Abstract
Objective
To derive a clinical decision rule to identify adult emergency department (ED) patients with traumatic intracranial hemorrhage (tICH) who are at low risk for requiring critical care resources during hospitalization.
Methods
This is a retrospective cohort study of patients (≥18 years) with tICH presenting to the ED. The need for intensive care unit (ICU) admission was defined as the presence of a critical care intervention including: intubation, neurosurgical intervention, blood product transfusion, vasopressor or inotrope administration, invasive monitoring for hemodynamic instability, emergent treatment for arrhythmia, therapeutic angiography, and cardiopulmonary resuscitation. The decision rule was derived using binary recursive partitioning.
Results
A total of 432 patients were identified (median age 48 years) of which 174 patients (40%) had a critical care intervention. We performed binary recursive partitioning with Classification and Regression Trees (CART) software to develop the clinical decision rule. Patients with a normal mental status (Glasgow Coma Score=15), isolated head injury, and age < 65 were considered low risk for a critical care intervention. The derived rule had a sensitivity of 98% (95% confidence interval [CI] 94–99), a specificity of 50% (95% CI 44–56), a positive predictive value of 57% (95% CI 51–62), and a negative predictive value of 97% (95% CI 93–99). The area under the curve for the decision rule was 0.74 (95% CI 0.70–0.77).
Conclusions
This clinical decision rule identifies low risk adult ED patients with tICH who do not need ICU admission. Further validation and refinement of these findings would allow for more appropriate ICU resource utilization.
Keywords: traumatic brain injury, intensive care, clinical decision rule
Introduction
The rising cost of medical care is a critical issue facing medicine today, particularly intensive care medicine which accounts for one-third of acute hospital charges.1 Appropriate utilization of intensive care unit (ICU) resources is important to provide safe and efficient health care. ICU admission decisions are highly variable and subjective, often leading to inappropriate admissions to both the ICU and hospital ward. Patients mis-triaged to a non-ICU setting, who then decompensate and require in-hospital transfer to the ICU, have an increased mortality.2–6 Patients initially admitted to the ICU but never requiring critical care resources are a poor utilization of health care resources. These inappropriate admissions lead to ICU and emergency department (ED) overcrowding, prolonged ED boarding times, and adverse patient outcomes.7, 8
Patients with traumatic intracranial hemorrhage (tICH) are often admitted to the ICU for early detection of secondary brain injury from cerebral edema, increased intracranial pressure, and cerebral ischemia.9 These secondary insults are the leading cause of inpatient death following tICH.10 The majority of patients with tICH, however, rarely develop hemorrhage progression or need acute neurosurgical intervention.11, 12 While guidelines suggest the need for in-patient observation for repeated neurological evaluations in patients with tICH, there are no clear recommendations identifying appropriate ICU admission.13–16 Moreover, admission criteria to ICUs vary widely and significant variability in the management of patients with tICH exists.17, 18
The objective of this study is to derive a clinical decision rule to predict the need for ICU admission in adult ED patients with tICH. We hypothesize that a clinical decision rule which identifies a group of patients with tICH, safely managed in a non-ICU setting can be created.
Materials and Methods
Study Design
This is a single center, retrospective cohort study of adult trauma patients who sustained a tICH on initial ED cranial computed tomography (CT) scan and were evaluated at a Level 1 trauma center from May 2006 through December 2008. The institutional review board at the study site approved the study.
Study Setting and Population
We searched the hospital trauma registry for International Statistical Classification of Diseases and Related Health Problems (ninth edition) (ICD-9) codes specific for tICH (codes 851–854) to identify the cohort of subjects. Eligible subjects included adult patients (18 years of age or older) who were identified with an acute tICH on cranial CT during ED evaluation. An acute tICH was defined as the presence of an acute subarachnoid hemorrhage, epidural hematoma, subdural hematoma, intraventricular hemorrhage, intraparenchymal hemorrhage/contusion, or diffuse axonal injury as interpreted by attending radiologist on cranial CT scan. We excluded patients with documented pre-existing “Do-Not-Resuscitate” (DNR) orders. At the study site, patients with tICH are routinely admitted under the trauma or neurological surgical service to the ICU for neurologic monitoring for a minimum of 24 hours following injury.
