Abstract
Purpose
The objective of this meta-analysis was to systematically evaluate the efficacy of pramipexole for the treatment of primary moderate to severe RLS.
Methods
Databases of PubMed, OVID, ScienceDirect, SpringerLink, Thomson Reuters Web of Science, the Cochrane Library, the Wiley Online Library, ArticleFirst, CALIS, Study, CNKI, and WanFang were searched to identify randomized controlled trials (RCTs) investigating pramipexole for the treatment of primary moderate to severe RLS. A meta-analysis was then conducted to pool results.
Findings
Twelve RCTs involving 3286 participants were included in this study. The average treatment duration was 11.12 (±5.72) weeks/person. The meta-analysis demonstrated that the post-treatment change in the International Restless Leg Syndrome Study Group Rating Scale (IRLS) score of pramipexole group was significantly superior to that of placebo group (weighted mean difference (WMD)=-4.64, 95% confidence intervals (CI) −5.95 to −3.33, n=8). More patients in pramipexole group showed at least a 50% reduction in the IRLS score after treatment (risk ratio [RR]) =1.57, 95% CI 1.43 to 1.73, n=8). In terms of the scores for the Clinical Global Impression of Improvement scale (CGI-I; RR=1.48, 95% CI 1.31 to 1.66, n=11) and the Patient Global Impression scale (PGI; RR=1.54, 95% CI 1.31 to 1.81, n=9), pramipexole group’s treatment outcomes were significantly superior to those of placebo group. In terms of the change in quality of life (WMD=5.39, 95% CI 2.28 to 8.50, n=4), change in daytime tiredness (WMD=-0.61, 95% CI −1.21 to −0.01, n=4), change in the number of periodic limb movements per hour of sleep (WMD=-35.95, 95% CI −56.42 to −15.48, n=3) and change in the quality of sleep (WMD=3.60, 95% CI 1.69 to 5.50, n=6), the treatment outcomes of pramipexole group were significantly superior to those of placebo group.
Implications
This meta-analysis study indicated that pramipexole could effectively improve the symptoms of primary moderate to severe RLS patients, although the quality of evidence was relatively low. Future clinical trials focusing on the medium-term and long-term treatment outcomes and using mainly objective indicators for evaluation are warranted. It is also necessary to pay close attention to augmentation during medication.
Keywords: Restless leg syndrome (RLS), pramipexole, efficacy, meta-analysis, clinical trials
INTRODUCTION
Primary restless legs syndrome (RLS) is a common sensorimotor disorder characterized by an irresistible urge to move one’s extremities to stop uncomfortable or odd sensations. It commonly affects the lower extremities. The symptom is more severe at rest or at night and can be temporarily relieved by moving the affected extremities. This disorder often interrupts patients’ sleep, thus affecting their quality of life.1–3 The prevalence rate of RLS in the normal adult population is approximately 3.9%-15%,4,5 and the prevalence increases with age.6 Approximately one-third of patients require treatment with medication,6 which leads to a heavy burden in their daily and social life.3 At present, the exact pathophysiology of primary RLS remains unclear.7 The results of animal models of RLS and biochemical, postmortem, and imaging studies in patients with the disease suggest that disruptions in brain iron trafficking lead to disturbances in striatal dopamine neurotransmission for at least some patients with RLS 7. Previous studies have shown that L-dopa can relieve the symptoms by 50% in approximately 90% of patients.8
In recent years, dopamine agonists have become the first-line drug for RLS treatment.9–11 However, this treatment does not achieve satisfactory efficacy in some patients.12,13 In addition, it is difficult to maintain the long-term effectiveness of this treatment, and sometimes it even leads to augmentation and/or rebound.10,14 Although several previous systematic reviews and meta-analyses have demonstrated that dopamine agonists, e.g., pramipexole and ropinirole, could relieve RLS symptoms and improve sleep15 and quality of life,6,16–19 selecting a suitable dopamine agonist for a given patient is challenging because one individual can only use one type of dopamine agonist at a time, e.g., pramipexole. At present, a full evaluation of the treatment efficacy of pramipexole is lacking because of the small sample size, limited number of events, and insufficient statistics for certain endpoints in the available trials of pramipexole.20–31 In addition, the results from different clinical trials are not completely consistent.20–31 To date, only one systematic review has specifically evaluated the efficacy of pramipexole;32 however, this review included only six trials22–26,29 and evaluated only two endpoints, and the quality of evidence was not classified according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE). Moreover, two relevant clinical trials were published at the beginning of 2014.30,31 Therefore, a re-evaluation of the efficacy of pramipexole is of great significance.
METHODS
This systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (PRISMA).33 There are no ethical issues involved in our study because our data were based on published studies.
Search Strategies
We searched databases of PubMed, OVID, ScienceDirect, SpringerLink, Thomson Reuters Web of Science, the Cochrane Library, the Wiley Online Library, ArticleFirst, CALIS, Study, CNKI, and WanFang. The search term for PubMed was “Search (random*) AND ((((restless leg* syndrome) OR Ekbom* syndrome)) AND (pramipexole OR mirapex OR sifrol OR mirapexin))”. Each database was searched from its inception date to June 17, 2014. No language restrictions were applied. In addition, we manually screened the reference lists of included trials and newly published reviews. We also screened the Clinical Trial Results web page on Boehringer Ingelheim’s website.34
Trial Selection
The inclusion criteria were as follows: (1) Participants: all included patients were older than 18 years and were diagnosed with RLS according to the International Restless Legs Syndrome Study Group diagnostic criteria35 or to the clinical version of the Hopkins telephone diagnostic interview,36 with a score of at least 15 on the International Restless Leg Syndrome Study Group Rating Scale (IRLS).37 The participants discontinued their RLS medications at least 2 weeks before starting the study medications. Pregnant women, substance abusers, and individuals with serious liver or kidney disease, severe insomnia, malignant tumors, Parkinson’s disease, or peripheral neuropathy were excluded. (2) Intervention: the trials used pramipexole in pramipexole group and placebo in placebo group, and all drugs were orally administered for at least seven days. No other drugs for RLS were jointly used. (3) Endpoints: the evaluated endpoints included IRLS score, Clinical Global Impression of Improvement scale (CGI-I),38 Patient Global Impression scale (PGI),38 quality of life and sleep quality. (4) Study type: randomized double-blinded controlled trials were included. We excluded duplicates literatures.
Endpoint Definitions
The IRLS was established by the International RLS Study Group. This scale contains 10 items and is used to rate the severity of RLS symptoms in the past week. Each item is scored on a range from 0 to 4, with higher numbers indicating greater severity. The highest possible score is 40. The higher scores indicate more severe symptoms.37 In the present study, we used post-treatment changes in the IRLS score to evaluate symptom changes, and we used the responder rates for the IRLS score to determine the proportion of patients whose IRLS scores were reduced by at least 50% after treatment. Based on a post-treatment global impression classification of RLS patients (very much improved/better, much improved/better, slightly improved, no change, slightly deteriorated, deteriorated and considerably deteriorated) proposed by the National Institute of Mental Health (NIMH),38 we used the responder rates for the CGI-I to investigate the proportion of patients whose symptoms were “very much improved” or “much improved” and used the responder rates for the PGI to investigate the proportion of patients whose symptoms were “very much better” or “much better”.
Augmentation was defined as symptomatic worsening of RLS, manifested with earlier onset of symptoms at afternoon or evening, rapid onset or shorter latent of symptoms at rest, severe symptoms, progression of RLS symptoms to other body parts (such as the upper extremities and body trunk, even face), and/or shortened effective duration of medication.39,40
Data Extraction
Using a unified form, two investigators independently extracted the data and created the data spreadsheet. Data accuracy was confirmed by these two investigators together, and discrepancies were resolved via discussion among all researchers participating in this study until a consensus was reached.
The extracted data mainly included the responder rates for the IRLS score, the responder rates for the CGI-I, the responder rates for the PGI, the change in the IRLS score, the change in quality of life, the quality of sleep, the change in the quality of sleep, the change in daytime tiredness, the change in periodic limb movements per hour of sleep, and the number of occurrences/rate of augmentation. To determine the post-treatment change from the baseline means, all of the increased data were presented as positive numbers, and all of the decreased data were presented as negative numbers on the data spreadsheet. All of the data were extracted based on an intention-to-treat analysis (ITT), and a portion of the data were also extracted based on the last observation carried forward (LOCF). In this study, we also included some of the data from the study by Scholz et al.6, in addition to the data extracted from original literature and the results reported on the Clinical Trial Results web page of Boehringer Ingelheim’s website.34
Publication Bias Evaluation and Data Quality Grading
Two investigators evaluated the publication bias of all included trials according to the Cochrane Collaboration’s tool for assessing bias (Reviewer’s Handbook41) and graded the evidence quality of all endpoints based on the GRADE profile version 3.6 provided by the GRADE study group. The risk of bias for certain studies was adopted from the study by Scholz et al.6
Statistical Analysis
The risk ratio (RR) was used to investigate the responder rates for the IRLS score, the responder rates for the CGI-I, the responder rates for the PGI, and augmentation. An RR higher than 1 indicated that pramipexole group’s response was superior to that of placebo group. The weighted mean difference (WMD) was used to evaluate continuous variables, including end-of-treatment data and changes from baseline means, which were expressed using the same measurement units. For the change in the IRLS scores, the change in daytime tiredness, and the change in periodic limb movements per hour of sleep, a negative WMD indicated that pramipexole group’s response was superior to that of placebo group. For the quality of sleep, the change in quality of life, and the change in the quality of sleep, a positive WMD indicated that pramipexole group’s response was superior to that of placebo group.
