HF data. This work was supported by National Institutes of Health grants HL105414 (D.G.T), HL091799 (A.M.F) and HL108213 (F.A.R.) and a PA Health Research Formula Fund (SAP#4100060220 to A.M.F.). D.G.T. and A.M.F. have equity in Renovacor, Inc., which has neither funded this work nor has a relevant related product. The remaining authors report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMurray JJ, et al. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J. 1998;19(Suppl P):P9–16. [PubMed] [Google Scholar]
- 3.Mozaffarian D, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Dzau VJ, et al. Prostaglandins in severe congestive heart failure. Relation to activation of the renin--angiotensin system and hyponatremia. N Engl J Med. 1984;310(6):347–52. doi: 10.1056/NEJM198402093100603. [DOI] [PubMed] [Google Scholar]
- 5.Benedict CR, et al. Relation of neurohumoral activation to clinical variables and degree of ventricular dysfunction: a report from the Registry of Studies of Left Ventricular Dysfunction. SOLVD Investigators. J Am Coll Cardiol. 1994;23(6):1410–20. doi: 10.1016/0735-1097(94)90385-9. [DOI] [PubMed] [Google Scholar]
- 6.Leier CV, Dei Cas L, Metra M. Clinical relevance and management of the major electrolyte abnormalities in congestive heart failure: hyponatremia, hypokalemia, and hypomagnesemia. Am Heart J. 1994;128(3):564–74. doi: 10.1016/0002-8703(94)90633-5. [DOI] [PubMed] [Google Scholar]
- 7.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20(1):248–54. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 8.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357(9266):1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 9.Vantrimpont P, et al. Additive beneficial effects of beta-blockers to angiotensin-converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) Study. SAVE Investigators. J Am Coll Cardiol. 1997;29(2):229–36. doi: 10.1016/s0735-1097(96)00489-5. [DOI] [PubMed] [Google Scholar]
- 10.Exner DV, et al. Beta-adrenergic blocking agent use and mortality in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a post hoc analysis of the Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1999;33(4):916–23. doi: 10.1016/s0735-1097(98)00675-5. [DOI] [PubMed] [Google Scholar]
- 11.Verdecchia P, et al. Effects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease. Circulation. 2009;120(14):1380–9. doi: 10.1161/CIRCULATIONAHA.109.865774. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 13.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) The CONSENSUS Trial Study Group. N Engl J Med. 1987;316(23):1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 14.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992;327(10):685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 15.Pfeffer MA, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med. 2001;134(7):550–60. doi: 10.7326/0003-4819-134-7-200104030-00008. [DOI] [PubMed] [Google Scholar]
- 18.Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA. 2002;287(7):883–9. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 19.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 20.Chavey WE, 2nd, et al. Guideline for the management of heart failure caused by systolic dysfunction: part II. Treatment. Am Fam Physician. 2001;64(6):1045–54. [PubMed] [Google Scholar]
- 21.Ashley EA, Neibauer J. Cardiology Explained. Remedica; London: 2004. Heart Failure. [PubMed] [Google Scholar]
- 22.Lee CR, et al. Vasopressin: a new target for the treatment of heart failure. Am Heart J. 2003;146(1):9–18. doi: 10.1016/S0002-8703(02)94708-3. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith SR, et al. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;1(6):1385–90. doi: 10.1016/s0735-1097(83)80040-0. [DOI] [PubMed] [Google Scholar]
