Abstract
Purpose
To report dosimetry and early toxicity data in breast cancer patients treated with postoperative proton radiation therapy.
Methods and Materials
From March 2013 to April 2014, 30 patients with nonmetastatic breast cancer and no history of prior radiation were treated with proton therapy at a single proton center. Patient characteristics and dosimetry were obtained through chart review. Patients were seen weekly while on treatment, at 1 month after radiation therapy completion, and at 3- to 6-month intervals thereafter. Toxicity was scored using Common Terminology Criteria for Adverse Events version 4.0. Frequencies of toxicities were tabulated.
Results
Median dose delivered was 50.4 Gy (relative biological equivalent [RBE]) in 5 weeks. Target volumes included the breast/chest wall and regional lymph nodes including the internal mammary lymph nodes (in 93%). No patients required a treatment break. Among patients with >3 months of follow-up (n = 28), grade 2 dermatitis occurred in 20 patients (71.4%), with 8 (28.6%) experiencing moist desquamation. Grade 2 esophagitis occurred in 8 patients (28.6%). Grade 3 reconstructive complications occurred in 1 patient. The median planning target volume V95 was 96.43% (range, 79.39%-99.60%). The median mean heart dose was 0.88 Gy (RBE) [range, 0.01–3.20 Gy (RBE)] for all patients, and 1.00 Gy (RBE) among patients with left-sided tumors. The median V20 of the ipsilateral lung was 16.50% (range, 6.1%–30.3%). The median contralateral lung V5 was 0.34% (range, 0%–5.30%). The median maximal point dose to the esophagus was 45.65 Gy (RBE) [range, 0–65.4 Gy (RBE)]. The median contralateral breast mean dose was 0.29 Gy (RBE) [range, 0.03–3.50 Gy (RBE)].
Conclusions
Postoperative proton therapy is well tolerated, with acceptable rates of skin toxicity. Proton therapy favorably spares normal tissue without compromising target coverage. Further follow-up is necessary to assess for clinical outcomes and cardiopulmonary toxicities.
Introduction
Adjuvant radiation therapy for breast cancer has been shown to improve overall survival after mastectomy (1–3) and contribute to longer breast cancer–specific survival after breast-conservation surgery (4). For patients with a high risk of nodal failure after surgery, comprehensive coverage of the axillary and supraclavicular lymph nodes within the radiation target is indicated. Recent data also suggest that inclusion of the internal mammary lymph nodes (IMNs) increases survival among select patients (5, 6).
Treatment to these areas with traditional irradiation techniques can encompass a significant amount of normal tissue, including the heart, lungs, and contralateral breast. Patients receiving radiation for breast cancer are subsequently at risk for cardiopulmonary toxicity (7–12) and secondary cancers (13, 14). Much effort has been directed at avoiding normal structures while still managing to target areas at risk for recurrence.
With traditional photon therapy techniques, target coverage can be compromised in efforts to minimize dose to the critical structures (15). Highly conformal techniques, such as intensity modulated radiation therapy (IMRT) and volumetric modulated arc therapy, can improve coverage but lead to higher levels of low and intermediate doses to surrounding organs. Proton therapy has the unique ability to achieve full coverage of the target tissue and simultaneous optimal organ sparing. This sparing is made possible by the rapid fall-off of dose distal to the target. Despite the potential benefits, clinical use of proton therapy for breast cancer is limited. Although several groups have reported outcomes for partial breast irradiation in early-stage breast cancer (16–18), the literature surrounding the use of proton therapy for locally advanced breast cancer consists of a single publication from the Massachusetts General Hospital on early outcomes for 12 patients treated on an institutional protocol (19). Furthermore, there are no published clinical data to date for the use of proton therapy in the treatment of the whole breast and regional lymph nodes. We report the early experience at a single proton center in treating breast cancer patients with adjuvant proton therapy, in both the postmastectomy and postlumpectomy setting for breast cancer.
Methods and Materials
Patient population
In 2013 a breast cancer treatment program was initiated at Procure Proton Therapy Center (Somerset, NJ). From March 2013 to April 2014, 30 consecutive patients with nonmetastatic breast cancer and no history of prior chest wall radiation therapy were treated with postoperative proton radiation therapy. These patients were not part of a clinical trial. Patients were generally referred because of unfavorable cardiopulmonary anatomy. Postlumpectomy patients were not offered treatment if large breast size (defined as having breast anatomy that was prone to significant interfraction mobility) would preclude accurate setup. Patient and treatment characteristics were extracted from a prospective database and are summarized in Table 1.
Table 1.
Patient characteristics
| Age (y), median (range) | 49 (29–86) |
| Stage | |
| II | 8 (26.7) |
| III | 20 (66.7) |
| Chest wall recurrence | 2 (6.7) |
| Histology | |
| IDC | 27 (90) |
| ILC | 3 (10) |
| Side | |
| Left | 27 (90) |
| Right | 3 (10) |
| Chemotherapy | |
| Neoadjuvant | 13 (43.3) |
| Adjuvant | 14 (46.7) |
| Anthracycline-based | 21 (70) |
| Concurrent herceptin | 4 (13.3) |
| None | 3 (10) |
| Surgery | |
| Lumpectomy (BCS) | 4 (13.3) |
| Chest wall wide local excision (recurrence) | 2 (6.7) |
| Mastectomy + implant reconstruction | 14 (46.7) |
| Mastectomy + autologous reconstruction | 1 (3.3) |
| Mastectomy no reconstruction | 9 (30) |
Abbreviations: BCS = breast-conserving surgery; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma.