Prior to the meta-analysis of each item, Chi-square tests were performed to test inter-trial heterogeneity; p≥0.10 and I2≤40% indicated the absence of significant heterogeneity, and a fixed-effects model was applied; otherwise, a random-effects model was applied for analysis. For pooling data, the Mantel-Haenszel method was applied for binary variables, and the Inverse-Variance method was applied for continuous variables. The sensitivity and publication bias of each endpoint with statistical significance were evaluated. Sensitivity was analyzed by removing each trial one at a time, and publication bias was detected using an Egger test42. Chi square tests were applied to compare the rate of loss to follow-up and the proportion of female patients in pramipexole group versus placebo group from the same trial. SPSS Predictive Analytics Software version 18.0 (SPSS, Inc., Chicago, IL, USA) was used for the chi square tests, and Stata Statistical Software version SE 12.0 (Stata Corp LP, College Station, TX, USA) was used for all other analyses.
RESULTS
Search Results and Trial Characteristics
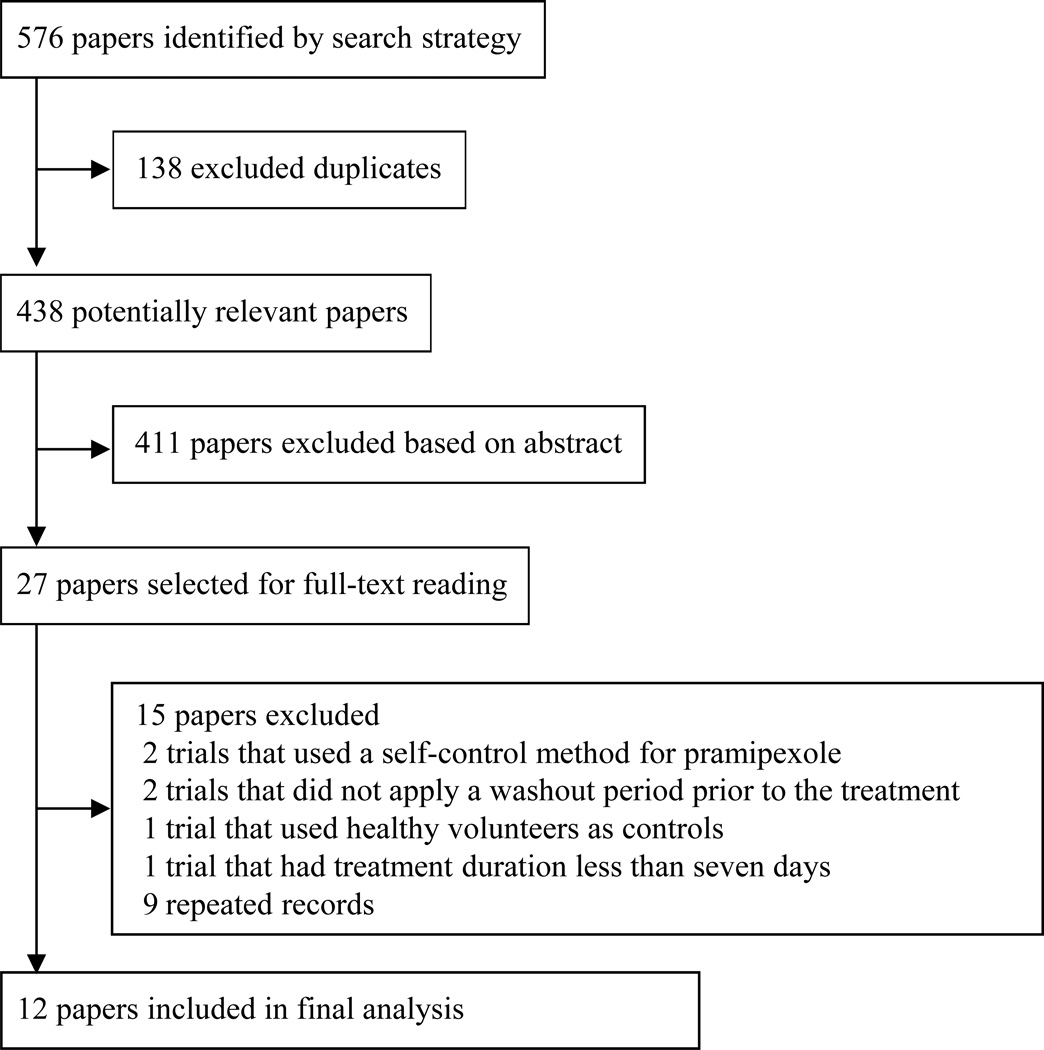
A total of 576 records were identified through database search, after we excluded two studies that used a self-control method before and after treatment,43,44 two trials that did not include a washout period prior to the treatment,45,46 one trial that used healthy volunteers as controls,47 one trial that had a treatment duration less than seven days,48 and nine repeated records,49–57 twelve trials were ultimately included in our study.20–31
All of the included trials were double-blinded randomized placebo-controlled trials, which included a total of 3286 participants who underwent an intention-to-treat analysis. Among the included trials, ten20,22–30 were parallel controlled, and two21,31 were cross-controlled. Eleven of the trials21–31 were published in peer-reviewed journals; the remaining trial20 was published at the Clinical Trial Results web page on Boehringer Ingelheim’s website.34 Eleven of the trials employed the international RLS diagnostic criteria,20–29,31 and the other adopted the clinical version of the Hopkins telephone diagnostic interview.30
The available data demonstrated that ten of the trials involved patients who had moderate to severe symptoms or an IRLS score ≥15.22–31 The trial by Montplaisir et al.21 reported that the RLS symptoms interrupted sleep more than three times per week. Participants were treated for three weeks in one trial,22 six to eight weeks in five trials,20,21,24,26,29 twelve weeks in five trials,23,25,28,30,31 and twenty-six weeks in one trial,27 yielding an average treatment duration of 11.12 (±5.72) (mean ± SD) weeks/person (median: 12 weeks/person). In all of the included trials, drugs were administered 2–3 h before sleep every day, with the minimal dose of 0.125 mg/day and the maximal dose of 1.5 mg/day. All of the trials were supported by pharmaceutical companies, including nine20,22–29 supported by Boehringer Ingelheim Co., two30,31 by Pfizer, and one21 by Pharmacia and Upjohn.
The rates of loss to follow-up (i.e. the proportion of participants who took the experimental drug at least once but never completed an effective endpoint assessment) in the included trials ranged from 0% to 9.76%, with an average rate of 2.62% (86/3286). With the exceptions of the trials by Inoue et al.26 (9.76%; 0% in pramipexole group and 19.05% in placebo group, p=0.040) and Ma et al.29 (6.23%; 3.96% in pramipexole group and 10.68% in placebo group, p=0.022), there was no statistically significant difference in the rate of loss to follow-up between the two groups (p>0.05). The LOCF method was employed for incomplete outcome data in three trials,23,26,28 and polysomnogram (PSG) was used in four trials.21,22,26,31
All of the included trials were multicenter trials; five were completed in Europe and North American,21,23,27,30,31 four in Europe,22,24,25,28 one in China,29 and one in Japan.26 Except for the trials by Ma et al.29 (p=0.048) and Oertel et al.24 (p=0.001), in which the females accounted for a significantly greater proportion in placebo group than that in pramipexole group, and the trial by Montagna et al.,28 in which the proportion of female participants in placebo group was slightly higher than that in pramipexole group (p=0.053), the baseline characteristics of all other included trials were comparable between the two groups (p>0.05).20–31 The screening process is presented in Figure 1, and the main characteristics of the 12 included trials are presented in Table 1.
Figure 1.
Flow diagram of the screening process
Table 1.