Study Protocol
Eligible patients underwent data abstraction following previously published guidelines on retrospective chart review.19 We abstracted data from the electronic medical record using a standardized data collection form with pre-defined variables by a single data abstractor trained to the methodology of data collection (DKN). The data abstractor was not blinded to study hypothesis. Variables collected included age, gender, mechanism of injury, prior anticoagulant use, co-morbidities, initial ED Glasgow Coma Scale (GCS), loss of consciousness, initial ED systolic blood pressure, heart rate, and respiratory rate, and alcohol intoxication. ED radiologic and laboratory variables collected included initial cranial CT results, initial platelet count, international normalized ratio (INR) level, and hematocrit. Additional variables collected during ED and hospital treatment include administration of packed red blood cells (PRBC), fresh frozen plasma (FFP), or recombinant activated factor VII (rFVIIa). ICU and hospital length of stay (days) were also recorded. Alcohol intoxication was considered positive if laboratory ethanol level ≥ 10 mg/dL. Cranial CT results were coded into anatomical categorical variables based on attending radiologist text report. ED data were abstracted prior to knowledge of patient outcomes. Data missing from the medical record were coded as missing in the dataset.
An Abbreviated Injury Score (AIS) for head and neck, face, chest, abdomen, extremities, and external body regions and the overall Injury Severity Score (ISS) were previously available from the trauma registry.20 These calculations were previously imputed into the trauma registry by data abstractors trained in these calculations. The AIS and ISS are scoring systems developed to measure injury severity based on anatomical injuries divided by body regions.20 A Marshall Score was calculated based on the initial cranial CT scan results following previously described methodology.21, 22 It is a validated classification system that predicts patient outcome based on cranial CT findings (Table 1).21, 22
Table 1.
Marshall score
| Class | Definition |
|---|---|
| CT Class 1 | No lesions present |
| CT Class 2 | Lesions and/or midline shift ≤ 5 mm, cisterns present; no high- or mixed-density lesions > 25 cm3 |
| CT Class 3 | Midline shift ≤ 5 mm, cisterns compressed or absent, no high- or mixed-density lesions > 25 cm3 |
| CT Class 4 | Midline shift > 5 mm, no high- or mixed-density lesions > 25 cm3 |
| CT Class 5 | Any surgically evacuated lesion |
| CT Class 6 | High- or mixed-density lesions > 25 cm3 not surgically evacuated |
Measurements
The primary outcome measure of this study is the presence of a critical care intervention at any point during ED care or hospitalization. The need for ICU admission was defined as the presence of a critical care intervention at any point during ED or hospitalization. The list of definitions of critical care interventions was adapted from the Task Force of American College of Critical Care Medicine Guidelines for ICU admission 23 and represented specific interventions or patient conditions that would warrant intensive care monitoring or management. Definitions of critical care interventions are included in Table 2.
Table 2.
Definitions of critical care interventions
| Critical care intervention | Definition |
|---|---|
| Mechanical ventilation | Mechanical ventilation for acute respiratory failure |
| RBC transfusion | Transfusion of packed red blood cells |
| FFP transfusion | Transfusion of fresh frozen plasma |
| Neurosurgical intervention | Craniotomy/craniectomy, burr hole evaluation of hematoma, placement of a subdural drain, placement of an intracranial pressure monitor/intraventricular catheter, or treatment with mannitol or hypertonic saline |
| Invasive monitoring | Use of central venous catheter to measure central venous pressure (not for venous access alone), or the use an arterial line to measure blood pressure, or the use of a pulmonary artery catheter to measure pulmonary artery wedge pressure |
| Arrhythmia | Non-sinus arrhythmia less than 40 or greater than 120 beats/minute with the need for urgent intervention |
| Vasopressor or inotrope use | Use of dopamine, norepinephrine, epinephrine, dobutamine, phenylephrine, or vasopressin for vasopressor or inotropic support |
| Interventional angiography | Use of interventional angiography for therapeutic purposes |
| Cardiac arrest | Cardiac arrest requiring cardiopulmonary resuscitation |
Predictor variables for analysis were determined a priori and were based on clinical sensibility and prior literature.24–29 These variables included: age, loss of consciousness, mechanism of injury, GCS score, prior anticoagulant medication use, isolated head injury (defined as non-head AIS < 3), initial systolic blood pressure, and Marshall score.
