Abstract
Radiotherapy for the treatment of cancer can cause a wide range of cellular effects, the most biologically potent of which is the double strand break in DNA. The process of repairing DNA double strand breaks involves one of two major mechanisms: non-homologous end-joining or homologous recombination. In this review, we review the molecular mechanisms of homologous recombination, in particular as it relates to the repair of DNA damage from ionizing radiation. We also present specific situations where homologous recombination may be dysfunctional in human cancers, and how this functional abnormality can be recognized. We also discuss the therapeutic opportunities that can be exploited based on deficiencies in homologous recombination at various steps in the DNA repair pathway. Side-by-side with these potential therapeutic opportunities, we review the contemporary clinical trials in which strategies to exploit these defects in homologous recombination can be enhanced by the use of radiotherapy in conjunction with biologically-targeted agents. We conclude that the field of radiation oncology has only scratched the surface of a potentially highly efficacious therapeutic strategy.
Ionizing Radiation Damage to DNA and Repair of Double-Strand Breaks
Ionizing radiation (IR) can cause a myriad of biologic effects throughout the cell, many of which result in loss of viability. This principle underlies the basis of radiotherapy (RT), a highly effective tool in the therapeutic armamentarium against cancer. In the nucleus, IR causes many types of damage to DNA, including ionization of bases and sugars, the formation of strand (DNA-DNA) and DNA-protein cross-links, as well as the formation of single and double DNA strand breaks. Therefore, IR exposure produces a heterogeneous cluster of DNA damage as a consequence of the spatial distribution of energy deposition. The most biologically significant effect of IR on the cell, in terms of cell survival and chromosomal breakage, is the formation of a DNA double strand break (DSB), which occurs in approximately 16-40 locations of a diploid genome for every 1 Gy of low linear energy transfer (LET) radiation absorbed by a cell. Although DSBs are therefore a relatively infrequent type of damage sustained by DNA after IR exposure, they are thought to be the primary lethal event in most cells1.
The repair of DNA DSBs is a complex biological process that is carried out by one of two pathways of DNA repair: non-homologous end-joining (NHEJ) or homologous recombination (HR). NHEJ is an error-prone method, in which the cells modify the broken ends of DNA before they are ligated. It can occur at any point in the cell cycle as it does not require a template of homologous DNA. In contrast, HR faithfully repairs DSBs through use of homologous DNA sequences primarily in the sister chromatids, which are present in the late S and G2 phases of the cell cycle. The process of HR is also used by cells to repair other types of DNA damage, including: stalled replication forks, inter-strand DNA cross-links, sites of meiotic DSBs and abortive topoisomerase II lesions. Although the predominant method of repairing DNA DSBs caused by IR is by NHEJ, there is a growing view that DSBs in cancer cells may be frequently repaired by HR2.
Over the last several years, many components of HR have been elucidated. The genes and proteins involved in this process have been comprehensively reviewed elsewhere3. Briefly, sensors of DNA DSBs are recruited to the site of damage, which activate a variety of cellular signaling pathways, including repair, checkpoints, senescence or cell death. The process of HR repair requires the generation of a 3’-single stranded DNA (ssDNA) by 5’ to 3’ resection at the site of the DSB, which is rapidly coated by replication protein A (RPA). The transition from RPA binding to the formation of RAD51 filaments, which is an essential step before strand exchange can occur, is a complex process controlled by many proteins. One of the key players in this transition is the BRCA2 protein, which appears to replace RPA on ssDNA and promotes the formation of Rad51 multimeric filaments on ssDNA.
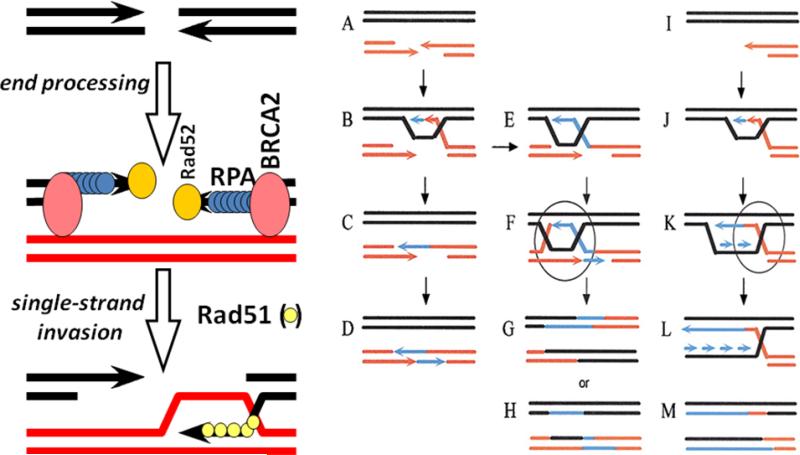
Once the Rad51 filament is formed, the process of invasion of an intact DNA duplex and the search for homology can be initiated. Strand invasion leads to displacement on one strand of the intact DNA duplex and the formation of a “D-loop”, which is the simplest form of HR and can directly act as the template for repairing the DSB (see Figure 1). DNA can then be synthesized using the homologous template from the D-loop, but how the D-loop is disengaged is less clearly understood. The front edge of the D-loop creates a structure that is similar to a replication fork, but the resolution of this structure to allow re-ligation of the two original duplexes is a mechanism which still needs to be elucidated. The ligation step to join the free DNA strands probably involves DNA ligase I (replication-like), but the use of DNA ligase III (single-strand break repair, the final step in base-excision repair) has not been excluded.
