Abstract
Photocatalytic organic transformations utilizing ruthenium and iridium complexes have garnered significant attention due to the access they provide to new synthetic spaces through new reaction mechanisms. A survey of the photophysical data and the diversity of transformations that may be accomplished utilizing commercially available photocatalysts is contained herein.
Keywords: Photocatalysis, photocatalysts, organic synthesis
Graphical Abstract

INTRODUCTION
Over the course of the preceding 30 years, there have been substantial developments utilizing cyclometalated ruthenium and iridum complexes in photochemistry.1–4 Historically these complexes have been primarily employed in solar cells,5 light emitting diodes (LEDs),6 and as initiators in free radical polymerizations.7 Both of the prototypical complexes, Ru(bpy)32+ and Ir(ppy)3 are d6, coordinatively saturated, 18-electron complexes. When excited by visible light they undergo a metal-to-ligand-charge transfer (MLCT) from the highest occupied molecular orbital of the metal (HOMO) to the lowest unoccupied molecular orbital of the ligand (LUMO).2,3 As a consequence, these complexes undergo both reductive and oxidative quenching pathways with relative ease,2–4 which can be rationally applied in organic transformations.8–12 Comparison of the potential of the photocatalyst to a substrate that may undergo a redox event can suggest the likelihood of an electron transfer event. However, caution should practiced because almost certainly the conditions under which the redox properties where determined are different than the reaction conditions and in addition may involve a nonreversible step. The former may affect the necessary potential and the latter can facilitate reactions that appear to have an underpotential.
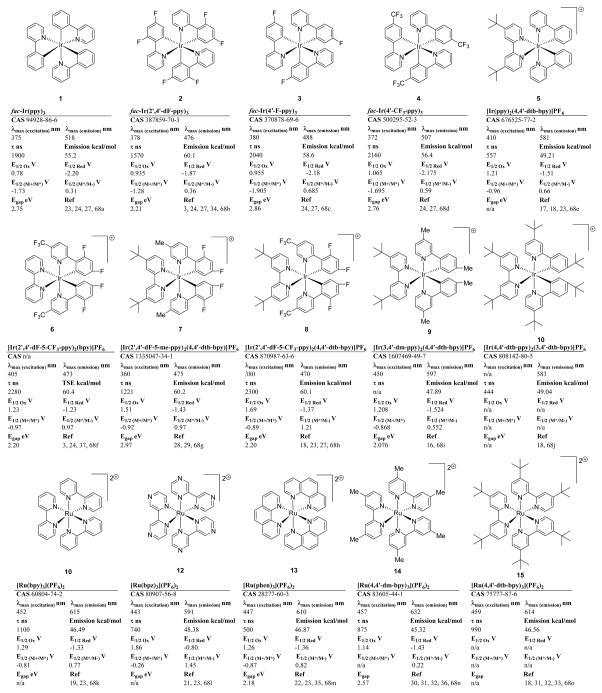
While electron transfer serves as a means for these complexes to return to the ground state, this can also be accomplished by energy transfer to other molecules with orbitals of the appropriate energy level. Importantly, modification of the ligand scaffold provides many opportunities to tune the photophysical properties of these complexes as can be seen in surveys of the various ruthenium and iridium complexes found in current literature.2–4 However, the number of complexes commercially available for use in catalysis is currently more limited. In this review, we will briefly discuss some of the electrochemical and photophysical properties of ruthenium and iridium photocatalysts which are commercially available65 (Table 1) and highlight a select number of the diverse organic transformations enabled by each catalyst.
TABLE 1.
PHOTOCATALYST DETAILS
PHOTOCATALYST 1
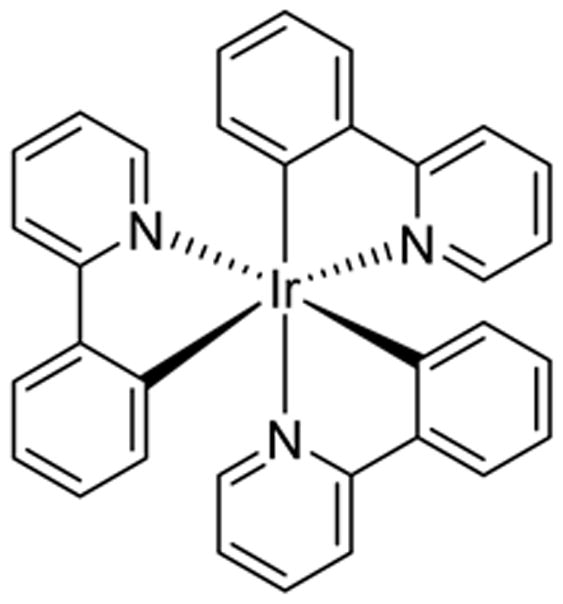
fac-Ir(ppy)3
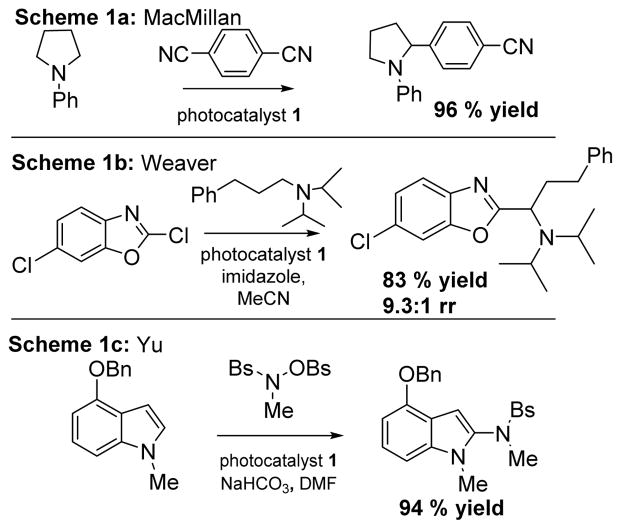
There has been significant progress in α-C–H functionalization of amines in photocatalysis, including the arylation of tertiary amines by MacMillan and coworkers,13 (Scheme 1a), the azoylation of aliphatic amines by our own lab14 (Scheme 1b), and the C-H amidation of unfunctionlized indoles with hydroxyl amines by Yu and coworkers15 (Scheme 1c), all of which use the prototypical catalyst 1.