Data Analysis
Data was entered into a spreadsheet and analyzed using STATA 10.0 statistical software (STATA Corp, College Station, TX). Interval data were reported as the median and interquartile range (IQRs). Proportions were presented with 95% confidence intervals. Clinically sensible cutoff values of predictor variables were determined a priori.
We derived the decision rule with binary recursive partitioning using Classification and Regression Trees (CART) software (Salford Systems, San Diego, CA).30 We used the Ginni splitting function in CART and set the misclassification cost for missing a patient with a critical care intervention at 20:1. This represents the relative cost of misclassifying 20 patients who did not receive a critical care intervention for 1 patient who did receive a critical care intervention. We internally validated the rule using 10-fold internal cross validation.
We required 10 subjects with the outcome of interest for each variable entered into the multivariate model, and planned to evaluate eight different clinical variables.31 Thus, we required 80 patients with the outcome of interest. Based on preliminary data, we estimated that 20% of eligible patients would receive a critical care intervention, requiring a cohort of 400 patients.
Results
A total of 432 subjects were identified from the trauma registry and met inclusion criteria. Median age was 48 years old (IQR 30–63) and 299 patients (69%; 95%CI 65–74) were male. The majority of patients were admitted to the ICU (407/432; 96%) with the remaining patients admitted to a non-ICU (floor) setting. None were discharged from the ED. The median GCS was 14 (IQR 11–15) with the majority of patients having a GCS 13–15 (71%; 95%CI 66–75). The most common tICHs were intraparenchymal hemorrhage (55%) and subarachnoid hemorrhage (46%). See Table 3 for complete patient characteristics.
Table 3.
Patient characteristics
| Characteristic | Patients | % or Median (95% CI or IQR) |
|---|---|---|
| History of anticoagulant use | 34/432 | 7.9% (95%CI 5.5–11) |
| Fall from standing | 103/432 | 24% (95%CI 20–28) |
| Fall from height | 31/432 | 7.2% (95%CI 4.9–10) |
| Motor vehicle collision | 170/432 | 39% (95%CI 35–44) |
| Pedestrian struck | 29/432 | 6.7% (95%CI 4.5–9.5) |
| Bicyclist struck | 18/432 | 4.2% (95%CI 2.5–6.5) |
| Gunshot wound | 7/432 | 1.6% (95%CI 0.65–3.3) |
| Direct blow | 61/432 | 14% (95%CI 11–18) |
| Unknown mechanism of injury | 13/432 | 3.0% (95%CI 1.6–5.1) |
| Alcohol intoxication | 137/430 | 32% (95%CI 27–36) |
| Initial systolic blood pressure (mmHg) | - | 134 (IQR 119–150) |
| Initial heart rate | - | 90 (IQR 70–102) |
| Initial respiratory rate | - | 18 (IQR 16–20) |
| Initial platelet count (per ml) | - | 232 (IQR 192–287) |
| Initial INR | - | 1.0 (IQR 0.95–1.1) |
| Initial hematocrit (%) | - | 40 (IQR 35–43) |
| Injury severity | ||
| Mild TBI: GCS 13–15 | 306/432 | 71% (95%CI 66–75) |
| Moderate TBI: GCS 9–12 | 31/432 | 7.2% (95%CI 4.9–10) |
| Severe TBI: GCS < 8 | 95/432 | 22% (95%CI 18–26) |
| Isolated head injury | 321/432 | 74% (95%CI 70–78) |
| Loss of consciousness reported | 175/421 | 42% (95%CI 37–46) |
| Abbreviated injury score, head | - | 4 (IQR 3–4) |
| Injury severity score | - | 17 (IQR 16–25) |
| Marshall Score | - | 2 (IQR 2-2) |
| Cranial CT scan injuries | ||
| Depressed skull fracture | 4/432 | 0.9% (95%CI 0.02–2.4) |
| Nondepressed skull fracture | 30/432 | 6.9% (95%CI 4.7–9.8) |
| Intraparenchymal hemorrhage | 238/432 | 55% (95%CI 50–60) |
| Subdural hemorrhage | 160/432 | 37% (95%CI 32–42) |
| Epidural hemorrhage | 19/432 | 4.4% (95%CI 2.7–6.8) |
