Abstract
Long-term heat acclimation (34 °C, 30d) alters the physiological responses and the metabolic state of organisms. It also improves ability to cope with hypoxic stress via a cross-tolerance mechanism. Within the brain, the hippocampal and frontal cortex neurons are the most sensitive to hypoxia and cell death is mainly caused by calcium influx via glutamate-gated ion channels, specifically NMDA and AMPA receptors. GluN1 subunit levels of NMDA-R correspond to NMDA-R levels. GluN2B/GluN2A subunit ratio is a qualitative index of channel activity; a higher ratio implies lower calcium permeability. The GluA2 subunit of AMPA-R controls channel permeability by inhibiting calcium penetration. Here, in rats model we (i)used behavioral-assessment tests to evaluate heat acclimation mediated hypoxic (15’ 4.5 ± 0.5% O2) neuroprotection, (ii) measured protein and transcript levels of NMDA-R and AMPA-R subunits before and after hypoxia in the hippocampus and the frontal cortex, to evaluate the role of Ca2+ in neuro-protection/cross-tolerance. Behavioral tests confirmed hypoxic tolerance in long-term (30d) but not in short-term (2d) heat acclimated rats. Hypoxic tolerance in the long-term acclimated phenotype was accompanied by a significant decrease in basal NMDA receptor GluN1 protein and an increase in its mRNA. The long-term acclimated rats also showed post ischemic increases in the GluN2B/GluN2A subunit ratio and GluA2 subunit of the AMPA receptor, supporting the hypothesis that reduced calcium permeability contributes to heat acclimation mediated hypoxia cross-tolerance. Abrupt post ischemic change in GluN2B/GluN2A subunit ratio with no change in NMDA-R subunits transcript levels implies that post-translational processes are inseparable acclimatory cross-tolerance mechanism.
Keywords: AMPA receptors, NMDA receptors, brain hypoxia, heat acclimation mediated cross-tolerance
Introduction
Acclimation to environmental stress involves a continuum of processes varying temporally in their appearance and duration. The process of heat acclimation is biphasic: during the initial phase (approximately 5 d) acute, temporary mechanisms compensate for perturbed cellular performance and heat dissipation mechanisms.1,2 These are replaced by long lasting processes optimizing the physiological and metabolic state of the organism and enabling it to cope in new environments.1,3 An inseparable effect of heat acclimation is protection against hypoxic/ischemic stress via the cross-tolerance mechanism. Cross-tolerance is brought about by using the signaling pathways that were reprogrammed during heat acclimation to protect the animal from heat stress4 for protection against the novel stressor. Heat acclimation mediated ischemic tolerance has been substantiated in the heart.5
In the brain, the most sensitive cells to hypoxic/ischemic stress are the neurons of the CA1 hippocampal layer and those of layers 3–6 of the frontal cortex.6 A massive glutamate discharge, which over-activates glutamate-gated ion channels7,8 is a major cause of death of these neurons following exposure to hypoxic/ischemic stress, and is mediated via calcium channel excitotoxicity, free radical formation, lactic acidosis and inhibition of protein synthesis.9,10 Although cell death starts two to three days after insult and may continue for several weeks, it is probably triggered by calcium influx via glutamate-gated channels in the first hours after the stress.11-13