Scheme 1.
PHOTOCATALYST 2

fac-Ir(2′,4′-dF-ppy)3
Lee and coworkers38 utilized 2 to initiate a C–H imidation of hetereoarenes with N-chlorophthalimide. After initially investigating 1, photocatalyst 2 proved to be the superior catalyst. The reaction likely proceeds through a N-radical intermediate initiated by electron transfer from excited 2, which undergoes radical addition to the arene partner. The hexadienyl radical serves to reduce the catalyst leading to rearomatization (Scheme 2). The scope consists of various substituted arenes with modest yields and regioselectivity.
Scheme 2.
Lee
PHOTOCATALYST 3

fac-Ir(4′-F-ppy)3
While current literature references are limited, and transformations that utilize photocatalyst 3 as an optimal catalyst are presently absent, it is noteworthy that the catalyst is being employed. Our group1,39 has routinely used 3 in our standard catalyst screen, and likewise Ooi and coworkers40 synthesized this catalyst in their asymmetric α-coupling of N-arylaminomethanes and used the photocatalyst in their optimization experiments. 3 is thus included in order to provide electrochemical and photophysical data.
PHOTOCATALYST 4
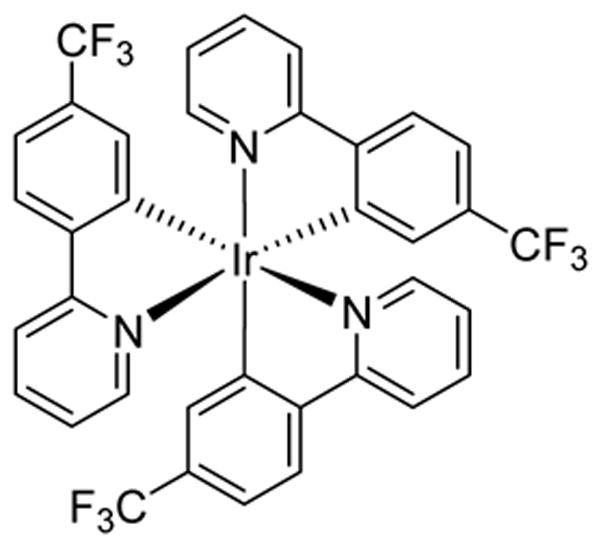
fac-Ir(4′-CF3-ppy)3
Photocatalyst 4 is another photocatalyst that has now become commercially available but has yet to have been demonstrated to be an optimal catalyst in current organic transformations. Our group1,41 has and will continue to employ 4 in our standard catalyst screens.
PHOTOCATALYST 5

[Ir(ppy)2 (4,4′-dtb-bpy)]PF6
Advances have been made recently in photocatalytic biomass remediation. Stephenson and coworkers42 utilized visible light and 5 in a photoflow reactor to degrade lignin biomass model substrates, in a two-step reaction sequence (not shown).
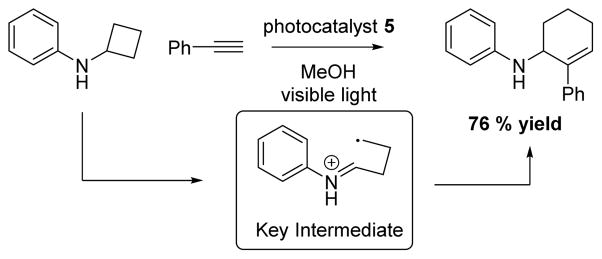
In 2015, Zheng43 demonstrated that cyclobutylanilines could undergo a ring-opening [4+2] reaction with alkynes using 5. They proposed that the reaction likely proceeds through a distonic radical cation. Excellent yields were achieved using various aliphatic cyclobutlyanilines and bicyclic amines (Scheme 3).
Scheme 3.
Zheng
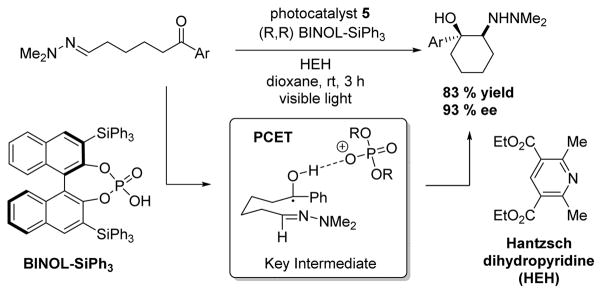
In 2013, Knowles and coworkers44 used 5 in concert with Binol-derived phosphoric acid to perform an asymmetric azapinacol cyclization with excellent yields and enantiomeric excess (ee) (Scheme 4). Electron transfer to the ketone only occurred when it was protonated by the chiral acid, allowing the C–C formation to take place enantioselectively.