| Subarachnoid hemorrhage | 199/432 | 46% (95%CI 41–51) |
| Interventricular hemorrhage | 41/432 | 9.5% (95%CI 6.9–13) |
| Diffuse axonal injury | 15/432 | 3.5% (95%CI 2.0–5.7) |
| Presence of cerebral shift | 44/432 | 10% (95%CI 7.5–13) |
| Presence of cerebral mass effect | 53/432 | 12% (95%CI 9.3–16) |
| Herniation | 24/432 | 5.6% (95%CI 3.6–8.2) |
Abbreviations: GCS, Glasgow Coma Score; TBI, traumatic brain injury; CT, computed tomography; INR, international normalized ratio; IQR, Interquartile range; CI, confidence interval
One hundred and seventy-four of 432 (40%; 95%CI 36–45) patients had a critical care intervention with mechanical ventilation (32%) and RBC transfusion (16%) the most common types of critical care interventions (Table 4). Differences in predictor variables frequencies between patients with and without a critical care intervention are presented in Table 5.
Table 4.
Critical care interventions
| Critical care intervention* | Patients | % (95% CI) |
|---|---|---|
| Mechanical ventilation | 137/432 | 32% (95%CI 27–36) |
| RBC transfusion | 68/432 | 16% (95%CI 12–20) |
| FFP transfusion | 44/432 | 10% (95%CI 7.5–13) |
| Neurosurgical intervention | 31/432 | 7.2% (95%CI 4.9–10) |
| Invasive monitoring | 6/432 | 1.4% (95%CI 0.51–3.0) |
| Arrhythmias requiring treatment | 4/432 | 0.93% (95%CI 0.25–2.4) |
| Vasopressor or inotrope use | 3/432 | 0.69% (95%CI 0.14–0.20) |
| Interventional angiography | 1/432 | 0.23% (95%CI 0–1.3) |
| Cardiac arrest | 0/432 | 0% (95%CI 0–0.85) |
Abbreviations: RBC, red blood cell; FFP, fresh frozen plasma; CI, confidence interval
see table 1 for definitions of critical care intervention
Table 5.
Bivariate analysis of tree predictor variables of critical care intervention
| Variable | Critical care intervention n=174 (%; 95% CI) | No critical care intervention n=258 (%, 95% CI) | Difference in proportions (95% CI) |
|---|---|---|---|
| Abnormal mental status | 147 (84%; 95%CI 78–90) | 77 (30%; 95%CI 24–36) | 54% (95%CI 45–60) |
| Marshall score > 2 | 59 (34%; 95%CI 27–41) | 10 (3.9%; 95%CI 1.9–7.0) | 30% (95%CI 27–41) |
| Non-isolated head injury | 75 (43%; 95%CI 36–51) | 36 (14%; 95%CI 10–19) | 29% (95%CI 21–38) |
| Non-fall from standing | 138 (79%; 95%CI 73–85) | 189 (73%; 95%CI 67–79) | 6.1% (95%CI −2.0–14) |
| Initial SBP < 90 mmHg | 10 (5.7%; 95%CI 2.8–10) | 2 (0.78%; 95%CI 0.09–2.8) | 5.0% (95%CI 1.4–8.6) |
| Age ≥ 65 years | 44 (25%; 95%CI 19–32) | 53 (21%; 95%CI 16–26) | 4.8% (95%CI −3.4–13) |
| Loss of consciousness* | 71 (42%; 95%CI 34–50) | 104 (41%; 95%CI 35–48) | 0.3% (95%CI −9.3–10) |
| Anti-coagulant use | 12 (6.9%; 95%CI 3.6–12) | 22 (8.5%; 95%CI 5.4–13) | −1.6% (95%CI −6.7–3.4) |
Abbreviations: SBP, systolic blood pressure; CI, confidence interval
Variable missing in 4 subjects with critical care intervention and 7 subjects without critical care intervention
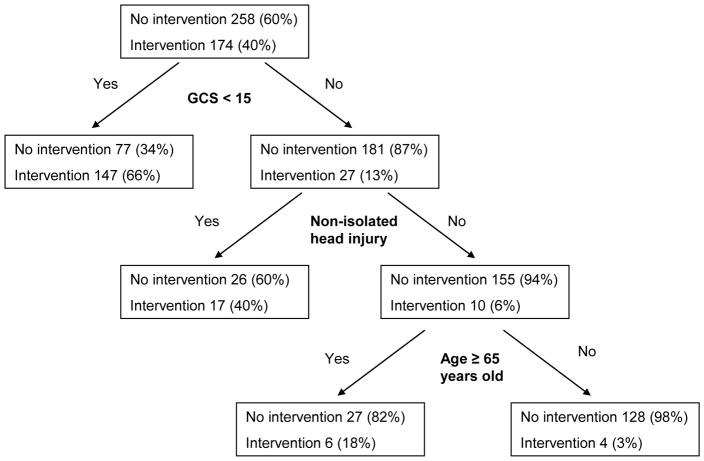
Binary recursive partitioning derived a rule with the following three predictor variables for requiring a critical care intervention: Abnormal mental status (GCS < 15), non-isolated head injury, and age ≥ 65 years of age (Figure). the generated rule had the following test characteristics: sensitivity = 170/174 (98%; 95%CI 94–99), specificity = 128/258 (50%; 95%CI 44–56), positive predictive value = 170/300 (57%; 95%CI 51–62), and negative predictive value = 128/132 (97%; 95%CI 93–99). The area under the curve for the decision rule was 0.74 (95%CI 0.70–0.77). Internal validation using 10-fold cross validation demonstrated a stable rule.
Figure.
Derived model from binary recursive partitioning
