Abstract
Plasmodium falciparum malaria is a deadly pathogen. The invasion of red blood cells (RBCs) by merozoites is a target for vaccine development. Although anti-merozoite antibodies can block invasion in vitro, there is no efficacy in vivo. To explain this discrepancy we hypothesized that complement activation could enhance RBC invasion by binding to the complement receptor 1 (CR1). Here we show that a monoclonal antibody directed against the merozoite and human polyclonal IgG from merozoite vaccine recipients enhanced RBC invasion in a complement-dependent manner and that soluble CR1 inhibited this enhancement. Sialic acid-independent strains, that presumably are able to bind to CR1 via a native ligand, showed less complement-dependent enhancement of RBC invasion than sialic acid-dependent strains that do not utilize native CR1 ligands. Confocal fluorescent microscopy revealed that complement-dependent invasion resulted in aggregation of CR1 at the RBC surface in contact with the merozoite. Finally, total anti-P. berghei IgG enhanced parasite growth and C3 deficiency decreased parasite growth in mice. These results demonstrate, contrary to current views, that complement activation in conjunction with antibodies can paradoxically aid parasites invade RBCs and should be considered in future design and testing of merozoite vaccines.
Keywords: Malaria, Complement, Red blood cells, Merozoites, CR1
Graphical Abstract
Highlights
-
•
Anti-merozoite antibodies and complement activation can paradoxically aid the malaria parasite invade RBCs.
-
•
Complement receptor 1 on RBCs mediates antibody and complement-dependent invasion.
-
•
The malaria parasite can use the immune response of the host to its own benefit.
-
•
This information should be used in the design and testing of future anti-merozoite vaccines.
The development of a malaria vaccine that blocks the invasion of red blood cells (RBCs) has been an elusive goal. While antibodies against merozoites, the invading stage of the parasite, can block RBC invasion in the test tube, tests in humans have been disappointing. We propose that one potential explanation is that the parasite is able to use part of the immune system to aid in the invasion of RBCs. Here we show that complement, a branch of the innate immune response, and anti-merozoite antibodies can enhance invasion of RBCs by malaria parasites. Therefore, this information should be taken into consideration in the future design and testing of anti-merozoite vaccines.
1. Introduction
Malaria, a mosquito-borne infectious disease caused by eukaryotic intracellular protists of the genus Plasmodium, kills close to one million people worldwide each year, predominantly children under 5 years of age (Murray et al., 2012, Wold Health Organization, 2013). Of the five species of Plasmodium that can infect humans, infection with Plasmodium falciparum accounts for the vast majority of deaths worldwide. Plasmodium's complex life cycle involves invasion of hepatocytes and red blood cells (RBCs); however, the clinical symptoms arise from the invasion of RBCs by the asexual blood stage parasite. Antibodies are thought to play an important role in natural immunity as demonstrated by the reduction in parasitemia and clinical symptoms in P. falciparum-infected individuals following passive transfer of immunoglobulins from semi-immune donors (COHEN et al., 1961, McGregor 1964a, Sabchareon et al., 1991). However, the effector mechanisms are poorly understood.
Development of a vaccine to block RBC invasion has proven to be an elusive goal. Much of the effort has been focused on the merozoite surface protein 1 (MSP1) and the apical membrane antigen 1 (AMA1). Multiple preclinical vaccine studies have demonstrated, using growth inhibition assays (GIA), that antibodies targeting MSP1 and AMA1 of P. falciparum have in vitro RBC invasion and growth inhibitory activity (Angov et al., 2003, Chang et al., 1992, Kennedy et al., 2016). In addition, some degree of protective immunity has been seen in some animal models (Darko et al., 2005, Singh et al., 2003, Singh et al., 2006). Unfortunately, to date, these studies have not translated into in vivo efficacy in human vaccine trials (Ogutu et al., 2009, Sagara et al., 2009, Spring et al., 2009). Thus, GIA is a poor predictor of blood stage protective immune responses despite the fact that antibodies do inhibit RBC invasion. The reasons for this discrepancy are unknown.
One possible explanation for this discrepancy came to light as the result of the discovery that the complement receptor 1 (CR1) is a sialic acid (SA)-independent receptor for P. falciparum (Spadafora et al., 2010, Tham et al., 2010). The complement system is part of the innate immune response and is an important effector arm of humoral immunity. It can be activated via three main pathways: the classical pathway (CP); the lectin pathway (LP); and the alternative pathway (AP) (Ricklin et al., 2010). Once activated, the complement system induces the formation of opsonins (C3b, C4b) that promote phagocytosis, induce lysis by formation of the terminal complement complex (TCC), and promote an inflammatory response (Ricklin et al., 2010). Once bound to the pathogen, surface C3b and C4b serve as ligands for CR1, which is present on RBCs as well as most leukocytes (Fearon, 1980, Tas et al., 1999). CR1 also binds complement factors C1q and mannan-binding lectin (MBL) (Ghiran et al., 2000, Tas et al., 1999).
We hypothesize that P. falciparum is capable of exploiting the opsonizing qualities of complement deposition on the merozoite surface which will allow it to bind to CR1 and invade via this invasion pathway. If we are correct, complement activation could negate the inhibitory activity of anti-merozoite neutralizing antibodies generated post vaccination or during natural infection.
2. Materials and Methods
2.1. Parasites, Parasite Culture, and RBC Treatment
SA-independent strains (7G8, 3D7, HB3, and Dd2NM) were obtained from the Walter Reed Army Institute of Research. SA-dependent strains (FVO, Camp, Dd2, and FCR3) were obtained from the Malaria Research and Reference Reagent Resource Center (BEI Resources, Manassas, VA). Parasite cultures were maintained at 1–4% hematocrit (Hct) in O + blood with 10% heat-inactivated (HI) plasma in RPMI 1640 Medium (Sigma-Aldrich, St. Louis, MO) with 25 μg/ml gentamicin, 20 μg/ml hypoxanthine, and 7.5% w/v NaHCO3 (complete media) in malaria gas (5% O2, 5% CO2, and 95% N) at 37 °C. Cultures were synchronized twice a week by 5% sorbitol lysis (Lambros and Vanderberg, 1979). Neuraminidase treatment of RBCs was carried out as described (Spadafora et al., 2010).
2.2. Sera, Complement Factors, and CH50/AH50 Assay
Non-hemolyzed whole blood was collected with a 21 gauge needle from two O + volunteers into glass tubes without additives (Becton Dickinson, Franklin Lakes, NJ) and allowed to clot at room temperature for 50 min. The samples were centrifuged at 1300 × g for 15 min and the serum was removed and re-centrifuged for 5 min again to pellet any residual RBCs or clot particles. The serum was aliquoted and stored at − 80 °C. Serum was used fresh (FS) or after heat inactivation (HIS) at 56 °C for 30 min in 200 μl aliquots in 1.5 ml polypropylene microcentrifuge tubes (Denville Scientific, South Plainfield, NJ). Serum C2 and Factor B (fB) were selectively inactivated by incubation of 200 μl serum aliquots in 1.5 ml microcentrifuge tubes in a 56 °C water bath for 3 min with constant mixing (Araujo et al., 1991). Purified complement factors (C1q, C2, C3, C4) and selectively depleted or inactivated sera were obtained commercially (CompTech, Tyler, TX). 50% CP and AP complement hemolytic (CH50 and AH50) assays were performed using the standard methods described in the literature (Morgan, 2000).