Figure 1.
The basic mechanism of homologous recombination (HR) involves processing a double-strand break to reveal a 3’-single strand tail. In the left panel, RPA binds to the single strand tail. By mechanisms that are still not fully understood, recombinase mediators such as BRCA2 and Rad52 help convert the RPA-coated ssDNA into Rad51 filaments. Rad51 filaments are capable of homology search and single strand invasion, which constitutes the basic mechanism of HR. In the right panel, the number of two duplex structures that can be encountered in the cell are shown. In A, the double-strand break is shown; in I, the collapsed replication fork is shown: both are substrates for HR. B-D represents synthesis-dependent strand annealing, in which no 4-strand structure is formed. B to E represents the formation of a Holliday junction, and E to F represents second-end capture and the formation of a double Holliday junction. I-L represents the restart of a collapsed replication fork, referred to as break-induced replication, in which strand invasion by HR recreates the replication fork structure. Holliday junction resolution is required for F-H and K-M.
As the region of strand exchange extends, at some point in this process the replication fork-like structure converts into a 4-strand structure behind the invading 3'strand, known as a Holliday Junction (Figure 1, B to E). In addition, if the 3’ invading strand continues to extend, a more complex structure occurs when the second broken-end is captured to form a second 4-strand structure, resulting in a “double Holliday Junction” at the site of recombination. The length of DNA over which a Holliday Junction can move is from 250 bp to many kilobases. Thus, if significant gaps in sequence have been created by damage to one DNA duplex, double-strand gap repair can occur by HR4.
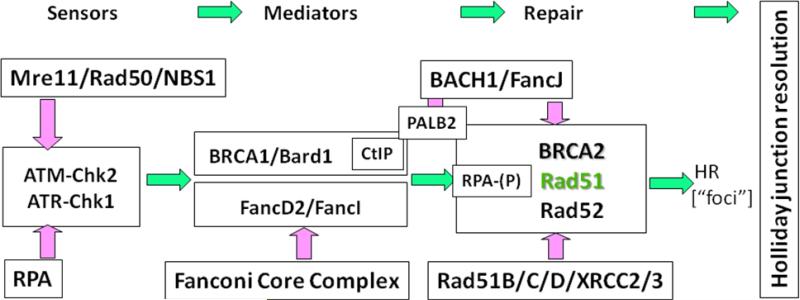
The complexity of the many steps involved in HR results in a large variety of proteins being utilized, which include: RAD51, RAD51-paralogues (XRCC2, XRCC3, RAD51B, RAD51C, and RAD51D), RAD52, RAD54, BRCA2, RPA and many other associated proteins (see Figure 2). The recruitment and function of these HR proteins is promoted by upstream proteins, which include the MRE11-RAD50-NBS1 (MRN) complex, CtIP, BRCA1, and other effectors downstream of the DNA damage sensor ATM. The final steps in HR, which mediate resolution of the 4-strand structures (resolvases), are specialized structure-specific endonucleases, such as Gen1, Mus81, Slx1/4 and XP-G. HR repair proteins are presented schematically in Figure 2.
Figure 2.
The key proteins controlling homologous recombination. The main engine of HR is the Rad51 recombinase, which can often be observed by the formation of Rad51 foci in cells. Immediately upstream of Rad51 are BRCA2 and Rad52, which mediates the conversion of RPA-coated ssDNA into Rad51 filaments. The Rad51 paralogues (Rad51B/C/D, XRCC2/3) assist in this process in ways that still need to be defined. Between BRCA1 and BRCA2, there are a number of mediator proteins that support Rad51 function, such as BACH1, PALB2 and CtIP. The Fanconi Anemia proteins are playing a role in HR, particularly for collapsed replication forks in ways that still need to be determined. Upstream damage sensors send important signals to mediators of HR, such as Chk2-mediated phosphorylation of BRCA1. Any of the points in this pathway of HR are potentially targetable sites for enhancing the therapeutic effect of ionizing radiation.