Four patients were misclassified by the rule (determined to be low risk but had a critical care intervention) (Table 6). Two patients received a critical care intervention due to head injury; one patient requiring endotracheal intubation for neurological deterioration during ED evaluation and another patient underwent craniotomy for an epidural hematoma. One patient received FFP transfusions for a pre-existing coagulopathy and another patient underwent endotracheal intubation on hospital day three for delirium tremens. All patients survived to hospital discharge.
Table 6.
Patients misclassified as low risk by the clinical decision rule
| Patient | Critical care intervention | LOS (ICU, hospital) |
|---|---|---|
| 62 year old male in MVC | Intubated on hospital day # 3 for delirium tremens | 13, 27 |
| 55 year old male with a ground level fall | Intubated in the ED after declining mental status upon return from computed tomography scan | 6, 17 |
| 21 year old male in MVC two days prior to ED arrival | Maintained normal mental status but taken for craniotomy due to size of epidural hematoma | 2, 7 |
| 41 year old male with a ground level fall and a history of end stage liver disease | Initial INR was 1.84 thus required 2 units of fresh frozen plasma in ED, no other blood product transfusion during hospitalization | 5, 59 |
Abbreviations: MVC, motor vehicle collision; ED, emergency department; INR, international normalized ratio; LOS, length of stay; ICU, intensive care unit; GCS, Glasgow Coma Score; ISS, injury severity score
Discussion
The primary objective of this study was to derive a clinical decision rule to identify a subset of low risk patients with tICH who do not need to be admitted to the ICU. Using binary recursive partitioning, we were able to develop a decision rule with excellent test characteristics. The results are promising for a number of reasons. First, the rule is highly sensitive in detecting a large number of patients with the outcome of interest (sensitivity 98%). Second, the high risk variables identified by the rule makes clinical sense as being predictive of requiring critical care interventions in patients with tICH. These variables previously have demonstrated to be prognostic in tICH.24–28 Third, a significant proportion of patients with tICH (31%) were low risk by the clinical decision rule. This suggests that successful validation and implementation of this rule will have a broad impact with significant resource sparing.32
Prior studies have evaluated baseline characteristics to predict outcome in traumatic patients with tICH.33–35 Trauma scoring systems including the ISS 36 and the Revised Trauma Score (RTS)37 predict mortality and other functional outcomes28 but are not designed as an ICU triage tool as they are calculated after the patient is discharged. A number of other prediction models specific to traumatic brain injury (TBI) exist, however the methodology is limited, and they are not clinically practical.28 Only one study to date attempts to predict the need for specialized ICU admission in patients with tICH evaluating baseline characteristics.34 Age and pupillary reactivity were significant predictors however the authors were unable to develop a sufficiently accurate prediction model. This study, however, did not evaluate patients with mild tICH (GCS scores 13–15) and had a high threshold for requiring ICU admission (raised intracranial pressure and need for neurosurgical intervention). Compared to these prior trauma scoring systems and prediction models, our decision rule is largely generalizable to all patients with tICH in the ED and uses data readily available to clinicians.