Trial Characteristics
| Trials | Participants (Pramipexole group vs. placebo group) |
Intervention (Pramipexole group) | Outcomes |
|---|---|---|---|
| Allen et al.30 | n=537 (excluding the patients treated with pregabalin), and the average age was 55.34 (±13.19) vs. 53.5 (±13.3); males accounted for 42.18% vs. 38.0%; the duration of symptoms was at least six months, and the baseline IRLS score was 22.25 (±5.30) vs. 22.4 (±5.6) |
Pramipexole 0.25 or 0.5 mg/day (once daily); the dose build-up phase was 2 weeks, and the maintenance period was 10 weeks |
12 weeks; IRLS score, responder rates on CGI-I, quality of life with a questionnaire65 quality of sleep with a questionnaire66 |
| BI248.61620 | N=373, and the average age was 48.69 (±1.49) vs. 49.6 (±1) years; males accounted for 29.74% vs. 39.39%; no indication was provided for the duration of symptoms; the baseline IRLS score was 25 vs. 24.9 |
Pramipexole at a fixed dose of 0.25 mg/day (once daily) with a treatment duration of 6 weeks for intervention group A; for intervention group B, 0.125 mg/day (once daily) for 1 week and 0.25 mg/day (once daily) for 5 weeks |
6 weeks; change in the IRLS; responder rates for the IRLS score; responder rates for the CGI-I; responder rates for the PGI |
| Ferini-Strambi et al.25 | n=357, and the average age was 56.3 (±12.4) vs. 56.9 (±13.0) years; males accounted for 27.5% vs. 36.4%; the duration of symptoms was 5.36 (±9.78) vs. 5.66 (±9.89) year; the baseline IRLS score was 24.2 (±5.2) vs. 24.6 (±5.7) |
Pramipexole 0.25, 0.5, or 0.75 mg/day (once daily); the dose build-up duration was 4 weeks, and the maintenance period was 8 weeks |
12 weeks; IRLS score; responder rates for the IRLS score; responder rates for the CGI-I; responder rates for the PGI; Medical Outcomes Study sleep scale;67 the Johns Hopkins RLS Quality of Life (RLS-QOL) questionnaire score68 |
| Garcia-Borreguero et al.31 |
n=148 (excluding the patients treated with pregabalin), and the average age ranged from 50.3 to 57.4 years; males accounted for 36%, and the duration of symptoms ranged from 2 to 11.9 years; no indication of the baseline IRLS score was provided |
Crossover trial; all participants were randomized across 6 treatment sequences, each comprising 3 double-blind treatment periods with pregabalin 300 mg/day (once daily), pramipexole 0.5 mg/day (once daily; dose began at 0.125 mg/day), and placebo. Each treatment period included 10 days’ dose escalation and 19 days’ fixed-dose treatment. Following each treatment period, the drug dosage was tapered over 6 days |
12 weeks; periodic limb movements during time in bed index (PLMI); responder rates for the CGI-I; quality of sleep score (Epworth Sleepiness scale (ESS)); quality of life (the Johns Hopkins RLS Quality of Life (RLS-QOL) questionnaire score) |
| Högl et al.27 | n=321, and the average age was 57.9 (±12.7) vs. 55.8 (±14.1) years; males accounted for 38.6% vs. 42.3%; the duration of symptoms was 6.0 (±9.6) vs. 5.4 (±8.5) years, and the baseline score was 23.9 (±5.3) vs. 23.5 (±5.4) |
Pramipexole; the initial dose was 0.12 mg/day (once daily), which was gradually adjusted to an acceptable dose of 0.125, 0.25, 0.5, or 0.75 mg/day (once daily); the dose build-up duration was 4 weeks, and the maintenance period was 22 weeks |
26 weeks; change in the IRLS; responder rates for the IRLS score; responder rates for the CGI-I; responder rates for the PGI; RLS-6 rating scales;69 the Johns Hopkins RLS Quality of Life (RLS-QOL) questionnaire score68 |
| Inoue et al.26 | n=37, and the average age was 48.7 (±16.1) vs. 62.3 (±11.9) years; males accounted for 45.0% vs. 52.4%; the duration of symptoms was 0.22 (±0.55) vs. 0.64 (±1.28) years; the baseline IRLS score was 23.4 (±6.4) vs. 25.1 (±5.8) |
Pramipexole; the initial dose was 0.12 mg/day (once daily), which was gradually increased to 0.25, 0.5, or 0.75 mg/day (once daily); the dose build-up duration was 1 week, and the maintenance period was 5 weeks |
6 weeks; IRLS score; responder rates for the CGI-I; responder rates for the PGI; Epworth Sleepiness Scale (ESS);70 the Pittsburgh Sleep Quality Index |
| Ma et al.29 | n=305, and the average age was 56.46 (±11.88) vs. 56.86 (±11.89) years; males accounted for 39.6% vs. 27.7%; the duration of symptoms was at least 3 months; the baseline IRLS score was at least 15 |
Pramipexole; the initial dose was 0.125 mg/day (once daily), which was increased to an “suitable dose” based on effectiveness and tolerance using a titration method; the dose build-up duration was 4 weeks, and the maintenance period was 2 weeks |
6 weeks; IRLS score; responder rates for the IRLS score; responder rates for the CGI-I; responder rates for the PGI; visual analog scales; Epworth sleepiness scale (ESS);70 RLS-6 rating scales69 |
| Montagna et al.28 | N=403, and the average age was 55.0 (±13.8) vs. 56.1 (±12.1) years; males accounted for 33.0% vs. 27%; the duration of symptoms was 3.5 (±7.2) vs. 3.3 (±6.5) year; the baseline IRLS score was 25.9 (±5.2) vs. 25.9 (±5.5) |
Pramipexole; the initial dose was 0.125 mg/day (once daily), which was increased to 0.125, 0.25, 0.5, or 0.75 mg/day (once daily) based on effectiveness and tolerance using a titration method; the dose build-up duration was 4 weeks, and the maintenance period was 8 weeks |
12 weeks; IRLS score; responder rates for the IRLS score; responder rates for the CGI-I; responder rates for the PGI; RLS-6 rating scales;69 the Johns Hopkins RLS Quality of Life (RLS-QOL) questionnaire score;68 the Hospital Anxiety and Depression Scale-Anxiety subscale (HADS-A) score |
| Montplaisir et al.21 | n=20, and the average age was 49.3 (±11.5) years; males accounted for 55.56%; the duration of symptoms was at least one year; the baseline IRLS score was not mentioned |
Crossover trial; pramipexole group received flexible up-titration of pramipexole (once daily) from 0.375 mg to 0.75 mg to 1.5 mg in 2 weeks and maintenance for 2 weeks; placebo group received placebo for 4 weeks; 2 week washout between phases |
8 weeks; periodic limb movements per hour of sleep measured under PSG monitoring |
| Oertel et al.24 | n=338, and the average age was 55.4 (±11.6) vs. 55.8 (±10.9) years; males accounted for 35.7% vs. 31.6%; the duration of symptoms was 4.95 (±9.21) vs. 9.06 (±5.63) years, the baseline IRLS score was 24.7 (±5.2) vs. 24.9 (±5.4) |
Pramipexole; the initial dose was 0.125 mg/day (once daily), which was increased to 0.25, 0.5, or 0.75 mg/day (once daily) based on effectiveness and tolerance using a titration method; the dose build-up duration and maintenance period were not mentioned |
6 weeks; IRLS score; responder rates for the CGI-I; responder rates for the IRLS score; responder rates for the PGI; visual analogue scales |
| Partinen et al.22 | n=108, and the average age was 56.2 (±10.9) years; males accounted for 38.71% vs. 19.05%, the duration of symptoms was 4.8 (±10.4) years, and the baseline IRLS score was 22.7 (±4.1) in total |
Pramipexole; the initial dose was 0.125 mg/day (once daily), which was increased to a pre-established dose of 0.125, 0.25, 0.5, or 0.75 mg/day (once daily) using a titration method; the dose build-up duration was 4 days, and the maintenance period was 17 days |
3 weeks; IRLS score; responder rates for the PGI; responder rates for the CGI-I; responder rates for the IRLS score; the Pittsburgh Sleep Quality Index; sleepiness and sleep quality (Epworth Sleepiness Scale (ESS)70); quality of life (Short Form 36 Health Survey questionnaire71); periodic limb movements during time in bed index (PLMI) |
| Winkelman et al.23 | n=339, and the average age was 51.4 (±13) years; males accounted for 38.19% vs. 36.47%, the duration of symptoms 5.1 (±15) years, the baseline IRLS score was 23.4 (±5.1) vs. 23.5 (±5.2) |
Pramipexole; the initial dose was 0.125 mg/day (once daily), which was increased to a pre-established dose of 0.25, 0.5 or 0.75 mg/day (once daily) based on the effectiveness and tolerance; the dose build-up duration was 3 weeks, and the maintenance period was 9 weeks |
12 weeks; IRLS score; responder rates for the CGI-I; responder rates for the PGI; responder rates for the IRLS score; daytime somnolence (Epworth sleepiness scale (ESS));70 quality of life (the Johns Hopkins RLS Quality of Life (RLS-QOL) questionnaire score)68 |
RLS: Restless Legs Syndrome; IRLS: International RLS Study Group Rating Scale; CGI-I: Clinical Global Impression of Improvement scale; PGI: Patient Global Impression scale; MOS: Medical Outcomes Study sleep disturbance score; RLS-QOL: Johns Hopkins RLS Quality of Life Scale; RLS-6: RLS 6-item questionnaire; ESS: Epworth sleepiness scale
Results are shown as mean ± standard deviation.
Risk of Bias
We adopted the risk of bias evaluation results for nine trials20–28 reported by Scholz et al.6 Except for BI248.61620 and the trial by Inoue et al.,26 in which the risk of “random sequences generation (selection bias)” was unclear, all other included trials had low risks of bias (Supplementary Materials: Figure S1 and Figure S2).