As expected, defects in any of the steps of HR can lead to impairment of the repair of DNA damage, including DSBs caused by IR. For example, cells from patients with genetic mutations in BRCA1 or BRCA2 are radiosensitive. Moreover, a radiosensitizing effect has been observed by down regulating elements of HR, such as decreasing the expression of RAD51. BRCA1 knockout cells are radiosensitive, which was initially thought to be due to defects in the G2/M cell cycle checkpoint. However, mutations of BRCA1 that produce defects in G2/M checkpoints do not necessarily produce defects either in HR or in radiosensitivity. Complementing cells containing BRCA1 mutations with functional wild-type BRCA1 does confer increased radioresistance; a similar finding has been also observed with BRCA2. Beyond alterations of the levels of HR related proteins, it has been shown that disrupting the interaction between RAD51 and BRCA2 can lead to defective HR and increased radiosensitivity2. It should be noted that the relative radiosensitivity of cells deficient in HR is less than cells deficient in NHEJ, but the difference in sensitivity could be clinically significant.
Recently, strategies to target cancers with defects in components of HR have been proposed5. This strategy, put forth over a decade ago, is often referred to as “synthetic lethality,” and refers to a phenomenon whereby the sum of two non-lethal genetic mutations results in cell death. This type of interaction is particularly appealing when one of the non-lethal genetic mutations is harbored by the tumor, and the second genetic lesion can be introduced therapeutically. By targeting the defects in a tumor that are not present in normal cells, therapeutic gains can be made that limit collateral damage in normal tissues. In the context of cancer cell defects in HR and the use of RT, several approaches have been conceived that would be therapeutically advantageous. The most simplistic approach to this would involve delivering RT to induce DNA DSBs while inhibiting HR. As tumor cells are always proliferating, but normal cells are not necessarily proliferating, the cancer cells are more likely to be affected by the inhibition of HR. In situations where tumors have an enhanced ability to perform HR repair in response to DNA DSBs introduced by RT, such as p53 inactivation, inhibiting the process of HR could further enhance therapeutic gain6. True synthetic lethality is when a drug inhibits a specific alternate repair pathway that is utilized by the cancer cell because of its defect in HR. An example of this is the use of poly-ADP-ribose-polymerase (PARP) inhibitors, which affect single-strand break repair. The loss of function of single-strand break repair produces more replication associated double-strand breaks, which require HR for repair7.
Situations rendering HR a possible target for therapy
There are some specific situations when known defects in HR could be exploited for therapeutic gain: patients with germline mutations in HR related genes and patients with tumors harboring defective HR processes. The former situation is still relatively infrequent, but given the known genomic instability associated with germline mutations in HR genes, this patient population warrants special consideration. Moreover, identifying patients on the basis of classical inheritance patterns or genetic testing will underestimate the number of patients whose cancers harbor abnormalities in components of HR, and could potentially benefit from this type of a targeted approach.
Mutations in the genes involved in HR can lead to syndromes predisposing to common cancers, as described in a recent review by Foulkes8. BRCA1 and BRCA2 are well-known tumor suppressor genes that, if mutated, can cause the Hereditary Breast and Ovarian Cancer (HBOC) syndrome. This syndrome is associated with not only early-onset breast cancer, but also an increased risk of ovarian, pancreatic, stomach, laryngeal, fallopian tube and prostate cancer. It should be noted that the estimated overall proportion of common malignancies, such as prostate and pancreatic cancer, associated with mutation in BRCA2 are only 0.1% and 0.5%, respectively, although the relative risk of developing the cancer (if a mutation is present) is significantly elevated (up to 20 to 10-fold, respectively)8. Likewise, mutations in the NBS1 gene, produce the Nijmegen Breakage Syndrome, a very rare autosomal recessive disorder that causes immunologic and neurologic dysfunction, which also predisposes patients to childhood lymphomas, leukemias, brain tumors and sarcomas9. NBS1 mutation also increases the risk of prostate cancer to a small degree (relative risk <1.5)8.
While most of the components of the HR pathway do not have specific cancers or syndromes associated with them, studies of single nucleotide polymorphisms or gene expression have implicated an association of many components of HR with various malignancies treated with radiotherapy. A sampling of the polymorphism associations is listed in Table 1, and more can be found in the National Institute of Health's “Genetic Association Database,” (http://geneticassociationdb.nih.gov). Miyagawa recently reviewed the association of abnormal expression of mRNA and/or protein elements of the HR pathway in common sporadic human cancers10. It is clear that inconsistent patterns of expression (increased and decreased) have been identified, often in the same type of cancer. It is likely that not all of these associations will be causally linked (i.e. they are false positive associations or the association is a true positive but the causative link is due to an adjacent genetic locus), but these observations suggest that there may be a significant number of true associations to be validated. The associated relative risk of cancer and the potential functional defect in HR remains to be determined.