The decision rule failed to identify four patients who ultimately underwent a critical care intervention which may be concerning to some clinicians. Closer inspection of these cases, however, indicates that the failure of the clinical decision rule to identify these patients would likely have little impact on the patients’ outcomes. One patient underwent endotracheal intubation in the ED for declining mental status after returning from CT scanning. A second patient was taken from the ED for neurosurgery for a large epidural. Thus, both these interventions occurred prior to disposition from the ED. The other two patients’ interventions were unrelated to their tICH. Thus, the clinical decision rule’s rule performance was perhaps better than reported.
The list of critical care interventions was adapted from prior published guidelines that broadly defined disease and physiological conditions that warranted ICU admission.23 This list was narrowed to represent critical care interventions specific for the injured patient. While most critical care interventions are universally equated to requiring ICU admission (i.e.: mechanical ventilation, neurosurgical intervention, invasive monitoring) certain interventions (specifically RBC and FFP transfusion) may be considered by clinicians to not require ICU admission. We opted to include blood product transfusion in our primary analysis since our aim was to provide clinicians with the most conservative model. Analysis without blood product transfusion derived the same model with similar test characteristics: sensitivity = 140/143 98%; 95% CI 94–100), specificity = 129/289 (45%; 95%CI 39–51), and AUC = 0.71 (95%CI 0.68–0.74).
Our study has several limitations. This study is retrospective and is subject to the limitations of medical record review. This study was conducted at a single, Level 1 trauma center where severity of injury, hospital resources, and management of TBI might not be generalizable to other settings. At the study site, the large majority of patients were admitted to the ICU and thus we are unable to appropriately evaluate patients with tICH that were managed in a non-ICU setting. Moreover, unexpected advantages with ICU care in these patients that prevented a critical care intervention from occurring would not be identified. For example, tICH patients admitted to the ICU at our center have neurological checks by nursing staff every one hour compared to every two hours or greater in non-ICU settings. Finally, the use of other multivariate analysis methodology may have produced a more accurate rule. We did conduct multivariate logistical regression which identified abnormal mental status (GCS < 15), non-isolated head injury, and Marshall score > 2 as predictive of a critical care intervention. Absence of any of these high risk criteria derived a rule that was less sensitive (93%; 95%CI 88–96) than the rule derived by binary recursive partitioning. This is consistent with other studies which demonstrate that logistic regression tends to derive a rule that has a higher overall accuracy (i.e.: better overall classification of patients) while binary recursive partitioning tends to have a higher overall sensitivity (though dependent on assigned misclassification costs).32, 38
Despite the promising results of this preliminary study, clinical decision rules are best derived and validated on prospectively collected data.32 Thus, these results require confirmation and potential refinement in a prospective study. First, clinical data is more reliable when collected prospectively. This is particularly true for the measurement of the outcome of interest (critical care interventions) which relies on accurate medical record documentation. Moreover prospective data has less missing data compared to retrospective data.32 Second, the predictor variable of isolated head injury was defined as non-head AIS < 3. AIS and ISS are generally not determined until the hospital course is completed, although calculation of this score may be done once the cranial CT scan results are obtained, during the patient’s ED evaluation. Thus, this predictor variable may have limited use by clinicians at the time the admission decision is being determined. Prospectively collected data allows a specifically defined isolated head injury definition based on actual injuries. Third, we were unable to collect data on physician gestalt in the retrospective study. Demonstrating that the clinical decision rule performs better than physician gestalt is important for successful implementation of the rule.32 Fourth, validation should be conducted on a separate dataset using the clinical decision rule.32 This would mitigate potential bias from evaluation of the same dataset. Finally, the retrospective data was obtained from a trauma registry which may not have captured all eligible patients making it prone to selection bias.