Values are number (percentage) unless otherwise noted.
Radiation therapy
Patients were simulated with a CT scan in the supine position using a custom mold for immobilization. Xio treatment planning software (Elekta, Stockholm, Sweden) was used for treatment planning. Artifacts seen on the planning CT scan were addressed on a per-patient basis with manual electron density override. Clinical target volumes (CTVs) and normal structures were contoured according to Radiation Therapy Oncology Group (RTOG) guidelines (20), with the exception of the supraclavicular fossa and exclusion of the ribs and intercostals. For the supraclavicular lymph node group, the volume was modified in the majority of cases to encompass the portion of the fossa that is deep and posterior to the sternocleidomastoid (analogous to level V of the neck). This area is a known site of nodal metastases and failure (21) and is usually covered with photons, because an anterior oblique field deposits a gradient of dose throughout the entire width of the patient. When using proton therapy to treat this area, it is important to extend the range of the en face beam to include the posterior supraclavicular fossa. We therefore routinely include this area in the treatment volume for patients undergoing postoperative proton therapy. This volume modification is illustrated in Figure 1. For planning target volume (PTV) expansion, the CTV was expanded by 7 mm laterally. In the distal/posterior direction, the plan was generated to cover the CTV, and the plan was evaluated for range uncertainty of ±(2.5% + 2 mm). A smear radius of 7 mm, equal to the lateral setup uncertainty, was also used. The PTV also flashed the skin anteriorly by 5 mm.
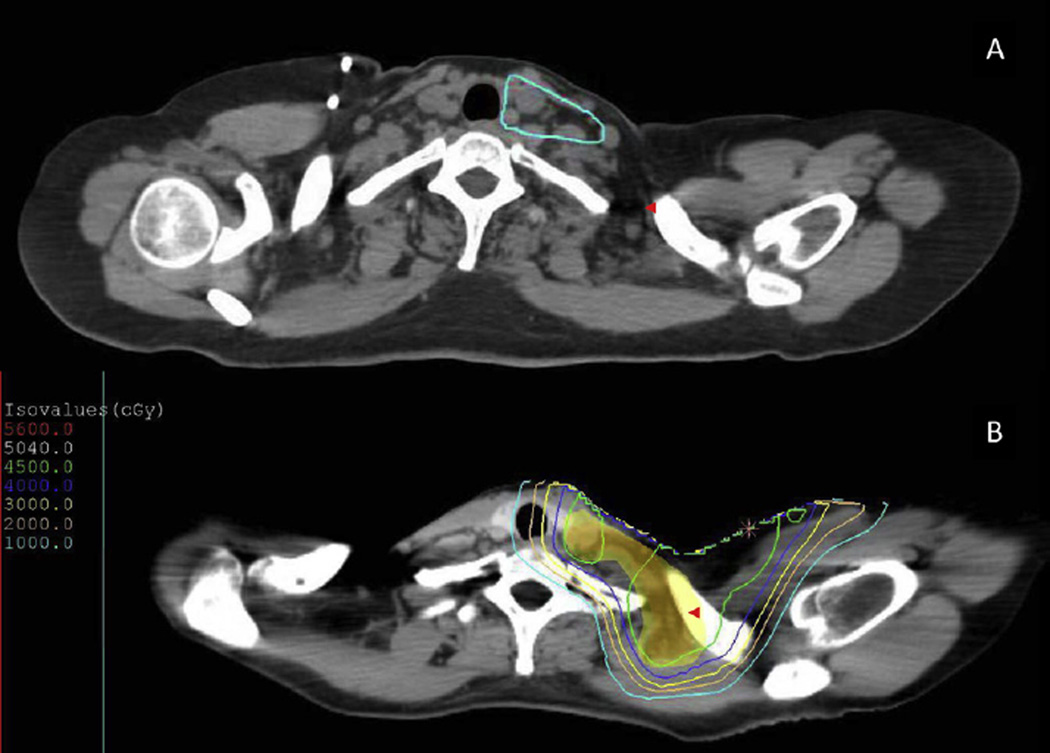
Fig. 1.
Lymph node groups were contoured per Radiation Therapy Oncology Group guidelines (A), with the exception of the posterior portion of the supraclavicular fossa (red arrowheads), which were included in the clinical target volume (B; red color wash) and planning target volume (B; yellow color wash).