The most important glutamate-gated ion channels that cause damage during and after the hypoxic insult are the N-methyl-D-aspartate receptor (NMDA-R) and the α-amino-3-hydroxy-5-methyl-4-isoazole-propionic acid receptor (AMPA-R).10 The NMDA-R has 3 gene families, each responsible for a group of subunits14: The GluN1 family - has one gene, GluN2 - has 4 genes encoding GluN2A-D and GluN3 has 2 genes, GluN3A-B. The GluN1 subunit is in all NMDA-R assemblies, and is essential for its function.15-17 GluN1 can be used as a marker of the presence of the NMDA-R on the external cell surface.11 The GluN2A-D profiles of the receptors vary according to brain area and developmental stage.18 GluN2A and GluN2B are abundant in the hippocampus and frontal cortex, whereas GluN2C is mainly expressed in the cerebellum and GluN2D in subcortical regions.19,20 Channels composed of the GluN1/GluN2A combination have a permeability to calcium that is 4 times greater, a higher opening probability and a quicker return from a state of desensitization than those made with the GluN1/GluN2B subunits.21,22 Therefore, changes in GluN2B/GluN2A ratio infer a change in channel activity. When the GluN2B/GluN2A ratio is greater than 1, there is a lower channel opening probability and less calcium penetration. Notably, this index is only relevant in brain areas where the presence of other NMDA-R subunits is negligible. The involvement of NMDA-R in the process of delayed cell death after hypoxic stress is well established, including its upregulation and increased activity in the post-stress period. Zhang et al.23 showed changes in GluN2A and GluN2B subunits in the adult rat hippocampus as early as 3 and 6 h after global hypoxia, and a decrease in channel function 24 h post stress.23,24 AMPA-R, another glutamate-gated ion channel, plays a central role in synaptogenesis, synaptic plasticity and construction of neuronal networks. These channels enter and leave the synapse according to neuronal activity, and are important in determining synaptic strength and adaptation.25 Excessive activation of these receptors is considered critical for neuro-degeneration following a variety of insults including ischemia.26 AMPA-R have four subunits, GluA1–4, surrounding a central pore. These subunits are expressed differently throughout the nervous system.27,28 Hippocampal and neocortical cells in adult mammals express GluA2 mRNA11,12 and have low calcium penetration,29 such that a minor reduction in GluA2 expression may have important physiological consequences.
AMPA-R plays a central role in delayed cell death following hypoxic/ischemic stress, and it is possible that blockade of these receptors within 24 h of the stress will prevent the delayed death of hippocampal CA1 cells.29 Transient global ischemia reduces the expression of GluA2 mRNA in hippocampal pyramidal cells as early as 12 h after the insult, reaching a 70% reduction at 24 h.30-32 Moreover, these studies found significant reductions in GluA2 protein in the CA1 region of the hippocampus 24 h after ischemic insult, without a change in the expression of GluA1 protein. These findings suggest a marked decrease in the GluA2 subunit without a change in the presence of the receptor after hypoxia, coinciding with increased calcium permeability of the AMPA-R channels measured in cortical neuron cell cultures.30-32
Given the heat acclimation-ischemic/hypoxic cross-tolerance phenomenon in the heart, we hypothesized that heat acclimation also induces hypoxic tolerance in the brain. The aim of this investigation was 2-fold (i) to determine, using behavioral-assessment tests, whether heat acclimation provides hypoxic-neuroprotection, and if protected (ii) to measure the protein and transcript levels of NMDA-R subunits and the AMPA-R GluA2 subunit before and after hypoxia in the hippocampus and the frontal cortex. The profile of these subunits may improve our understanding of cellular calcium management during insult and neuro-protection conferred by heat acclimation.
Results
Behavioral test
The response to transient hypoxic stress was assessed and Figure 1 shows clear behavioral effects: all exposed rats performed significantly worse than controls (C) (P < 0.0005). Following hypoxic stress, neurological function of the long-term heat acclimated group (HyAc30d) was significantly better than the non-acclimated control (HyC) and the short-term acclimated (HyAc2d) groups (4.96 ± 0.28 vs. 3.8 ± 0.48; P < 0.05 and 3.5 ± 0.22; P < 0.005 respectively). The HyAc2d group had the lowest post hypoxic behavioral score (average 3.5 ± 0.22), indicating that there is no cross-tolerance after this stressful, short acclimation phase.