2.3. In Vitro Invasion Assays
Invasion assays were carried out in triplicate wells of a 96-well plate containing sorbitol-synchronized cultures of late trophozoites or schizonts at 0.5% to 2% parasitemia in 2–4% hematocrit. RBCs had a mean CR1 expression of 600 molecules per RBC as measured by flow cytometry (Spadafora et al., 2010). Inhouse prepared serum (FS or HIS) was always autologous to the RBCs used in the assays. Complement-depleted or C3/C4-inactivated sera were always commercially-obtained (CompTech, TX) and came from pooled donor blood, and was thus, heterologous to the RBCs in the assays. Sera were added to a final concentration of 10%. The cyclical C3 inhibitor peptide compstatin (NH3-IIe-Cys-Val-Val-Gln-Asp-Trp-Gly-His-His-Arg-Cys-Thr-COOH) (Tocris Bioscience, Bristol, UK) (Mastellos et al., 2015), was used to determine the effect of blocking C3. A peptide derived from the linearized and scrambled sequence of compstatin without cysteins (NH3-Arg-Thr-Ala-Trp-Gln-His-Asp-Ala-lle-His-Val-Gly-Val-COOH) was synthesized and used as a control. sCR1 was used as an inhibitor (Celldex Therapeutics, Hampton, NH) and fetuin (Sigma-Aldrich, St. Louis, MO), an inert glycoprotein with no complement activity, was used as negative control protein where appropriate. Antibodies were added at different concentrations. Mouse monoclonal antibody mAb5.2 was raised against the 19 kDa subunit of the merozoite surface protein 1 (MSP119) (Siddiqui et al., 1986) and was purified from cultured hybridoma by protein A/G chromatography (Thermo Fisher Scientific, Rockford, IL). Mouse IgG2b Clone eBMG2b (eBioscience, San Diego, CA) was used as isotype control. IgG from individuals vaccinated with MSP142 (Otsyula et al., 2013), comprising the C-terminal 42-kDa portion of the FVO variant of P. falciparum MSP1, was purified by protein A/G chromatography. The plate was placed in a gas-impermeable heat-sealable bag and inflated with malaria culture gas (Haynes et al., 2002). As an alternative procedure we used filter-purified merozoites as described by Boyle et al., 2010, Boyle et al., 2015 with the exception that late trophozoite/schizonts were enriched using a Percoll gradient (Moll et al., 2008). After 20 to 24 h, 5 μl aliquots of individual wells were added to 100 μl 5 μg/ml Hoechst 33342 (Life Technologies, Grand Island, NY) in PBS containing 2% paraformaldehyde (Sigma-Aldrich). At least 100,000 RBCs were acquired for each sample. Acquisition was done using a LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ) equipped with a violet laser and analysis was performed using FCS Express (De novo Software, Glendale, CA). RBCs with Hoechst-positive ring stage parasitemia (early trophozoite) were used as endpoint for 24-h invasion assays. The background staining of an uninfected RBC sample was subtracted. The majority of the experiments were repeated 2–3 times.
2.4. Merozoite Attachment Assay
Unless otherwise stated, all the centrifugation steps were at 770 × g for 5 min at room temperature (RT). Attachment assays were carried out as invasion assays except that highly synchronous late stage parasite cultures were incubated with 10 μg/ml of leupeptin (Sigma-Aldrich) for 6 to 8 h followed by three washes with RPMI 1640. The hematocrit was adjusted to 2% by the addition of fresh RBCs. The final culture medium also contained 2 μM cytochalasin D (Sigma-Aldrich) and 10% complement deficient or reconstituted serum in the presence or absence of mouse mAb5.2 or IgG2b. The cultures were incubated again for 3–4 h at 37 °C in malaria gas followed by centrifugation at 400 × g for 1 min at 4 °C and two washes with 100 μl of 1% BSA/PBS blocking buffer. mAb5.2 (2 mg/ml), if not added previously, and goat polyclonal anti-C3 (MP Biomedicals, Santa Ana, CA) antibodies diluted 1:33 in blocking buffer were added for 30 min at 4 °C. Following three washes with blocking buffer, the pellets were resuspended in 1:100 dilution of donkey anti-goat IgG-PerCP R&D Systems, Minneapolis, MN) and goat anti-mouse DyLight 488 (KPL, Gaithersburg, MD) in blocking buffer and incubated for 30 min at 4 °C. After an additional three washes with blocking buffer the pellets were resuspended in 2% paraformaldehyde/PBS with 5 μg/ml Hoechst 33342 (Life Technologies) solution. Acquisition by flow cytometry was carried out as above.
2.5. Confocal Microscopy of Merozoite Attachment
For confocal microscopy of the interaction of merozoites with RBCs the culture conditions were the same as for attachment assays. After incubation, the pellets were resuspended in 1% BSA/PBS blocking buffer containing 130 μg/ml mouse anti-MSP1 mAb5.2 (if not present during the assay), 190 μg/ml of chicken polyclonal anti-CR1 (Gallus Immunotech, ON, Canada), and 30 μg/ml of mouse monoclonal 1H8 anti-C3 IgG1 (Kerafast, Boston, MA) and incubated for 30 min at 4 °C. The specificity of each primary antibody was verified by the use of negative control antibodies. After three washes with blocking buffer, the pellets were resuspended in 100 μl of blocking buffer containing 1:500 dilutions of goat anti-mouse IgG2b-DyLight 488, goat anti-chicken Alexa Fluor 546, and goat anti-mouse IgG1 Alexa Fluor 594 (Thermo Fisher Scientific) in the presence of 30 μM Vybrant DID cell labeling solution (Life Technologies) followed by an incubation for 30 min at 4 °C. After an additional three washes, the pellets were resuspended in 4% paraformaldehyde with 10 μg/ml Hoechst 33342 (Life Technologies). Prior to imaging, 10 μl of the cell suspension was transferred to 0.6 ml microcentrifuge tubes and centrifuged at 200 × g for 1 min and the pellet was resuspended in 10 μl of VectorShield Hard Set (Vector Labs, Inc., Burlingame, CA). 4 μl of this suspension was mounted on a slide, covered with a cover slip, and allowed to harden for a few minutes. Fluorescence z-series were collected using a LEICA SP8 confocal microscope (Leica Microscopes Systems Inc., Buffalo Grove, IL) at 40 × oil (HC PL APO CS2 40 ×/1.30 Oil) immersion. The images were processed using Imaris (Bitplane, Concord, MA) image processing software.
2.6. Passive Transfer Experiments
Polyclonal anti-Plasmodium berghei antibody was generated by three cycles of infection and treatment of C57BL/6 J mice (The Jackson Laboratory, Bar Harbor, ME). For the initial infection mice were injected intraperitoneally with 1 × 106 P. berghei ANKA IRBC. Parasitemia was monitored with Giemsa smears and once it reached (5%–10%) the mice were treated with sub-curative one intramuscular dose of chloroquine (500 μg/mouse) for three consecutive days. The parasitemia was allowed to rebound and the cycle was repeated for a total of 3 times. For the final treatment, mice were treated daily for one week and allowed to rest for two weeks. Plasma was collected from mice by cardiac puncture in citrate phosphate dextrose solution (CPD) (Sigma-Aldrich) and total antibody was purified using protein A/G columns (GenScript, Piscataway, NJ). Antibody purified from uninfected mice served as control. For passive transfer experiments wild type or C3 deficient mice of C57BL/6J background were injected with 1.5 × 107 IRBCs in 100 μl of RPMI 1640 in one retro-orbital plexus and with 100 μl of anti-Pb, control antibodies, or 100 μl of PBS in the contralateral plexus. Parasitemia was monitored by staining tail vein blood with Hoechst 33342 (2 μg/ml in PF) (Life Technologies) at pre-determined intervals until day 7 post-infection. Acquisition and analysis were performed as described above.