- 24.Szatalowicz VL, et al. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305(5):263–6. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 25.Francis GS, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82(5):1724–9. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 26.Lanfear DE, et al. Association of arginine vasopressin levels with outcomes and the effect of V2 blockade in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. 2013;6(1):47–52. doi: 10.1161/CIRCHEARTFAILURE.112.970012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farmakis D, et al. Hyponatremia in heart failure. Heart Fail Rev. 2009;14(2):59–63. doi: 10.1007/s10741-008-9109-7. [DOI] [PubMed] [Google Scholar]
- 28.Sica DA. Hyponatremia and heart failure--pathophysiology and implications. Congest Heart Fail. 2005;11(5):274–7. doi: 10.1111/j.1527-5299.2005.04180.x. [DOI] [PubMed] [Google Scholar]
- 29.De Luca L, et al. Hyponatremia in patients with heart failure. Am J Cardiol. 2005;96(12A):19L–23L. doi: 10.1016/j.amjcard.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 30.Lee DS, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290(19):2581–7. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 31.Land H, et al. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- 32.Aoyagi T, Koshimizu TA, Tanoue A. Vasopressin regulation of blood pressure and volume: findings from V1a receptor-deficient mice. Kidney Int. 2009;76(10):1035–9. doi: 10.1038/ki.2009.319. [DOI] [PubMed] [Google Scholar]
- 33.Hupf H, et al. Evidence for a vasopressin system in the rat heart. Circ Res. 1999;84(3):365–70. doi: 10.1161/01.res.84.3.365. [DOI] [PubMed] [Google Scholar]
- 34.Chandrashekhar Y, et al. The role of arginine vasopressin and its receptors in the normal and failing rat heart. J Mol Cell Cardiol. 2003;35(5):495–504. doi: 10.1016/s0022-2828(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura Y, et al. Hypertrophic growth of cultured neonatal rat heart cells mediated by vasopressin V(1A) receptor. Eur J Pharmacol. 2000;391(1–2):39–48. doi: 10.1016/s0014-2999(99)00775-x. [DOI] [PubMed] [Google Scholar]
- 36.Xu YJ, Gopalakrishnan V. Vasopressin increases cytosolic free [Ca2+] in the neonatal rat cardiomyocyte. Evidence for V1 subtype receptors. Circ Res. 1991;69(1):239–45. doi: 10.1161/01.res.69.1.239. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Hirano Y, Hiraoka M. Arginine vasopressin-induced potentiation of unitary L-type Ca2+ channel current in guinea pig ventricular myocytes. Circ Res. 1995;76(4):592–9. doi: 10.1161/01.res.76.4.592. [DOI] [PubMed] [Google Scholar]
- 38.Tanoue A. New topics in vasopressin receptors and approach to novel drugs: effects of vasopressin receptor on regulations of hormone secretion and metabolisms of glucose, fat, and protein. J Pharmacol Sci. 2009;109(1):50–2. doi: 10.1254/jphs.08r15fm. [DOI] [PubMed] [Google Scholar]
- 39.Aoyagi T, et al. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology. 2007;148(5):2075–84. doi: 10.1210/en.2006-1315. [DOI] [PubMed] [Google Scholar]
- 40.Birumachi J, et al. Impaired arginine-vasopressin-induced aldosterone release from adrenal gland cells in mice lacking the vasopressin V1A receptor. Eur J Pharmacol. 2007;566(1–3):226–30. doi: 10.1016/j.ejphar.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Egashira N, et al. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav Brain Res. 2007;178(1):123–7. doi: 10.1016/j.bbr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Tomasiak M, et al. Vasopressin acts on platelets to generate procoagulant activity. Blood Coagul Fibrinolysis. 2008;19(7):615–24. doi: 10.1097/MBC.0b013e328309905d. [DOI] [PubMed] [Google Scholar]
- 43.Wun T, Paglieroni T, Lachant NA. Physiologic concentrations of arginine vasopressin activate human platelets in vitro. Br J Haematol. 1996;92(4):968–72. doi: 10.1046/j.1365-2141.1996.436975.x. [DOI] [PubMed] [Google Scholar]
- 44.Oshikawa S, et al. Vasopressin stimulates insulin release from islet cells through V1b receptors: a combined pharmacological/knockout approach. Mol Pharmacol. 2004;65(3):623–9. doi: 10.1124/mol.65.3.623. [DOI] [PubMed] [Google Scholar]