Table 1.
Examples of homologous recombination genes with polymorphisms associated with cancers/tumors treated with radiation therapy. Data obtained from the National Institute of Health's “Genetic Association Database,” (http://geneticassociationdb.nih.gov).
| Gene | Cancer/tumor treated with radiation therapy |
|---|---|
| RPA1 | head and neck cancer |
| RPA3 | lung cancer |
| MRE11 | non-Hodgkin lymphoma |
| RAD51 | breast cancer, acute myelogenous leukemia, gastric cancer |
| RAD51B, RAD51C, RAD51D | breast cancer, meningioma |
| XRCC2 | breast cancer, basal cell carcinoma, rectal cancer |
| XRCC3 | breast cancer, bladder cancer, head and neck cancer |
| RAD52 | breast cancer, papillary thyroid cancer |
| RAD54L | meningioma |
Beyond the association of malignancies and gene polymorphisms or expression abnormalities in components of HR, several studies have been conducted associating radiotherapeutic response and elements of HR, both therapeutic and toxic. A few studies have documented improved clinical outcomes after radiotherapy in association with polymorphisms of HR-related genes. Two such studies from the MD Anderson Cancer Center have shown that patients with polymorphisms of XRCC2, XRCC3, and RAD54L had significant variations in survival after chemoradiation with gemcitabine for resectable pancreatic adenocarcinoma11-12. As recently reviewed by Barnett and colleagues, components of HR identified through genome-wide association studies (GWAS) have been associated with radiotherapy toxicity and include RAD51, BRCA1, BRCA2, and XRCC313. The prediction would be that these toxicities occur in tissues with significant numbers of proliferating stem cells, such as epithelial, rather than predominantly tissues with mostly post-mitotic cells, such as neural tissues.
While studies associating HR genes with malignancies and response to radiotherapy are thought provoking, applying therapies that specifically target HR will require identification of biomarkers that can predict response. Several studies have been performed demonstrating the feasibility of this concept. As recently reviewed by Liu and colleagues14, biomarkers of DNA DSB repair can be (1) assays of enzymatic activity, expression or post-translational modification, (2) direct measures of DNA DSB repair complexes as foci, or (3) assays of chromosomal damage or breaks. Several studies have found associations between biomarkers of HR and outcome after radiotherapy. Soderlund and colleagues found that the presence of the MRE11/RAD50/NBS1 and BRCA1/BRCA2/RAD51 complexes was prognostic and predictive of response to radiotherapy in early stage breast cancer patients, treated in a randomized controlled trial15-16. Similarly, two studies of patients with squamous cell carcinoma of the head and neck treated with radiotherapy have identified components of the HR process that are associated with treatment response; in one study increased NBS1 was associated with inferior survival17, while in another study, high levels of RAD51 was associated with inferior survival18. Willers and colleagues recently reported a novel study of breast cancer patients who underwent ex vivo assessment of tumor biopsies for IR-induced components of HR (BRCA1 and Rad51). The authors confirmed that this approach was feasible and informative of the functional status of components of the HR pathway, which may represent a more meaningful and robust biologic end point than those mentioned previously19.
Thus, although there are encouraging preliminary data suggesting association between alterations in components of the HR pathway and cancer induction, as well as response to cancer therapy, individual polymorphisms have rarely been confirmed as functionally defective mutations. The combination of unbiased whole genome polymorphism analyses with good quality functional data is needed to develop a catalogue of prevalent polymorphisms that actually directly affect HR function.
Methods of exploiting HR to enhance radiotherapeutic gain
Non-drug based approaches
There are a number of ways that the process of HR can be exploited to enhance radiotherapeutic gain. At this juncture, many of these techniques are in preclinical development, but may become clinically relevant in the future. Down-regulation of components of the HR pathway is one such example. Collis and colleagues demonstrated the radiosensitizing effect of a Rad51-targeted ribozyme minigene in preclinical studies20. In a similar study, Yu and colleagues found that siRNA targeting BRCA2 effectively radiosensitized cells in vitro and in vivo, and suggested this type of approach may be beneficial clinically21. At this juncture, the ability to deliver targeted oligonucleotides to patients in vivo is limited, but with advances in delivery techniques this approach may become a viable option for HR modulation in the future.