2.7. Ethics Statement
The use of human serum and plasma reported here was carried under human subject research exemptions by the Walter Reed Army Institute of Research Human Subject Research Review Board and by the Penn State Hershey Medical Center Institutional Review Board that allowed the use of unidentified serum and plasma samples. The malaria vaccine serum samples were collected originally using protocols approved by the Walter Reed Army Institute of Research Human Subject Research Review Board and adhered to the principles of the Declaration of Helsinki. All animal experiments were carried under protocols approved by the Penn State Hershey Medical Center Institutional Animal Care and Use Committee and adhered to NIH guidelines for care and use of laboratory animals.
2.8. Statistical Analysis
Statistical analysis was done with SigmaPlot v11.2 (Systat Software, San José, CA). Means of normally distributed continuous numeric variables from two groups were compared using t-test or paired t-test, whichever was appropriate. Comparisons across more than two groups were done using analysis of variance with post-hoc Holm-Sidak tests. Differences in categorical variables were analyzed using chi-square with Fisher's exact test. All tests were two-tailed α < 0.05.
3. Results
3.1. Invasion of RBCs is Enhanced by Fresh Serum and Anti-merozoite Monoclonal Antibody mAb5.2 Relative to Heat-inactivated Serum
To test the hypothesis that complement can enhance RBC invasion, we first evaluated the effect of heat inactivation of serum on invasion. Although traditional methods of complement inactivation utilize 30 min heat treatment at 56 °C, this duration of heat treatment likely results in the inactivation of multiple enzymes in the complement pathway. Thus, in order to achieve a more selective inactivation, we also incubated serum for 3 min and 5 min at 56 °C (Araujo et al., 1991) and confirmed inactivation by measuring CH50 and AH50 (Fig. S1A and S1B). Fig. 1a shows the ring parasitemia of overnight RBC invasion in the presence of 3, 5 or 30 min HIS or FS. Since heat inactivation of serum is standard in most malaria cultures or GIA (Moll et al., 2008), from this point forward we present the data as enhancement relative to 30 min HIS (Fig. 1b). The use of FS resulted in a 40% invasion enhancement relative to 30 min HIS. This enhancement was not due to excessive RBC lysis in the FS wells since the level of free hemoglobin was very low and similar between HIS and FS (Fig. S2). A drastic reduction in invasion enhancement was observed with just 3 or 5 min HIS (Fig. 1b). Additional replicates are shown in Fig. S3. These results suggest that complement may be involved in mediating the enhancement of invasion observed in FS relative to HIS. Further, we were also able to identify enhanced C3 deposition on single merozoites after incubation in FS but not in 30 min HIS (Fig. S4).
Fig. 1.
FS and anti-merozoite monoclonal antibody mAb5.2 enhance P. falciparum invasion of RBCs relative to HIS. a) Two SA-independent strains of P. falciparum, 7G8 and 3D7, were tested in the presence of 10% FS, 3 min HIS, 5 min HIS, or 30 min HIS. Invasion of RBCs decreased with progressively longer heat inactivation times. b) Invasion of RBCs in the same experiment as panel A expressed as percent enhancement relative to 30 min HIS. See Fig. S3 for additional replicates. c) Invasion of P. falciparum strain 7G8 was tested in the presence of monoclonal anti-merozoite surface protein 1(MSP1) mAb5.2 (40 μg/ml), an isotype control antibody (IgG2b), or PBS in 10% FS or 30 min HIS. Invasion of RBCs is expressed as percent enhancement in FS relative to 30 min HIS. Each panel depicts a representative experiment. See Fig. S5 for absolute parasitemia and one additional replicate. Error bars represent standard deviations for triplicate wells. P values were obtained by one-way ANOVA with post-hoc comparisons.
In order to determine whether activation of complement via the classical pathway by anti-merozoite antibodies could enhance invasion, we used mAb5.2, raised against the C-terminal 19 kD fragment of P. falciparum Uganda-Palo Alto MSP1 (Kaslow et al., 1994, Siddiqui et al., 1986). This antibody was selected due to its availability and relative ease of production. Fig. 1c shows that addition of mAb5.2 resulted in significant enhancement of invasion above fresh serum. Fig. S5 shows the absolute parasitemia for Fig. 1c and one additional replicate.
3.2. Enhancement of Invasion by Anti-merozoite Monoclonal Antibody mAb5.2 is Complement-dependent
Using CH50 we confirmed that we could reverse the 3 min heat inactivation of complement by addition of C2 (Fig. S1A) (Araujo et al., 1991). Therefore, we tested the effect of 3 min HIS on the ability of mAb5.2 to enhance parasite invasion of RBCs. Use of 3 min HIS abolished the antibody-mediated enhancement of invasion (Figs. 2a and S6). Addition of purified C2 and fB together, but not separately (Fig. S6), to 3 min HIS rescued antibody-mediated enhancement of invasion but had no effect in the absence of antibody or in the presence of an isotype control (Figs. 2a and S7). Interestingly, C1q or C2-repleted pooled heterologous serum produced minimal level of RBC invasion enhancement relative to depleted serum (Fig. S8). On the other hand, autologous FS produced significant amount of enhancement relative to HIS (Fig. S8). The reasons for the lack of enhancement with heterologous serum are unclear. However, one possible factor that we are currently investigating is the role of minor incompatibilities with the donor RBCs.
Fig. 2.
Enhancement of RBC invasion by anti-merozoite monoclonal antibody mAb5.2 is complement dependent. a) Addition of C2 and fB rescued RBC invasion enhancement properties of mAb5.2 in 3 min HIS. Invasion of P. falciparum strain 7G8 was tested in the presence of 40 μg/ml anti-MSP1 mAb5.2, 40 μg/ml IgG2b isotype control, or PBS in the presence of 10% FS, 3 min HIS, or 30 min HIS. C2 and fB were added to a final concentration of 25 μg/ml and 200 μg/ml respectively. The glycoprotein fetuin was used as a control at 225 μg/ml. Fig. S6A shows the absolute parasitemia. Additional replicates are shown in Fig. S7. b) Enhancement of RBC invasion by anti-MSP1 mAb5.2 was eliminated by the peptide complement inhibitor compstatin but not by control peptide. Conditions were the same as panel A. Invasion of RBCs is expressed as percent enhancement relative to 30 min HIS. Fig. S9A shows the absolute parasitemia. Additional replicates are shown in Fig. S9B and S9C·Error bars represent standard deviations of triplicate wells. P is based on two-sample t-test for the comparison between mAb5.2 and IgG2b or PBS.