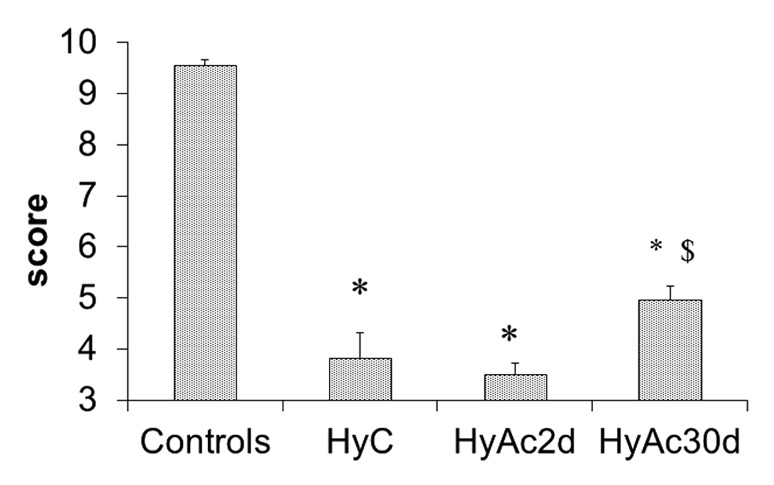
Figure 1. Clinical Score. Prolonged exposure to heat improves the ability to cope with hypoxic stress. The rats were examined 1 min after hypoxic stress. Each bar presents M ± SE, n = 12. HyC -hypoxic stress only; HyAc2d - hypoxic stress in rats acclimated for 2 d; HyAc30d - hypoxic stress in rats acclimated for 30 d; * P < 0.005 vs. C. $ P < 0.05 HyAc30d vs. HyC.
Protein Expression
GluN1 Subunit
A resemblance between the GluN1 membrane protein profiles in the frontal cortex and the hippocampus was found in response to acclimation and hypoxic stress (Fig. 2). Hypoxic stress caused a significant rise in GluN1 levels in non-acclimated rats: Δ59 ± 29% in the frontal cortex, and Δ63 ± 24% in the hippocampus (P < 0.05), compared with the untreated C group. The basal levels of the GluN1 subunit were significantly lower in the Ac30d rats than the C group (63 ± 13% and 35 ± 15% of the C levels, in the frontal cortex and the hippocampus respectively; P < 0.05). The GluN1 subunits levels in the HyAc30d group were higher than Ac30d, (by 40.6 ± 10% and 20.6 ± 10% in the frontal cortex and hippocampus, respectively). Yet, HyAc30d GluN1 levels were significantly lower than those of the untreated C rats (by 74 ± 10% and 76 ± 10% in the frontal cortex and hippocampus respectively; P < 0.05 in both cases). GluN1 levels following 2 d of acclimation were insignificantly different than the C rats. HyAc2d GluN1 levels were lower than HyC in both the hippocampus and frontal cortex (76 ± 21% and 71 ± 15%, respectively; P < 0.05).
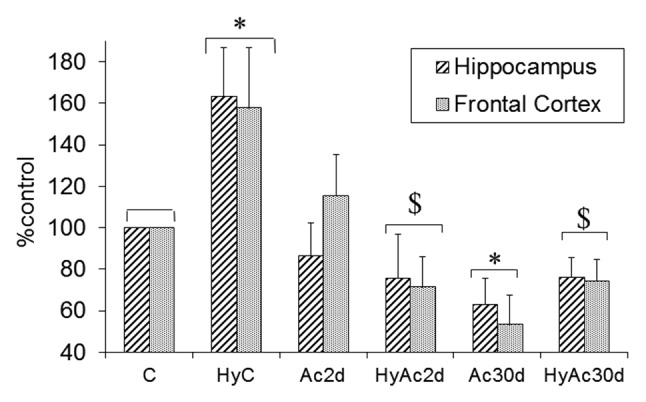
Figure 2. GluN1 Hippocampus Vs. Frontal Cortex. Changes in GluN1 subunit levels in enriched membranal lysates of the hippocampus and frontal cortex after each treatment are shown. No significant differences were found between the frontal cortex and the hippocampus. Each bar represents M ± SE, n = 8. C-controls, Ac2d-heat acclimation for 2 d, Ac30d - heat acclimation for 30d, HyC -hypoxic stress only; HyAc2d - hypoxic stress in rats acclimated for 2 d; HyAc30d - hypoxic stress in rats acclimated for 30 d; * P < 0.05 vs. C; $ P < 0.05 - HyAc30d vs. HyC.