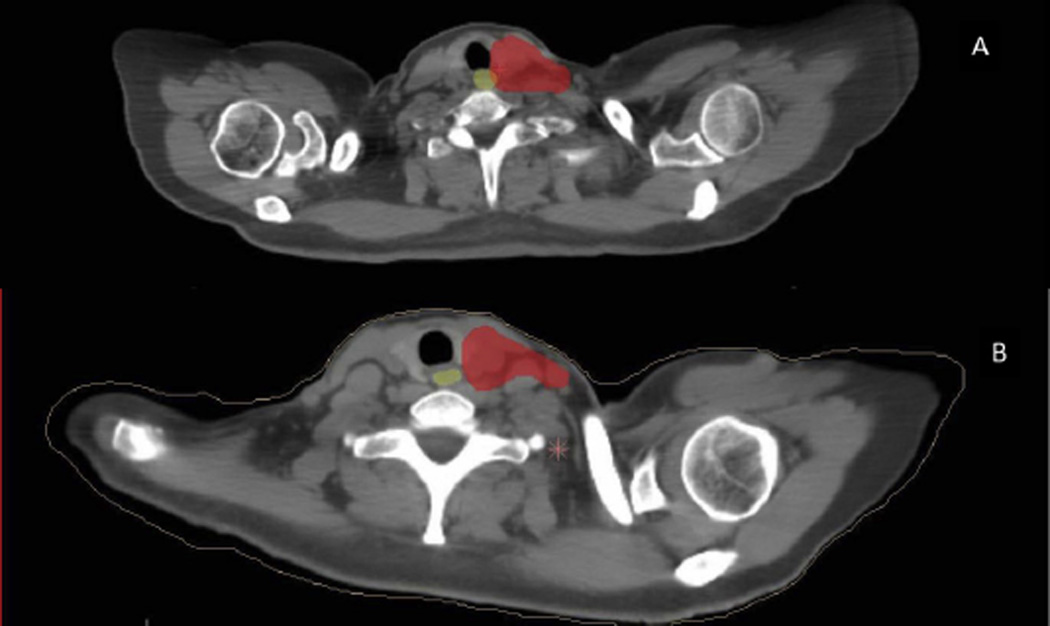
In the initial experience the CTV was drawn medially to the edge of the esophagus. An additional margin was added medially for PTV, which subsequently overlapped with the esophagus. Because of detection of mild esophagitis in 3 of the first 5 patients treated, the contouring technique was later modified so that no PTV margin was added around the esophagus, and PTV overlap was eliminated. This modification is illustrated in Figure 2.
Fig. 2.
(A) The initial Proton Collaborative Group protocol included the clinical target volume contoured to the edge of the esophagus, with expansion causing planning target volume (red color wash) overlap with the esophagus (yellow color wash). (B) The protocol was later modified to minimize planning target volume expansion and overlap around the esophagus.
Proton therapy was delivered with uniform scanning beams. The beam arrangement included an anteriorly oriented field encompassing the chest wall and regional lymph nodes, which was matched to an anteriorly directed supraclavicular fossa field. To feather the match, a set of beams with the same orientation and targets but with a match line that was shifted approximately 1 cm in the superior/inferior direction was also used. A typical beam arrangement is shown in Figure 3. The daily fraction was delivered using all 4 fields daily. In general, 45 Gy (relative biological effectiveness [RBE]) was delivered to the chest wall, regional nodes, and supraclavicular fossa, followed by a cone down of an additional 5.4 Gy (RBE) to the chest wall using an RBE value of 1.1. An additional boost to involved lymph nodes, mastectomy scar, and tumor bed was used for select patients at physician discretion. The median dose delivered to the comprehensive PTV was 50.4 Gy (RBE) [range, 45–65 Gy (RBE)]. Setup accuracy was confirmed with daily X-ray verification of the isocenter based on bony anatomy. For postlumpectomy patients, low-dose verification CTs were obtained 3 times during the course of treatment to ensure accurate setup and plan the tumor bed boost: once on the day of simulation, another on week 1, and a third the week before the boost.
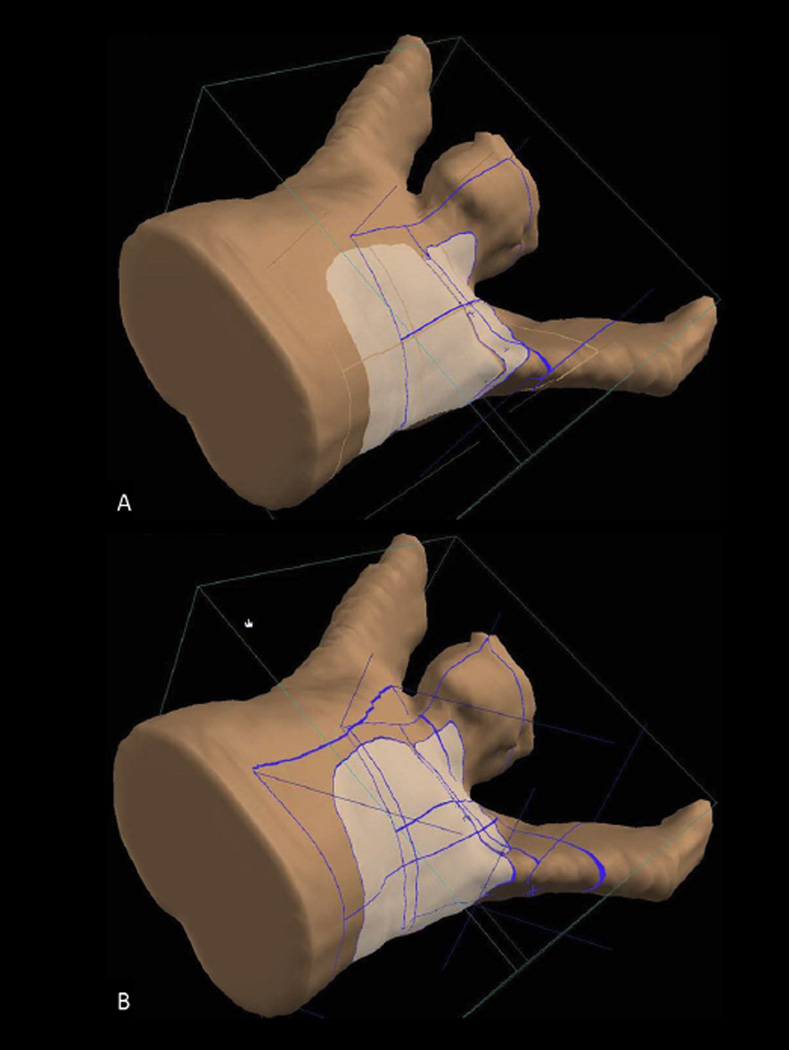
Fig. 3.