Scheme 4.
Knowles
In 2015, Yu45 reported a visible light mediated remote C–H chlorination of aliphatic and benzylic amines (Scheme 5a) using 5 which took advantage of the ease of N-chlorination to functionalize a distant and far less reactive C–H bond on the same molecule. The reaction likely takes place via a 1,5-atom transfer, which upon addition of NaOH provides the subsequent cyclization product. Yu went on to apply the methodology to the late stage functionalization of the antitumor drug (+)-dehydroabietyamine (Scheme 5b) to produce a chlorinated version of the pharmacophore.
Scheme 5.
PHOTOCATALYST 6

[Ir(2′,4′-dF-5-CF3-ppy)2(bpy)]PF6
While photocatalysis has resulted in a number of enabling methods, there has also been a significant increase in the development and use of photocatalysis in concert with other transition-metal chemistry, which is just beginning to reveal new mechanistic possibilities. Molander46 demonstrated a recent example of photocatalysis being used in concert with other transition-metal catalysis. The group demonstrated a single-electron transmetalation in organoboron cross-coupling using Ni(0). The traditional two-electron transmetallation cleaves the C–B bond hetereolytically, giving lower or no reactivity in regards to sp3 hybridized carbons.47 Molander and coworkers demonstrated that a photocatalytic single-electron transfer (SET) using 6 enables cleavage of the C–B bond to occur homolytically, effectively reversing the order of reactivity (sp3>sp2>sp) and correlates to radical stability.48 A wide variety of aryl halide partners were shown to be compatible (Scheme 6a). Furthermore, it was demonstrated that stereoselectivity is possible with help of photoredox cross-coupling and a chiral ligand (Scheme 6b).
Scheme 6.
PHOTOCATALYST 7

[Ir(2′,4′-dF-5-me-ppy)2 (4,4′-dtb-bpy)]PF6
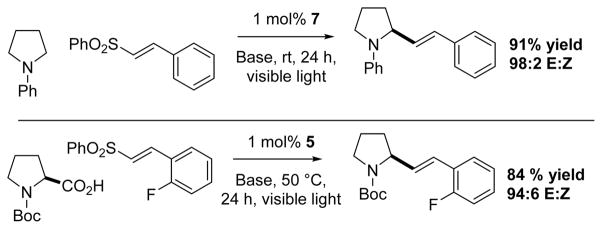
MacMillan et al. showed that N-aryl amines could undergo selective C–H vinylation utilizing vinyl sulfones and photocatalyst 7 in good yields and E:Z selectivity. The methodology could be extended to N-Boc α-amino acids as well (via decarboxylation), though a change to catalyst 5 was necessary (Scheme 7).49
Scheme 7.
MacMillan
PHOTOCATALYST 8
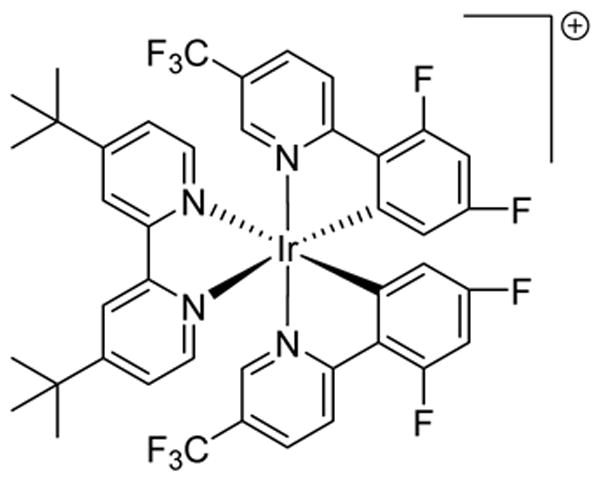
[Ir(2′,4′-dF-5-CF3-ppy)2(4,4′-dtb-bpy)]PF6
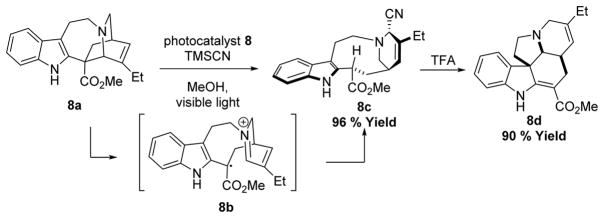
Photocatalysis has also been used in the derivatization of natural products into biologically interesting molecules in both early and late stages. In 2014, Stephenson utilized 8 to access a key intermediate via an oxidative ring fragmentation of (+)-catharanthine (8a) in a photoflow reactor early in the synthetic process (Scheme 8), presumably generating a transient cyclic iminium (8b) which underwent cyanation to produce the intermediate species (8c) in 96% yield, followed by acid mediated rearrangement to reach (−)-pseudotabersonine (8d) in 90% yield.50
Scheme 8.
Stephenson
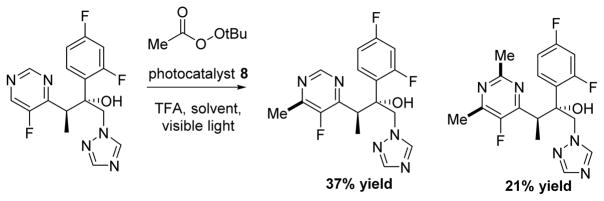
DiRocco and coworkers demonstrated the efficacy of the same photocatalyst to perform late stage functionalization (LSF) by methylation, ethylation, or cyclopropylation of various complex medicinal and agrochemical molecules by visible light photocatalysis (Scheme 9).51 Established methods such as Minisci’s persulfate mediated decarboxylation required high temperatures52 and are often too harsh for large, complex molecules. While increases to the scope have been made,53 DiRocco’s method to overcome these issues represents a significant advancement in LSF, in part, because the catalyst can help regulate the concentration of the reactive species.