To further confirm that complement is responsible for antibody-mediated enhancement of RBC invasion, we used the C3-selective inhibitor compstatin, a cyclic 13-amino acid peptide that inhibits C3 cleavage and activation in human serum (Sahu et al., 1996). Enhancement of invasion was observed in FS in the presence of mAb5.2. The addition of compstatin eliminated this enhancement (Figs. 2b and S9). Interestingly, compstatin had no effect on the enhancement of invasion by FS alone. These results demonstrate that activation of the CP can result in enhancement of RBC invasion by merozoites.
3.3. Soluble CR1 Blocks Enhancement of Invasion by Fresh Serum and Anti-merozoite Antibodies
To test whether CR1 is involved in complement and antibody-mediated enhancement of RBC invasion by P. falciparum we used soluble CR1 (sCR1) as an inhibitor. Anti-merozoite antibody-mediated enhancement of RBC invasion was not only negated in the presence of sCR1 but declined below the level of the isotype control IgG2b (Figs. 3 and S10). There was no effect in the absence of mAb5.2 (Figs. 3 and S10).
Fig. 3.
Enhancement of RBC invasion by mAb5.2 is abolished in the presence of sCR1. Invasion of P. falciparum strain 7G8 was tested in the presence of 40 μg/ml anti-MSP1 mAb5.2 or 40 μg/ml IgG2b isotype control in the presence of 10% FS or 30 min HIS. The final concentration of sCR1 or fetuin was 80 μg/ml. Fig. S10 shows the absolute parasitemia and additional replicates. Error bars represent standard deviations of triplicate wells. P is based on two-sample t-test.
3.4. SA-dependent Strains Demonstrate Greater Enhancement of Invasion in the Presence of Complement than SA-independent Strains
P. falciparum can be subdivided into SA-dependent strains that invade neuraminidase (NA)-treated RBCs at a low level and SA-independent strains that are able to invade NA-treated RBCs at a higher level (Mitchell et al., 1986) via CR1 and the merozoite ligand PfRh4 (Tham et al., 2010). Since SA-dependent strains are unable to utilize PfRh4 for CR1-mediated invasion (Stubbs et al., 2005), we hypothesized that these strains may be more reliant on complement to accomplish this function. Hence, we compared invasion levels of SA-dependent and SA-independent strains in fresh and 30 min HIS in untreated and NA-treated RBCs. SA-dependent strains demonstrated greater enhancement of invasion with fresh serum compared to HIS in NA-treated RBCs than SA-independent strains, median enhancement 54.8% vs. 31.5%, P < 0.01 by two-sample t-test (Figs. 4a, S11, and S12). Similar results, although of lower magnitude, were observed in the presence of C3/C4-inactivated vs C3/C4-reconstituted heterologous pooled serum (Fig. 4b and S11C).
Fig. 4.
SA-dependent strains of P. falciparum are more reliant on complement to invade NA-treated RBCs than SA-independent strains. a) SA-dependent strains show greater enhancement of invasion into NA-treated RBC (median 54.8%) in the presence of FS relative to 30 min HIS than SA-independent strains (median 31.5%), P < 0.01 by two-sample t-test. Fig. S11 shows absolute parasitemias. Fig. S12 shows one additional replicate. b) Enhancement of invasion into NA-treated RBCs in the presence of C3/C4-reconstituted serum relative to C3/C4-inactivated serum. Fig. S11C shows absolute parasitemias. Error bars are standard deviations of triplicate wells. P values are based on two-sample t-test.
3.5. Anti-merozoite mAb5.2 Enhances Merozoite Attachment to RBCs and Induces CR1 Aggregation in a Complement-dependent Manner
To address the question of whether the presence of complement enhances the binding of merozoites to the RBC surface we performed attachment assays. In order to inhibit merozoite invagination we allowed merozoites to egress in the presence of cytochalasin D (Miller et al., 1979), complement deficient or reconstituted serum, and either mAb5.2 or IgG2b isotype control. We then detected RBCs with surface merozoites with mAb5.2 by flow cytometry. As expected, in the presence of mAb5.2 and deficient serum reconstituted with either C3/C4 or C3, we observed a significantly greater percent of RBCs with attached merozoites and surface C3 than in the presence of IgG2b isotype control and complement deficient serum (Fig. 5a andb, and S13), P ≤ 0.01 by two-sample t-test.
Fig. 5.
Antibody and complement-mediated attachment of merozoites to RBC CR1. Attachment of P. falciparum strain 3D7 was tested in the presence of anti-MSP1 mAb5.2 or IgG2b isotype control, in the presence of a) C3/C4-inactivated or C3/C4-reconstituted serum and b) C3-depleted or C3-reconstituted serum. Fig. S13 shows additional replicates. P is based on two-sample t-test. Error bars represent standard deviations for triplicate wells. c) In the presence of C3 and anti-MSP1 mAb5.2 merozoites attach to RBCs via CR1 (yellow) which shows intense aggregation at the site of merozoite contact. This area is also positive for C3 (cyan) and shows lipid accumulation by DID staining (red). Hoechst 33,342 was used to stain the DNA (blue) and MSP1 staining shows green. On the other hand, use of C3-depleted serum resulted in absence of CR1 aggregation, C3 deposition, or lipid accumulation. Scale bar is 2 μm. d) Zoomed view of C3 deposition (i and iii) and CR1 co-localization (ii and iv) in C3-reconstituted and depleted serum. e) Quantitation of the merozoites with C3 deposition and CR1 co-localization in C3-reconstituted and depleted serum in the presence of anti-MSP1 mAb5.2. *P < 0.001, by chi-square test with Fisher's exact test, under the null hypothesis that the CR1 and C3b co-localization are equal in complement sufficient and deficient serum. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the interest of depicting the direct interaction of merozoites with RBCs in the presence of complement and mAb5.2 we performed immunofluorescence microscopy. Attachment assays were setup in the presence of C3-depleted and reconstituted heterologous serum, with or without mAb5.2. In the presence of C3 and mAb5.2 there was dense aggregation of CR1 and lipids, and C3 deposition at the site of merozoite contact with the RBC but these findings were absent in C3-depleted serum, P < 0.001 by chi-square test (Fig. 5c, d, and e).
3.6. Complement Decreases Invasion Inhibitory Activity of Human Anti-MSP1 Antibodies
To assess the role of human antibody-mediated complement activation on RBC invasion we purified total IgG from MSP142 vaccines (Otsyula et al., 2013) and tested it in invasion assays using C3/C4-inactivated or reconstituted sera. Percent inhibition was calculated relative to PBS-only since we did not have pre-immunization serum. The median inhibitory activity in C3/C4-inactivated serum was negligible but upon reconstitution of serum with the missing complement factors the inhibitory activity became negative, signifying invasion enhancement (Fig. 6). There was no difference between C3/C4-inactivated and reconstituted serum in the presence of IgG from unvaccinated controls.
Fig. 6.
Complement decreases inhibitory activity of polyclonal IgG from MSP142 vaccine recipients. a) RBC invasion of P. falciparum strain FVO in the presence of purified total IgG (2.5 mg/ml) from MSP142 vaccine recipients and either C3/C4-inactivated or C3/C4-reconstituted serum, N = 13. b) RBC invasion in the presence of total IgG from malaria naïve non-vaccinee controls, N = 14. Horizontal markers represent medians. P is by paired t-test.