(A) A typical beam arrangement used to treat the unreconstructed chest wall and regional lymph nodes. (B) Feathered junction with the match line shift superiorly by 1 cm for the same patient as in (A).
Follow-up
Patients were seen weekly while on treatment, at 1 month after radiation therapy completion, and at 3- to 6-month intervals thereafter. Skin toxicity, fatigue, esophagitis, lymphedema, reconstructive complications, chest wall pain, and rib fracture were assessed using Common Terminology Criteria for Adverse Events version 4.0. Toxicities were defined as occurring any time after treatment initiation.
Results
Progression or recurrence
Median follow-up was 9.3 months (range, 2.3–18.6 months) from the beginning of radiation therapy. No patient experienced disease progression or recurrence during treatment. During the follow-up period, 1 patient with cT3N3cM0, ypT1micN1M0 invasive ductal carcinoma was treated with postmastectomy radiation therapy and developed isolated distant liver metastases 10 months after completion of radiation therapy.
Dosimetry
There were 26 patients treated to the postmastectomy chest wall and regional nodes and 4 treated to the whole breast and regional lymphatics after lumpectomy. The internal mammary nodes were treated at the physician’s discretion and included in the target in 28 patients (93.0%).
Proton therapy generally achieved full coverage of the PTV (assessed by PTV V100 > 90% and V95 > 95%). Protons also significantly spared organs at risk, including the heart, lungs, and contralateral breast. The esophagus was constrained to a max point dose <100%, which was achieved in the majority of patients. Dosimetry data are summarized in Table 2.
Table 2.
Dosimetry values
| PTV | |
| V100 (%) | 89.20 (68.56–96.30) |
| V95 (%) | 96.43 (79.39–99.60) |
| V110 (%) | 13.30 (3.02–34.98) |
| Max point dose, Gy (RBE) | 58.84 (50.8–70.5) |
| Heart (left-sided tumors, n=27) | |
| Mean dose, Gy (RBE) | 1.0 (0.09–3.20) |
| V20 (%) | 1.16 (0–6.0) |
| V5 (%) | 5.00 (0.17–14.40) |
| Max point dose, Gy (RBE) | 22.80 (2.48–43.70) |
| Lungs | |
| Total V20 (%) | 7.31 (0.14–13.2) |
| Ipsilateral V20 (%) | 16.50 (6.1–30.3) |
| Ipsilateral V5 (%) | 34.35 (22.5–53.8) |
| Contralateral V5 (%) | 0.34 (0–5.30) |
| Contralateral breast | |
| Mean dose, Gy (RBE) | 0.29 (0.03–3.50) |
| V5 (%) | 1.46 (0–9.90) |
| Spinal cord | |
| Max point dose, Gy (RBE) | 1.24 (0–28.1) |
| Esophagus | |
| Mean dose, Gy (RBE) | 7.50 (0–19.59) |
| V30 (%) | 10.80 (0–37.0) |
| V40 (%) | 3.40 (0–28.9) |
| Max point dose, Gy (RBE) | 45.65 (0–65.4) |
Abbreviation: RBE = relative biological effectiveness.
Values are median (range).
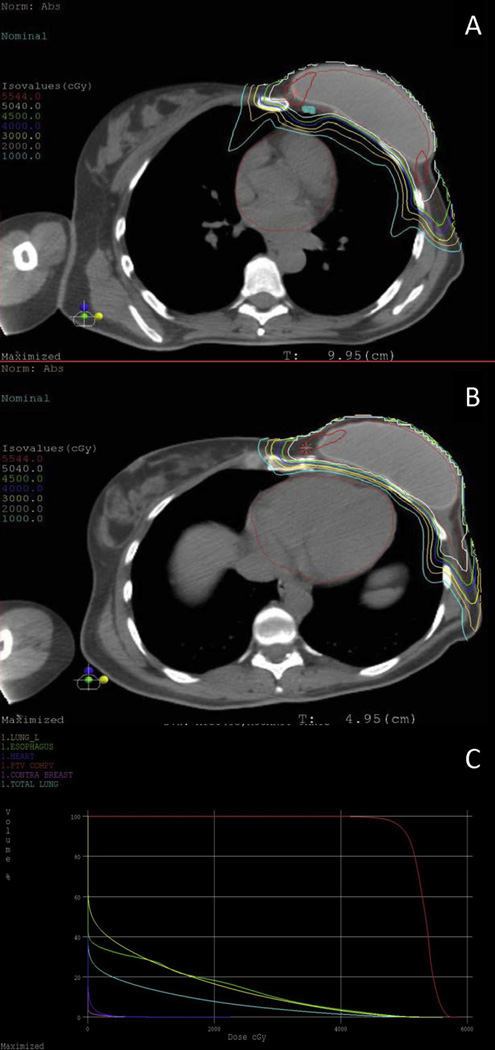
A representative dose distribution and dose–volume histogram of a treated patient are shown in Figure 4.