Another approach to disrupting the process of HR involves combining hyperthermia with radiotherapy. As recently reviewed by Iliakis and colleagues, heat has been shown to interfere with all aspects of the DNA DSB repair process, including HR22. Although the specific mechanisms of heat-induced HR impairment have yet to be elucidated, a number of clinical trials have demonstrated the efficacy of combining hyperthermia and radiotherapy. On the basis of preclinical data, it appears unlikely that clinically relevant non-conventional fractionation or dose-rate schemes are likely to alter the repair of DNA DSBs by HR. However, using high linear energy transfer (LET) radiotherapy (i.e., neutrons, low-energy protons, alpha-particles) with inhibitors of HR may be a viable strategy. Several preclinical studies have demonstrated that high LET radiation does not have a significant impact on the sensitivity of cells deficient in NHEJ; however, cells deficient in HR are significantly more radiosensitive23.
Drug based approaches
Recently, several novel classes of drugs have been developed that inhibit HR, and have been studied in conjunction with ionizing radiation. Because of space limitations, drugs known to have a radiation modulating effect, that have been shown to have an effect on HR, and are commonly employed with radiotherapy, such as topoisomerase II inhibitors (e.g., etoposide), anti-metabolites (i.e., gemcitabine, 5-fluorouracil), microtubule inhibitors (e.g., paclitaxel, vinorelbine), cross-linking agents (e.g., platinum compounds and mitomycin C), alkylating agents (e.g., temozolomide), will not be considered further here, as they were discussed in a series of excellent reviews published in 2007, in the Journal of Clinical Oncology (volume 25, number 26). A listing of the clinical studies which combined RT and the novel agents discussed here can be found in the supplement to this review.
Several studies have demonstrated the radiation modulating effect of the heat-shock protein 90 (HSP90) inhibitors, through multiple putative targets, as reviewed by Camphausen and Tofilon24. Interestingly, Noguchi and colleagues demonstrated that tumor cells exposed to the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17AAG), have reduced expression of BRCA1 and RAD51, but no alterations in Ku70 or Ku80, suggesting that the in vitro radiosensitizing effect observed may be due to impairment of HR and not NHEJ; moreover, the radiosensitizing effect was not observed in normal cells, suggesting opportunity for therapeutic gain25. The feasibility of treating patients with a HSP90 inhibitor and radiotherapy has not been assessed in clinical studies.
Imatinib (Gleevec, Novartis, East Hanover, NJ) and dasatinib (Sprycel, Bristol-Myers Squibb, Plainsboro, NJ) are inhibitors of c-Abl kinase, which has been suggested to be involved in the radiation-induced assembly of RAD51 and RAD5226. For this reason, investigators at the National Cancer Institute studied the radiosensitizing effect of imatinib on glioma and fibroblast cell lines. The authors found that imatinib was able to reduce expression of RAD51 in the tumor, but not the normal cell lines. In addition, they observed a radiation dose enhancing effect of 1.3-1.5 in the tumor cell lines, with no radiosensitization in the normal cell lines27. Several studies have confirmed the radiosensitizing effect in vitro and in vivo. Interestingly, a study from Germany found that imatinib conferred a radioprotective effect in human squamous carcinoma cell lines. This effect was in part explained by the induction of RAD51 after exposure to the drug28. Taken together, these results suggest that evaluating components of HR would be critical during a clinical study with this c-Abl kinase inhibitors and RT. However, none of the clinical studies that have been performed involved correlative studies of HR repair elements. Phase I/II clinical trials of imatinib and RT have suggested a radiation modifying effect, however, no therapeutic gain has been realized yet. Clinical studies are now focusing on dasatinib in combination with RT in several early phase trials.
Erlotinib (Tarceva, Genentech, South San Francisco, CA) and gefitinib (Iressa, AstraZeneca, Wilmington, DE) inhibit the intracellular tyrosine kinase domain of the epidermal growth factor receptor (EGFR). Erlotinib has been found to cause several cellular effects which could modify the cellular response to radiation, including attenuation of RAD51 expression29. Studies by Li et al. confirmed that erlotinib attenuated the HR response in vitro through BRCA130. Of all the novel agents that modulate the HR pathway, and have been combined with RT, erlotinib has been studied most extensively. Three phase II trials of patients with newly diagnosed glioblastoma multiforme have been performed31-33. When compared to historical results using RT and temozolomide concurrently, only one of the trials appeared to demonstrate a survival advantage for patients treated with RT, temozolomide, and erlotinib33. Unfortunately, no biologic correlative studies were performed to assess for HR related factors that might predict response to therapy, and no further studies in glioblastoma patients are ongoing. Several other phase I and/or II trials of erlotinib and RT have been completed, with mixed results in terms of toxicity and efficacy. There are currently three phase III trials combining RT and erlotinib ongoing. Several phase I and/or II studies with gefitinib have been reported, also with mixed results. There are currently no phase III trials ongoing with RT and this agent.