GRADE
The GRADE rating revealed that no endpoints had high-quality evidence, two endpoints had medium-quality evidence, six had low-quality evidence, and one had extremely low-quality evidence (Table 2).
Table 2.
Summary of the rating results regarding the quality of evidence
| Pramipexole compared with placebo for primary moderate to severe restless leg syndrome | ||||||
|---|---|---|---|---|---|---|
| Patient or population: primary moderate to severe restless leg syndrome, according the International Restless Legs Syndrome Study Group criteria; 18 years or older Settings: Outpatient settings in Europe, North America, China, Japan Intervention: Pramipexole Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pramipexole | |||||
| Change in the IRLS20,22–25,27–29 International Restless Leg Syndrome Study Group Rating Scale. Scale from 0 to 40. Follow-up: 3–26 weeks |
The mean change on IRLS ranged across control groups from -5.7 to −11.35 points |
The mean change on IRLS in pramipexole groups was 4.64 lower (5.95 to 3.33 lower) |
- | 2420 (8 studies) |
⊕⊕⊝⊝ low1, 2 |
The difference in the treatment duration was the main cause of the heterogeneity |
| Responder rates for the IRLS score22–29 Count Follow-up: 3–26 weeks |
38 per 100 | 60 per 100 (55 to 66) |
RR 1.57 (1.43 to 1.73) |
2188 (8 studies) |
⊕⊕⊕⊝ moderate2 |
- |
| Responder rates for the CGI-I20,22–31 Count Follow-up: 3–26 weeks |
44 per 100 | 66 per 100 (58 to 74) |
RR 1.48 (1.31 to 1.66) |
3234 (11 studies) |
⊕⊕⊝⊝ low1, 2 |
BI248.61620 was the main contributor for heterogeneity. |
| Responder rates for the PGI20,22–29 Count Follow-up: 3–26 weeks |
41 per 100 | 63 per 100 (54 to 74) |
RR 1.54 (1.31 to 1.81) |
2568 (9 studies) |
⊕⊕⊝⊝ low1, 2 |
BI248.61620 was the main contributor for heterogeneity. |
| Change in quality of life23,25,27,28 The Johns Hopkins RLS Quality of Life questionnaire score Follow-up: 12–26 weeks |
The mean change in quality of life ranged across control groups from 12.3 to 14.5 points |
The mean change in quality of life in pramipexole groups was 5.39 higher (2.28 to 8.5 higher) |
- | 1397 (4 studies) |
⊕⊕⊝⊝ low1, 2 |
Hogl et al.27 was the main contributor for heterogeneity. |
| Quality of sleep30,31 Quality of sleep with a Questionnaire Follow-up: mean 12 weeks |
The mean quality of sleep ranged across control groups from 6–57.7 points |
The mean quality of sleep in pramipexole groups was 0.51 higher (0.03 lower to 1.06 higher) |
- | 685 (2 studies) |
⊕⊕⊕⊝ moderate2 |
- |
| Change in daytime tiredness24,25,27,28 Medical Outcomes Study sleep disturbance score; RLS-6 item scores Follow-up: 6–26 weeks |
The mean change in daytime tiredness ranged across control groups from −0.8 to −1.3 points |
The mean change in daytime tiredness in pramipexole groups was 0.61 lower (1.21 to 0.01 lower) |
- | 1411 (4 studies) |
⊕⊕⊝⊝ low1, 2 |
The differences in treatment duration and the proportion of female patients in placebo group might be the main cause of heterogeneity. |
| Change in periodic limb movements per hour of sleep21,22,26 Epworth Sleepiness Scale; The Pittsburgh Sleep Quality Index Follow-up: 3–8 weeks |
The mean change in periodic limb movements in sleep per hour of sleep ranged across control groups from −1.54 to −7.21 points |
The mean change in periodic limb movements in sleep per hour of sleep in pramipexole groups was 35.95 lower (56.42 to 15.48 lower) |
- | 168 (3 studies) |
⊕⊝⊝⊝ very low1, 2, 3 |
The difference in the drug dosage might be responsible for heterogeneity. |
| Change in quality of sleep22–26,28 Medical Outcomes Study sleep scale; Epworth Sleepiness Scale; the Pittsburgh Sleep Quality Index; RLS-6 rating scales Follow-up: 3–12 weeks |
The mean change in quality of sleep ranged across control groups from 0.4 to 25.9 points |
The mean change in quality of sleep in pramipexole groups was 3.60 higher (1.69 to 5.5 higher) |
- | 1574 (6 studies) |
⊕⊕⊝⊝ low1, 2 |
The difference in the evaluation tool of quality of sleep might be responsible for heterogeneity. |
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio; IRLS: International RLS Study Group Rating Scale; CGI-I: Clinical Global Impression of Improvement Scale; PGI: Patient Global Impression Scale; RLS-6: RLS six-item questionnaire; RLS: Restless legs syndrome.
GRADE working group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
The variation in point estimates among different trials was relatively large, and the heterogeneity test showed results of p<0.10 and I2 >40%.
All of the trials were supported by pharmaceutical companies.
The sample size was small.
Change in the IRLS
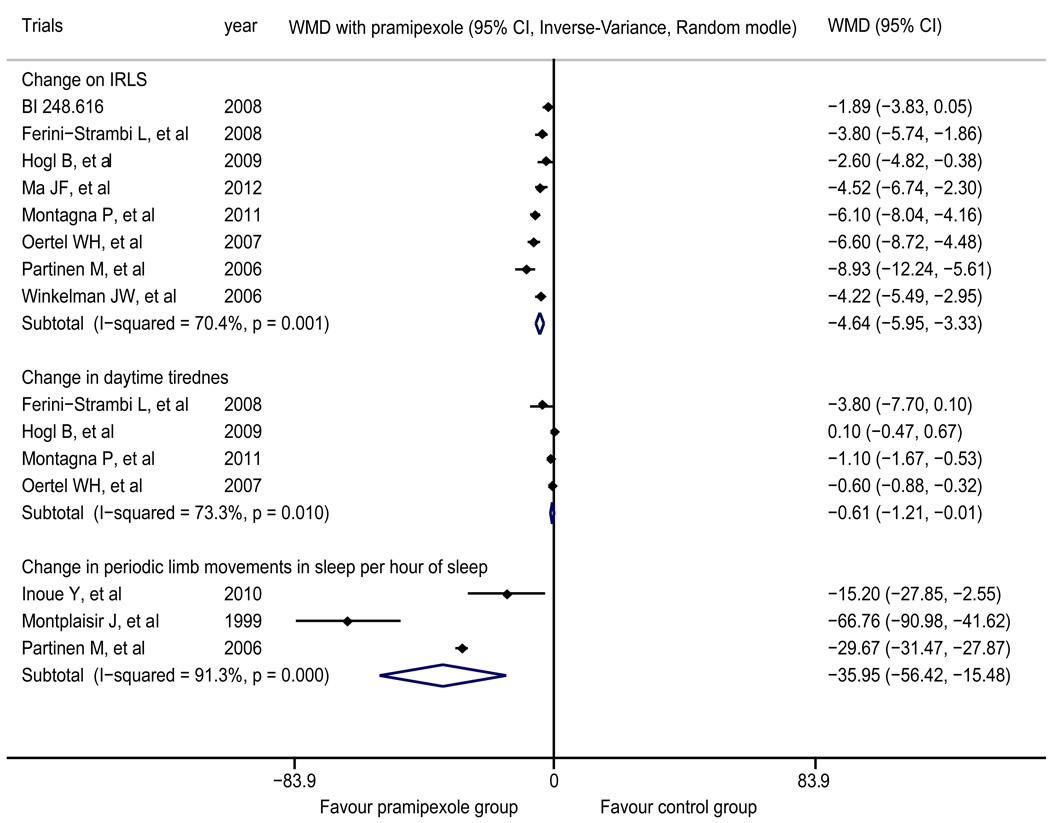
The meta-analysis of eight trials20,22–25,27–29 demonstrated that the post-treatment change in the IRLS score of pramipexole group was significantly superior to that of placebo group (WMD=-4.64, 95% CI −5.95 to −3.33). The intragroup heterogeneity test yielded results of I2=70.4% and p=0.001 (Figure 2).
Figure 2.