3.7. Passive Transfer of Anti-P. berghei Antibodies Enhances Parasitemia and C3 Deficiency Decreases Parasitemia in Mice
To validate the observed in vitro antibody-mediated complement enhancement of RBC invasion in an in vivo setting we infected C57BL/6 mice with P. berghei ANKA and administered varying concentrations of polyclonal total anti-P. berghei IgG (anti-Pb), control total IgG from naïve mice, or PBS. The anti-Pb antibody resulted in enhancement of endpoint parasitemia in a reverse dose-dependent manner when compared to the PBS control group (Fig. 7A and B). Additional replicates are found in Fig. S14. Surprisingly, control antibodies, independent of the dose administered, produced an inhibitory effect when compared to the PBS control, P = 0.06 by two-sample t-test.
Fig. 7.
Anti-P. berghei antibodies and complement enhance parasite growth in mice. a) Time course of parasite growth in mice injected with anti-P. berghei antibody (Anti-Pb), control antibody (Cont Ab), or PBS. b) Day 7 enhancement of parasitemia from panel A relative to mice that received PBS. P value is based on analysis of variance. c) Time course of P. berghei parasitemia in C3–/− (C3 KO) and wild type mice that received 250 μg of Anti-Pb or Cont Ab, or equivalent volume of PBS. d) Day 7 enhancement of parasitemia from panel c. P is based on two-sample t-test. Additional replicates are shown in Fig. S14. Error bars represent standard errors for each group of 4–5 mice.
To further explore the role of C3 in in vivo parasite growth, we subjected C3–/− mice to P. berghei infection in the presence of anti-Pb, control antibodies, or PBS. Fig. 7C and 7D show that C3–/− mice had blunted parasite growth relative to their wild type counterparts across all groups although it was only statistically significant in the case of PBS. C3–/− mice that received anti-Pb antibody still showed enhancement of parasitemia although it did not reach statistical significance. C3–/− mice that received control IgG had an intermediate response.
4. Discussion
Antibodies that coat pathogens in the circulation induce activation of the complement cascade and opsonization with C4b and C3b. Opsonized pathogens can bind to CR1 on RBCs via C4b and C3b. When the RBCs traverse through the liver or spleen and encounter macrophages, attached pathogens are removed by phagocytosis and the RBCs are spared and recirculate (Davies et al., 1990). This process of binding to RBCs and removal of opsonized pathogens is beneficial to the host. However, unlike other pathogens, opsonization of malaria merozoites may facilitate binding to their ultimate target cell, the RBC. Recently, two groups (Kennedy et al., 2016, Rosa et al., 2016) reported that the fluid phase complement regulator Factor H can bind to IRBCs and to merozoites. Factor H functions to accelerate the degradation of the AP convertase and as cofactor for the Factor I-mediated cleavage of C3b to iC3b (Pouw et al., 2015). Since CR1 is also able to bind iC3b (Nilsson et al., 2011), Factor H activity could lead to the accumulation of this molecule on the merozoite surface and enhance binding to CR1. Therefore, we reasoned that opsonization of merozoites may enhance RBC invasion. Enhancement of invasion by complement has been reported in other pathogens such as Leishmania (Da Silva et al., 1989, Datta and Rappaport, 2006, Schlesinger et al., 1990, Schlesinger, 1993).
To investigate the role of complement in RBC invasion by merozoites, we evaluated the effect of serum in the absence or presence of anti-merozoite antibodies. Because heat treatment at 56 °C is the traditional way to inactivate complement, we first compared FS to 56 °C HIS. We showed that FS enhances RBC invasion relative to 3, 5, and 30 min HIS. Although we were able to restore the CH50 activity of 3 min HIS by addition of C2 (Fig. S1A), addition of C2 and/or fB to 3 min HIS did not rescue RBC invasion enhancing activity. One possible explanation is that 3 min heat inactivation may also inactivate elements of the LP upstream of C2 that are activated by merozoites such as mannose binding lectin associated serine proteases (MASPs).
In order to test the effect of anti-merozoite antibodies in complement-mediated RBC invasion we used two types of antibodies, an anti-merozoite mouse monoclonal (mAb5.2) and polyclonal IgG from MSP142 vaccines (Otsyula et al., 2013). Use of mAb5.2 resulted in enhancement of RBC invasion above that of FS alone. 3 min HIS eliminated this enhancement and addition of both C2 and fB was required for rescue. Because C2 is an integral part of the CP and fB of the AP amplification loop, these data provide strong evidence that both of these pathways of complement activation play a role in antibody-mediated enhancement of RBC invasion. The involvement of the CP is not surprising given its reliance on antibodies. However, antibodies are also known to play an important role in stabilizing the nascent AP convertase (Lutz and Jelezarova, 2006). In addition, the AP serves as the main amplification loop for the majority of complement deposition even when the CP is the initial source of complement activation (Harboe et al., 2004, Harboe and Mollnes, 2008). The important role of C3 was further demonstrated by the effect of the C3 specific inhibitor compstatin. Use of this inhibitor eliminated antibody-mediated enhancement of invasion (Fig. 2b). Although compstatin had no effect on enhancement by FS alone, we cannot rule out that other complement factors such as C1q, MBL, or C4 also play a role here. Our experiments using single factor depleted and reconstituted heterologous serum (Fig. S8) suggest that heterologous serum is not as effective as autologous FS in producing enhancement of invasion. Possible reasons include the presence of minor incompatibilities between serum and RBCs or residual effects from the method of depletion. Thus, further studies should be done with single factor-depleted autologous serum. Enhancement of invasion has been described with the use of antibodies that block other antibodies that inhibit the processing of MSP1 (Guevara Patino et al., 1997). However, the mechanism that we describe here is clearly different.
In an effort to validate the relevance of antibody and complement-mediated enhancement of RBC invasion in humans we purified total IgG from serum from MSP142 vaccine recipients collected two weeks after three doses of vaccine (Otsyula et al., 2013). Due to the limited amounts of sample obtained we were unable to carry out extensive assays. Nonetheless, we measured the RBC invasion inhibitory activity of total IgG in C3/C4-inactivated or reconstituted serum. Consistent with previous results (Otsyula et al., 2013), we found very little inhibitory activity in total IgG. However, unlike IgG from non-immunized controls, IgG from vaccine recipients showed reversal of inhibitory activity and enhancement of RBC invasion upon reconstitution of serum with C3/C4. Thus, these results demonstrate that enhancement of RBC invasion can occur with human anti-merozoite antibodies.
Contrary to our data, Boyle et al. (Boyle et al., 2015) recently reported that complement activation enhances the inhibitory activity of human anti-merozoite antibodies. They determined that the critical inhibitory step was fixation of C1q on the merozoite surface. One major difference between the approach of these investigators and ours is their use of filter-purified merozoites as opposed to allowing merozoites to naturally egress from schizonts. Consequently, we carried out invasion assays with filter-purified merozoites as described by Boyle et al. (Boyle et al., 2015). We showed that filtered-purified merozoites appear to be highly defective and more sensitive to complement activation than naturally egressed merozoites (Fig. S15).