Inhibitors of the ubiquitin/proteasome system are in various phases of preclinical and clinical development, and several have been shown to modulate the biologic response to IR, including bortezomib and nelfinavir34. Recently, data from Murkawa et al. has emerged suggesting that modulators of the proteasome may affect HR35. Research from Zhang and collaborators suggests that proteasome inhibitors block MDC1 degradation, which in turn leads to abrogation of BRCA1 recruitment, leading to impairment in HR36. In clinical studies, bortezomib has been studied in combination with radioimmunotherapy, and been found to have an acceptable toxicity profile37; phase II studies are currently underway. Varied levels of toxicity have been reported in phase I trials using external beam RT and bortezomib. Nelfinavir was recently reported to demonstrate safety and favorable responses when combined with chemoradiation for locally advanced pancreatic cancer38. Further studies are underway.
Members of a relatively new class of drugs, the histone deacetylase inhibitors (HDACis) have demonstrated a range of radiation modulating effects in a number of studies39. Radiosensitizing effects have been observed in all subclasses of HDACis, with many different tumor cell types, both in vitro and in vivo39. A study by Adimoolam et al. demonstrated that the HDACi PCI-24781 decreased expression of BRCA1, BRCA2, and RAD51, and that in a functional assay, HR was impaired in a dose dependent fashion; a radiosensitizing effect was noted in tumor cells in culture40. Phase I and/or II studies of the HDACis valproic acid, vorinostat (Zolinza, Merck, Whitehouse Station, NJ), and panobinostat (LBH-589, Novartis, East Hanover, NJ) and RT, are ongoing.
While PARP is not an essential element of the HR pathway, stalled replication forks are frequently repaired by HR, and thus, as a critical component of this process, PARP may be an attractive target for combined therapy with radiation. Several preclinical studies have demonstrated the radiation modulating effect of PARP, by pharmacologic inhibition, in cells and animal models with first and second generation agents. This effect has been demonstrated under hypoxic conditions and rapidly proliferating cells, suggesting an opportunity for radiotherapeutic gain. There are currently three ongoing phase I and/or II clinical trials studying the combination of RT and PARP inhibitors.
Although several drugs with pre-clinical evidence of affecting the HR pathway and modulating the biologic response to IR are being studied in clinical trials, there has been relatively sparse biomarker collection to confirm this effect. In the more recently planned trials (for example, NCT00983268), gathering biomarkers that assess DNA damage repair is becoming more common, but whether these markers will be sufficiently useful to distinguish tumors that are more suitable for HR targeting strategies is not clear. The development of functional assays for measuring HR in clinical samples is needed before advances in this targeting strategy can be successfully employed.
Supplementary Material
Table 2.
Altered expression levels of molecules involved in homologous recombination in cancers treated with radiation therapy. Expression of mRNA and/or protein was evaluated in human cancer specimens. Studies of cultured cells or in non-sporadic (familial or syndromic) cancer were excluded. Reproduced from Table 1, Kiyoshi Miyagawa, “Clinical relevance of the homologous recombination machinery in cancer therapy,” Cancer Sci 2008, page 190 (in Cancer Science, Volume 99, Issue 2, 2008, pages 187-194), permission pending (requested 1/27/10, may take 20 days).
| Gene/protein | Increased expression | Decreased expression |
|---|---|---|
| RAD51 | pancreatic cancer breast cancer non-small cell lung cancer head and neck cancer soft tissue sarcoma |
colorectal cancer breast cancer |
| RAD51C | breast cancer | |
| RAD52 | colorectal cancer | |
| BRCA1 | lung cancer ovarian cancer lung cancer |
breast cancer |
| BRCA2 | ovarian cancer | |
| MRE11 | breast cancer colorectal cancer |
|
| RAD50 | breast cancer | |
| NBS1 | head and neck cancer melanoma melanoma |
breast cancer colorectal cancer |
| BLM | chronic myelogenous leukemia lymphoma breast cancer breast cancer lung cancer renal cell cancer seminoma |
|
| WRN | chronic myelogenous leukemia colorectal cancer |
gastric cancer |
| FANCF | ovarian cancer cervical cancer |
|
| ERCC1 | non-small cell lung cancer ovarian cancer colorectal cancer |
non-small cell lung cancer gastric cancer colorectal cancer |