Forest plot of the change in the IRLS score, the change in daytime tiredness and the change in periodic limb movements per hour of sleep
The treatment outcomes for pramipexole were superior to those for placebo. IRLS: International RLS Study Group Rating Scale
Responder rates for the IRLS score
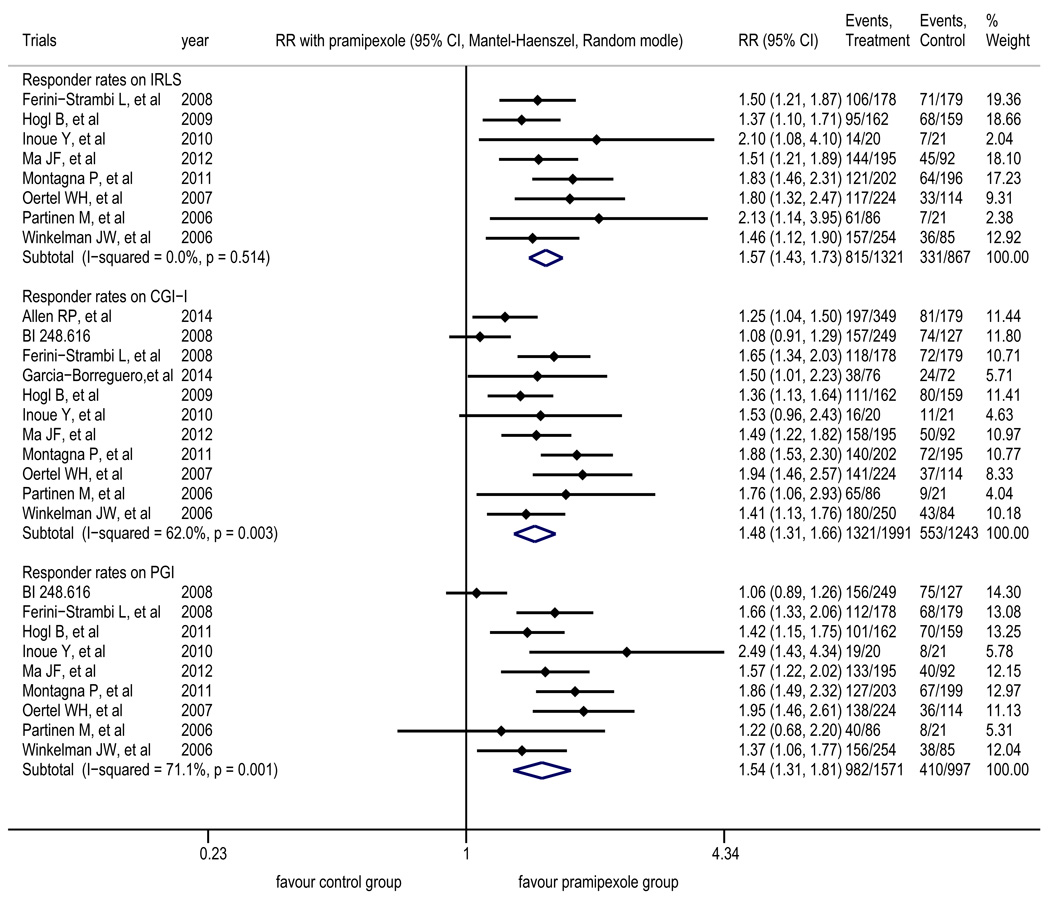
The meta-analysis of eight trials22–29 revealed that compared with placebo group, pramipexole group had a significantly higher proportion of patients whose IRLS score decreased by at least 50% after treatment (RR=1.57, 95% CI 1.43 to 1.73). The intragroup heterogeneity test yielded results of I2=0 and p=0.514 (Figure 3).
Figure 3.
Forest plot of the responder rates for the IRLS score, the responder rates for the CGI-I, and the responder rates for the PGI
The treatment outcomes for pramipexole were superior to those for placebo. IRLS: International RLS Study Group Rating Scale; CGI-I: Clinical Global Impression of Improvement scale; PGI: Patient Global Impression scale
Responder rates for the CGI-I
The meta-analysis of 11 trials20,22–31 showed that compared with placebo group, pramipexole group had a significantly higher proportion of patients whose symptoms were “very much improved” or “much improved” after treatment according to doctors (RR=1.48, 95% CI 1.31 to 1.66). The intragroup heterogeneity test yielded results of I2=62.0% and p=0.003 (Figure 3); after the removal of BI248.616,20 the intragroup heterogeneity test yielded results of I2=36.2% and p=0.118.
Responder rates for the PGI
The meta-analysis of nine trials20,22–29 showed that compared with placebo group, pramipexole group had a significantly higher proportion of patients whose symptoms were “very much better” or “much better” after treatment, as perceived by patients themselves (RR=1.54, 95% CI 1.31 to 1.81). The intragroup heterogeneity test yielded results of I2=71.1% and p=0.001 (Figure 3); after the removal of BI248.616,20 the intragroup heterogeneity test yielded results of I2=26.5% and p=0.217.
Quality of life
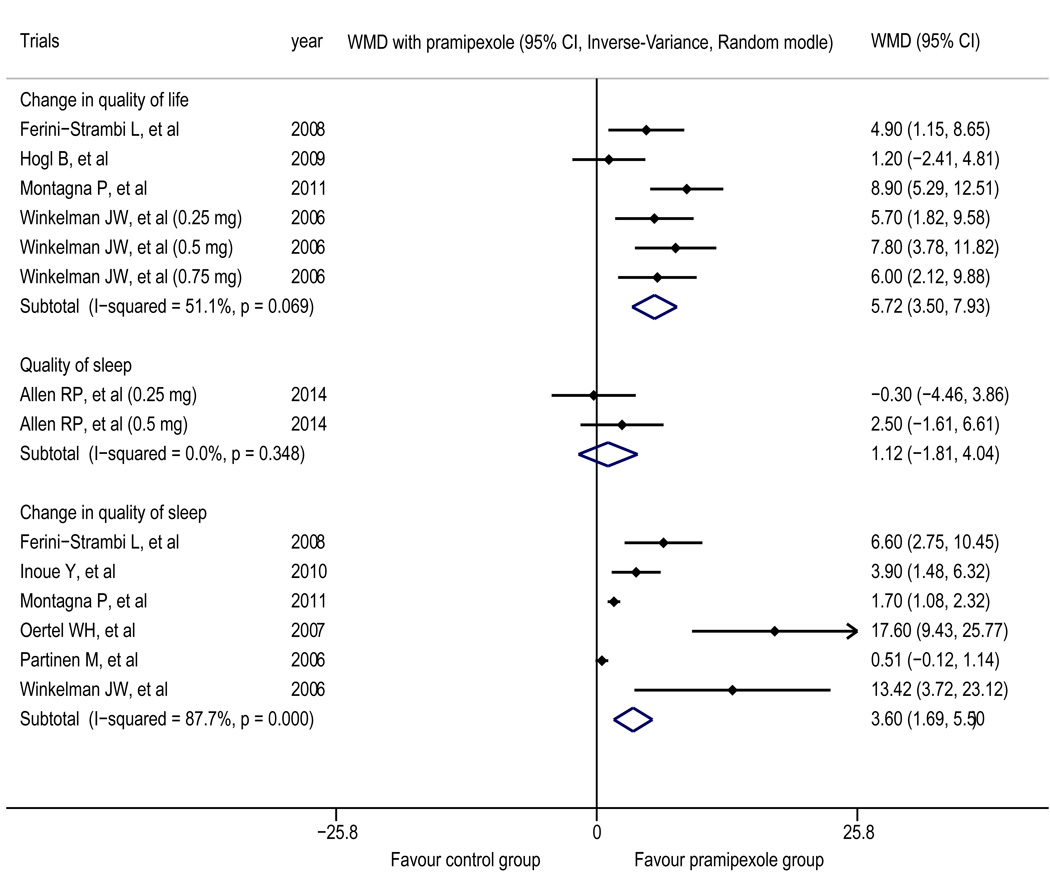
The results reported in the trials by Ferini-Strambi et al.25 and Montagna et al.28 demonstrated that compared with placebo group, pramipexole group showed significantly decreased in the RLS-QOL score after treatment (p=0.01; p<0.001). However, in one trial,27 the improvement in the RLS-QOL score did not differ significantly between the two groups after treatment (p=0.591). The results reported in the trial by Partinen et al.22 demonstrated that other than the social function score (a subscale), which was higher in pramipexole group than in placebo group, all of the other scores related to life quality (SF-36) did not show a statistically significant difference between the two groups after treatment (p>0.05). Only the trial by Allen et al.30 reported scores for the quality of life; thus, the data were insufficient for pooling. The meta-analysis of four trials23,25,27,28 revealed that in terms of the change in quality of life, the treatment outcome of pramipexole group was superior to that of placebo group (WMD=5.39, 95% CI 2.28 to 8.50). The intragroup heterogeneity test yielded results of I2=67.7% and p=0.026 (Figure 4); after the trial by Hogl et al.27 was removed, the results were I2=14.0% and p=0.313.
Figure 4.
Forest plot of the change in quality of life, the quality of sleep and the change in quality of sleep The treatment outcomes for pramipexole were superior to those for placebo for the change in quality of life and the change in quality of sleep.
Quality of sleep
The meta-analysis of two trials30,31 indicated that the current evidence was insufficient to prove that the improvement of sleep quality was greater in pramipexole group compared with placebo group (WMD=0.51, 95% CI −0.03 to 1.06; Figure 4). The trial by Montplaisir et al.21 revealed that compared with placebo group, pramipexole group experienced a 98% decrease in periodic limb movement in sleep index, and the number of periodic limb movements, the number of sleep interruptions related to periodic limb movement, and the number of periodic waking at night all significantly decreased (all p<0.01).
