Abstract
Objectives
Tanzania has the 3rd highest birth rate of sickle cell anaemia (SCA) in Africa, but few studies describe severity of complications or available treatments, especially in North West Tanzania around Lake Victoria where the sickle gene is most prevalent. This is a report of the spectrum of clinical disease and range of interventions available at Bugando Medical Centre (Bugando) in North West Tanzania in Africa.
Methods
A cross-sectional study was carried out in Bugando between August 1, 2012 and September 30, 2012. Children (< 15 years old) with SCA attending Bugando were sequentially enrolled. A trained research assistant completed a Swahili questionnaire with the parent or guardian of each participant concerning demographic information, clinical features of disease, and treatments received.
Results
Among the 124 participants enrolled, the median age was 6 years [IQR 4-8.5], and only 13 (10.5%) were < 3 years old. Almost all participants (97.6%) had a prior history of a vaso-occlusive episode, 83 (66.9%) had prior acute chest syndrome, and 21 (16.9%) had prior stroke. In the preceding 12 months, 120 (96.8%) had been hospitalized, and a vaso-occlusive episode was the most common reason for hospitalization (35.5%). Prescriptions for folic acid (92.7%) and malaria prophylaxis (84.7%) were common, but only one had received a pneumococcal vaccine, and none had received hydroxyurea or prophylactic penicillin.
Conclusion
Children with SCA receiving care in Tanzania are diagnosed late, hospitalized frequently, and have severe complications. Opportunities exist to improve care through wider access to screening and diagnosis as well as better coordination of comprehensive care.
Keywords: Sickle cell anaemia, Tanzania, Sub-Saharan Africa, Vaso-occlusive episode, Acute chest syndrome, Stroke, Children
Introduction
Sickle cell anaemia (SCA) is a significant problem in Africa. Of 300,000 annual births with haemoglobin SS disease, 75% are born in Africa.1 By the year 2015, there will be a 50% increase in the number of affected births.2 Tanzania has the 3rd highest number of SCA births in Africa after Nigeria and the Democratic Republic of Congo.1 Mortality from SCA in Tanzania is ten-fold greater than high income countries.3
The natural history of SCA has been described outside of Africa. Clinical features include painful episodes, infections, anaemia, central nervous system complications such as stroke and increased risk of mortality.4,5 The introduction of newborn screening (NBS) and early initiation of comprehensive care with penicillin prophylaxis, pneumococcal vaccination, hydroxyurea and transfusion protocols has significantly decreased morbidity and mortality.6,7 Access to NBS has been limited in Africa, and the severity of disease and available treatments are not well described.
Tanzania has recognized the public health significance of SCA and is introducing appropriate interventions,8 but research describing SCA mortality rates and the burden of malaria in this population has been limited to the coastal city of Dar es Salaam.3,9 The sickle gene is most prevalent in North Western Tanzania, particularly around Lake Victoria,10,11 and few studies have described the morbidity and mortality of SCA in this region.
We conducted a cross-sectional study at a tertiary-level hospital in Mwanza, the largest town in North Western Tanzania on the shore of Lake Victoria. Our primary aim was to assess the lifetime prevalence of SCA-related complications as well as the interventions used to treat children with SCA. We hypothesized that painful vaso-occlusive episodes would be the most common cause of hospitalization, that at least half of children would have been diagnosed at least once with acute chest syndrome,12,13 and that less than 10% were receiving either penicillin or pneumococcal vaccine. Our secondary aim was to evaluate factors associated with stroke because it is a common cause of long term morbidity and acute chest syndrome because it is a common cause of mortality.14–16
Methods
Study Design
This was a cross-sectional study designed to evaluate the lifetime prevalence of SCA-related complications in children attending a tertiary care centre in Tanzania as well as the interventions used to treat children with SCA.
Study Area/Setting
This study was conducted in the outpatient paediatric clinic of Bugando Medical Centre (BMC) in Mwanza, Tanzania. Mwanza is the second largest city in Tanzania located on the shores of Lake Victoria, in North Western Tanzania (see Figure 1). The Sukuma tribe is the most prevalent ethnic group, making up more than 50% of the population in Mwanza region, and approximately 15% of the population nationally.17 The prevalence of sickle cell trait (SCT) is high, reaching 27%.11
Figure 1. Map of Tanzania.
Derived from http://commons.wikimedia.org/wiki/File:Tanzania_location_map.svg © Sémhur / Wikimedia Commons / CC-BY-SA-3.0 (or Free Art License).
BMC is a 900-bed referral and teaching hospital. It serves a catchment area of 13 million people. The department of paediatrics has a capacity of 121 inpatient beds with approximately 10 hospitalizations daily. Daily clinics provide outpatient care to children. One day each week is designated for follow up of children with SCA. Approximately 225 children with SCA are registered in the clinic.
Study Population and Procedures
From August 1, 2012 until September 30, 2012, all children < 15 years of age with SCA who attended the BMC paediatric clinic were serially enrolled. After informed written consent was obtained from the parent or guardian, a trained research assistant interviewed the parent or guardian in Swahili and completed a questionnaire containing demographic information, the reason for most recent hospitalization in the past 12 months, lifetime prevalence of having experienced SCA complications, and prior therapies received by participants in the clinic or during prior hospitalization.