To demonstrate the role of CR1 in the process of complement-mediated invasion we used sCR1 as a competitor. We observed that in the presence of sCR1 there was not only complete reversal of antibody-mediated enhancement in FS but significant amount of inhibition was observed in comparison to the isotype (Fig. 3). The inhibitory activity of sCR1 may be attributed to its diverse functions. Firstly, sCR1 competes for binding sites with erythrocyte surface CR1, which may potentially form large C3b-sCR1 complexes on the merozoite surface and induce steric hindrance of ligand receptor interactions in the presence of these complexes. Secondly, sCR1 is able to bind to PfRh4 on the merozoite surface thereby inhibiting the direct interaction of erythrocyte surface CR1 with PfRh4. Thirdly, sCR1 possesses complement regulatory activity by destabilizing membrane assembled C3 convertases through its intrinsic decay accelerating activity and by acting as a cofactor for Factor I, which breaks down C3b into iC3b. The role of CR1 in complement-dependent invasion was further supported by our co-localization studies that clearly show aggregation of CR1 at the site of merozoite attachment but only in the presence of C3.
PfRh4 has been proposed as the native merozoite ligand that binds to CR1. SA-dependent strains of P. falciparum are unable to use this ligand (Stubbs et al., 2005). Therefore, we hypothesized that SA-dependent strains may be more reliant on complement to use this invasion pathway. Although the invasion of NA-treated RBCs by SA-dependent strains is low, the percent enhancement of invasion in the presence of FS is significantly greater than SA-independent strains. These data demonstrate that, for some strains, C3 can act as the sole CR1 ligand.
Lastly, our passive transfer experiments in mice confirm that anti-malaria antibody and complement can benefit parasite growth. Administration of anti-Pb IgG enhanced parasite growth in a reverse dose-dependent manner. At first look, these results stand in contrast to previous studies that have shown that antibody from immune rodents inhibits growth in vivo when given to naïve animals (Cavinato et al., 2001, Jarra et al., 1986, Rotman et al., 1998, Waki et al., 1995, Yoneto et al., 2001). However, the doses of antibody in those studies were much higher, usually given in milligram amounts and sometimes in several doses prior to and after infection. While mice do not express CR1 on their RBCs, they do express other complement receptors that can bind complement such as Crry (Molina, 2002). Several studies have also shown that passive transfer of total IgG from individuals living in endemic areas to acutely infected children can reduce parasitemia, but this was achieved at 5–10 fold higher doses per Kg than the ones we have used in our studies (COHEN et al., 1961, McGregor 1964b, Sabchareon et al., 1991). It is unlikely that these levels of antibodies can be achieved by natural infection or by immunization. Thus, it is possible that low doses of anti-merozoite antibodies enhance parasite growth in vivo while very high doses are required for true in vivo inhibitory activity. By contrast, the inhibitory activity of control polyclonal antibody may be due to its complement scavenging properties (Arumugam et al., 2007, Hartung, 2008).
C3–/− mice consistently showed significant blunting of parasitemia relative to wild type mice. Yet, they still showed enhancement of parasite growth in response to anti-Pb IgG, probably due to residual C4b opsonizing activity. Previous studies of the role of complement in the growth of rodent malaria have been inconsistent. Use of cobra venom factor (CVF) as a complement depleting agent resulted in increased parasitemia (Ward et al., 1981). However, CVF exerts its action by inducing AP convertase formation and C3 activation which could result in parasite growth enhancement by increased deposition of C3 fragments on merozoites. Unlike our study, a previous study (Ramos et al., 2012) reported no change in P. berghei parasitemia in C3–/− mice compared to wild type mice, but in that study quantitation was done by microscopy and infection was by intraperitoneal injection. C1q and C2/fB deficiency resulted in minimal increase in Plasmodium chabaudi parasitemia (Taylor et al., 2001). Thus, further studies of these models are needed to fully understand the role of complement activation in parasite growth.
5. Conclusions
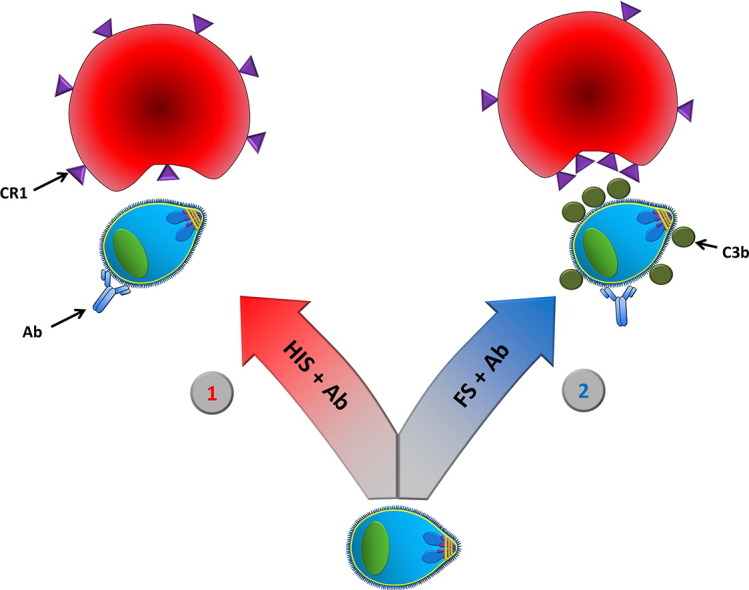
We have demonstrated that merozoites are capable of invading RBCs via a previously unrecognized antibody and complement-mediated pathway by binding to CR1. Our findings are especially relevant to the efforts of developing an anti-merozoite malaria vaccine since some antibodies may actually enhance RBC invasion. Based on our findings we propose a model where the presence of anti-merozoite antibodies can activate complement and lead to binding to and aggregation of RBC CR1 (Fig. S16). The absence of complement could artificially enhance the inhibitory activity of some antibodies. Thus, complement activity should be included in GIA to measure the inhibitory activity of anti-merozoite antibodies.
Funding Sources
This work was funded by Grant P131040 from the Congressionally Directed Medical Research Program, PI José A. Stoute. The funding agency played no role in the collection or interpretation of the data, the preparation of the manuscript, and the decision to publish.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Authorship Contributions
SB and JAS designed all the experiments. SB, JAS, and MEL carried out all the experiments. MDS, CFO, and EA carried out the malaria vaccine studies and provided the human serum.
Acknowledgments
We thank Dr. Henry Marsh, Celldex Therapeutics, for the gift of soluble CR1.
We are grateful to Mr. Wade Edris and the microscopy core facility at Penn State College of Medicine for assistance with confocal microscopy. We thank Nate Sheaffer, Jade Vogel, and Joseph Bednarzyk from the Penn State Hershey Flow Cytometry Core Facility for assistance with flow cytometry acquisition.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.05.015.
Appendix A. Supplementary data
A model of antibody and complement-mediated enhancement of RBC invasion by Plasmodium merozoites, see discussion. 1) In the absence of complement, such as via heat inactivation of serum, the merozoites depend on interactions between the RBC receptors and the parasite ligands. Antibody (Ab) directed against merozoites may have inhibitory effect, and thereby block RBC invasion. 2) In the presence of complement, such as in fresh serum (FS), the merozoites may utilize not only specific receptor ligand interactions, as described in 1, but also a complement receptors on RBCs via complement opsonins. Complement opsonins (C3b) on the merozoite surface may act as an alternative, host derived, parasite ligand that the merozoite can bind to the RBC CR1, and counteract some of the invasion blocking effects of anti-merozoite antibodies. Binding of C3b to CR1 causes aggregation of CR1.