- 45.Stewart LQ, et al. Pituitary-adrenal response to acute and repeated mild restraint, forced swim and change in environment stress in arginine vasopressin receptor 1b knockout mice. J Neuroendocrinol. 2008;20(5):597–605. doi: 10.1111/j.1365-2826.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 46.Knepper MA, Kwon TH, Nielsen S. Molecular physiology of water balance. N Engl J Med. 2015;372(14):1349–58. doi: 10.1056/NEJMra1404726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasuoka Y, et al. Decreased expression of aquaporin 2 in the collecting duct of mice lacking the vasopressin V1a receptor. Clin Exp Nephrol. 2013;17(2):183–90. doi: 10.1007/s10157-012-0686-3. [DOI] [PubMed] [Google Scholar]
- 48.Ostrowski NL, et al. Expression of vasopressin V1a and V2 receptor messenger ribonucleic acid in the liver and kidney of embryonic, developing, and adult rats. Endocrinology. 1993;133(4):1849–59. doi: 10.1210/endo.133.4.8404628. [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi K, et al. Control of renal medullary blood flow by vasopressin V1 and V2 receptors. Am J Physiol. 1995;269(1 Pt 2):R193–200. doi: 10.1152/ajpregu.1995.269.1.R193. [DOI] [PubMed] [Google Scholar]
- 50.Zhu W, et al. Arginine vasopressin enhances cell survival via a G protein-coupled receptor kinase 2/beta-arrestin1/extracellular-regulated kinase 1/2-dependent pathway in H9c2 cells. Mol Pharmacol. 2013;84(2):227–35. doi: 10.1124/mol.113.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowen-Pidgeon D, et al. Arrestin effects on internalization of vasopressin receptors. Mol Pharmacol. 2001;59(6):1395–401. doi: 10.1124/mol.59.6.1395. [DOI] [PubMed] [Google Scholar]
- 52.Koshimizu TA, et al. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev. 2012;92(4):1813–64. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- 53.Innamorati G, Sadeghi H, Birnbaumer M. Transient phosphorylation of the V1a vasopressin receptor. J Biol Chem. 1998;273(12):7155–61. doi: 10.1074/jbc.273.12.7155. [DOI] [PubMed] [Google Scholar]
- 54.Berrada K, et al. Dynamic interaction of human vasopressin/oxytocin receptor subtypes with G protein-coupled receptor kinases and protein kinase C after agonist stimulation. J Biol Chem. 2000;275(35):27229–37. doi: 10.1074/jbc.M002288200. [DOI] [PubMed] [Google Scholar]
- 55.Innamorati G, Sadeghi H, Birnbaumer M. Phosphorylation and recycling kinetics of G protein-coupled receptors. J Recept Signal Transduct Res. 1999;19(1–4):315–26. doi: 10.3109/10799899909036654. [DOI] [PubMed] [Google Scholar]
- 56.Thibonnier M, et al. The basic and clinical pharmacology of nonpeptide vasopressin receptor antagonists. Annu Rev Pharmacol Toxicol. 2001;41:175–202. doi: 10.1146/annurev.pharmtox.41.1.175. [DOI] [PubMed] [Google Scholar]
- 57.Yip KP. Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol. 2002;538(Pt 3):891–9. doi: 10.1113/jphysiol.2001.012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Innamorati G, et al. Phosphorylation of the V2 vasopressin receptor. J Biol Chem. 1997;272(4):2486–92. doi: 10.1074/jbc.272.4.2486. [DOI] [PubMed] [Google Scholar]
- 59.Innamorati G, et al. A serine cluster prevents recycling of the V2 vasopressin receptor. Proc Natl Acad Sci U S A. 1998;95(5):2222–6. doi: 10.1073/pnas.95.5.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feinstein TN, et al. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem. 2013;288(39):27849–60. doi: 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oakley RH, et al. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274(45):32248–57. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 62.Finley JJt, Konstam MA, Udelson JE. Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia. Circulation. 2008;118(4):410–21. doi: 10.1161/CIRCULATIONAHA.108.765289. [DOI] [PubMed] [Google Scholar]