Fig. 4.
Representative dose distribution demonstrating full coverage of (A) internal mammary lymph nodes and (B) heart sparing of a patient treated with postmastectomy proton radiation to the left reconstructed chest wall, axilla, supraclavicular fossa, and internal mammary lymph nodes. (C) Dose–volume histogram.
Toxicity
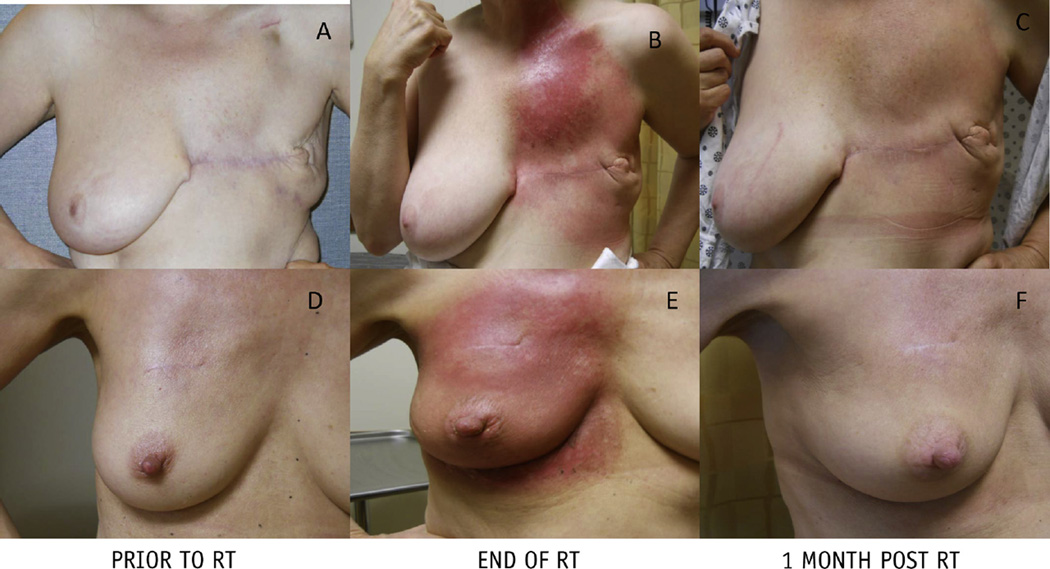
No patient required a treatment break. Among patients with >3 months of follow-up (n = 28), grade 2 dermatitis occurred in 20 patients (71.4%), and the incidence of moist desquamation was 28.6% (n = 8). Figure 5 shows typical courses of the skin reactions for patients undergoing treatment. Grade 2 skin pain occurred in 7 patients (25%). There were 8 patients (28.6%) who developed grade 2 esophagitis. All cases of esophagitis resolved within the first month of follow-up. There were no cases of lung toxicity or cardiac toxicity. One patient experienced a grade 3 reconstructive complication requiring removal of implant and bilateral reconstruction in the setting of recurrent cellulitis and implant asymmetry. There were no cases of chest wall pain or rib fracture.
Fig. 5.
Skin reactions of patients undergoing proton therapy to the chest wall and regional nodes after mastectomy without reconstruction (top row) and after lumpectomy (bottom row). (A, D) Baseline. (B, E) End of radiation therapy (RT). (C, F) One month follow-up after radiation therapy.
Toxicities are summarized in Table 3.
Table 3.
Acute toxicities
| Toxicity | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Dermatitis | 7 (25) | 20 (71.4) | 0 |
| Moist desquamation | N/A | 8 (28.6) | 0 |
| Skin pain | 3 (10.7) | 7 (25) | 0 |
| Fatigue | 13 (46.4) | 1 (3.6) | 0 |
| Esophagitis | 11 (39.3) | 8 (28.6) | 0 |
| Lymphedema | 8 (28.6) | 0 | 0 |
| Reconstructive complications | 1 (3.6) | 0 | 1 (3.6) |
| Chest wall pain | 1 (3.6) | 1 (3.6) | 0 |
| Rib fracture | 0 | 0 | 0 |
Values are number (percentage).