The meta-analysis of four trials24,25,27,28 showed that regarding the change in daytime tiredness, pramipexole group had a superior treatment outcome compared with placebo group (WMD=-0.61, 95% CI −1.21 to −0.01); the intragroup heterogeneity test yielded results of I2=73.3% and p=0.010 (Figure 2). The result obtained by Garcia-Borreguero et al.31 indicated that compared with placebo group, pramipexole group showed significantly improved results of periodic limb movement in sleep index (pramipexole group vs. placebo group (mean ± standard error): 8.0 (±2.4) vs. 37.0 (±2.5), p<0.05). The meta-analysis of three trials21,22,26 showed that regarding the change in periodic limb movements per hour of sleep, pramipexole group had a superior treatment outcome compared with placebo group (WMD=-35.95, 95% CI −56.42 to −15.48); the intragroup heterogeneity test yielded results of I2=91.3% and p<0.001 (Figure 2). The meta-analysis of six trials22–26,28 showed that in terms of the change in quality of sleep, pramipexole group had a superior treatment outcome compared with placebo group (WMD=3.60, 95% CI 1.69 to 5.50); the intragroup heterogeneity test yielded results of I2=87.7% and p<0.001 (Figure 4).
Augmentation
Among the included trials, two27,30 assessed the occurrence of augmentation, and one30 evaluated the rate of augmentation in the pramipexole and pregabaline group without mentioning the placebo group. The analysis result of one trial27 demonstrated that the risk of augmentation in patients treated with pramipexole was comparable to that of the placebo group (pramipexole vs. placebo: RR=1.40, 95% CI, 0.62 to 3.13).
Sensitivity analysis
The sensitivity analysis indicated that for the change in daytime tiredness, after the removal of any one trial other than the one by Hogl et al.,27 the upper limit of the 95% CI of WMD was higher than zero, indicating that the results for this endpoint were affected by the other three trials. For the change in periodic limb movements per hour of sleep, the upper limit of the 95% CI of WMD was higher than zero after the removal of any one trial, indicating that the results for this endpoint were affected by the other trials (Supplementary Materials:Figure S3 and S4).
For the change in the IRLS score, the upper limit of the 95% CI of WMD was lower than zero after the removal of any one trial. For the change in quality of life and the change in quality of sleep, the lower limit of the 95% CI of WMD remained higher than zero after the removal of any one trial. For the responder rates for the IRLS scores, the responder rates for the CGI-I, and the responder rates for the PGI, the lower limit of the 95% CI of RR remained higher than 1 after the removal of any one trial. These results indicated that the results for these endpoints were robust (Supplementary Materials: Figure S5–S10).
Publication Bias
The Egger test derived a p value less than 0.05 for the change in quality of sleep, indicating the existence of a publication bias for this endpoint. For the other endpoints, the p values were all greater than 0.05, indicating the absence of evident publication bias for these endpoints (Table 3).
Table 3.
Results of the Egger test
| Results | Change in IRLS |
Responder rates for IRLS |
Responder rates for CGI-I |
Responder rates for PGI |
Change in quality of life |
Change in daytime tiredness |
Change in PLMS |
Change in quality of sleep |
|---|---|---|---|---|---|---|---|---|
| P value | 0.364 | 0.110 | 0.226 | 0.228 | 0.768 | 0.663 | 0.795 | 0.017 |
| Interval (95% CI) |
−9.16 to 3.89 |
−0.50 to 3.78 | −1.50 to 5.57 | −1.95 to 6.90 | −76.59 to 65.45 | −9.91 to 7.83 | −48.67 to 46.19 |
0.99 to 5.71 |
RLS: Restless Legs Syndrome; IRLS: International RLS Study Group Rating Scale; CGI-I: Clinical Global Impression of Improvement scale; PGI: Patient Global Impression scale; PLMS: Periodic limb movements in sleep per hour of sleep
RR is used as an effect size index for binary variables, and WMD is used as an effect size index for continuous variables.
DISCUSSION
This study demonstrated that for patients with primary moderate to severe RLS, pramipexole showed a higher treatment efficacy compared with placebo for eight endpoints, including the change in the IRLS scores, the proportion of patients whose IRLS score decreased by at least 50% after treatment, CGI-I, PGI, the change in quality of life, the change in daytime tiredness, the change in periodic limb movements during sleep and the change in quality of sleep.
This study has the several strengths. Firstly, the literature search was comprehensive and reproducible. We searched twelve major databases and screened the Clinical Trial Results web page on Boehringer Ingelheim’s website.34 This study included all RCTs that were completed.
Secondly, all of the included trials had a relatively low risk of bias. All trials were multi-center, placebo-controlled, randomized and double-blinded international trials. Each participant was assigned a serial number that was randomly generated by computers at the pharmaceutical company and was randomly allocated based on the participant’s location and the proportion of study subjects recruited from that site. The researchers did not know the details of the randomized allocation and were not able to change the randomizing process. The data in each trial were managed by specific personnel to ensure the blinding of the participants, doctors and outcome evaluators. Therefore, these trials had low risks of bias.
Thirdly, the trial results were reliable. Except for the two trials26,29 in which the rate of loss to follow-up of placebo group was higher than that of pramipexole group, and the two trials24,29 in which the proportion of females in placebo group was higher than the proportion in pramipexole group, the baseline data of all other trials were completely comparable between the two groups. Moreover, the highest rate of loss to follow-up (9.76%26) among all of the included trials was far below 20%, and the average rate was only 2.62%. Therefore, the results reported in those trials were trustworthy.
Fourthly, our conclusions were representative and generalizable. The involved participants were outpatients who had moderate to severe symptoms, and the participants included Caucasians, Asians, and black people from Europe, North America and Asia. Therefore, the data from these participants could effectively reflect the treatment needs of patient populations from the abovementioned races and areas, and consequently, our conclusions from the present study are extensively applicable.
It is worth noting that except for the responder rates for the IRLS score and quality of sleep, there were different levels of heterogeneity for the endpoints (I2 62.0% to 91.3%; Figure 2–4). The trial by Hogl et al.27 was the major contributor to the heterogeneity of the change in quality of life, possibly because it used a significantly longer treatment duration compared with other trials. In other words, compared with a 12-week treatment, using pramipexole for 26 consecutive weeks was not more effective for improving quality of life. The BI248.616 trial20 was the major contributor to the heterogeneity of the responder rates for the PGI and the responder rates for the CGI-I, which could be attributed to its treatment regimen in which half of the patients in pramipexole group were treated with a fixed drug dose. In other words, the administration of pramipexole at a fixed dose of 0.25 mg/day reduced the possibility that a portion of patients would achieve satisfactory outcomes; administering the drug at a dose adjusted according to the individual’s response could provide more satisfactory symptom improvement.
Regarding the publication bias related to the change in sleep quality22–26,28, possible explanations were as follows: Firstly, the patients who have poor treatment outcomes are more likely to be lost to follow-up. A significantly higher rate of loss to follow-up in placebo group compared with pramipexole group in the trial by Inoue et al.26 might contribute to the occurrence of bias. Secondly, all of the trials received financial support from pharmaceutical companies. Finally, similar to the statement of Scholz et al.,6 we cannot completely rule out the potential influence of other factors, such as older age, more severe symptoms, and a stronger response to the treatment.
Unlike the study conducted by Zhang et al.,32 the present study included a greater number of existing trials, rated the quality of evidence for all endpoints according to GRADE, performed a sensitivity analysis, conducted publication bias identification and source analysis of heterogeneity. In addition, this study confirmed the efficacy of pramipexole from various perspectives, such as the proportion of patients with an IRLS score reduction of at least 50% after treatment, CGI-I, PGI, and the change in quality of life. Compared with previous studies6,16–19, this study specifically evaluated the efficacy of pramipexole, included a larger number of clinical trials, involved a larger sample size, and conducted in-depth analyses of each endpoint regarding sensitivity, publication bias, and heterogeneity sources. This study revealed that pramipexole could reduce the IRLS score by 4.64 points (pramipexole vs. placebo: WMD=-4.64, 95% CI −5.95 to −3.33), showing a smaller point estimated value and a slightly expanded 95% CI compared with previous studies in which the IRLS score was reduced by 5.74 points (dopamine agonists vs. placebo: WMD=-5.74, 95% CI −6.74 to −4.74)17 and by 5.47 points (dopamine agonists vs. placebo: WMD=-5.47 95% CI −6.40 to −4.54)6,19 after treatment with dopamine agonists. This result might be attributable to the single type of dopamine receptor agonist used (only pramipexole) and the smaller number of clinical trials included.