Ethical approval for the study was obtained from the ethical committees of Muhimbili University of Health and Allied Sciences and Bugando Medical Centre, as well as the Institutional Review Board of Weill Cornell Medical College. Informed written consent was obtained in Swahili from the parent or guardian of participants before participation. Patient care was managed by health workers according to BMC management protocols.
Definitions
Complications of SCA were based on those proposed by the Comprehensive Sickle Cell Centers in 2010.18
Acute chest syndrome: acute respiratory illness with fever and/or respiratory symptoms such as cough, dyspnea, tachypnea, or hypoxia requiring hospitalization.
Dactylitis: a new episode of acute pain and swelling in the fingers or toes with no clear source other than vaso-occlusion.
Hyperhaemolysis: an episode of marked anaemia with evidence of increased red blood cell destruction.
Leg ulcers: ulceration of the skin of the legs with prolonged wound healing requiring medical attention for wound treatment, debridement, or dressings.
Priapism: painful persistent, prolonged erection of the penis.
Sequestration crisis: an episode of marked anemia and enlargement of the spleen requiring hospitalization.
Stroke: acute focal neurological deficit in a pattern consistent with a stroke syndrome.
Vaso-occlusive episode: a new episode of acute pain with no clear source other than vaso-occlusion that requires hospitalization.
Vision problem: participant complaining of vision problem that was confirmed by any defect in visual field testing.
Statistical Analysis
The primary study outcome was the lifetime prevalence of individual SCA-related complications. Secondary outcomes included the cause of most recent hospitalization in the past 12 months, and interventions received by participants during hospitalization and at clinic. Possible explanatory variables for stroke and acute chest syndrome were sex, age, tribe, age at diagnosis, number of hospitalizations in the past 12 months, or a family member with SCA. The tribe of the patient was selected as a possible explanatory variable because a tendency to marry within one's own tribe may perpetuate a higher prevalence of SCA among one tribe and may also perpetuate genetic modifiers of disease phenotype within one tribe.
Data were entered into Microsoft Excel and analysed using STATA version 13 (San Antonio, Texas). Categorical variables were described as proportions (percentages), and continuous variables were described as medians [interquartile range]. Differences between proportions were calculated using Fisher's exact test. P-values of less than 0.05 were considered statistically significant. Univariable and multivariable logistic regression were used to identify factors associated with stroke and acute chest syndrome. For associated factors, odds ratios (OR) were determined with 95% confidence intervals [95% CI].
Results
Baseline characteristics
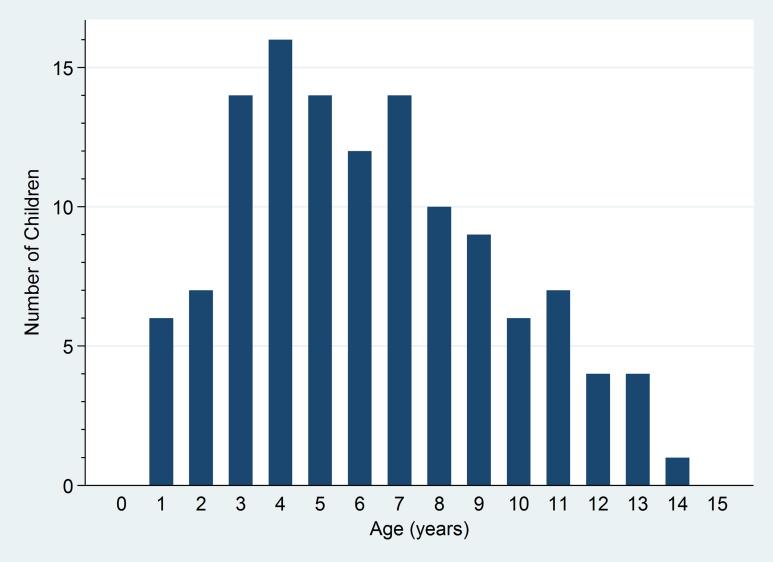
Among the 124 participants, 72 (58.1%) were male, and the median age was 6 years [IQR 4-8.5] (see Table 1). Only 13 (10.5%) were under 3 years old (see Figure 2). More than ten different ethnic groups were represented. Sukuma was the most prevalent (55.7%). The proportion of Sukuma in the participants was similar to the proportion in the general population. The median age at diagnosis was 3 years old [IQR 1-4]. A family member diagnosed with SCA was reported by 69 (55.7%), and 23 (18.6%) reported the death of a family member from SCA.
Table 1.
Characteristics of 124 children with sickle cell anaemia seen at Bugando Medical Centre in Mwanza, Tanzania, between August and September 2012
| Characteristic | Frequency (%) or Median [IQR] |
|---|---|
| Male | 72 (58.1) |
| Age (years) | 6 [4-8.5] |
| 0-2 years | 13 (10.5) |
| 3-5 years | 44 (35.5) |
| 6-8 years | 36 (29.0) |
| ≥ 9 years | 31 (25.0) |
| Tribe | |
| Sukuma | 69 (55.7) |
| Jita | 16 (12.9) |
| Muha | 11 (8.9) |
| Kurya | 7 (5.6) |
| Jaluo | 4 (3.2) |
| Kerewe | 4 (3.2) |
| Nyamwezi | 4 (3.2) |
| Other | 9 (7.3) |
| Age at diagnosis (years) | 3 [1-4] |
| Ever hospitalized | 123 (99.2) |
| Hospitalizations in the past 12 months | 1 [1-2] |
| 0 | 4 (3.2) |
| 1 | 86 (69.4) |
| 2 | 26 (21.0) |
| 3 | 7 (5.7) |
| 4 | 1 (0.8) |
| Lifetime hospitalizations | 4 [3-5] |
| 0-4 | 74 (59.7) |
| 5-9 | 49 (39.5) |
| > 10 | 1 (0.8) |
| Family history of SCA diagnosis | 69 (55.7) |
| Family history of SCA death | 23 (18.6) |
SCA: sickle cell anaemia
Figure 2.