Conclusion
In conclusion, we were able to derive a clinical decision rule which accurately identifies a low risk subset of patients with tICH that do not require ICU admission. Further validation and refinement of these findings would allow for more appropriate ICU resource utilization.
Acknowledgments
We thank Ayan Patel, UC Davis Clinical and Translational Science Center for his work on database development.
Footnotes
Conflict of interest: None
Address for reprints: Reprints not available from the authors
References
- 1.Groeger JS, Guntupalli KK, Strosberg M, et al. Descriptive analysis of critical care units in the United States: patient characteristics and intensive care unit utilization. Crit Care Med. 1993;21:279–91. doi: 10.1097/00003246-199302000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Frost SA, Alexandrou E, Bogdanovski T, Salamonson Y, Parr MJ, Hillman KM. Unplanned admission to intensive care after emergency hospitalisation: risk factors and development of a nomogram for individualising risk. Resuscitation. 2009;80:224–30. doi: 10.1016/j.resuscitation.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Escarce JJ, Kelley MA. Admission source to the medical intensive care unit predicts hospital death independent of APACHE II score. JAMA. 1990;264:2389–94. [PubMed] [Google Scholar]
- 4.Sax FL, Charlson ME. Medical patients at high risk for catastrophic deterioration. Crit Care Med. 1987;15:510–5. doi: 10.1097/00003246-198705000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Goldhill DR, Sumner A. Outcome of intensive care patients in a group of British intensive care units. Crit Care Med. 1998;26:1337–45. doi: 10.1097/00003246-199808000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Hillman KM, Bristow PJ, Chey T, et al. Duration of life-threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28:1629–34. doi: 10.1007/s00134-002-1496-y. [DOI] [PubMed] [Google Scholar]
- 7.Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35:1477–83. doi: 10.1097/01.CCM.0000266585.74905.5A. [DOI] [PubMed] [Google Scholar]
- 8.Escher M, Perneger TV, Chevrolet JC. National questionnaire survey on what influences doctors’ decisions about admission to intensive care. BMJ. 2004;329:425. doi: 10.1136/bmj.329.7463.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham DI, Ford I, Adams JH, et al. Ischaemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry. 1989;52:346–50. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall LFGT, Klauber MR. The outcome of severe closed head injury. J Neurosurg. 1991;75:S28–36. [Google Scholar]
- 11.Huynh T, Jacobs DG, Dix S, Sing RF, Miles WS, Thomason MH. Utility of neurosurgical consultation for mild traumatic brain injury. Am Surg. 2006;72:1162–5. discussion6–7. [PubMed] [Google Scholar]
- 12.Sifri ZC, Livingston DH, Lavery RF, et al. Value of repeat cranial computed axial tomography scanning in patients with minimal head injury. Am J Surg. 2004;187:338–42. doi: 10.1016/j.amjsurg.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Vos PE, Battistin L, Birbamer G, et al. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207–19. doi: 10.1046/j.1468-1331.2002.00407.x. [DOI] [PubMed] [Google Scholar]
- 14.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58:S25–46. doi: 10.1227/01.NEU.0000210365.36914.E3. discussion Si–iv. [DOI] [PubMed] [Google Scholar]
- 15.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58:S16–24. discussion Si–iv. [PubMed] [Google Scholar]
- 16.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute epidural hematomas. Neurosurgery. 2006;58:S7–15. discussion Si–iv. [PubMed] [Google Scholar]