Figure 3 shows the kinetics of GluN1 protein levels following hypoxia and highlights the differences between the C and the heat acclimated groups. The frontal cortex and hippocampus demonstrated similar profiles. In non-acclimated animals (Fig. 3A), GluN1 levels increased immediately after hypoxic stress in both the frontal cortex and the hippocampus (Δ26 ± 12%, Δ20 ± 8%, respectively; P < 0.05), peaking at 4 h. At 24h, a significant drop was noted (-25 ± 9% and -39 ± 15% in the frontal cortex and hippocampus, respectively; P < 0.05). In contrast, in the acclimated group (Fig. 3B), GluN1 levels in the frontal cortex and hippocampus were significantly lower than the C group (heat acclimation vs. C - basal: -56 ± 12%, -59 ± 9%, respectively; P < 0.05) and remained relatively stable around the mean over the recovery period (e.g., 4hr 74 ± 10%, 76 ± 10%; for the frontal cortex and the hippocampus, respectively (P < 0.05.
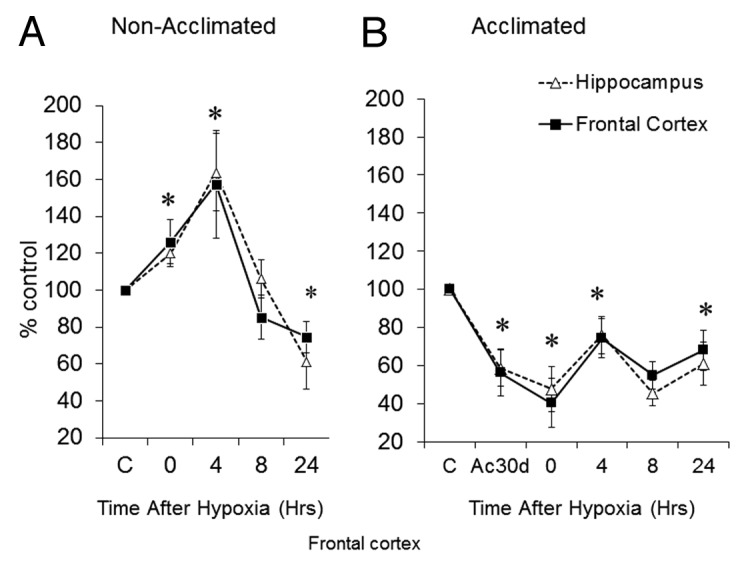
Figure 3. Post-hypoxia GluN1 Kinetics. GluN1 subunit levels in (A) non-acclimated and (B) heat-acclimated for 30d before and following hypoxic stress in the frontal cortex and the hippocampus. Each data point represents M ± SE, n = 8, * P < 0.05 vs. C group.
GluN2B/GluN2A Subunit Ratio
The GluN2B/GluN2A protein ratio may reflect changes in the calcium permeability of the NMDA-R channel. Subjection to hypoxia alone (HyC, Figure 4) lead to a small, but significant reduction in the GluN2B/GluN2A ratio; 92% ± 7 and 84% ± 10 of the basal ratio in the frontal cortex and hippocampus, respectively (P < 0.05). Interestingly, Ac30d alone significantly increased the GluN2B/GluN2A ratio in the frontal cortex (Δ22 ± 4% vs. C; P < 0.05) but not in the hippocampus. In the HyAc30d group, unlike the C and the Ac2d groups, both the frontal cortex and the hippocampus showed a significant increase in their GluN2B/GluN2A ratio (Δ25 ± 4% and Δ10 ± 6%, respectively; P < 0.05).