Age distribution of 124 children with sickle cell anaemia seen at Bugando Medical Centre in Mwanza, Tanzania between August and September 2012
Hospitalizations
All but 1 of the participants (99.2%) reported a history of being hospitalized at least once for the management of their disease (see Table 1). Within the preceding 12 months, 86 (69.4%) had been hospitalized once and 34 (27.4%) had been hospitalized 2 or more times. The number of hospitalizations in the preceding 12 months did not differ according to the age (P=0.636) or the sex (P=0.655) of the participant. In the participants’ lifetimes, 50 (40.3%) had been hospitalized ≥ 5 times, and 1 subject (0.8%) had been hospitalized > 10 times. As expected, the number of hospitalizations in participants’ lifetimes was significantly higher if the subject was older (P<0.001), but did not differ according to sex (P=0.736).
Lifetime Prevalence of Complications and Reason for Most Recent Hospitalization
The most prevalent prior complication of SCA was a vaso-occlusive episode which had been diagnosed in 121 participants (97.6%) (see Table 2). Acute chest syndrome had been diagnosed in 83 participants (66.9%), and stroke had been diagnosed in 21 participants (16.9%). Of the 21 participants who had been diagnosed with a stroke, 17/21 (81.0%) also reported a history of seizure. The lifetime prevalence of complications was similar across age groups for dactylitis (P=0.175), sequestration crisis (P=0.488), and vaso-occlusive episodes (P=0.548). The prevalence of all other complications increased in successive age groups.
Table 2.
Cause of most recent hospitalization within the past 12 months and lifetime prevalence of any complication stratified by age group among 124 children with sickle cell anaemia seen at Bugando Medical Centre in Mwanza, Tanzania between August and September 2012
| 0-2 years old (n=13) Frequency (%) | 3-5 years old (n=44) Frequency (%) | 6-8 years old (n=36) Frequency (%) | ≥ 9 years old (n=31) Frequency (%) | Total (n=124) Frequency (%) | P-value by Fisher's exact test | |
|---|---|---|---|---|---|---|
| Lifetime Prevalence of Complications | ||||||
| Acute chest syndrome | 5 (38.5) | 22 (50.0) | 29 (80.6) | 27 (87.1) | 83 (66.9) | <0.001* |
| Dactylitis | 11 (84.6) | 35 (79.6) | 22 (61.1) | 20 (64.5) | 88 (71.0) | 0.175 |
| Hyperhaemolysis | 2 (15.4) | 34 (77.3) | 33 (91.7) | 29 (93.7) | 98 (79.0) | <0.001* |
| Leg ulcers | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (9.7) | 3 (2.4) | 0.043* |
| Priapism† | 0 (0.0) | 5 (15.6) | 9 (40.9) | 8 (66.7) | 22 (30.6) | 0.002* |
| Sequestration crisis | 4 (30.8) | 20 (45.5) | 12 (33.3) | 9 (29.0) | 45 (36.3) | 0.488 |
| Stroke | 0 (0.0) | 3 (6.8) | 5 (13.9) | 13 (41.9) | 21 (16.9) | <0.001* |
| Vaso-occlusive episode | 12 (92.3) | 43 (97.7) | 35 (97.2) | 31 (100.0) | 121 (97.6) | 0.548 |
| Vision problem | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.0 (0.0) | 0 (0.0) | N/A |
| Cause of most recent hospitalization in past 12 months | ||||||
| Acute chest syndrome | 0 (0.0) | 10 (22.7) | 7 (19.4) | 7 (22.6) | 24 (19.4) | 0.063 |
| Anaemia | 3 (23.1) | 2 (4.6) | 0 (0.0) | 2 (6.5) | 7 (5.7) | |
| Fever | 5 (38.5) | 18 (40.9) | 11 (30.6) | 5 (16.1) | 39 (31.5) | |
| Stroke | 0 (0.0) | 0 (0.0) | 1 (2.8) | 3 (9.7) | 4 (3.2) | |
| Vaso-occlusive episode | 4 (30.8) | 12 (27.3) | 15 (41.7) | 12 (38.7) | 43 (34.7) | |
| Other | 1 (7.7) | 0 (0.0) | 1 (2.8) | 1 (3.2) | 3 (2.4) | |
| Not hospitalized | 0 (0.0) | 2 (4.6) | 1 (2.8) | 1 (3.2) | 4 (3.23) |
Out of 72 males
Among children hospitalized in the past 12 months the most common cause of hospitalization was a vaso-occlusive episode in 43 (34.7%). Fever was the reason for hospitalization in 39 (31.5%), and acute chest syndrome was the reason for hospitalization in 24 (19.4%). The cause of most recent hospitalization in the past 12 months did not differ significantly according to sex (P=0.861) or age group (P=0.063).