- 17.Hesdorffer DC, Ghajar J. Marked improvement in adherence to traumatic brain injury guidelines in United States trauma centers. J Trauma. 2007;63:841–7. doi: 10.1097/TA.0b013e318123fc21. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 18.Hesdorffer DC, Ghajar J, Iacono L. Predictors of compliance with the evidence-based guidelines for traumatic brain injury care: a survey of United States trauma centers. J Trauma. 2002;52:1202–9. doi: 10.1097/00005373-200206000-00031. [DOI] [PubMed] [Google Scholar]
- 19.Worster A, Bledsoe RD, Cleve P, Fernandes CM, Upadhye S, Eva K. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. 2005;45:448–51. doi: 10.1016/j.annemergmed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Copes WS, Champion HR, Sacco WJ, Lawnick MM, Keast SL, Bain LW. The Injury Severity Score revisited. J Trauma. 1988;28:69–77. doi: 10.1097/00005373-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–92. [PubMed] [Google Scholar]
- 22.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–82. doi: 10.1227/01.neu.0000186013.63046.6b. discussion -82. [DOI] [PubMed] [Google Scholar]
- 23.Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27:633–8. [PubMed] [Google Scholar]
- 24.Brown AW, Malec JF, McClelland RL, Diehl NN, Englander J, Cifu DX. Clinical elements that predict outcome after traumatic brain injury: a prospective multicenter recursive partitioning (decision-tree) analysis. J Neurotrauma. 2005;22:1040–51. doi: 10.1089/neu.2005.22.1040. [DOI] [PubMed] [Google Scholar]
- 25.Demetriades D, Kuncir E, Murray J, Velmahos GC, Rhee P, Chan L. Mortality prediction of head Abbreviated Injury Score and Glasgow Coma Scale: analysis of 7,764 head injuries. J Am Coll Surg. 2004;199:216–22. doi: 10.1016/j.jamcollsurg.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Hukkelhoven CW, Steyerberg EW, Habbema JD, et al. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J Neurotrauma. 2005;22:1025–39. doi: 10.1089/neu.2005.22.1025. [DOI] [PubMed] [Google Scholar]
- 27.Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–9. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perel P, Edwards P, Wentz R, Roberts I. Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak. 2006;6:38. doi: 10.1186/1472-6947-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mina AA, Knipfer JF, Park DY, Bair HA, Howells GA, Bendick PJ. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma. 2002;53:668–72. doi: 10.1097/00005373-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Brieman LFJ, Olshen RA, et al. Classification and Regression Trees. Washington, DC: Chapman & Hall; 1984. [Google Scholar]
- 31.Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol. 2005;58:475–83. doi: 10.1016/j.jclinepi.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Stiell IG, Wells GA. Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerg Med. 1999;33:437–47. doi: 10.1016/s0196-0644(99)70309-4. [DOI] [PubMed] [Google Scholar]
- 33.Andrews PJ, Sleeman DH, Statham PF, et al. Predicting recovery in patients suffering from traumatic brain injury by using admission variables and physiological data: a comparison between decision tree analysis and logistic regression. J Neurosurg. 2002;97:326–36. doi: 10.3171/jns.2002.97.2.0326. [DOI] [PubMed] [Google Scholar]
- 34.Hukkelhoven CW, Steyerberg EW, Habbema JD, Maas AI. Admission of patients with severe and moderate traumatic brain injury to specialized ICU facilities: a search for triage criteria. Intensive Care Med. 2005;31:799–806. doi: 10.1007/s00134-005-2628-y. [DOI] [PubMed] [Google Scholar]
- 35.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:329–37. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 36.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- 37.Champion HR, Sacco WJ, Copes WS, Gann DS, Gennarelli TA, Flanagan ME. A revision of the Trauma Score. J Trauma. 1989;29:623–9. doi: 10.1097/00005373-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med. 1990;113:664–70. doi: 10.7326/0003-4819-113-9-664. [DOI] [PubMed] [Google Scholar]