References
- Angov E., Aufiero B.M., Turgeon A.M., Van H.M., Ockenhouse C.F., Kester K.E., Walsh D.S., McBride J.S., Dubois M.C., Cohen J. Development and pre-clinical analysis of a Plasmodium falciparum Merozoite Surface Protein-1(42) malaria vaccine. Mol. Biochem. Parasitol. 2003;128:195–204. doi: 10.1016/s0166-6851(03)00077-x. [DOI] [PubMed] [Google Scholar]
- Araujo M.N., Leser P.G., Gabriel J.A., Assad R.L., Atra E. A simple radial immunohemolysis assay for the measurement of functional complement C2 activity. Braz. J. Med. Biol. Res. 1991;24:49–57. [PubMed] [Google Scholar]
- Arumugam T.V., Tang S.C., Lathia J.D., Cheng A., Mughal M.R., Chigurupati S., Magnus T., Chan S.L., Jo D.G., Ouyang X. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14104–14109. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M.J., Wilson D.W., Richards J.S., Riglar D.T., Tetteh K.K., Conway D.J., Ralph S.A., Baum J., Beeson J.G. Isolation of viable plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14378–14383. doi: 10.1073/pnas.1009198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M.J., Reiling L., Feng G., Langer C., Osier F.H., Aspeling-Jones H., Cheng Y.S., Stubbs J., Tetteh K.K., Conway D.J. Human antibodies fix complement to inhibit plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. 2015;42:580–590. doi: 10.1016/j.immuni.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavinato R.A., Bastos K.R., Sardinha L.R., Elias R.M., Alvarez J.M., d'Imperio Lima M.R. Susceptibility of the different developmental stages of the asexual (schizogonic) erythrocyte cycle of Plasmodium chabaudi chabaudi to hyperimmune serum, immunoglobulin (Ig)G1, IgG2a and F(ab′)2 fragments. Parasite Immunol. 2001;23:587–597. doi: 10.1046/j.1365-3024.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Chang S.P., Gibson H.L., Lee-Ng C.T., Barr P.J., Hui G.S. A carboxyl-terminal fragment of plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J. Immunol. 1992;149:548–555. [PubMed] [Google Scholar]
- Cohen S., McGregor I.A., Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Da Silva R.P., Hall B.F., Joiner K.A., Sacks D.L. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J. Immunol. 1989;143:617–622. [PubMed] [Google Scholar]
- Darko C.A., Angov E., Collins W.E., Bergmann-Leitner E.S., Girouard A.S., Hitt S.L., McBride J.S., Diggs C.L., Holder A.A., Long C.A. The clinical-grade 42-kilodalton fragment of merozoite surface protein 1 of Plasmodium falciparum strain FVO expressed in Escherichia coli protects Aotus nancymai against challenge with homologous erythrocytic-stage parasites. Infect. Immun. 2005;73:287–297. doi: 10.1128/IAI.73.1.287-297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P.K., Rappaport J. HIV and complement: hijacking an immune defense. Biomed. Pharmacother. 2006;60:561–568. doi: 10.1016/j.biopha.2006.07.087. [DOI] [PubMed] [Google Scholar]
- Davies K.A., Hird V., Stewart S., Sivolapenko G.B., Jose P., Epenetos A.A., Walport M.J. A study of in vivo immune complex formation and clearance in man. J. Immunol. 1990;144:4613–4620. [PubMed] [Google Scholar]
- Fearon D.T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J. Exp. Med. 1980;152:20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiran I., Barbashov S.F., Klickstein L.B., Tas S.W., Jensenius J.C., Nicholson-Weller A. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J. Exp. Med. 2000;192:1797–1808. doi: 10.1084/jem.192.12.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara Patino J.A., Holder A.A., McBride J.S., Blackman M.J. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 1997;186:1689–1699. doi: 10.1084/jem.186.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Mollnes T.E. The alternative complement pathway revisited. J. Cell. Mol. Med. 2008;12:1074–1084. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Ulvund G., Vien L., Fung M., Mollnes T.E. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin. Exp. Immunol. 2004;138:439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H.P. Advances in the understanding of the mechanism of action of IVIg. J. Neurol. 2008;255(Suppl. 3):3–6. doi: 10.1007/s00415-008-3002-0. [DOI] [PubMed] [Google Scholar]
- Haynes J.D., Moch J.K., Smoot D.S. Erythrocytic malaria growth or invasion inhibition assays with emphasis on suspension culture GIA. Methods Mol. Med. 2002;72:535–554. doi: 10.1385/1-59259-271-6:535. [DOI] [PubMed] [Google Scholar]
- Jarra W., Hills L.A., March J.C., Brown K.N. Protective immunity to malaria. Studies with cloned lines of Plasmodium chabaudi chabaudi and P. berghei in CBA/Ca mice. II. The effectiveness and inter- or intra-species specificity of the passive transfer of immunity with serum. Parasite Immunol. 1986;8:239–254. doi: 10.1111/j.1365-3024.1986.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Kaslow D.C., Hui G., Kumar S. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP1(19)) variants secreted from Saccharomyces cerevisiae. Mol. Biochem. Parasitol. 1994;63:283–289. doi: 10.1016/0166-6851(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Kennedy M.C., Wang J., Zhang Y., Miles A.P., Chitsaz F., Saul A., Long C.A., Miller L.H., Stowers A.W. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 2002;70:6948–6960. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A.T., Schmidt C.Q., Thompson J.K., Weiss G.E., Taechalertpaisarn T., Gilson P.R., Barlow P.N., Crabb B.S., Cowman A.F., Tham W.H. Recruitment of factor H as a novel complement evasion strategy for blood-stage Plasmodium falciparum infection. J. Immunol. 2016;196:1239–1248. doi: 10.4049/jimmunol.1501581. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Lutz H.U., Jelezarova E. Complement amplification revisited. Mol. Immunol. 2006;43:2–12. doi: 10.1016/j.molimm.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Mastellos D.C., Yancopoulou D., Kokkinos P., Huber-Lang M., Hajishengallis G., Biglarnia A.R., Lupu F., Nilsson B., Risitano A.M., Ricklin D. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur. J. Clin. Investig. 2015;45:423–440. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor I.A. The passive transfer of human malarial immunity. Am.J.Trop. Med. Hyg. 1964;13:237–239. doi: 10.4269/ajtmh.1964.13.237. [DOI] [PubMed] [Google Scholar]
- McGregor I.A. The passive transfer of human malarial immunity. Am.J.Trop. Med. Hyg. 1964;13:237–239. doi: 10.4269/ajtmh.1964.13.237. [DOI] [PubMed] [Google Scholar]
- Miller L.H., Aikawa M., Johnson J.G., Shiroishi T. Interaction between cytochalasin B-treated malarial parasites and erythrocytes. Attachment and junction formation. J. Exp. Med. 1979;149:172–184. doi: 10.1084/jem.149.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G.H., Hadley T.J., McGinniss M.H., Klotz F.W., Miller L.H. Invasion of erythrocytes by Plasmodium falciparum malaria parasites: evidence for receptor heterogeneity and two receptors. Blood. 1986;67:1519–1521. [PubMed] [Google Scholar]
- Molina H. The murine complement regulator Crry: new insights into the immunobiology of complement regulation. Cell. Mol. Life Sci. 2002;59:220–229. doi: 10.1007/s00018-002-8418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll K., Ljungstrom I., Perlmann H., Scherf A., Wahlgren M. In: Methods in Malaria Research. fifth ed. Moll K., Ljungstrom I., Perlmann H., Scherf A., Wahlgren M., editors. MR4/ATCC; Manassas: 2008. [Google Scholar]