Interventions
At the clinic, folic acid and instructions to drink extra fluids were frequently provided (92.7% and 93.6%) (see Table 3). Malaria prophylaxis in the form of chloroquine once per week was prescribed to 105 (84.7%). Pneumococcal vaccination was only given to one subject, and neither prophylactic penicillin nor Hydroxyurea were given to any participants. During hospitalization, analgesics, IV fluids, antibiotics, and blood transfusion were provided to > 90% of all participants.
Table 3.
Interventions received by 124 children with sickle cell anaemia seen at Bugando Medical Centre in Mwanza, Tanzania, between August and September 2012
| Frequency (%) | |
|---|---|
| Interventions during hospitalization | |
| Analgesics | 123 (99.2) |
| Antibiotics | 120 (96.8) |
| Blood transfusion | 112 (90.3) |
| IV fluids | 122 (98.4) |
| Interventions at clinic | |
| Folic acid | 115 (92.7) |
| Hydroxyurea | 0 (0.0) |
| Malaria prophylaxis | 101 (81.5) |
| Mebendazole | 35 (28.2) |
| Penicillin prophylaxis | 0 (0.0) |
| Pneumococcal vaccine | 1 (0.8) |
| Told to drink extra fluids | 116 (93.6) |
Factors Associated with Stroke & Acute Chest Syndrome
Using univariable logistic regression, several factors were found to be associated with stroke. Family history of SCA had the strongest association (OR 4.17, 95% CI 1.31-13.24, P=0.015) (see Table 4). Increasing age (OR 1.49 per year of increased age, 95% CI 1.24-1.79, P<0.001), and a higher number of lifetime hospitalizations (OR 1.85 per hospitalization, 95% CI 1.34-2.54, P<0.001) were also associated with stroke. Using multivariable logistic regression adjusted for age, sex, Sukuma tribe, age at diagnosis, number of lifetime hospitalizations and family history of SCA, only two factors remained significantly associated with stroke: the age of the participant and the age at diagnosis. Older age increased the odds of stroke (OR 1.75 per additional year of age, 95% CI 1.31-2.35, p<0.001) and older age at diagnosis decreased the odds of stroke (OR 0.62 per every year delay in diagnosis, 95% CI 0.40-0.94, P=0.025).
Table 4.
Baseline characteristics associated with stroke among 124 children with sickle cell anaemia seen at Bugando Medical Centre in Mwanza, Tanzania between August and September 2012 by univariable logistic regression
| Variable | Stroke | No Stroke | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| n (%) or median [IQR] | OR [95% CI] | P-value | OR [95% CI] | P-value | ||
| Total | 21 (16.9) | 103 (83.1) | ||||
| Gender | ||||||
| Female | 6 (11.5) | 46 (88.5) | ||||
| Male | 15 (20.8) | 57 (11.5) | 2.02 [0.73-5.61] | 0.179 | 2.94 [0.73-11.80] | 0.104 |
| Age (years) | 10 [8-12] | 5 [3-7] | 1.49 [1.24-1.79] | <0.001* | 1.75 [1.31-2.35] | <0.001* |
| Tribe | ||||||
| Other | 6 (10.9) | 49 (89.1) | ||||
| Sukuma | 15 (21.7) | 54 (78.3) | 2.27 [0.82-6.31] | 0.116 | 0.96 [0.26-3.54] | 0.952 |
| Age at diagnosis (years) | 3 [2-3] | 3 [1-5] | 0.94 [0.74-1.19] | 0.622 | 0.62 [0.40-0.94] | 0.025* |
| Hospitalizations past 12 month | 1 [1-2] | 1 [1-2] | 1.66 [0.88-3.14] | 0.121 | 0 | |
| Lifetime hospitalizations | 5 [4-7] | 4 [3-5] | 1.85 [1.34-2.54] | <0.001* | 1.32 [0.89-1.98] | 0.169 |
| Family history of SCA diagnosis | ||||||
| SCA not in family | 4 (7.3) | 51 (92.7) | ||||
| SCA in family | 17 (24.6) | 52 (75.4) | 4.17 [1.31-13.24] | 0.015* | 3.06 [0.69-13.60] | 0.141 |
| Family history of SCA death | ||||||
| No death in family | 14 (13.9) | 87 (86.1) | ||||
| Death in family | 7 (30.4) | 16 (69.6) | 2.72 [0.95-7.79] | 0.062 | ||
Factors associated with the complication of acute chest syndrome were also analysed using univariable logistic regression (see Table 5). Male gender (OR 0.44, 95% CI 0.20-0.99, P=0.047) was negatively associated with acute chest syndrome. Older age (OR 1.39 per additional year of age, 95% CI 1.18-1.64, P<0.001), and higher number of lifetime hospitalizations (OR 1.97 per an additional hospitalization, 95% CI 1.43-2.71, P<0.001) family history of SCA (OR 4.46, 95% CI 2.00-9.96, P<0.001), and family history of death from SCA (OR 6.60, 95% CI 1.47-29.74, P=0.014), were significantly associated with acute chest syndrome. Using multivariable logistic regression adjusted for age, sex, Sukuma tribe, age at diagnosis, number of lifetime hospitalizations and family history of SCA, three factors remained associated with acute chest syndrome: older age (OR 1.35 per additional year of age, 95% CI 1.04-1.75, P<0.001), a higher number of lifetime hospitalizations (OR 1.69, 95% CI 1.17-2.48, P=0.005), and a family history of SCA (OR 2.92, 95% CI 1.13-2.75, P=0.026).
