Abstract
The brain influences immune function through a powerful neural reflex that suppresses the release of a key pro-inflammatory cytokine, tumor necrosis factor α, after immune challenge. The efferent motor pathway of this reflex is in the splanchnic nerves, not the vagi. This reflex regulates inflammation but does not suppress fever.
Keywords: endotoxemia, fever, greater splanchnic nerve, inflammation, lipopolysaccharide (LPS), sympathetic nervous system, tumor necrosis factor α (TNFα), vagus nerve
With the present “discovery article” we would like to introduce to the readers of Temperature our recent study that focused on a neural reflex that controls inflammation.1
The idea of a neural reflex that controls the degree of inflammation induced by an immune challenge has been around since the beginning of the last decade when the group of researchers led by Kevin Tracey in New York introduced the concept of the inflammatory reflex. The authors discovered that electrically stimulating the vagus nerve during endotoxemia resulted in a profound inhibition of plasma tumor necrosis factor α (TNFα) levels.2 Since then a detailed description, based on a series of brilliant and original studies (for a comprehensive review, see ref. 3), of the efferent motor pathway of this reflex, termed the cholinergic anti-inflammatory pathway, has been completed and generally accepted by the majority of the international scientific community. The pathway proposed is complex: (1) the vagus nerves, activated by an immune insult, drive the splenic sympathetic nerves to release noradrenaline in the spleen; (2) this noradrenaline then activates a population of T lymphocytes that release acetylcholine (ACh); (3) ACh binds to nicotinic ACh receptors containing the α7 subunit present on macrophages to inhibit the release of TNFα.
The lack of a clear enhancement of the inflammatory response to endotoxin after vagotomy, together with the absence of any direct neural link between the vagus and splenic post-ganglionic sympathetic neurons4,5 prompted us to reconsider this model. On consideration, we believed that Tracey and colleagues could have misinterpreted their findings: they demonstrated that electrically stimulating the vagus nerves can have a powerful anti-inflammatory action, but this finding is insufficient to prove that a vagal anti-inflammatory pathway is physiologically active under conditions of immune challenge and mediates the inflammatory reflex.
With our recent study1 we set out to prove the existence of a neural reflex that controls inflammation and identify the autonomic nerves involved in this action. Initially we confirmed that endotoxemia, induced by intravenous injection of Lipopolysaccharide (LPS, 60μg/kg), activates the sympathetic post-ganglionic nerves to the spleen. However, the critical finding that led us to reject the Tracey model was that this splenic nerve activity was not driven from the vagi: cutting the vagi had no effect on it. By contrast, even unilateral section of its sympathetic preganglionic supply, the greater splanchnic nerve, profoundly reduced splenic nerve activity. This means that the splenic nerve response to immune challenge was driven entirely by the conventional sympathetic pathway, not the vagi. We then demonstrated the effect of this pathway on inflammation: bilateral section of the greater splanchnic sympathetic nerves enhanced the TNFα response to LPS by four to 5-fold when compared with sham treated animals. Sectioning the cervical vagi had no effect. Plasma corticosterone levels were unaffected by nerve sections, so could not explain the result. Interestingly, the increases in body temperature and heart rate were unaltered by splanchnic nerve section, confirming previous findings.6 While the higher levels of TNFα in splanchnicectomized rats might have been expected to exacerbate fever, they evidently made no measurable difference within the time course of our experiment.