Scheme 9.
DiRocco
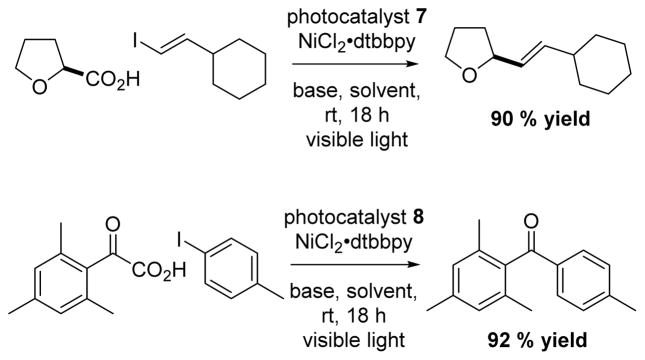
In 2015, MacMillan showed that photocatalysts 754 or 855 could be combined synergistically with a NiCl2•dtbbpy catalyst for the formation of olefins and aryl ketones (Scheme 10).
Scheme 10.
MacMillan
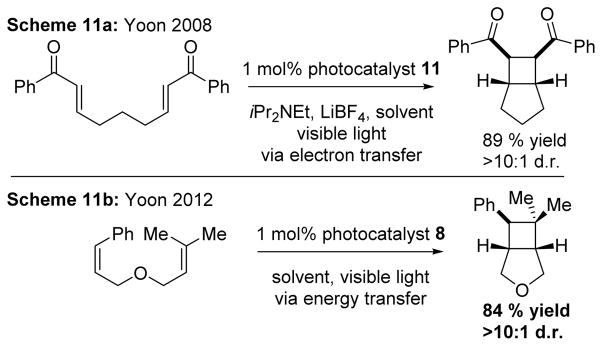
In 2008, Yoon demonstrated the [2+2] intramolecular cycloaddition took place via an electron transfer mechanism with catalytic amounts of 11, vide infra, (Scheme 11a).8 Then, in 2012, he also demonstrated that an energy transfer pathway could be used for substrates that were unlikely to undergo electron transfer (Scheme 11b). 8 was selected not only because the excited state oxidation potential cannot initialize radical cation cycloadditions, but also because the energy of the catalyst’s excited state is comparable to the triplet state energy of excited styrenes.56
Scheme 11.
PHOTOCATALYST 9

[Ir(3,4′-dm-ppy)2(4,4′-dtb-bpy)]PF6
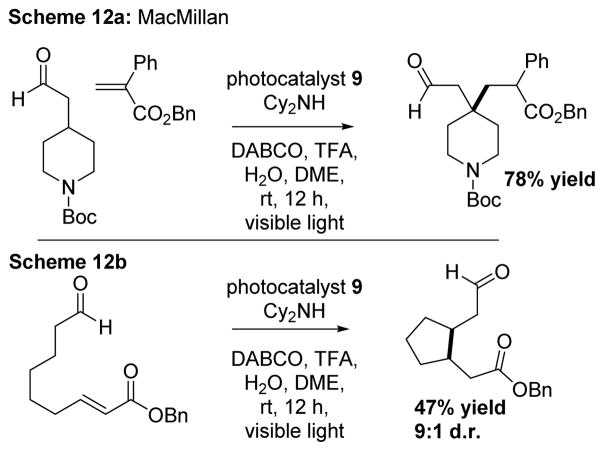
In 2014, MacMillan and coworkers reported a method that utilizes 9 to directly couple a Michael acceptor and an aldehyde at the β-position (Scheme 12a). The β-functionalization of the aldehydes (as opposed to α-functionalization) via any method has little precedence and demonstrates the enabling nature of photocatalysis. It also allows for intramolecular 5- or 6-exo-trig radical cyclizations onto aldehydes that are unsaturated and possess a terminal electron withdrawing group (Scheme 12b).16
Scheme 12.
PHOTOCATALYST 10
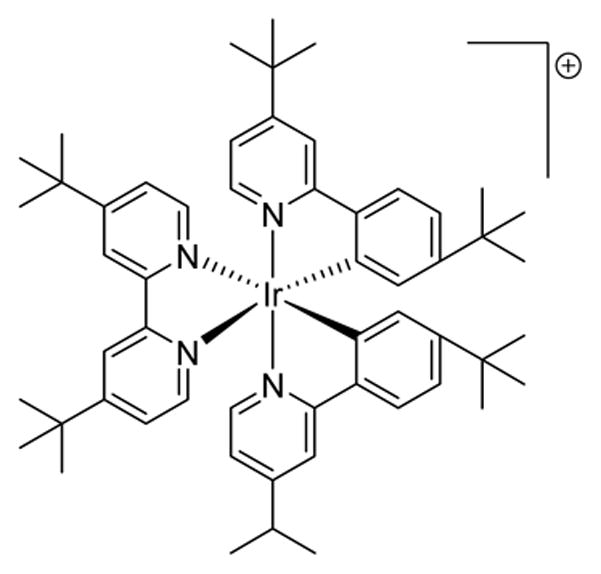
[Ir(4,4′-dtb-ppy)2(3,4′-dtb-bpy)]PF6
Current literature references for catalyst 10 are limited and it is included in order to provide electrochemical and photophysical data.