Acknowledgments
1Supported in part by the ASTRO Resident in Radiation Oncology Research Seed Grant
2Supported in part by the National Cancer Institute and the Susan G. Komen Breast Cancer Research Foundation
References
- 1.Ward JF. The Yield of DNA Double-Strand Breaks Produced Intracellularly by Ionizing-Radiation - a Review. International Journal of Radiation Biology. 1990;57:1141–1150. doi: 10.1080/09553009014551251. [DOI] [PubMed] [Google Scholar]
- 2.Powell SN, Kachnic LA. Therapeutic exploitation of tumor cell defects in homologous recombination. Anticancer Agents Med Chem. 2008;8:448–60. doi: 10.2174/187152008784220267. [DOI] [PubMed] [Google Scholar]
- 3.Filippo JS, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annual Review of Biochemistry. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 4.Szostak JW, Orr-Weaver TL, Rothstein RJ, et al. The double-strand-break repair model for recombination. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 5.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 6.Willers H, Dahm-Daphi J, Powell SN. Repair of radiation damage to DNA. British Journal of Cancer. 2004;90:1297–1301. doi: 10.1038/sj.bjc.6601729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 8.Foulkes WD. Molecular Origins of Cancer: Inherited Susceptibility to Common Cancers. New England Journal of Medicine. 2008;359:2143–2153. doi: 10.1056/NEJMra0802968. [DOI] [PubMed] [Google Scholar]
- 9.Hiel JA, Weemaes CM, van den Heuvel LP, et al. Nijmegen breakage syndrome. Archives of Disease in Childhood. 2000;82:400–406. doi: 10.1136/adc.82.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyagawa K. Clinical relevance of the homologous recombination machinery in cancer therapy. Cancer Sci. 2008;99:187–94. doi: 10.1111/j.1349-7006.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Liu H, Jiao L, et al. Significant effect of homologous recombination DNA repair gene polymorphisms on pancreatic cancer survival. Cancer Research. 2006;66:3323–30. doi: 10.1158/0008-5472.CAN-05-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Frazier M, Evans DB, et al. Single nucleotide polymorphisms of RecQ1, RAD54L, and ATM genes are associated with reduced survival of pancreatic cancer. Journal of Clinical Oncology. 2006;24:1720–8. doi: 10.1200/JCO.2005.04.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett GC, West CML, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nature Reviews Cancer. 2009;9:134–142. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu SK, Olive PL, Bristow RG. Biomarkers for DNA DSB inhibitors and radiotherapy clinical trials. Cancer and Metastasis Reviews. 2008;27:445–458. doi: 10.1007/s10555-008-9137-8. [DOI] [PubMed] [Google Scholar]
- 15.Soderlund K, Skoog L, Fornander T, et al. The BRCA1/BRCA2/Rad51 complex is a prognostic and predictive factor in early breast cancer. Radiotherapy and Oncology. 2007;84:242–251. doi: 10.1016/j.radonc.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Soderlund K, Stal O, Skoog L, et al. Intact Mre11/Rad50/Nbs1 complex predicts good response to radiotherapy in early breast cancer. International Journal of Radiation Oncology Biology Physics. 2007;68:50–58. doi: 10.1016/j.ijrobp.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Yang MH, Chiang WC, Chou TY, et al. Increased NBS1 expression is a marker of aggressive head and neck cancer and overexpression of NBS1 contributes to transformation. Clinical Cancer Research. 2006;12:507–515. doi: 10.1158/1078-0432.CCR-05-1231. [DOI] [PubMed] [Google Scholar]
- 18.Connell PP, Jayathilaka K, Haraf DJ, et al. Pilot study examining tumor expression of RAD51 and clinical outcomes in human head cancers. International Journal of Oncology. 2006;28:1113–1119. [PubMed] [Google Scholar]
- 19.Willers H, Taghian AG, Luo CM, et al. Utility of DNA Repair Protein Foci for the Detection of Putative BRCA1 Pathway Defects in Breast Cancer Biopsies. Molecular Cancer Research. 2009;7:1304–1309. doi: 10.1158/1541-7786.MCR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collis SJ, Tighe A, Scott SD, et al. Ribozyme minigene-mediated RAD51 down-regulation increases radiosensitivity of human prostate cancer cells. Nucleic Acids Research. 2001;29:1534–1538. doi: 10.1093/nar/29.7.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu D, Sekine E, Fujimori A, et al. Down regulation of BRCA2 causes radio-sensitization of human tumor cells in vitro and in vivo. Cancer Science. 2008;99:810–815. doi: 10.1111/j.1349-7006.2008.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iliakis G, Wu WQ, Wang ML. DNA double strand break repair inhibition as a cause of heat radiosensitization: Re-evaluation considering backup pathways of NHEJ. International Journal of Hyperthermia. 2008;24:17–29. doi: 10.1080/02656730701784782. [DOI] [PubMed] [Google Scholar]