Discussion
Radiation therapy for breast cancer decreases postoperative local and regional recurrence, thereby increasing overall survival. The targets to be covered for locally advanced breast cancer are controversial, especially the IMN chain. All of the randomized trials that established the role of postmastectomy radiation therapy (1–3) and the role of comprehensive nodal treatment after lumpectomy (5, 6) included the IMN chain in the target volume. However, targeting the IMNs with conventional techniques of photons or photon-electrons often leads to significant exposure to the lung and heart, and the potential benefit in terms of disease control is offset by the increased risk of morbidity and mortality.
The first source of this risk is cardiopulmonary toxicity. There is a well-established incidence of cardiovascular events and coronary artery stenosis attributable to radiation therapy, especially among patients with left-sided tumors (9–12). All levels of dose, including intermediate and low doses, seem to drive the risk of cardiovascular disease. Darby et al (7) recently demonstrated a linear correlation between the mean dose to the heart and subsequent rates of ischemic heart disease among a large cohort of patients treated with conventional radiation therapy for breast cancer, without any apparent threshold. There is a similar risk pattern for radiation-induced pneumonitis. Although the data in breast cancer patients are limited (22), several authors have found low-dose lung parameters, including the volume receiving 10 Gy (V10) and 5 Gy (V5), to be significant predictors in patients treated for breast and lung cancer (23–25).
These data highlight the importance of heart- and lung-sparing approaches for breast radiation therapy. With conventional techniques, limiting dose to these organs is mainly achieved with the use of CT-guided planning, blocking, breath-hold techniques, and beam angling. However, this often comes with a price of compromised target coverage, as shown by Fontanilla et al (15). In that study the authors demonstrated that conventional photon techniques with traditional field borders only cover approximately 75% of the chest wall, 84% of level 1, 88% of level 2, 96% of level 3, 84% of the supraclavicular lymph nodes, and 80% of the IMNs, as delineated by RTOG consensus guidelines.
To counter this inadequate target coverage while still sparing the normal structures, some practitioners have incorporated IMRT techniques for breast cancer. However, the increase in homogeneity and target coverage from multifield IMRT results in a “low-dose bath” to surrounding organs and creates a higher integral dose, mean lung dose, and mean heart dose. Jagsi et al (26) recently compared 4 IMRT techniques for use in breast cancer, all of which showed significant dose to the underlying heart and lungs (26). These high integral doses and low-dose parameters may be associated with increased cardiopulmonary sequelae and secondary cancers.
Protons are able to achieve homogenous target coverage without the low-dose exposure to surrounding organs (27–29). The lower integral doses achieved with proton therapy has translated into a significantly lower rate of secondary cancers when compared with photon radiation therapy as assessed in one matched pair analysis of patients with a variety of histologies treated to a variety of sites (30). Whether the sparing of the normal tissues by proton therapy for breast cancer leads to reduced cardiovascular disease and secondary cancers remains to be seen and is a topic of ongoing studies.
The number of patients requiring treatment to the IMNs is likely to increase owing to the insights gained from the recently published MA.20 and European Organization for Research and Treatment of Cancer 22922 trials (5, 6). The projected expansion of indications for comprehensive nodal irradiation requires a thorough evaluation of the safety and efficacy of proton therapy as a treatment option. Reported clinical outcomes of protons for breast cancer remain limited, especially in the treatment of patients with locally advanced disease and an intact breast. To date, the published experience in the literature for patients with locally advanced disease consists of a single phase 1/2 trial that showed proton therapy to be well tolerated in the postmastectomy setting, with excellent target coverage and near-complete sparing of organs at risk (19). However, this series had only 12 patients and did not include any postlumpectomy patients. The present report represents the largest series to date of patients with breast cancer treated with protons. Proton therapy consistently achieved excellent coverage of the target, which included the IMNs in 93% of patients. A limited number of patients were treated with an intact breast and to the regional lymph nodes, again with complete coverage of the target volumes. The sparing of the heart, lung, and breast tissue was dramatic and comparable to previously published proton reports, as shown in Table 4.
Table 4.
Organ at risk median dosimetry for various techniques targeting internal mammary lymph nodes
| Parameter | Electrons (30) |
Photon/ electron patch (31) |
5-Field mixed photon/ electron (32) |
IMRT after immediate reconstruction* (33) |
IMRT photons (34) |
9-Field IMRT (25) |
Tangential beamlet IMRT (25) |
Protons (previous series) (19, 26) |
Protons (present series) |
|---|---|---|---|---|---|---|---|---|---|
| Heart (mean dose) | NR | 7.5 Gy | NR | 3.50 Gy | 8.69 Gy | 7.18 Gy | 2.60 Gy | 0.44 Gy (RBE) |
1.00 Gy (RBE) |
| Heart V20 (%) | NR | NR | 46.00 | NR | 13.00 | 0.31 | 0.40 | 1.60 | 1.16 |
| Heart V5 (%) | NR | NR | 65.00† | NR | 56.00 | 80.18 | 10.15 | 4.10 | 5.00 |
| Ipsilateral lung V20 (%) |
38.03 | NR | 28.00 | 25.30 | 28.00 | 34.46 | 29.49 | 16.20 | 16.50 |
| Ipsilateral lung V5 (%) |
NR | NR | 65.00† | NR | 65.00 | 93.45 | 54.00 | 25.20 | 34.35 |
| Total lung V20 (%) | 20.39 | 14.4 | NR | NR | NR | NR | NR | 12.70 | 7.31 |
Abbreviations: IMRT = intensity modulated radiation therapy; NR = not reported.