PHOTOCATALYST 11
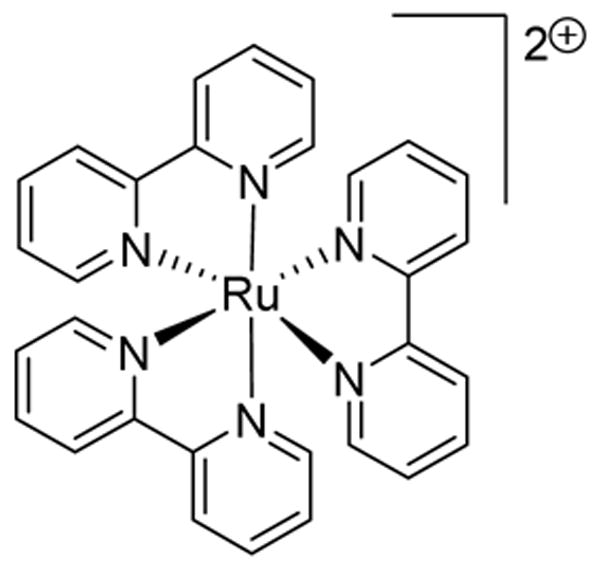
[Ru(bpy)3](PF6)2
In another example of merging catalytic cycles of transition-metal catalysis and photocatalysis, Glorius and coworkers57 merged gold catalysis and photocatalysis (Scheme 13), with oxidation of the gold complex by 11 during the reaction. Moderate to good yields of oxy- and amino arylated alkenes were realized using [Ph3PAu]NTf2 and vinyl alcohols.
Scheme 13.
Glorius
Our group58 has shown that visible light and 1 can facilitate (E) to (Z) isomerizations of substituted styrenes. Osawa’s group59 has also shown isomerizations of stilbene derivatives with photocatalyst 11. Rueping60 and coworkers produced an economical and practical two-phase photoflow setup using 11, achieving quantitative yields of cis-stilbene on a 1.8 g scale (Scheme 14). The catalyst/DMF mixture and (E) stilbene/pentane was circulated through an illuminated glass microreactor. As the volatile (Z) stilbene was evaporated, the catalyst and the unreacted high boiling (E) stilbene were pumped back into system for further isomerization.
Scheme 14.
Rueping
PHOTOCATALYST 12
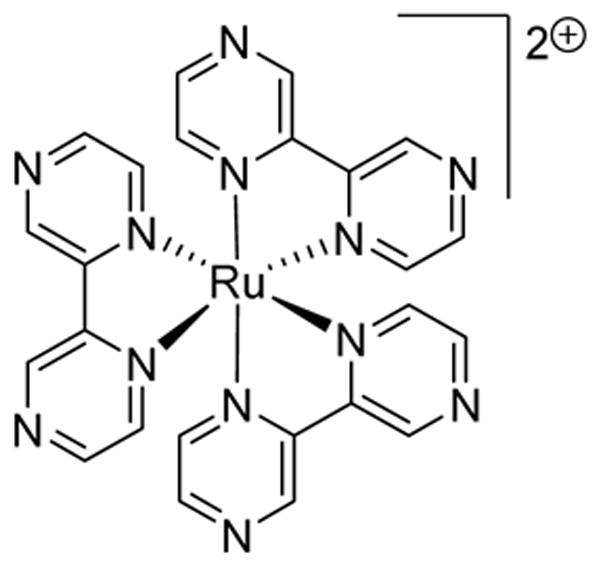
[Ru(bpz)3](PF6)2
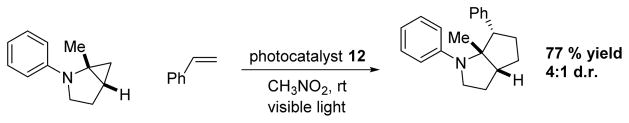
In 2012, Zheng and coworkers61 utilized 12 for an intermolecular [3+2] cycloaddition of olefins with cyclopropylanilines, monocyclic, and bicyclic amines, giving good yields and diastereomeric ratios (Scheme 15).
Scheme 15.
Zheng
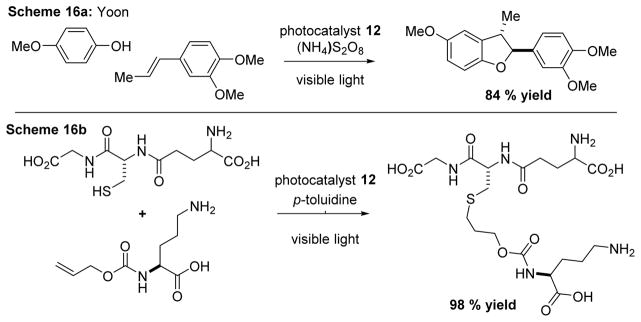
In 2014, utilizing 12 with an external oxidant Yoon and cowokers62 detailed an oxidative [3+2] cycloaddition of phenols with alkenes (Scheme 16a) which demonstrated a modular synthesis of dihyrobenzofurans. In the same year they developed a thio-ene “click” reaction of thiols with alkenes (Scheme 16b).63
Scheme 16.