Because of the limitations of data availability of the existing trials, this study did not find a higher risk of augmentation among patients treated with pramipexole compared with patients treated with placebo. However, other studies did indicate that pramipexole could result in a high augmentation rate of 7.83%-47.06%30,58–62 and could even lead to termination of treatment in some patients.59 Therefore, during medication, particularly during long-term treatment, it is necessary to pay close attention to the occurrence of augmentation. Recent guidelines have also included similar warnings.63
Our study has several limitations which should be acknowledged. Firstly, except for one endpoint (the change in periodic limb movements per hour of sleep) measured under PSG monitoring, other endpoints were mainly subjective evolution indicators. Secondly, because each trial had its specific focus, not all endpoints were reported in all trials, leading to an insufficient statistical assessment for these different endpoints. For example, pramipexole did not show the anticipated advantage in terms of quality of sleep (n=2), but it showed significant superiority to placebo in terms of the change in quality of sleep (n=6; Figure 4). In addition, the insufficiency of the relevant trials and relatively small sample size also led to an increased sensitivity of the change in daytime tiredness and change in periodic limb movements per hour of sleep. Thirdly, because of the existence of heterogeneity and the financial sponsorship of pharmaceutical companies, the quality of evidence based on GRADE was relatively low. Fourthly, the medium-term and long-term efficacy was not evaluated because of the short average treatment duration of only 11.12 (±5.72) weeks/person.
It is worth emphasizing that because the pathogenesis and mechanisms of RLS remain unclear and because the drugs targeting the dopamine system dysfunction can only reduce the symptoms by 50% in approximately 90% patients,8 it is not practical to anticipate that the use of dopamine agonists could eliminate all symptoms of RLS. For patients who have severe symptoms and do not respond to first-line drugs, it may be warranted to jointly take multiple drugs.10,64
CONCLUSIONS
This meta-analysis demonstrated that pramipexole use could effectively improve the symptoms of primary moderate to severe RLS patients, Due to the fact that the quality of evidence was relatively low, future clinical trials focusing on the medium-term and long-term outcomes and using mainly objective indicators for evaluation are warranted to replicate our findings.
Supplementary Material
Acknowledgments
We sincerely thank Mr. Ya Jun Li (the Library of Hubei University of Medicine) for his help with the literature searches. We also greatly appreciate the help of Dr. Hui Nie (diyahui912@gmail.com) from Durham of North Carolina, U.S.A. with the translation of this manuscript.
Footnotes
CONFLICT OF INTEREST
The authors report no disclosures relevant to the manuscript
AUTHOR CONTRIBUTIONS
GJL, LW, YFW, LYC designed the study. GJL and LW conducted the literature search and extracted data. SLW and LLX conducted the quality assessment. GJL and LW conducted the statistical analyses. All authors participated in the data interpretation. GJL and LW drafted the first version of the report. All authors revised and approved the final draft of the report.
References
- 1.Innes KE, Selfe TK, Agarwal P. Restless legs syndrome and conditions associated with metabolic dysregulation, sympathoadrenal dysfunction, and cardiovascular disease risk: a systematic review. Sleep Med Rev. 2012;16:309–339. doi: 10.1016/j.smrv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99–111. doi: 10.3810/pgm.2013.05.2636. [DOI] [PubMed] [Google Scholar]
- 3.Comella CL. Treatment of restless legs syndrome. Neurotherapeutics. 2014;11:177–187. doi: 10.1007/s13311-013-0247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohayon MM, O'Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283–295. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012;16:987–1007. doi: 10.1007/s11325-011-0606-x. [DOI] [PubMed] [Google Scholar]
- 6.Scholz H, Trenkwalder C, Kohnen R, Riemann D, Kriston L, Hornyak M. Dopamine agonists for restless legs syndrome. Cochrane Database Syst Rev. 2011:CD006009. doi: 10.1002/14651858.CD006009.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earley CJ, Connor J, Garcia-Borreguero D, et al. Altered Brain iron homeostasis and dopaminergic function in Restless Legs Syndrome (Willis-Ekbom Disease) Sleep Med. 2014 doi: 10.1016/j.sleep.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Stiasny-Kolster K, Kohnen R, Moller JC, Trenkwalder C, Oertel WH. Validation of the "L-DOPA test" for diagnosis of restless legs syndrome. Mov Disord. 2006;21:1333–1339. doi: 10.1002/mds.20969. [DOI] [PubMed] [Google Scholar]
- 9.Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults--an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35:1039–1062. doi: 10.5665/sleep.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchfuhrer MJ. Strategies for the treatment of restless legs syndrome. Neurotherapeutics. 2012;9:776–790. doi: 10.1007/s13311-012-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi KD, Mehta SH. A clinical primer on restless legs syndrome: what we know, and what we don't know. Am J Manag Care. 2012;18:S83–S88. [PubMed] [Google Scholar]
- 12.Earley CJ, Silber MH. Restless legs syndrome: understanding its consequences and the need for better treatment. Sleep Med. 2010;11:807–815. doi: 10.1016/j.sleep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Godau J, Spinnler N, Wevers AK, Trenkwalder C, Berg D. Poor effect of guideline based treatment of restless legs syndrome in clinical practice. J Neurol Neurosurg Psychiatry. 2010;81:1390–1395. doi: 10.1136/jnnp.2010.211417. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Borreguero D, Williams AM. Dopaminergic augmentation of restless legs syndrome. Sleep Med Rev. 2010;14:339–346. doi: 10.1016/j.smrv.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Hansen RA, Song L, Moore CG, et al. Effect of ropinirole on sleep outcomes in patients with restless legs syndrome: meta-analysis of pooled individual patient data from randomized controlled trials. Pharmacotherapy. 2009;29:255–262. doi: 10.1592/phco.29.3.255. [DOI] [PubMed] [Google Scholar]
- 16.Zintzaras E, Kitsios GD, Papathanasiou AA, et al. Randomized trials of dopamine agonists in restless legs syndrome: a systematic review, quality assessment, and meta-analysis. Clin Ther. 2010;32:221–237. doi: 10.1016/j.clinthera.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Hornyak M, Trenkwalder C, Kohnen R, Scholz H. Efficacy and safety of dopamine agonists in restless legs syndrome. Sleep Med. 2012;13:228–236. doi: 10.1016/j.sleep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Wilt TJ, MacDonald R, Ouellette J, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta-analysis. JAMA Intern Med. 2013;173:496–505. doi: 10.1001/jamainternmed.2013.3733. [DOI] [PubMed] [Google Scholar]
- 19.Hornyak M, Scholz H, Kohnen R, Bengel J, Kassubek J, Trenkwalder C. What treatment works best for restless legs syndrome? Meta-analyses of dopaminergic and non-dopaminergic medications. Sleep Med Rev. 2014;18:153–164. doi: 10.1016/j.smrv.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Boehringer Ingelheim. [last accessed 21 June 2014];BI 248.616. A Phase IV randomised, double-blind, active and placebo-controlled, 6-week trial to investigate the efficacy and safety of a starting (and fixed) dose 0.25 mg pramipexole (Mirapex®) in patients with idiopathic Restless Legs Syndrome. 2008 Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/248/248.616_U08-3876.pdf.
- 21.Montplaisir J, Nicolas A, Denesle R, Gomez-Mancilla B. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. 1999;52:938–943. doi: 10.1212/wnl.52.5.938. [DOI] [PubMed] [Google Scholar]
- 22.Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study--the PRELUDE study. Sleep Med. 2006;7:407–417. doi: 10.1016/j.sleep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology. 2006;67:1034–1039. doi: 10.1212/01.wnl.0000231513.23919.a1. [DOI] [PubMed] [Google Scholar]
- 24.Oertel WH, Stiasny-Kolster K, Bergtholdt B, et al. Efficacy of pramipexole in restless legs syndrome: a six-week, multicenter, randomized, double-blind study (effect-RLS study) Mov Disord. 2007;22:213–219. doi: 10.1002/mds.21261. [DOI] [PubMed] [Google Scholar]
- 25.Ferini-Strambi L, Aarskog D, Partinen M, et al. Effect of pramipexole on RLS symptoms and sleep: a randomized, double-blind, placebo-controlled trial. Sleep Med. 2008;9:874–881. doi: 10.1016/j.sleep.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Inoue Y, Hirata K, Kuroda K, et al. Efficacy and safety of pramipexole in Japanese patients with primary restless legs syndrome: A polysomnographic randomized, double-blind, placebo-controlled study. Sleep Med. 2010;11:11–16. doi: 10.1016/j.sleep.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Hogl B, Garcia-Borreguero D, Trenkwalder C, et al. Efficacy and augmentation during 6 months of double-blind pramipexole for restless legs syndrome. Sleep Med. 2011;12:351–360. doi: 10.1016/j.sleep.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Montagna P, Hornyak M, Ulfberg J, et al. Randomized trial of pramipexole for patients with restless legs syndrome (RLS) and RLS-related impairment of mood. Sleep Med. 2011;12:34–40. doi: 10.1016/j.sleep.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Ma JF, Wan Q, Hu XY, et al. Efficacy and safety of pramipexole in chinese patients with restless legs syndrome: results from a multi-center, randomized, double-blind, placebo-controlled trial. Sleep Med. 2012;13:58–63. doi: 10.1016/j.sleep.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Allen RP, Chen C, Garcia-Borreguero D, et al. Comparison of pregabalin with pramipexole for restless legs syndrome. N Engl J Med. 2014;370:621–631. doi: 10.1056/NEJMoa1303646. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Borreguero D, Patrick J, DuBrava S, et al. Pregabalin versus pramipexole: effects on sleep disturbance in restless legs syndrome. Sleep. 2014;37:635–643. doi: 10.5665/sleep.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Wang Y, Cong SY, Nao JF, Feng J, Bi GR. Efficacy and tolerability of pramipexole for the treatment of primary restless leg syndrome: a meta-analysis of randomized placebo-controlled trials. Neuropsychiatr Dis Treat. 2013;9:1035–1043. doi: 10.2147/NDT.S49454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boehringer Ingelheim. [Last accessed 21 May 2014]; http://trials.boehringer-ingelheim.com/trial_results/clinical_trials_overview/clinical_trial_result.c=n.i=.html.