Forward planned simplified IMRT using opposed lateral beams.
Extrapolated from figure in publication.
In the present study, the rate of moist desquamation was 28.6%, and there were no cases of grade 3 dermatitis. This compares favorably to rates seen with both IMRT photons (31) and conventional electrons (32) used in the postmastectomy setting. For patients who have undergone postmastectomy reconstruction and require full dose to the skin, protons may offer an advantage over photons with bolus. With uniform scanning proton therapy there is 100% dose at the skin without having to use bolus on the chest wall, which can lead to inter- and intratreatment variability because most bolus is not conformal over the implant. However, this warrants further study, because there are also long-term concerns associated with high surface doses to patients with implants. For those patients who do not require full dose delivered to the skin, pencil beam scanning can offer proximal range modulation and selective skin sparing, analogous to a photon beam without bolus.
Notably, a significant number of patients experienced moderate esophagitis. The rates of esophagitis among patients treated with sequential chemoradiation therapy for breast cancer are not well described, but the 33% in the current series is presumed to be higher than what would be expected with conventional techniques. There are 3 possible factors that contributed to this relative increase. First, our current contouring practice of the supraclavicular fossa covers the internal jugular vein and common carotid artery, in accordance with the RTOG atlas for IMRT for breast cancer. This portion of the fossa does not normally receive full dose when using conventional photon field borders (15). The typical medial field edge of anterior oblique beam used in the conventional treatment of the supraclavicular fossa is placed at the pedicle of vertebral body. By doing so, the esophagus is almost completely blocked out of the field. However, this also creates significant underdosing of the medial aspect of the supraclavicular fossa, although the concerns for failure in this site are low. Second, in proton plans a portion of the upper breast or chest wall is included in the supraclavicular field, and beams require a more medial orientation. As a result, the distal edge of the beam ends near the esophagus. Given the uncertainty of the RBE at the distal edge of the Bragg peak, there could be slightly higher dose than anticipated. This is in contrast to the laterally angled oblique portal used to treat the supraclavicular field using photons. Last, the PTV expansion in the initial experience resulted in a nontrivial amount of the upper esophagus being included within the PTV, and likely contributed to the increased level of low-grade esophagitis seen in the present study. As previously noted, our technique was later modified, and PTV overlap with the esophagus was minimized. Importantly, all cases of esophagitis were easily managed in the outpatient setting and had resolved by the first follow-up appointment.
Although patient characteristics and treatment parameters were prospectively collected, this study is retrospective in nature and conclusions are therefore limited. The number of patients treated in the postlumpectomy setting was limited, and only those patients with small breasts were selected owing to concerns with setup in larger-breasted women. In-room imaging (such as AlignRT) would be helpful for a more robust setup for these patients. In addition, follow-up is short, and further study is needed to assess for both locoregional control rates and long-term toxicity. Additional follow-up is planned to further investigate the findings of this report.
Conclusion
In a series including 30 breast cancer patients treated with postoperative proton therapy to the breast/chest wall and regional nodes, protons achieved excellent coverage of the target volume, including the IMNs. The heart, lungs, and contralateral breast were well spared. Integral doses to these organs were significantly lower than what would be expected from conventional techniques. Treatment was well tolerated, with no grade 3 toxicities. The rate of moist desquamation was acceptable and comparable to rates observed after photon irradiation. Additional follow-up is planned to assess for long-term outcomes and toxicity. Further study is needed to accurately select which patients stand to benefit from proton therapy for breast cancer.
Summary.
This report represents the largest series of patients treated with proton therapy for breast cancer. Dosimetry analysis showed excellent target coverage, which included the IMNs in the majority of patients. The heart and lungs were effectively spared. Treatment was well tolerated, and early toxicity was acceptable. Further study is planned to assess for long-term outcomes and toxicity.
Footnotes
Conflict of interest: B.C., H.T., and O.C. have minority investment in ProCure Proton Therapy Center, Somerset, NJ.