PHOTOCATALYST 13

[Ru(phen)3](PF6)2
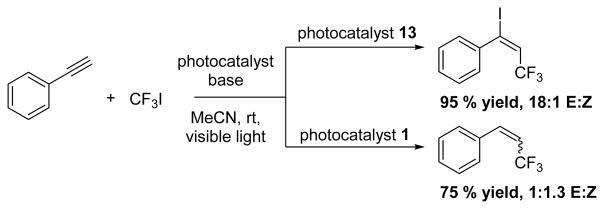
In 2014, using 13, Jung and Han reported a convenient method to trifluoromethylate and vicinally chlorinate both internal and terminal alkenes (Scheme 17). 1 gave similar conversion in initial screening.64 In the same year, Cho and coworkers synthesized difunctionalized alkenes from alkynes using CF3I.65 In Cho’s work, however, it was shown that a switch in catalyst from 13 to 1, resulted in the hydrotrifluoromethylated product, albeit with poor E:Z selectivity (Scheme 18).
Scheme 17.
Jung & Han
Scheme 18.
Cho
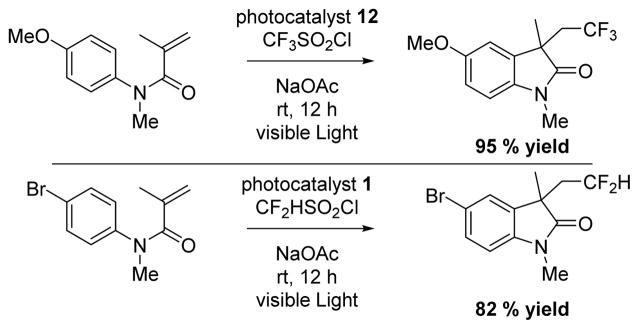
Dolbier and coworkers showed that with 13 and a trifluoromethylsulfonyl chloride, N-arylacrylamides could be cyclized through the trifluoromethyl radical to produce 3,3-disubstituted 2-oxoindoles (Scheme 19). The scope was expanded to include the use of the CF2H radical, though it required a change of photocatalyst to 1.66
Scheme 19.
Dolbier
PHOTOCATALYST 14

[Ru(4,4′-dm-bpy)3](PF6)2
Investigating the radical mediated C-C bond formation between an α-glucosylbromide and methyl acrylate in 2011, Gagné et al. reported that while the common photocatalyst 11 accomplished the desired transformation, the rate was exceptionally slow. By changing the solvent and the catalyst to 14 (Scheme 20), the rates of conversion were enhanced.67 In 2012, utilizing similar conditions, Gagné investigated the permeation of light into the reaction mixture using a photoflow reactor and also expanded the scope to include acroleins.
Scheme 20.
Gagné
PHOTOCATALYST 15

[Ru(4,4′-dtb-bpy)3](PF6)2
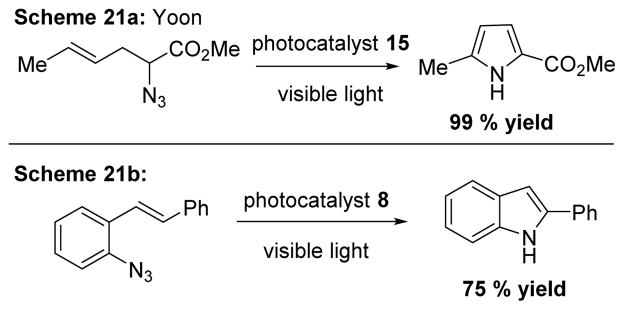
In 2014, Yoon and coworkers utilized the energy transfer mechanism of 15. Following energy transfer, the substrate expelled the N2 to produce a nitrene intermediate, which underwent subsequent intramolecular insertion to produce pyrroles in good yields (Scheme 21a). Similarly, photocatalyst 8 transformed ortho alkenylated aryl azides to provide access to substituted indoles (Scheme 21b). 24
Scheme 21.
CONCLUSION
We have shown a handful of examples of the rapidly growing and diverse repertoire of reactions and collected electro- and photophysical data for a set of commercially available photocatalysts. We have shown some of the distinct mechanisms in which these catalysts can participate and based on this versatility, we expect to see new directions continue to emerge from this powerful new set of catalysts.
Acknowledgments
JDW gratefully acknowledges the NIH NIGMS (GM115697) for financial support of this work.