- 63.Feldman AM, et al. Vasopressin antagonists for patients with acute heart failure: interpreting new clinical and translational data. Clin Pharmacol Ther. 2014;95(4):373–5. doi: 10.1038/clpt.2013.240. [DOI] [PubMed] [Google Scholar]
- 64.Gheorghiade M, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004;291(16):1963–71. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- 65.Gheorghiade M, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation. 2003;107(21):2690–6. doi: 10.1161/01.CIR.0000070422.41439.04. [DOI] [PubMed] [Google Scholar]
- 66.Rossi GP. Arginine vasopressin receptor antagonists for heart failure: a winter climbing to the Everest’s tip? J Am Coll Cardiol. 2007;49(22):2160–2. doi: 10.1016/j.jacc.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 67.Konstam MA, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 68.Udelson JE, et al. Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol. 2007;49(22):2151–9. doi: 10.1016/j.jacc.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 69.Gheorghiade M, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297(12):1332–43. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 70.Vaduganathan M, et al. The disconnect between phase II and phase III trials of drugs for heart failure. Nat Rev Cardiol. 2013;10(2):85–97. doi: 10.1038/nrcardio.2012.181. [DOI] [PubMed] [Google Scholar]
- 71.Alehagen U, et al. Association of copeptin and N-terminal proBNP concentrations with risk of cardiovascular death in older patients with symptoms of heart failure. JAMA. 2011;305(20):2088–95. doi: 10.1001/jama.2011.666. [DOI] [PubMed] [Google Scholar]
- 72.Voors AA, et al. C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J. 2009;30(10):1187–94. doi: 10.1093/eurheartj/ehp098. [DOI] [PubMed] [Google Scholar]
- 73.Neuhold S, et al. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal proB-type natriuretic peptide in patients with chronic heart failure: prediction of death at different stages of the disease. J Am Coll Cardiol. 2008;52(4):266–72. doi: 10.1016/j.jacc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 74.Pozsonyi Z, et al. Copeptin (C-terminal pro Arginine-Vasopressin) is an Independent Long-Term Prognostic Marker in Heart Failure with Reduced Ejection Fraction. Heart Lung Circ. 2014 doi: 10.1016/j.hlc.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Balling L, Gustafsson F. Copeptin as a biomarker in heart failure. Biomark Med. 2014;8(6):841–54. doi: 10.2217/bmm.14.50. [DOI] [PubMed] [Google Scholar]
- 76.Goldsmith SR, et al. Hemodynamic effects of infused arginine vasopressin in congestive heart failure. J Am Coll Cardiol. 1986;8(4):779–83. doi: 10.1016/s0735-1097(86)80417-x. [DOI] [PubMed] [Google Scholar]
- 77.Goldsmith SR, et al. Efficacy and safety of the vasopressin V1A/V2-receptor antagonist conivaptan in acute decompensated heart failure: a dose-ranging pilot study. J Card Fail. 2008;14(8):641–7. doi: 10.1016/j.cardfail.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 78.Zmily HD, Alani A, Ghali JK. Evaluation of lixivaptan in euvolemic and hypervolemic hyponatremia and heart failure treatment. Expert Opin Drug Metab Toxicol. 2013;9(5):645–55. doi: 10.1517/17425255.2013.783566. [DOI] [PubMed] [Google Scholar]
- 79.Morooka H, et al. Chronic administration of oral vasopressin type 2 receptor antagonist tolvaptan exerts both myocardial and renal protective effects in rats with hypertensive heart failure. Circ Heart Fail. 2012;5(4):484–92. doi: 10.1161/CIRCHEARTFAILURE.111.965392. [DOI] [PubMed] [Google Scholar]
- 80.Zhu W, et al. Increased vasopressin 1A receptor expression in failing human hearts. J Am Coll Cardiol. 2014;63(4):375–6. doi: 10.1016/j.jacc.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ikeda T, et al. Chronic blockade of vasopressin type1a receptor and/or type2 receptor improves both cardiac and renal damage and dysfunction in hypertensive rats. 2013;34 [Google Scholar]