- 23.Frankenberg-Schwager M, Gebauer A, Koppe C, et al. Single-Strand Annealing, Conservative Homologous Recombination, Nonhomologous DNA End Joining, and the Cell Cycle-Dependent Repair of DNA Double-Strand Breaks Induced by Sparsely or Densely Ionizing Radiation. Radiation Research. 2009;171:265–273. doi: 10.1667/RR0784.1. [DOI] [PubMed] [Google Scholar]
- 24.Camphausen K, Tofilon PJ. Inhibition of Hsp90: A multitarget approach to radiosensitization. Clinical Cancer Research. 2007;13:4326–4330. doi: 10.1158/1078-0432.CCR-07-0632. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi M, Yu D, Hirayama R, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochemical and Biophysical Research Communications. 2006;351:658–663. doi: 10.1016/j.bbrc.2006.10.094. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Yuan SSF, Liu W, et al. Radiation-induced assembly of Rad51 and Rad52 recombination complex requires ATM and c-Abl. Journal of Biological Chemistry. 1999;274:12748–12752. doi: 10.1074/jbc.274.18.12748. [DOI] [PubMed] [Google Scholar]
- 27.Russell JS, Brady K, Burgan WE, et al. Gleevec-mediated inhibition of Rad51 expression and enhancement of tumor cell radiosensitivity. Cancer Research. 2003;63:7377–7383. [PubMed] [Google Scholar]
- 28.Bartkowiak D, Hipp PR, Mendonca MS, et al. A radioprotective effect of imatinib (Gleevec((R))) in human squamous carcinoma cells. Strahlentherapie Und Onkologie. 2007;183:432–439. doi: 10.1007/s00066-007-1680-7. [DOI] [PubMed] [Google Scholar]
- 29.Chinnaiyan P, Huang SM, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by Erlotinib (Tarceva). Cancer Research. 2005;65:3328–3335. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- 30.Li LP, Wang H, Yang ES, et al. Erlotinib Attenuates Homologous Recombinational Repair of Chromosomal Breaks in Human Breast Cancer Cells. Cancer Research. 2008;68:9141–9146. doi: 10.1158/0008-5472.CAN-08-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prados MD, Chang SM, Butowski N, et al. Phase II Study of Erlotinib Plus Temozolomide During and After Radiation Therapy in Patients With Newly Diagnosed Glioblastoma Multiforme or Gliosarcoma. Journal of Clinical Oncology. 2009;27:579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2009 doi: 10.1007/s11060-009-0067-2. [DOI] [PubMed] [Google Scholar]
- 33.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II Trial of Erlotinib and Temozolomide With Radiation Therapy in the Treatment of Newly Diagnosed Glioblastoma Multiforme: North Central Cancer Treatment Group Study N0177. Journal of Clinical Oncology. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBride WH, Iwamoto KS, Syljuasen R, et al. The role of the ubiquitin/proteasome system in cellular responses to radiation. Oncogene. 2003;22:5755–5773. doi: 10.1038/sj.onc.1206676. [DOI] [PubMed] [Google Scholar]
- 35.Murakawa Y, Sonoda E, Barber LJ, et al. Inhibitors of the proteasome suppress homologous DNA recombination in mammalian cells. Cancer Research. 2007;67:8536–8543. doi: 10.1158/0008-5472.CAN-07-1166. [DOI] [PubMed] [Google Scholar]
- 36.Shi W, Ma ZF, Willers H, et al. Disassembly of MDC1 Foci Is Controlled by Ubiquitin-Proteasome-dependent Degradation. Journal of Biological Chemistry. 2008;283:31608–31616. doi: 10.1074/jbc.M801082200. [DOI] [PubMed] [Google Scholar]
- 37.Berenson JR, Yellin O, Patel R, et al. A phase I study of samarium lexidronam/bortezomib combination therapy for the treatment of relapsed or refractory multiple myeloma. Clinical Cancer Research. 2009;15:1069–75. doi: 10.1158/1078-0432.CCR-08-1261. [DOI] [PubMed] [Google Scholar]
- 38.Brunner TB, Geiger M, Grabenbauer GG, et al. Phase I trial of the human immunodeficiency virus protease inhibitor Nelfinavir and chemoradiation for locally advanced pancreatic cancer. Journal of Clinical Oncology. 2008;26:2699–2706. doi: 10.1200/JCO.2007.15.2355. [DOI] [PubMed] [Google Scholar]
- 39.De Schutter H, Nuyts S. Radiosensitizing Potential of Epigenetic Anticancer Drugs. Anti-Cancer Agents in Medicinal Chemistry. 2009;9:99–108. doi: 10.2174/187152009787047707. [DOI] [PubMed] [Google Scholar]
- 40.Adimoolam S, Sirisawad M, Chen J, et al. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19482–19487. doi: 10.1073/pnas.0707828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.