- Morgan B.P. In: Complement Methods and Protocols. Morgan B.P., editor. Humana Press; Totowa, New Jersey: 2000. pp. 61–75. [Google Scholar]
- Murray C.J., Rosenfeld L.C., Lim S.S., Andrews K.G., Foreman K.J., Haring D., Fullman N., Naghavi M., Lozano R., Lopez A.D. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- Nilsson U.R., Funke L., Nilsson B., Ekdahl K.N. Two conformational forms of target-bound iC3b that distinctively bind complement receptors 1 and 2 and two specific monoclonal antibodies. Ups. J. Med. Sci. 2011;116:26–33. doi: 10.3109/03009734.2010.528465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogutu B.R., Apollo O.J., McKinney D., Okoth W., Siangla J., Dubovsky F., Tucker K., Waitumbi J.N., Diggs C., Wittes J. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsyula N., Angov E., Bergmann-Leitner E., Koech M., Khan F., Bennett J., Otieno L., Cummings J., Andagalu B., Tosh D. Results from tandem Phase 1 studies evaluating the safety, reactogenicity and immunogenicity of the vaccine candidate antigen Plasmodium falciparum FVO merozoite surface protein-1 (MSP1(42)) administered intramuscularly with adjuvant system AS01. Malar. J. 2013;12:29. doi: 10.1186/1475-2875-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouw R.B., Vredevoogd D.W., Kuijpers T.W., Wouters D. Of mice and men: the factor H protein family and complement regulation. Mol. Immunol. 2015;67:12–20. doi: 10.1016/j.molimm.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Ramos T.N., Darley M.M., Weckbach S., Stahel P.F., Tomlinson S., Barnum S.R. The C5 convertase is not required for activation of the terminal complement pathway in murine experimental cerebral malaria. J. Biol. Chem. 2012;287:24734–24738. doi: 10.1074/jbc.C112.378364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa T.F., Flammersfeld A., Ngwa C.J., Kiesow M., Fischer R., Zipfel P.F., Skerka C., Pradel G. The Plasmodium falciparum blood stages acquire factor H family proteins to evade destruction by human complement. Cell. Microbiol. 2016;18:573–590. doi: 10.1111/cmi.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman H.L., Daly T.M., Clynes R., Long C.A. Fc receptors are not required for antibody-mediated protection against lethal malaria challenge in a mouse model. J. Immunol. 1998;161:1908–1912. [PubMed] [Google Scholar]
- Sabchareon A., Burnouf T., Ouattara D., Attanath P., Bouharoun-Tayoun H., Chantavanich P., Foucault C., Chongsuphajaisiddhi T., Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am.J.Trop. Med. Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- Sagara I., Dicko A., Ellis R.D., Fay M.P., Diawara S.I., Assadou M.H., Sissoko M.S., Kone M., Diallo A.I., Saye R. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine. 2009;27:3090–3098. doi: 10.1016/j.vaccine.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A., Kay B.K., Lambris J.D. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- Schlesinger L.S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- Schlesinger L.S., Bellinger-Kawahara C.G., Payne N.R., Horwitz M.A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- Siddiqui W.A., Tam L.Q., Kan S.C., Kramer K.J., Case S.E., Palmer K.L., Yamaga K.M., Hui G.S. Induction of protective immunity to monoclonal-antibody-defined Plasmodium falciparum antigens requires strong adjuvant in Aotus monkeys. Infect. Immun. 1986;52:314–318. doi: 10.1128/iai.52.1.314-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Kennedy M.C., Long C.A., Saul A.J., Miller L.H., Stowers A.W. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect. Immun. 2003;71:6766–6774. doi: 10.1128/IAI.71.12.6766-6774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Miura K., Zhou H., Muratova O., Keegan B., Miles A., Martin L.B., Saul A.J., Miller L.H., Long C.A. Immunity to recombinant plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect. Immun. 2006;74:4573–4580. doi: 10.1128/IAI.01679-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadafora C., Awandare G.A., Kopydlowski K.M., Czege J., Moch J.K., Finberg R.W., Tsokos G.C., Stoute J.A. Complement receptor 1 is a sialic acid-independent erythrocyte receptor of Plasmodium falciparum. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring M.D., Cummings J.F., Ockenhouse C.F., Dutta S., Reidler R., Angov E., Bergmann-Leitner E., Stewart V.A., Bittner S., Juompan L. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J., Simpson K.M., Triglia T., Plouffe D., Tonkin C.J., Duraisingh M.T., Maier A.G., Winzeler E.A., Cowman A.F. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science. 2005;309:1384–1387. doi: 10.1126/science.1115257. [DOI] [PubMed] [Google Scholar]
- Tas S.W., Klickstein L.B., Barbashov S.F., Nicholson-Weller A. C1q and C4b bind simultaneously to CR1 and additively support erythrocyte adhesion. J. Immunol. 1999;163:5056–5063. [PubMed] [Google Scholar]
- Taylor P.R., Seixas E., Walport M.J., Langhorne J., Botto M. Complement contributes to protective immunity against reinfection by Plasmodium chabaudi chabaudi parasites. Infect. Immun. 2001;69:3853–3859. doi: 10.1128/IAI.69.6.3853-3859.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham W.H., Wilson D.W., Lopaticki S., Schmidt C.Q., Tetteh-Quarcoo P.B., Barlow P.N., Richard D., Corbin J.E., Beeson J.G., Cowman A.F. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17327–17332. doi: 10.1073/pnas.1008151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki S., Uehara S., Kanbe K., Nariuch H., Suzuki M. Interferon-gamma and the induction of protective IgG2a antibodies in non-lethal Plasmodium berghei infections of mice. Parasite Immunol. 1995;17:503–508. doi: 10.1111/j.1365-3024.1995.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Ward P.A., Sterzel R.B., Lucia H.L., Campbell G.H., Jack R.M. Complement does not facilitate plasmodial infections. J. Immunol. 1981;126:1826–1828. [PubMed] [Google Scholar]
- Wold Health Organization . 2013. World Malaria Report 2013. (Geneva) [Google Scholar]
- Yoneto T., Waki S., Takai T., Tagawa Y., Iwakura Y., Mizuguchi J., Nariuchi H., Yoshimoto T. A critical role of Fc receptor-mediated antibody-dependent phagocytosis in the host resistance to blood-stage Plasmodium berghei XAT infection. J. Immunol. 2001;166:6236–6241. doi: 10.4049/jimmunol.166.10.6236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A model of antibody and complement-mediated enhancement of RBC invasion by Plasmodium merozoites, see discussion. 1) In the absence of complement, such as via heat inactivation of serum, the merozoites depend on interactions between the RBC receptors and the parasite ligands. Antibody (Ab) directed against merozoites may have inhibitory effect, and thereby block RBC invasion. 2) In the presence of complement, such as in fresh serum (FS), the merozoites may utilize not only specific receptor ligand interactions, as described in 1, but also a complement receptors on RBCs via complement opsonins. Complement opsonins (C3b) on the merozoite surface may act as an alternative, host derived, parasite ligand that the merozoite can bind to the RBC CR1, and counteract some of the invasion blocking effects of anti-merozoite antibodies. Binding of C3b to CR1 causes aggregation of CR1.