- 82.Udelson JE, et al. Acute hemodynamic effects of conivaptan, a dual V(1A) and V(2) vasopressin receptor antagonist, in patients with advanced heart failure. Circulation. 2001;104(20):2417–23. doi: 10.1161/hc4501.099313. [DOI] [PubMed] [Google Scholar]
- 83.Goldsmith SR, Elkayam U. Abstract 2732: A Dose-Ranging Pilot Study of Conivaptan in Acute Decompensated Heart Failure. Circulation. 2007;114(18 Supplement):II_570. [Google Scholar]
- 84.Koshimizu TA, et al. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci U S A. 2006;103(20):7807–12. doi: 10.1073/pnas.0600875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aoyagi T, et al. Vasopressin regulates the renin-angiotensin-aldosterone system via V1a receptors in macula densa cells. Am J Physiol Renal Physiol. 2008;295(1):F100–7. doi: 10.1152/ajprenal.00088.2008. [DOI] [PubMed] [Google Scholar]
- 86.Oikawa R, et al. Decreased susceptibility to salt-induced hypertension in subtotally nephrectomized mice lacking the vasopressin V1a receptor. Cardiovasc Res. 2010;87(1):187–94. doi: 10.1093/cvr/cvq034. [DOI] [PubMed] [Google Scholar]
- 87.Goldsmith SR. The role of vasopressin in congestive heart failure. Cleve Clin J Med. 2006;73(Suppl 3):S19–23. doi: 10.3949/ccjm.73.suppl_3.s19. [DOI] [PubMed] [Google Scholar]
- 88.Li X, et al. Controlled and cardiac-restricted overexpression of the arginine vasopressin V1A receptor causes reversible left ventricular dysfunction through Galphaq-mediated cell signaling. Circulation. 2011;124(5):572–81. doi: 10.1161/CIRCULATIONAHA.111.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hiroyama M, et al. Vasopressin promotes cardiomyocyte hypertrophy via the vasopressin V1A receptor in neonatal mice. Eur J Pharmacol. 2007;559(2–3):89–97. doi: 10.1016/j.ejphar.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Tilley DG, et al. beta-Adrenergic Receptor-Mediated Cardiac Contractility is Inhibited via Vasopressin Type 1A-Receptor-Dependent Signaling. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clair MJ, et al. Selective vasopressin, angiotensin II, or dual receptor blockade with developing congestive heart failure. J Pharmacol Exp Ther. 2000;293(3):852–60. [PubMed] [Google Scholar]
- 92.Kim JK, et al. Arginine vasopressin gene expression in chronic cardiac failure in rats. Kidney Int. 1990;38(5):818–22. doi: 10.1038/ki.1990.276. [DOI] [PubMed] [Google Scholar]
- 93.Muders F, et al. The central vasopressinergic system in experimental left ventricular hypertrophy and dysfunction. Prog Brain Res. 2002;139:275–9. doi: 10.1016/s0079-6123(02)39023-x. [DOI] [PubMed] [Google Scholar]
- 94.Dunlay SM, et al. Risk factors for heart failure: a population-based case-control study. Am J Med. 2009;122(11):1023–8. doi: 10.1016/j.amjmed.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levy D, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557–62. [PubMed] [Google Scholar]
- 96.Burrell LM, et al. Attenuation of genetic hypertension after short-term vasopressin V1A receptor antagonism. Hypertension. 1995;26(5):828–34. doi: 10.1161/01.hyp.26.5.828. [DOI] [PubMed] [Google Scholar]
- 97.Sladek CD, Blair ML, Mangiapane M. Evidence against a pressor role for vasopressin in spontaneous hypertension. Hypertension. 1987;9(4):332–8. doi: 10.1161/01.hyp.9.4.332. [DOI] [PubMed] [Google Scholar]
- 98.Donald RA, et al. Plasma corticotrophin releasing hormone, vasopressin, ACTH and cortisol responses to acute myocardial infarction. Clin Endocrinol (Oxf) 1994;40(4):499–504. doi: 10.1111/j.1365-2265.1994.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 99.Yamazaki T, et al. Tolvaptan improves left ventricular dysfunction after myocardial infarction in rats. Circ Heart Fail. 2012;5(6):794–802. doi: 10.1161/CIRCHEARTFAILURE.112.968750. [DOI] [PubMed] [Google Scholar]