References
- 1.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82B trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 3.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy:20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative G. Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelan TJ, Olivatto I, Ackerman I, et al. NCIC-CTG MA.20: An intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol. 2011;29(18 suppl) Abstract LBA 1003. [Google Scholar]
- 6.Poortmans PSH, Kirkove C, Budach V, et al. Irradiation of the internal mammary and medial supraclavicular lymph nodes in stage I to III breast cancer: 10 years’ results of the EORTC radiation oncology and breast cancer groups phase III trial 22922/10925. EJC. 2013;47 [Google Scholar]
- 7.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 8.Taghian AG, Assaad SI, Niemierko A, et al. Risk of pneumonitis in breast cancer patients treated with radiation therapy and combination chemotherapy with paclitaxel. J Natl Cancer Inst. 2001;93:1806–1811. doi: 10.1093/jnci/93.23.1806. [DOI] [PubMed] [Google Scholar]
- 9.Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 10.Correa CR, Litt HI, Hwang WT, et al. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25:3031–3037. doi: 10.1200/JCO.2006.08.6595. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol. 2012;30:380–386. doi: 10.1200/JCO.2011.34.5900. [DOI] [PubMed] [Google Scholar]
- 12.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 13.Grantzau T, Mellemkjaer L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: A national population based study under the Danish Breast Cancer Cooperative Group (DBCG) Radiother Oncol. 2013;106:42–49. doi: 10.1016/j.radonc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Berrington de Gonzalez A, Curtis RE, Kry SF, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: A cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12:353–360. doi: 10.1016/S1470-2045(11)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontanilla HP, Woodward WA, Lindberg ME, et al. Current clinical coverage of Radiation Therapy Oncology Group-defined target volumes for postmastectomy radiation therapy. Pract Radiat Oncol. 2012;2:201–209. doi: 10.1016/j.prro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Kozak KR, Smith BL, Adams J, et al. Accelerated partial-breast irradiation using proton beams: Initial clinical experience. Int J Radiat Oncol Biol Phys. 2006;66:691–698. doi: 10.1016/j.ijrobp.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Bush DA, Slater JD, Garberoglio C, et al. Partial breast irradiation delivered with proton beam: Results of a phase II trial. Clin Breast Cancer. 2011;11:241–245. doi: 10.1016/j.clbc.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Chang JH, Lee NK, Kim JY, et al. Phase I trial of proton beam accelerated partial breast irradiation in breast cancer. Radiother Oncol. 2013;108:209–214. doi: 10.1016/j.radonc.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald SM, Patel SA, Hickey S, et al. Proton therapy for breast cancer after mastectomy: Early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2013;86:484–490. doi: 10.1016/j.ijrobp.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 20.White J, Tai A, Arthur D, et al. Breast Cancer Atlas for Radiation Therapy Planning: Consensus Definitions. Radiation Therapy Oncology Group. 2009 [Google Scholar]
- 21.Reed VK, Cavalcanti JL, Strom EA, et al. Risk of subclinical micrometastatic disease in the supraclavicular nodal bed according to the anatomic distribution in patients with advanced breast cancer. Int J Radiat Oncol Biol Phys. 2008;71:435–440. doi: 10.1016/j.ijrobp.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recht A, Ancukiewicz M, Alm El-Din MA, et al. Lung dose-volume parameters and the risk of pneumonitis for patients treated with accelerated partial-breast irradiation using three-dimensional conformal radiotherapy. J Clin Oncol. 2009;27:3887–3893. doi: 10.1200/JCO.2008.20.0121. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2006;66:1399–1407. doi: 10.1016/j.ijrobp.2006.07.1337. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Hong SE, Kong M, et al. Predictive factors for radiation pneumonitis in lung cancer treated with helical tomotherapy. Cancer Res Treat. 2013;45:295–302. doi: 10.4143/crt.2013.45.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jo IY, Kay CS, Kim JY, et al. Significance of low-dose radiation distribution in development of radiation pneumonitis after helicaltomotherapy-based hypofractionated radiotherapy for pulmonary metastases. J Radiat Res. 2014;55:105–112. doi: 10.1093/jrr/rrt080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagsi R, Moran J, Marsh R, et al. Evaluation of four techniques using intensity-modulated radiation therapy for comprehensive locoregional irradiation of breast cancer. Int J Radiat Oncol Biol Phys. 2010;78:1594–1603. doi: 10.1016/j.ijrobp.2010.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald SM, Jimenez R, Paetzold P, et al. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiat Oncol. 2013;8:71. doi: 10.1186/1748-717X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagundes MA, Pankuch M, Hartsell W, et al. Cardiac-sparing post-mastectomy proton radiation therapy for women with stage III, loco-regional, breast cancer: A dosimetric comparison study. Int J Radiat Oncol. 2013;87:S245. [Google Scholar]
- 29.Ares C, Khan S, Macartain AM, et al. Postoperative proton radiotherapy for localized and locoregional breast cancer: Potential for clinically relevant improvements? Int J Radiat Oncol Biol Phys. 2010;76:685–697. doi: 10.1016/j.ijrobp.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 30.Chung CS, Yock TI, Nelson K, et al. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87:46–52. doi: 10.1016/j.ijrobp.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Li J, Xie J, et al. Post mastectomy linac IMRT irradiation of chest wall and regional nodes: Dosimetry data and acute toxicities. Radiat Oncol. 2013;8:81. doi: 10.1186/1748-717X-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spierer MM, Hong LX, Wagman RT, et al. Postmastectomy CT-based electron beam radiotherapy: Dosimetry, efficacy, and toxicity in 118 patients. Int J Radiat Oncol Biol Phys. 2004;60:1182–1189. doi: 10.1016/j.ijrobp.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 33.Ohri N, Cordeiro PG, Keam J, et al. Quantifying the impact of immediate reconstruction in postmastectomy radiation: A large, dose-volume histogram-based analysis. Int J Radiat Oncol Biol Phys. 2012;84:e153–e159. doi: 10.1016/j.ijrobp.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Li J, Xie J, et al. Post mastectomy linac IMRT irradiation of chest wall and regional nodes: Dosimetry data and acute toxicities. Radiat Oncol. 2013;8:81. doi: 10.1186/1748-717X-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]