Table 5.
Baseline characteristics associated with acute chest syndrome among 124 children with sickle cell anaemia seen at Bugando Medical Centre in Mwanza, Tanzania between August and September 2012 by univariable logistic regression
| Variable | Acute Chest | No Acute Chest | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| n (%) or median [IQR] | OR [95% CI] | P-value | OR [95% CI] | P-value | ||
| Total | 83 (66.9) | 41 (33.1) | ||||
| Gender | ||||||
| Female | 40 (76.9) | 12 (23.1) | ||||
| Male | 43 (59.7) | 29 (40.3) | 0.44 [0.20-0.99] | 0.047* | 0.38 [0.14-1.04] | 0.061 |
| Age (years) | 7 [5-9] | 4 [3-6] | 1.39 [1.18-1.64] | <0.001* | 1.35 [1.04-1.75] | 0.023 |
| Tribe | ||||||
| Other | 34 (61.8) | 21 (38.2) | ||||
| Sukuma | 49 (71.0) | 20 (29.0) | 1.51 [0.71-3.21] | 0.116 | 1.15 [0.45-2.95] | 0.776 |
| Age at diagnosis (years) | 3 [1-5] | 2 [1-4] | 1.13 [0.93-1.37] | 0.214 | 0.88 [0.65-1.19] | 0.409 |
| Hospitalizations past 12 month | 1 [1-2] | 1 [1-1] | 1.95 [0.99-3.83] | 0.054 | ||
| Lifetime hospitalizations | 5 [4-6] | 3 [3-4] | 1.97 [1.43-2.71] | <0.001* | 1.69 [1.17-2.48] | 0.005* |
| Family history of SCA diagnosis | ||||||
| SCA not in family | 27 (49.1) | 28 (50.9) | ||||
| SCA in family | 56 (81.2) | 13 (18.8) | 4.47 [2.00-9.96] | <0.001* | 2.92 [1.13-2.75] | 0.026 |
| Family history of SCA death | ||||||
| No death in family | 62 (61.4) | 39 (38.6) | ||||
| Death in family | 21 (91.3) | 2 (8.7) | 6.60 [1.47-29.74] | 0.014* | ||
Discussion
This study describes the demographics, range of SCA complications and available treatments for children with SCA living in North Western Tanzania where the highest prevalence of haemoglobin S has been reported.10,11 A slight majority of the participants were male (58.1%), and the median age of children attending clinic was six years. There were only 6 children less than 2 years old, and no children less than 12 months old. A similar age distribution has been described in sickle cell clinics within other African countries. In Nigeria, the median age was 5.9 years, but there were a higher number of children under 1 year of age (3.8%).19 In a clinic in Democratic Republic of Congo (DRC), the median age of children was even higher at 10 years old.13 At the initiation of a non-birth prospective cohort in Dar es Salaam, Tanzania, only 10% of children were less than 2 years old.3 The lack of a newborn screening program, the lower incidence of complications during the first year of life, and poor community awareness about the early symptoms of sickle cell disease likely contribute to the older age of those attending the sickle cell clinic.
The median age of diagnosis was 3 years old [IQR 1-4] for children at our centre. Only 4 children (3.2%) were diagnosed in infancy (before 12 months of life). This is largely due to the lack of newborn screening in the country. A sickling test is the primary method of diagnosis and is unreliable in the neonatal period, yielding many false negatives. Haemoglobin electrophoresis is available only at the national hospital in Dar es Salaam. Children are typically diagnosed after having one or more SCA related complications. Other countries without newborn screening report an age of diagnosis at or above one year of age. In Brazil, the median age of diagnosis was 2 years old for those in their second decade of life.20 Studies from Nigeria, report a median age of diagnosis from 2-2.4 years old, but in contrast to our study, approximately 20% of children were diagnosed before their first birthday.19,21 Even in Toronto, Canada, those who were not screened at birth were diagnosed at a median age of 2 years old.22
Approximately half of the participants had a family history of SCA (69/124, 55.7%), but their median age of diagnosis (3 years [IQR 1-4]) was not significantly different than those without a family history of disease (2 years [IQR 1-5], P=0.654). Having multiple family members with the same disease is expected to increase awareness of symptoms and prompt higher quality care for subsequent children, but this effect depends on proper education of patients and their family members as well as good access to health care services. In the DRC only 67% of patients reported that their family members had an awareness of sickle cell anaemia.13 A thorough family history should always be obtained for children and, in areas where haemoglobin S is highly prevalent, ought to specifically include family history of sickle cell disease and the death of any family members from sickle cell disease. A vigilant family history may be the only way to identify individuals who are at risk of developing stroke and/or acute chest syndrome. Family education is also an essential intervention in this setting where early recognition of symptoms may be the fastest way to diagnose a child with SCA.