- 100.Nazari A, et al. The cardioprotective effect of different doses of vasopressin (AVP) against ischemia-reperfusion injuries in the anesthetized rat heart. Peptides. 2011;32(12):2459–66. doi: 10.1016/j.peptides.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 101.Milik E, et al. Down-regulation of V1a vasopressin receptors in the cerebellum after myocardial infarction. Neurosci Lett. 2011;499(2):119–23. doi: 10.1016/j.neulet.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 102.Oikawa R, et al. Vasopressin V1A receptor enhances baroreflex via the central component of the reflex arc. Eur J Pharmacol. 2007;558(1–3):144–50. doi: 10.1016/j.ejphar.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 103.Dobruch J, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E. Enhanced involvement of brain vasopressin V1 receptors in cardiovascular responses to stress in rats with myocardial infarction. Stress. 2005;8(4):273–84. doi: 10.1080/10253890500456287. [DOI] [PubMed] [Google Scholar]
- 104.Cudnoch-Jedrzejewska A, et al. Brain vasopressin V(1) receptors contribute to enhanced cardiovascular responses to acute stress in chronically stressed rats and rats with myocardial infarcton. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R672–80. doi: 10.1152/ajpregu.00543.2009. [DOI] [PubMed] [Google Scholar]
- 105.Milik E, et al. Altered expression of V1a receptors mRNA in the brain and kidney after myocardial infarction and chronic stress. Neuropeptides. 2014;48(5):257–66. doi: 10.1016/j.npep.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 106.Bax WA, et al. [Arg8]vasopressin-induced responses of the human isolated coronary artery: effects of non-peptide receptor antagonists. Eur J Pharmacol. 1995;285(2):199–202. doi: 10.1016/0014-2999(95)00503-d. [DOI] [PubMed] [Google Scholar]
- 107.Szczepanska-Sadowska E, et al. Prolonged stimulation of intrarenal V1 vasopressin receptors results in sustained hypertension. Am J Physiol. 1994;267(5 Pt 2):R1217–25. doi: 10.1152/ajpregu.1994.267.5.R1217. [DOI] [PubMed] [Google Scholar]
- 108.Lankhuizen IM, et al. Vascular and renal effects of vasopressin and its antagonists in conscious rats with chronic myocardial infarction; evidence for receptor shift. Eur J Pharmacol. 2001;423(2–3):195–202. doi: 10.1016/s0014-2999(01)01092-5. [DOI] [PubMed] [Google Scholar]
- 109.Lankhuizen IM, et al. [Arg8]-vasopressin-induced responses on coronary and mesenteric arteries of rats with myocardial infarction: the effects of V1a- and V2-receptor antagonists. J Cardiovasc Pharmacol. 2000;36(1):38–44. doi: 10.1097/00005344-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Mentzelopoulos SD, et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310(3):270–9. doi: 10.1001/jama.2013.7832. [DOI] [PubMed] [Google Scholar]
- 111.Gueugniaud PY, et al. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359(1):21–30. doi: 10.1056/NEJMoa0706873. [DOI] [PubMed] [Google Scholar]
- 112.Ong ME, et al. A randomised, double-blind, multi-centre trial comparing vasopressin and adrenaline in patients with cardiac arrest presenting to or in the Emergency Department. Resuscitation. 2012;83(8):953–60. doi: 10.1016/j.resuscitation.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 113.Mentzelopoulos SD, et al. Vasopressin for cardiac arrest: meta-analysis of randomized controlled trials. Resuscitation. 2012;83(1):32–9. doi: 10.1016/j.resuscitation.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 114.Coute RA, Mader TJ, Sherman LD. Outcomes by rescue shock number during the metabolic phase of porcine ventricular fibrillation resuscitation. Am J Emerg Med. 2014;32(6):586–91. doi: 10.1016/j.ajem.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 115.Varvarousi G, et al. Combination pharmacotherapy improves neurological outcome after asphyxial cardiac arrest. Resuscitation. 2012;83(4):527–32. doi: 10.1016/j.resuscitation.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 116.Mauch J, et al. Resuscitation strategies from bupivacaine-induced cardiac arrest. Paediatr Anaesth. 2012;22(2):124–9. doi: 10.1111/j.1460-9592.2011.03688.x. [DOI] [PubMed] [Google Scholar]