References
- 1.Singh A, Kubik JJ, Weaver JD. Chemical Science. 2015 doi: 10.1039/c5sc03013g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campagna S, Puntoriero F, Nastasi F, Bergamini G, Balzani V. Top Curr Chem. 2007;280:117. [Google Scholar]
- 3.Flamigni L, Barbieri A, Sabatini C, Ventura B, Barigelletti F. Top Curr Chem. 2007;281:143. [Google Scholar]
- 4.Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalyanasundaram K, Grätzel M. Coord Chem Rev. 1998;177:347. [Google Scholar]
- 6.Lowry MS, Bernhard S. Chem Eur J. 2006;12:7970. doi: 10.1002/chem.200600618. [DOI] [PubMed] [Google Scholar]
- 7.Lalevee J, Peter M, Dumur F, Gigmes D, Blanchard N, Tehfe MA, Morlet-Savary F, Fouassier JP. Chem Eur J. 2011;17:15027. doi: 10.1002/chem.201101445. [DOI] [PubMed] [Google Scholar]
- 8.Ischay MA, Anzovino ME, Du J, Yoon TP. J Am Chem Soc. 2008;130:12886. doi: 10.1021/ja805387f. [DOI] [PubMed] [Google Scholar]
- 9.Nicewicz DA, MacMillan DWC. Science. 2008;322:77. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pac C, Ihama M, Yasuda M, Miyauchi Y, Sakurai H. J Am Chem Soc. 1981;103:6495. [Google Scholar]
- 11.Fukuzumi S, Mochizuki S, Tanaka T. J Phys Chem. 1990;94:722. [Google Scholar]
- 12.Narayanam JMR, Stephenson CRJ. Chem Soc Rev. 2011;40:102. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]
- 13.McNally A, Prier CK, MacMillan DWC. Science. 2011;334:1114. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A, Arora A, Weaver JD. Org Lett. 2013;15:5390. doi: 10.1021/ol402751j. [DOI] [PubMed] [Google Scholar]
- 15.Qin Q, Yu S. Org Lett. 2014;16:3504. doi: 10.1021/ol501457s. [DOI] [PubMed] [Google Scholar]
- 16.Terrett JA, Clift MD, MacMillan DWC. J Am Chem Soc. 2014;136:6858. doi: 10.1021/ja502639e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slinker JD, Gorodetsky AA, Lowry MS, Wang J, Parker S, Rohl R, Bernhard S, Malliaras GG. J Am Chem Soc. 2004;126:2763. doi: 10.1021/ja0345221. [DOI] [PubMed] [Google Scholar]
- 18.Lowry MS, Hudson WR, Pascal RA, Bernhard S. J Am Chem Soc. 2004;126:14129. doi: 10.1021/ja047156+. [DOI] [PubMed] [Google Scholar]
- 19.Juris A, Balzani V, Belser P, von Zelewsky A. HeIv Chim Acta. 1981;64:2175. [Google Scholar]
- 20.Rillema DP, Allen G, Meyer TJ, Conrad D. Inorg Chem. 1983;22:1617. [Google Scholar]
- 21.Haga M, Dodsworth ES, Eryavec G, Seymour P, Lever ABP. Inorg Chem. 1985;24:1901. [Google Scholar]
- 22.Young RC, Meyer TJ, Whitten DG. J Am Chem Soc. 1976;98:286. [Google Scholar]
- 23.Prier CK, Rankic DA, MacMillan DW. Chem Rev. 2013;113:5322. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farney EP, Yoon TP. Angew Chem, Int Ed. 2014;53:793. doi: 10.1002/anie.201308820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dedeian K, Djurovich PI, Garces FO, Carlson G, Watts RJ. Inorg Chem. 1991;30:1685. [Google Scholar]
- 26.Grushin VV, Herron N, LeCloux DD, Marshall WJ, Petrov VA, Wang Y. Chem Commun. 2001:1494. [Google Scholar]
- 27.Singh A, Teegardin K, Kelly M, Prasad KS, Krishnan S, Weaver JD. J Organomet Chem. 2015;776:51. [Google Scholar]
- 28.Ladouceur S, Fortin D, Zysman-Colman E. Inorg Chem. 2011;50:11514. doi: 10.1021/ic2014013. [DOI] [PubMed] [Google Scholar]
- 29.Swanick KN, Ladouceur S, Zysman-Colman E, Ding Z. RSC Adv. 2013;3:19961. [Google Scholar]
- 30.Anderson BL, Maher AG, Nava M, Lopez N, Cummins CC, Nocera DG. J Phys Chem. 2015;119:7422. doi: 10.1021/jp5110505. [DOI] [PubMed] [Google Scholar]
- 31.Zou YQ, Lu LQ, Fu L, Chang NJ, Rong J, Chen JR, Xiao WJ. Angew Chem, Int Ed. 2011;50:7171. doi: 10.1002/anie.201102306. [DOI] [PubMed] [Google Scholar]
- 32.Damrauer NH, Boussie TR, Devenney M, McCusker JK. J Am Chem Soc. 1997;119:8253. [Google Scholar]
- 33.Bernhard S, Barron JA, Houston PL, Abruña HD, Ruglovksy JL, Gao X, Malliaras GG. J Am Chem Soc. 2002;124:13624. doi: 10.1021/ja0270524. [DOI] [PubMed] [Google Scholar]
- 34.Lv H, Cai YB, Zhang JL. Angew Chem, Int Ed. 2013;52:3203. doi: 10.1002/anie.201208364. [DOI] [PubMed] [Google Scholar]
- 35.Kalyanasundaram K. Coord Chem Rev. 1982;46:159. [Google Scholar]
- 36.Bryant GM, Fergusson JE, Powell HKJ. Aust J Chem. 1971;24:257. [Google Scholar]
- 37.Bueldt LA, Prescimone A, Neuburger M, Wenger OS. Eur J Inorg Chem. 2015;2015:4666. [Google Scholar]
- 38.Kim H, Kim T, Lee DG, Roh SW, Lee C. Chem Commun. 2014;50:9273. doi: 10.1039/c4cc03905j. [DOI] [PubMed] [Google Scholar]
- 39.Arora A, Teegardin KA, Weaver JD. Org Lett. 2015;17:3722. doi: 10.1021/acs.orglett.5b01711. [DOI] [PubMed] [Google Scholar]