Early death may contribute to both the older age of participants and older age of diagnosis in our study. Before the use of antimalarials, SCA in sub-Saharan Africa was almost universally fatal before the age of five.23 This may still be true in malaria endemic areas with poor access to health care. Children may present multiple times with anaemia and fever and be misdiagnosed with malaria. Despite their higher morbidity, they may not be referred to a tertiary care centre. More recent studies have demonstrated that children with SCA in Africa can survive childhood,24 but the mortality rates are still ten times higher than those found in high-income countries, and the highest rates are found in those younger than 5 years old.3 In the USA the greatest improvement in mortality after implementation of comprehensive care was in the youngest age group,25 providing a major impetus for similar programmes in sub-Saharan Africa where substantially more people with SCA reside. Newborn cohort studies are needed to better understand the natural history of SCA in Africa, particularly in rural areas that are malaria endemic with poor access to advanced medical care.
All but 4 participants in our study were hospitalized in the past 12 months, and the median number of hospitalizations among these children was 1 [IQR 1-2]. The most common causes of hospitalization were pain (35.5%), fever (33.1%) and ACS (19.4%). Pain is more common cause of hospitalization in high income countries accounting for up to two-thirds of hospitalizations.26,27 The lower prevalence of hospitalization for pain is likely due to the elevated prevalence of fever. Malaria and bacterial infections are common causes of fever in people with SCA in sub-Saharan Africa. Both are important contributors to morbidity and mortality among people with SCA in this setting and likely contribute to the higher frequency hospitalization for fever.9,28
The lifetime prevalence of complications related to SCA was significant. Almost all of the participants (97.6%) had experienced a prior vaso-occlusive episode, regardless of age. This is much higher than the amount of vaso-occlusive episodes reported in cohort studies from developed countries. In the USA, for example, only half of children with SCA have experienced a vaso-occlusive episode by 5 years old,12 and the rate of vaso-occlusive episodes steadily increases from 0.4 per person year in those younger than five to a peak of 1.21 per person year in those 25-29 years old.29 The higher prevalence of vaso-occlusive episodes in our study is similar to other hospitals in Africa.13 Risk factors that are more prevalent in sub-Saharan Africa and can trigger a vaso-occlusive episode include malaria, as well as arboviruses. Until eradication of these risk factors, clinicians should strive for early, aggressive, treatment of pain.
Half of the participants had experienced an episode of acute chest syndrome by the age of five years, and prior stroke was reported by 1/6 of all participants and in almost half of those older than 9 years old. This prevalence of acute chest syndrome is twice as high as the USA,30 and may be due to lack of immunization, late presentation, and concomitant malnutrition.31 The incidence of stroke in children with SCA is much lower in the USA, with only 11% having stroke before age 20 and 24% before age 45.16 Despite the high prevalence of stroke in our study, no one had undergone screening transcranial Doppler to identify a higher risk of stroke. Such a screening protocol would provide an opportunity to initiate children on chronic transfusion protocols that have successfully been used in developed countries for both primary prevention in high-risk individuals and secondary prevention in those with prior stroke.32 If a screening program were implemented and a transfusion protocol started, the current blood supply system might struggle to support chronic transfusion protocols for multiple children with SCA. More practical approaches, such as the use of hydroxyurea for primary stroke are currently being investigated in Africa.33
In the paediatric clinic, a large majority of participants received instructions about how to prevent vaso-occlusive disease (93.6%) as well as treatment with folic acid (92.7%). Many also were given antimalarial for prophylaxis (81.5%), and more than a quarter (28.2%) were given medication to treat intestinal worms. The local clinic staff successfully counsel and educate families about these basic preventive measures for SCA. However, at the time of the study, no children were receiving either prophylactic penicillin or pneumococcal vaccine despite their effectiveness in improving mortality34,35 and the fact that fever was the cause of one third of hospitalizations. The same bacteria affecting children in high income countries are present in East Africa28 suggesting a role for both of these preventive measures in this setting.
Since the completion of this study, Tanzania has broadened its national immunization policy to include pneumococcal conjugate vaccine. In addition the sickle cell centre at the national hospital has started and maintained a centre for comprehensive sickle cell care3 and lain the groundwork for a national sickle cell health policy.8 A national guideline for sickle cell treatment has been published and disseminated for use by physicians around the country36 that includes instructions for both prophylactic penicillin and appropriate vaccination of all children with SCA.
Hydroxyurea was not prescribed to any children at our centre. As the primary disease modifying drug for SCA, hydroxurea is proven to decrease vaso-occlusive episodes, acute chest syndrome, and mortality37–39 in high income countries. Prescription of this life saving therapy in Africa has been hampered by the cost of the drug, the ability to monitor its side effects, and compliance problems. In addition, prior studies sparked debate about its safety in areas with high malaria prevalence.40,41 Several clinical studies are underway in Africa to help answer these questions, clearing the way for access to this important therapy.