- 117.Krismer AC, et al. The effects of endogenous and exogenous vasopressin during experimental cardiopulmonary resuscitation. Anesth Analg. 2001;92(6):1499–504. doi: 10.1097/00000539-200106000-00029. [DOI] [PubMed] [Google Scholar]
- 118.Voelckel WG, et al. Comparison of epinephrine and vasopressin in a pediatric porcine model of asphyxial cardiac arrest. Crit Care Med. 2000;28(12):3777–83. doi: 10.1097/00003246-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 119.Mayr VD, et al. Developing a vasopressor combination in a pig model of adult asphyxial cardiac arrest. Circulation. 2001;104(14):1651–6. doi: 10.1161/hc3901.095896. [DOI] [PubMed] [Google Scholar]
- 120.Voelckel WG, et al. Effects of epinephrine and vasopressin in a piglet model of prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2002;30(5):957–62. doi: 10.1097/00003246-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 121.Lopez-Herce J, et al. Terlipressin versus adrenaline in an infant animal model of asphyxial cardiac arrest. Intensive Care Med. 2010;36(7):1248–55. doi: 10.1007/s00134-010-1828-2. [DOI] [PubMed] [Google Scholar]
- 122.Indrambarya T, et al. Low-dose vasopressin infusion results in increased mortality and cardiac dysfunction following ischemia-reperfusion injury in mice. Crit Care. 2009;13(3):R98. doi: 10.1186/cc7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laugwitz KL, et al. Adenoviral gene transfer of the human V2 vasopressin receptor improves contractile force of rat cardiomyocytes. Circulation. 1999;99(7):925–33. doi: 10.1161/01.cir.99.7.925. [DOI] [PubMed] [Google Scholar]
- 124.Brostrom MA, et al. Vasopressin-induced hypertrophy in H9c2 heart-derived myocytes. Int J Biochem Cell Biol. 2000;32(9):993–1006. doi: 10.1016/s1357-2725(00)00037-6. [DOI] [PubMed] [Google Scholar]
- 125.Xu Y, et al. Vasopressin accelerates protein synthesis in neonatal rat cardiomyocytes. Mol Cell Biochem. 1999;195(1–2):183–90. doi: 10.1023/a:1006961330375. [DOI] [PubMed] [Google Scholar]
- 126.Tahara A, et al. Effect of YM087, a potent nonpeptide vasopressin antagonist, on vasopressin-induced protein synthesis in neonatal rat cardiomyocyte. Cardiovasc Res. 1998;38(1):198–205. doi: 10.1016/s0008-6363(97)00324-6. [DOI] [PubMed] [Google Scholar]
- 127.Sharma A, et al. Protein kinase C regulates internal initiation of translation of the GATA-4 mRNA following vasopressin-induced hypertrophy of cardiac myocytes. J Biol Chem. 2007;282(13):9505–16. doi: 10.1074/jbc.M608874200. [DOI] [PubMed] [Google Scholar]
- 128.Gassanov N, et al. Arginine vasopressin-mediated cardiac differentiation: insights into the role of its receptors and nitric oxide signaling. J Biol Chem. 2007;282(15):11255–65. doi: 10.1074/jbc.M610769200. [DOI] [PubMed] [Google Scholar]
- 129.Gutkowska J, et al. Functional arginine vasopressin system in early heart maturation. Am J Physiol Heart Circ Physiol. 2007;293(4):H2262–70. doi: 10.1152/ajpheart.01320.2006. [DOI] [PubMed] [Google Scholar]
- 130.Yamamoto K, et al. Arginine vasopressin increases nitric oxide synthesis in cytokine-stimulated rat cardiac myocytes. Hypertension. 1997;30(5):1112–20. doi: 10.1161/01.hyp.30.5.1112. [DOI] [PubMed] [Google Scholar]
- 131.Brostrom MA, Meiners S, Brostrom CO. Functional receptor for platelet-derived growth factor in rat embryonic heart-derived myocytes: role of sequestered Ca2+ stores in receptor signaling and antagonism by arginine vasopressin. J Cell Biochem. 2002;84(4):736–49. doi: 10.1002/jcb.10085. [DOI] [PubMed] [Google Scholar]
- 132.Saeedi R, et al. AMP-activated protein kinase influences metabolic remodeling in H9c2 cells hypertrophied by arginine vasopressin. Am J Physiol Heart Circ Physiol. 2009;296(6):H1822–32. doi: 10.1152/ajpheart.00396.2008. [DOI] [PubMed] [Google Scholar]