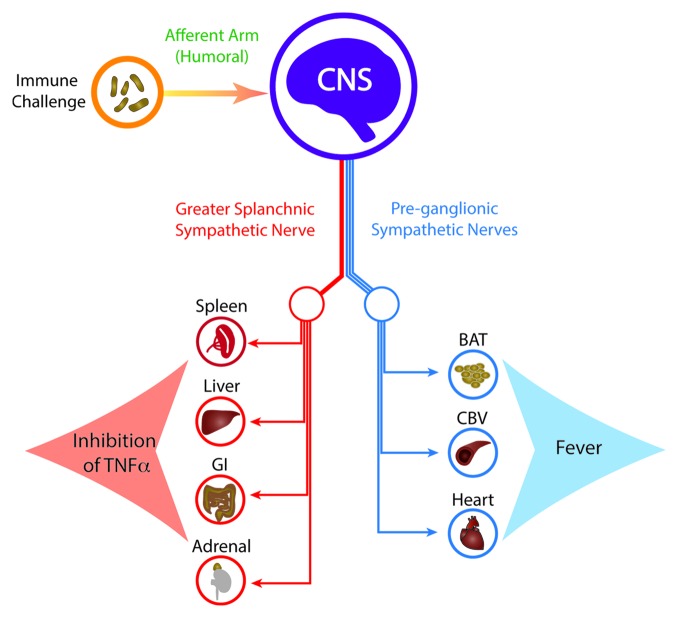
These results demonstrate the existence of a neural reflex that exerts a significant influence on inflammation, and thereby, immune function. Activity in the greater splanchnic nerves regulates the levels of a key pro-inflammatory cytokine, TNFα, which is a ‘necessary and sufficient’ mediator of inflammation. Inflammation, in turn, is the gateway to innate and adaptive immunity. Our results also reinforce the concept that fever and the inflammatory reflex are two distinct physiological responses, mediated by distinct sympathetic pathways, triggered by the same immune challenge (Fig. 1). On one side, activation of the sympathetic drives to brown adipose tissue, cutaneous vasoconstriction and the heart produce the classic febrile response.7 On the other, it activates the efferent motor pathway of the inflammatory reflex, via the greater splanchnic sympathetic nerves to the spleen and probably other abdominal visceral organs. The afferent arm of the inflammatory reflex, though not investigated in this study, is likely to be humoral, similar to that established for fever.7
Figure 1. Immune challenges such as i.v. LPS are sensed peripherally but are relayed to the central nervous system (CNS) mainly by humoral signals. In response to immune challenge, the brain activates two distinct sets of sympathetic pathways: the efferent motor pathway of the inflammatory reflex and the sympathetic outputs that cause tachycardia and fever. The efferent motor pathway of the inflammatory reflex traverses the greater splanchnic sympathetic nerves, which in turn drive the post-ganglionic sympathetic nerves to the spleen, liver, gastro-intestinal (GI) tract and adrenal glands. The final effect of this reflex is to inhibit TNFα production. Fever is caused by activation of sympathetic pathways to brown adipose tissue (BAT), cutaneous blood vessels (CBV) and the heart, raising body temperature and heart rate.
Glossary
Abbreviations:
- ACh
acetylcholine
- LPS
lipopolysaccharide
- TNFα
tumor necrosis factor α
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Martelli D, et al. . J Physiol 2014; 592:1677 - 86; http://dx.doi.org/ 10.1113/jphysiol.2013.268573; PMID: 24421357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borovikova LV, et al. . Nature 2000; 405:458 - 62; http://dx.doi.org/ 10.1038/35013070; PMID: 10839541 [DOI] [PubMed] [Google Scholar]
- 3.Andersson U, et al. . Annu Rev Immunol 2012; 30:313 - 35; http://dx.doi.org/ 10.1146/annurev-immunol-020711-075015; PMID: 22224768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martelli D, et al. . Auton Neurosci 2014; 182:65 - 9; http://dx.doi.org/ 10.1016/j.autneu.2013.12.007; PMID: 24411268 [DOI] [PubMed] [Google Scholar]
- 5.Bratton BO, et al. . Exp Physiol 2012; 97:1180 - 5; PMID: 22247284 [DOI] [PubMed] [Google Scholar]
- 6.Dogan MD, et al. . Brain Res 2003; 993:227 - 9; http://dx.doi.org/ 10.1016/j.brainres.2003.09.010; PMID: 14642851 [DOI] [PubMed] [Google Scholar]
- 7.McAllen RM, et al. . Eur J Appl Physiol 2010; 109:27 - 33; http://dx.doi.org/ 10.1007/s00421-009-1295-z; PMID: 19949811 [DOI] [PubMed] [Google Scholar]