During hospitalization, more than 90% of participants received antibiotics and blood transfusions, regardless of the reason for their most recent hospitalization. In addition to using antibiotics for children with objective fever at time of hospitalization, antibiotics are frequently prescribed to children who report subjective fever when they are hospitalized for a vaso-occlusive crisis. Physicians are concerned about infections precipitating painful crisis since historically, few children have been appropriately immunized or received prophylactic penicillin. The use of transfusion at our centre is also much higher than among children with SCA hospitalized in the USA where only 28.8% receive blood transfusions.42 The frequent use of blood transfusions is likely related to the severity of anaemia in hospitalized children with SCA. At our centre, almost two thirds of hospitalized children with SCA have a haemoglobin less than 7 g/dL.43
This study has several limitations. Since the study site was a tertiary care centre, enrolled participants may have better access to medical care in general or may have a higher prevalence of complications that provoked them to seek medical attention. Since the data was collected retrospectively, diagnoses and treatments could not be confirmed. As with other questionnaire based studies, parental education and recall bias may influence the reported presence and number of events and hospitalizations. The short duration of enrolment may have led to an unrepresentative sample, although the results are consistent with our experience. Mortality was not assessed since participants were only included if they were alive at the time of interview.
Conclusion
Children with SCA in Tanzania are diagnosed late and do have access to many basic preventive measures. They have a high burden of complications, are frequently hospitalized for vaso-occlusive episode and fever and commonly receive both antibiotics and blood transfusions. Opportunities exist to improve the care of children with SCA through wider access to newborn screening and diagnosis, better coordination of comprehensive care, and provision of proven preventive therapies such as prophylactic penicillin, pneumococcal vaccination, and hydroxyurea. The burden of stroke is high and could be decreased through the implementation of transcranial Doppler screening and transfusion therapy..
Acknowledgments
LRS has received support from the United States Institutes of Health Fogarty International Center (TW009337).
Footnotes
Financial Disclosure
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest Statement
The authors declare no conflicts of interest.
References
- 1.Piel FB, Patil AP, Howes RE, Nyangiri O a., Gething PW, Dewi M, et al. Lancet. 9861. Vol. 381. Elsevier Ltd; 2013. Global epidemiology of Sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. pp. 142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global Burden of Sickle Cell Anaemia in Children under Five, 2010-2050: Modelling Based on Demographics, Excess Mortality, and Interventions. PLoS Med. 2013;10(7):e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, et al. Mortality in sickle cell anemia in africa: A prospective cohort study in Tanzania. PLoS One. Jan. 2011;6(2):e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatrics. 1989;84(3):500–8. [PubMed] [Google Scholar]
- 5.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 6.Telfer P, Coen P, Chakravorty S, Wilkey O, Evans J, Newell H, et al. Clinical outcomes in children with sickle cell disease living in England: A neonatal cohort in East London. Haematologica. 2007;92(7):905–12. doi: 10.3324/haematol.10937. [DOI] [PubMed] [Google Scholar]
- 7.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–52. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makani J, Soka D, Rwezaula S, Krag M, Mghamba J, Ramaiya K, et al. Health policy for sickle cell disease in Africa : experience from Tanzania on interventions to reduce under-five mortality. Trop Med Int Heal. 2014 doi: 10.1111/tmi.12428. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makani J, Komba AN, Cox SE, Oruo J, Mwamtemi K, Kitundu J, et al. Malaria in patients with sickle cell anemia: Burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010;115(2):215–20. doi: 10.1182/blood-2009-07-233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piel FB, Patil AP, Howes RE, Nyangiri O a, Gething PW, Williams TN, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun. 2010;1:104. doi: 10.1038/ncomms1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison A. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg. 1954;48(4):312–8. doi: 10.1016/0035-9203(54)90101-7. [DOI] [PubMed] [Google Scholar]
- 12.Gill FM, Sleeper L a, Weiner SJ, Brown a K, Bellevue R, Grover R, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86(2):776–83. [PubMed] [Google Scholar]
- 13.Aloni MN, Nkee L. Challenge of Managing Sickle Cell Disease in a Pediatric Population Living in Kinshasa , Democratic Republic of Congo: A Sickle Cell Center Experience. Hemoglobin. 2014;38(3):196–200. doi: 10.3109/03630269.2014.896810. [DOI] [PubMed] [Google Scholar]
- 14.Gray A, Anionwu EN, Davies SC, Brozovic M. Patterns of mortality in sickle cell disease in the United Kingdom. J Clin Pathol. 1991;44(6):459–63. doi: 10.1136/jcp.44.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas a N, Pattison C, Serjeant GR. Causes of death in sickle-cell disease in Jamaica. Br Med J (Clin Res Ed) 1982;285(6342):633–5. doi: 10.1136/bmj.285.6342.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohene-Frempong K, Weiner SJ, Sleeper L a, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–94. [PubMed] [Google Scholar]
- 17.Research on Poverty Alleviation (REPOA) & Michigan State University (MSU). Round 5 Afrobarometer Survey in Tanzania, 2012. 2012 [Google Scholar]