- 133.Woischwill C, et al. Regulation of the human atrial myosin light chain 1 promoter by Ca2+-calmodulin-dependent signaling pathways. FASEB J. 2005;19(6):503–11. doi: 10.1096/fj.04-2201com. [DOI] [PubMed] [Google Scholar]
- 134.Matsui H, et al. Effects of a nonpeptide vasopressin antagonist (OPC-21268) on cytosolic Ca2+ concentration in vascular and cardiac myocytes. Hypertension. 1992;19(6 Pt 2):730–3. doi: 10.1161/01.hyp.19.6.730. [DOI] [PubMed] [Google Scholar]
- 135.Kurata S, Ishikawa K, Iijima T. Enhancement by arginine vasopressin of the L-type Ca2+ current in guinea pig ventricular myocytes. Pharmacology. 1999;59(1):21–33. doi: 10.1159/000028302. [DOI] [PubMed] [Google Scholar]
- 136.He YP, et al. Involvement of ERK and AKT signaling in the growth effect of arginine vasopressin on adult rat cardiac fibroblast and the modulation by simvastatin. Mol Cell Biochem. 2008;317(1–2):33–41. doi: 10.1007/s11010-008-9802-9. [DOI] [PubMed] [Google Scholar]
- 137.Ishikawa S, Kawasumi M, Saito T. Simvastatin inhibits the cellular signaling and proliferative action of arginine vasopressin in cultured rat glomerular mesangial cells. Endocrinology. 1995;136(5):1954–61. doi: 10.1210/endo.136.5.7720643. [DOI] [PubMed] [Google Scholar]
- 138.He YP, et al. Arginine vasopressin stimulates proliferation of adult rat cardiac fibroblasts via protein kinase C-extracellular signal-regulated kinase 1/2 pathway. Sheng Li Xue Bao. 2008;60(3):333–40. [PubMed] [Google Scholar]
- 139.Yan-ping H, et al. Mitogenic effect of arginine vasopressin on adult rat cardiac fibroblast: involvement of PKC-erk1/2 pathway. J Cardiovasc Pharmacol. 2008;52(1):72–81. doi: 10.1097/FJC.0b013e31817f36b8. [DOI] [PubMed] [Google Scholar]
- 140.Yang XD, et al. Effects of arginine vasopressin on growth of rat cardiac fibroblasts: role of V1 receptor. J Cardiovasc Pharmacol. 2003;42(1):132–5. doi: 10.1097/00005344-200307000-00020. [DOI] [PubMed] [Google Scholar]
- 141.Liu S, et al. Caveolin-1 restoration by cholesterol enhances the inhibitory effect of simvastatin on arginine vasopressin-induced cardiac fibroblasts proliferation. Mol Cell Biochem. 2009;331(1–2):173–80. doi: 10.1007/s11010-009-0155-9. [DOI] [PubMed] [Google Scholar]
- 142.Niu X, et al. Effects of angiotensin-(1-7) on the proliferation and collagen synthesis of arginine vasopressin-stimulated rat cardiac fibroblasts: role of mas receptor-calcineurin-NF-kappaB signaling pathway. J Cardiovasc Pharmacol. 2014;64(6):536–42. doi: 10.1097/FJC.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 143.Shang FJ, et al. Inhibitory effect of cyclosporin A on growth and collagen synthesis of rat cardiac fibroblasts induced by arginine vasopressin. Yao Xue Xue Bao. 2006;41(11):1044–9. [PubMed] [Google Scholar]
- 144.Fan YH, et al. Arginine vasopressin increases iNOS-NO system activity in cardiac fibroblasts through NF-kappaB activation and its relation with myocardial fibrosis. Life Sci. 2007;81(4):327–35. doi: 10.1016/j.lfs.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 145.Fan YH, Zhao LY, Wang HC. Effects of IL-1beta on inducible nitric oxide synthase-nitric oxide system activity in arginine vasopressin-induced rat cardiac fibroblasts. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2007;23(1):70–3. [PubMed] [Google Scholar]
- 146.Yan-Hong F, et al. Effects of arginine vasopressin on differentiation of cardiac fibroblasts into myofibroblasts. J Cardiovasc Pharmacol. 2010;55(5):489–95. doi: 10.1097/FJC.0b013e3181d706ae. [DOI] [PubMed] [Google Scholar]
- 147.Takase T, et al. Abstract 14201: Vasopressin Type 1a Receptor Deficiency Prevents the Onset of Heart Failure: Possible Link with Myocardial Fibrosis. Circulation. 2010;122(21 Supplement):A14201. [Google Scholar]
- 148.Mitacchione G, et al. The gut hormone ghrelin partially reverses energy substrate metabolic alterations in the failing heart. Circ Heart Fail. 2014;7(4):643–51. doi: 10.1161/CIRCHEARTFAILURE.114.001167. [DOI] [PMC free article] [PubMed] [Google Scholar]