- 40.Uraguchi D, Kinoshita N, Kizu T, Ooi T. J Am Chem Soc. 2015;137:13768. doi: 10.1021/jacs.5b09329. [DOI] [PubMed] [Google Scholar]
- 41.Senaweera S, Weaver JD. J Am Chem Soc. 2016;138:2520. doi: 10.1021/jacs.5b13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen JD, Matsuura BS, Stephenson CRJ. J Am Chem Soc. 2014;136:1218. doi: 10.1021/ja4113462. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Zheng N. Angew Chem, Int Ed. 2015;54:11424. doi: 10.1002/anie.201504076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rono LJ, Yayla HG, Wang DY, Armstrong MF, Knowles RR. J Am Chem Soc. 2013 doi: 10.1021/ja4100595. [DOI] [PubMed] [Google Scholar]
- 45.Qin Q, Yu S. Org Lett. 2015;17:1894. doi: 10.1021/acs.orglett.5b00582. [DOI] [PubMed] [Google Scholar]
- 46.Tellis JC, Primer DN, Molander GA. Science. 2014;345:433. doi: 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Labadie JW, Stille JK. J Am Chem Soc. 1983;105:6129. [Google Scholar]
- 48.Finch A, Gardner PJ, Pearn EJ, Watts GB. T Faraday Soc. 1967;63:1880. [Google Scholar]
- 49.Noble A, MacMillan DWC. J Am Chem Soc. 2014;136:11602. doi: 10.1021/ja506094d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beatty JW, Stephenson CRJ. J Am Chem Soc. 2014;136:10270. doi: 10.1021/ja506170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Rocco DA, Dykstra K, Krska S, Vachal P, Conway DV, Tudge M. Angew Chem, Int Ed. 2014;53:4802. doi: 10.1002/anie.201402023. [DOI] [PubMed] [Google Scholar]
- 52.Minisci F, Bernardi R, Bertini F, Galli R, Perchinummo M. Tetrahedron. 1971;27:3575. [Google Scholar]
- 53.Molander GA, Colombel V, Braz VA. Org Lett. 2011;13:1852. doi: 10.1021/ol2003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noble A, McCarver SJ, MacMillan DWC. J Am Chem Soc. 2015;137:624. doi: 10.1021/ja511913h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu L, Lipshultz JM, MacMillan DW. Angew Chem, Int Ed. 2015;54:7929. doi: 10.1002/anie.201501908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Z, Yoon TP. Angew Chem, Int Ed. 2012;51:10329. doi: 10.1002/anie.201204835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahoo B, Hopkinson MN, Glorius F. J Am Chem Soc. 2013;135:5505. doi: 10.1021/ja400311h. [DOI] [PubMed] [Google Scholar]
- 58.Singh K, Staig SJ, Weaver JD. J Am Chem Soc. 2014;136:5275. doi: 10.1021/ja5019749. [DOI] [PubMed] [Google Scholar]
- 59.Osawa M, Hoshino M, Wakatsuki Y. Angew Chem, Int Ed. 2001;40:3472. doi: 10.1002/1521-3773(20010917)40:18<3472::aid-anie3472>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 60.Fabry DC, Ronge MA, Rueping M. Chem Eur J. 2015;21:5350. doi: 10.1002/chem.201406653. [DOI] [PubMed] [Google Scholar]
- 61.Maity S, Zhu M, Shinabery RS, Zheng N. Angew Chem, Int Ed. 2012;51:222. doi: 10.1002/anie.201106162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blum TR, Zhu Y, Nordeen SA, Yoon TP. Angew Chem, Int Ed. 2014;53:11056. doi: 10.1002/anie.201406393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyson EL, Niemeyer ZL, Yoon TP. J Org Chem. 2014;79:1427. doi: 10.1021/jo500031g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh SH, Malpani YR, Ha N, Jung YS, Han SB. Org Lett. 2014;16:1310. doi: 10.1021/ol403716t. [DOI] [PubMed] [Google Scholar]
- 65.Iqbal N, Jung J, Park S, Cho EJ. Angew Chem, Int Ed. 2014;53:539. doi: 10.1002/anie.201308735. [DOI] [PubMed] [Google Scholar]
- 66.Tang XJ, Thomoson CS, Dolbier WR., Jr Org Lett. 2014;16:4594. doi: 10.1021/ol502163f. [DOI] [PubMed] [Google Scholar]
- 67.Andrews RS, Becker JJ, Gagné MR. Org Lett. 2011;13:2406. doi: 10.1021/ol200644w. [DOI] [PubMed] [Google Scholar]
- 68.These catalysts are commercially available from: (a) Aspira Scientific, Cat # 300895, Sigma-Aldrich, Cat # 688096, and many others, (b) Aspira Scientific, Cat # 300896, Sigma-Aldrich, Cat # 682594, (c) Aspira Scientific, Cat # 300997, (d) Aspira Scientific, Cat # 300998, (e) Aspira Scientific, Cat # 300897, Sigma-Aldrich, Cat # 747769, and many others, (f) Sigma-Aldrich, Cat # 804215, (g) Aspira Scientific, Cat # 300981, (h) Aspira Scientific, Cat # 300898, Strem Chemicals Inc., Cat # 77-0425, (i) Aspira Scientific, Cat # 300899 (j) Aspira Scientific, Cat # 300900, (k) Aspira Scientific, Cat # 300891, Sigma-Aldrich, Cat # 754730, (l) Aspira Scientific, Cat # 300892, Sigma-Aldrich, Cat # 747777, (m) Aspira Scientific, Cat # 300901, and many others, (n) Aspira Scientific, Cat # 300893, (o) Aspira Scientific, Cat # 300894