- 18.Ballas SK, Lieff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, et al. Definitions of the phenotypic manifestations of sickle cell disease. American Journal of Hematology. 2010:6–13. doi: 10.1002/ajh.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adegoke SA, Adeodu OO, Adekile AD. Sickle cell disease clinical phenotypes in children from South-Western, Nigeria. Niger J Clin Pract. 2015;18(1):95–101. doi: 10.4103/1119-3077.146987. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes TAADM, Medeiros TMD De, Alves JJP, Bezerra CM, Fernandes JV, Serafim ÉSS, et al. Socioeconomic and demographic characteristics of sickle cell disease patients from a low-income region of northeastern Brazil. Rev Bras Hematol Hemoter. Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. 2015;37(3):172–7. doi: 10.1016/j.bjhh.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown BJ, Akinkunmi BF, Fatunde OJ. Age at diagnosis of sickle cell disease in a developing country. Afr J Med Med Sci. 2010;39(3):221–5. [PubMed] [Google Scholar]
- 22.Lieberman L, Kirby M, Ozolins L, Mosko J, Friedman J. Initial presentation of unscreened children with sickle cell disease: The Toronto experience. Pediatr Blood Cancer. 2009;53(3):397–400. doi: 10.1002/pbc.22023. [DOI] [PubMed] [Google Scholar]
- 23.Fleming AF, Storey J, Molineaux L, Iroko EA, Attai ED. Abnormal haemoglobins in the Sudan savanna of Nigeria. I. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol. 1979;73(2):161–72. doi: 10.1080/00034983.1979.11687243. [DOI] [PubMed] [Google Scholar]
- 24.Rahimy MC. Effect of a comprehensive clinical care program on disease course in severely ill children with sickle cell anemia in a sub-Saharan African setting. Blood. 2003;102(3):834–8. doi: 10.1182/blood-2002-05-1453. [DOI] [PubMed] [Google Scholar]
- 25.Yanni E, Grosse SD, Yang Q, Olney RS. Trends in Pediatric Sickle Cell Disease- Related Mortality in the United States, 1983-2002. J Pediatr. 2009;154:541–5. doi: 10.1016/j.jpeds.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 26.Teoh Y, Greenway A, Savoia H, Monagle P, Roy J, Barnes C. Hospitalisations for sickle-cell disease in an Australian paediatric population. J Paediatr Child Health. 2013;49(1):68–71. doi: 10.1111/jpc.12018. [DOI] [PubMed] [Google Scholar]
- 27.Fosdal MB, Wojner-Alexandrov AW. Events of Hospitalization Among Children With Sickle Cell Disease. J Pediatr Nurs. 2007;22(4):342–6. doi: 10.1016/j.pedn.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Williams TN, Uyoga S, Macharia A, Ndila C, McAuley CF, Opi DH, et al. Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. Lancet. 2009;374:1364–70. doi: 10.1016/S0140-6736(09)61374-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–6. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 30.Castro O, Brambilla DJ, Thorington B, Reindorf C a, Scott RB, Gillette P, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84(2):643–9. [PubMed] [Google Scholar]
- 31.Cox SE, Makani J, Fulford AJ, Komba AN, Soka D, Williams TN, et al. Nutritional status, hospitalization and mortality among patients with sickle cell anemia in Tanzania. Haematologica. Jul. 2011;96(7):948–53. doi: 10.3324/haematol.2010.028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang WC, Dwan K. Blood transfusion for preventing primary and secondary stroke in people with sickle cell disease. Cochrane database Syst Rev. 2013;11(11):1–31. doi: 10.1002/14651858.CD003146.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galadanci N, Abdullahi S, Tabari M, Abubakar S, Belonwu R, Salihu A, et al. Primary stroke prevention in Nigerian children with sickle cell disease (SPIN): challenges of conducting a feasibility trial. Pediatr Blood Cancer. 2015;62(3):395–401. doi: 10.1002/pbc.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirst C, Owusu-Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database Syst Rev. 2014;(11):CD003427. doi: 10.1002/14651858.CD003427.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Halasa NB, Shankar SM, Talbot TR, Arbogast PG, Mitchel EF, Wang WC, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428–33. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 36.Hassell J, Makani J, Punnialingam S, Smart L, Soka D. Management of Sickle Cell Disease. Dar es Salaam: 2014. pp. 1–40. [Google Scholar]
- 37.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert S V, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. The New England journal of medicine. 1995 doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 38.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–51. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 39.Thornburg CD, Files B a., Luo Z, Miller ST, Kalpatthi R, Iyer R, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120:4304–10. doi: 10.1182/blood-2012-03-419879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pino P, Taoufiq Z, Brun M, Tefit M, Franetich JF, Ciceron L, et al. Effects of hydroxyurea on malaria, parasite growth and adhesion in experimental models. Parasite Immunol. 2006;28(12):675–80. doi: 10.1111/j.1365-3024.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- 41.Brun M, Bourdoulous S, Couraud PO, Elion J, Krishnamoorthy R, Lapoumeroulie C. Hydroxyurea downregulates endothelin-1 gene expression and upregulates ICAM-1 gene expression in cultured human endothelial cells. Pharmacogenomics J. 2003;3(4):215–26. doi: 10.1038/sj.tpj.6500176. [DOI] [PubMed] [Google Scholar]
- 42.Raphael JL, Oyeku SO, Kowalkowski M a, Mueller BU, Ellison AM. Trends in blood transfusion among hospitalized children with sickle cell disease. Pediatr Blood Cancer. 2013;60(11):1753–8. doi: 10.1002/pbc.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simbauranga R. Prevalence of anemia and factors associated with severe anemia among under five children admitted at Bugando Medical Centre. Catholic University of Health and Allied Sciences Bugando; Mwanza, Tanzania: 2013. [Google Scholar]