- 35.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria special considerations and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 36.Hening WA, Allen RP, Washburn M, Lesage S, Earley CJ. Validation of the Hopkins telephone diagnostic interview for restless legs syndrome. Sleep Med. 2008;9:283–289. doi: 10.1016/j.sleep.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 38.National Institute of Mental Health (NIMH) Early clinical drug evaluation unit (ECDEU). Clinical global impressions. In: Guy W, editor. ECDEU assessment manual for psychopharmacology (rev.) NIMH USA, Rockville, MD: 1976. [Google Scholar]
- 39.Garcia-Borreguero D, Allen RP, Kohnen R, et al. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: Report from a World Association of Sleep Medicine - International Restless Legs Syndrome Study Group consensus conference at the Max Planck Institute. Sleep Med. 2007;8:520–530. doi: 10.1016/j.sleep.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Borreguero D, Kohnen R, Hogl B, et al. Validation of the Augmentation Severity Rating Scale (ASRS): a multicentric, prospective study with levodopa on restless legs syndrome. Sleep Med. 2007;8:455–463. doi: 10.1016/j.sleep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JPT, Green S. [Accessed November 11, 2014];The Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. The Cochrane Collaboration Web site. 2011 http://handbook.cochrane.org/
- 42.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ClinicalTrials.gov (A service of the U.S. National Institutes of Health) [last accessed 21 June 2014];A Randomised, Comparing Fixed Doses of Pramipexole to Investigate the Efficacy and Safety in Patients with RLS. 2013 Available from: http://clinicaltrials.gov/ct2/show/NCT00390689?term=pramipexole&rank=20.
- 44.Inoue Y, Kuroda K, Hirata K, Uchimura N, Kagimura T, Shimizu T. Efficacy, safety and dose-response of pramipexole in Japanese patients with primary restless legs syndrome: randomized trial. Neuropsychobiology. 2011;63:35–42. doi: 10.1159/000322289. [DOI] [PubMed] [Google Scholar]
- 45.ClinicalTrials.gov (A service of the U.S. National Institutes of Health) [last accessed 21 June 2014];Polysomnography Study of Pregabalin and Pramipexole versus Placebo in Patients with Restless Legs Syndrome and Associated Sleep Disturbance. 2012 Available from: http://clinicaltrials.gov/ct2/show/NCT00991276?term=pramipexole&rank=35.
- 46.Trenkwalder C, Stiasny-Kolster K, Kupsch A, Oertel WH, Koester J, Reess J. Controlled withdrawal of pramipexole after 6 months of open-label treatment in patients with restless legs syndrome. Mov Disord. 2006;21:1404–1410. doi: 10.1002/mds.20983. [DOI] [PubMed] [Google Scholar]
- 47.Ferri R, Manconi M, Plazzi G, et al. Leg movements during wakefulness in restless legs syndrome: time structure and relationships with periodic leg movements during sleep. Sleep Med. 2012;13:529–535. doi: 10.1016/j.sleep.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Manconi M, Ferri R, Zucconi M, et al. Preferential D2 or preferential D3 dopamine agonists in restless legs syndrome. Neurology. 2011;77:110–117. doi: 10.1212/WNL.0b013e3182242d91. [DOI] [PubMed] [Google Scholar]
- 49.Partinen M, Hirvonen K, Jama L, et al. Effects of pramipexole on periodic limb movements (PLMs) in restless legs syndrome (RLS): A polysomnographic study. Sleep Med. 2006;7(Suppl):126–127. [Google Scholar]
- 50.Partinen M, Hirvonen K, Jama L, et al. Clinician ratings and patient self-ratings of improvement after 3 weeks of double-blind placebo-controlled pramipexole for restless legs syndrome (RLS) Sleep Med. 2006;7(Suppl):126. [Google Scholar]
- 51.Hirvonen k, Partinen M, Jama L, Alakuijala A, Hublin C. Efficacy of pramipexole by clinician and patient assessment during a polysomnographic (PSG) study of restless legs syndrome (RLS) Sleep Med. 2007;7(Suppl):71. [Google Scholar]
- 52.Jama L, Partinen M, Hirvonen K, et al. Pramipexole significantly reduces periodic limb movement index (PLMI) in restless legs syndrome (RLS) Sleep Med. 2007;8(Suppl):70. [Google Scholar]
- 53.Partinen M, Hirvonen K, Jama L, et al. In patients with restless legs syndrome (RLS), pramipexole significantly improves periodic limb movements during time in bed index (PLMI) Sleep Med. 2007;8(Suppl):72. [Google Scholar]
- 54.Stiasny-Kolster K, Oertel WH, Bergtholdt B, et al. Sleep self-assessment by visual analogue scales (VASs) in a 6-week European trial of pramipexole for restless legs syndrome (RLS) Sleep Med. 2007;8(Suppl):71. [Google Scholar]
- 55.Hornyak M, Sohr M, Busse M. Evaluation of painful sensory symptoms in restless legs syndrome: experience from two clinical trials. Sleep Med. 2011;12:186–189. doi: 10.1016/j.sleep.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Borreguero D, Chen C, Allen RP, et al. Long-Term Efficacy and Augmentation Assessment of a Dopamine Agonist(Pramipexole) Compared with an Alpha-2-Delta Ligand (Pregabalin) in Restless Legs Syndrome: Results of a Randomized, Double-Blinded, Placebo-Controlled Trial. Neurology. 2012;79:e87–e87e91. [Google Scholar]
- 57.Garcia-Borreguero D, Chen C, Allen RP, et al. Effects of pregabalin and pramipexole on sleep and quality of life in patients with restless legs syndrome: results from a long-term, randomised, double-blinded, parallel-group, placebo-controlled, active-comparator trial. J Sleep Res. 2012;21(Suppl):1. [Google Scholar]
- 58.Ferini-Strambi L. Restless legs syndrome augmentation and pramipexole treatment. Sleep Med. 2002;3(Suppl 3):23S–25S. doi: 10.1016/s1389-9457(02)00144-2. [DOI] [PubMed] [Google Scholar]
- 59.Silber MH, Girish M, Izurieta R. Pramipexole in the management of restless legs syndrome: an extended study. Sleep. 2003;26:819–821. doi: 10.1093/sleep/26.7.819. [DOI] [PubMed] [Google Scholar]
- 60.Stiasny-Kolster K, Oertel WH. Low-dose pramipexole in the management of restless legs syndrome. An open label trial. Neuropsychobiology. 2004;50:65–70. doi: 10.1159/000077943. [DOI] [PubMed] [Google Scholar]
- 61.Winkelman JW, Johnston L. Augmentation and tolerance with long-term pramipexole treatment of restless legs syndrome (RLS) Sleep Med. 2004;5:9–14. doi: 10.1016/j.sleep.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Lipford MC, Silber MH. Long-term use of pramipexole in the management of restless legs syndrome. Sleep Med. 2012;13:1280–1285. doi: 10.1016/j.sleep.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14:675–684. doi: 10.1016/j.sleep.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 64.Sun Y, van Valkenhoef G, Morel T. A mixed treatment comparison of gabapentin enacarbil, pramipexole, ropinirole and rotigotine in moderate-to-severe restless legs syndrome. Curr Med Res Opin. 2014;30:2267–2278. doi: 10.1185/03007995.2014.946124. [DOI] [PubMed] [Google Scholar]
- 65.Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26:925–935. doi: 10.1016/s0149-2918(04)90136-1. [DOI] [PubMed] [Google Scholar]
- 66.Hays RD, Stewart AL, Stewart AL, Ware JE. Measuring functioning and well-being: the medical outcomes study approach. Durham, NC: Duke University Press; 1992. Sleep measures; pp. 235–259. [Google Scholar]
- 67.Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005;6:41–44. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Abetz L, Vallow SM, Kirsch J, Allen RP, Washburn T, Earley CJ. Validation of the Restless Legs Syndrome Quality of Life questionnaire. Value Health. 2005;8:157–167. doi: 10.1111/j.1524-4733.2005.03010.x. [DOI] [PubMed] [Google Scholar]
- 69.Allen R, Oertel W, Walters A, et al. Relation of the International Restless Legs Syndrome Study Group rating scale with the Clinical Global Impression severity scale, the restless legs syndrome 6-item questionnaire, and the restless legs syndrome-quality of life questionnaire. Sleep Med. 2013;14:1375–1380. doi: 10.1016/j.sleep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 71.Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306:1437–1440. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.