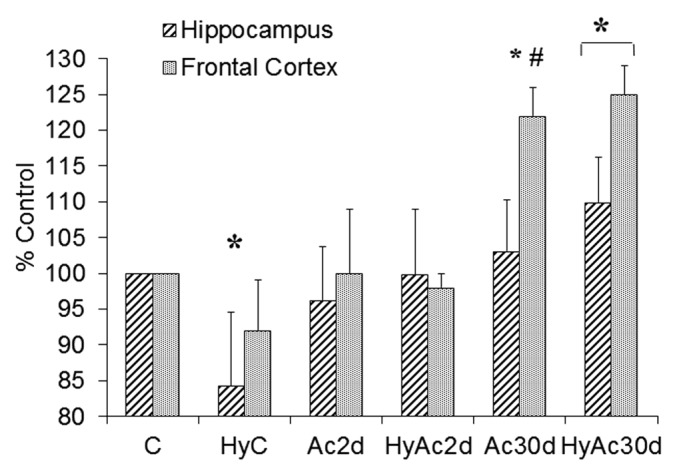
Figure 4. Changes in the GluN2B/GluN2A ratio after short- and long-term heat acclimation, with or without hypoxia in the frontal cortex and the hippocampus. Each bar represents M ± SE, n = 8. * P < 0.05 vs. the C group for both areas; # P < 0.05 hippocampus vs. frontal cortex within the same treatment group. For abbreviations see legend of Figure 2.
Following hypoxia, the GluN2B/GluN2A ratio in the non-acclimated group (Fig. 5A) dropped immediately in the frontal cortex and the hippocampus (by 7 ± 2% and 14% ± 3 respectively; P < 0.05). This difference remained over the 24h follow-up, except at 24h in the hippocampus. In the acclimated rats, the GluN2B/GluN2A subunit ratio was site specific (Fig. 5B). In the frontal cortex, acclimation per se, elevated the GluN2B/GluN2A ratio (Δ15 ± 6% (P < 0.05). Hypoxic stress induced an immediate further elevation, peaking 4 h after the stress (Δ20 ± 7%, P < 0.05). This difference subsided 24 h after the stress. Ac30d hippocampus (Fig. 5B) showed a delayed but statistically significant GluN2B/GluN2A ratio elevation following hypoxic stress peaking 4 h after the stress (13 ± 6%, P < 0.05).
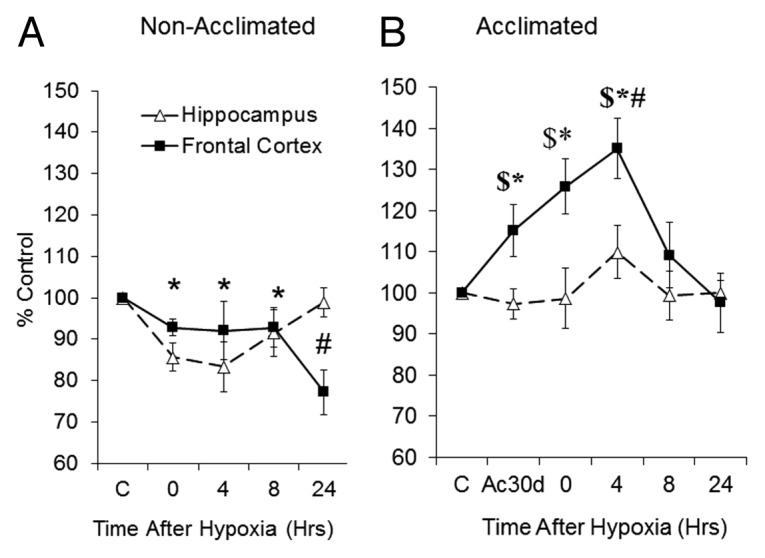
Figure 5. Post-hypoxic GluN2B/GluN2A kinetics. Changes in GluN2B/GluN2A subunit ratio over time in (A) non-acclimated and (B) heat acclimated groups. Each data point represents M ± SE, n = 8. * P < 0.05 both areas vs. C, # P < 0.05 frontal cortex vs. matched basal. $ P < 0.05 frontal cortex vs. hippocampus within the same group.
GluA2 Subunit
The GluA2 subunit of AMPA-R is important in determining channel calcium permeability. We hypothesize that a decrease in the GluA2 subunit increases calcium influx after hypoxic stress, leading to more cytotoxic damage. Figure 6 shows that there were no differences between the Ac30d and the C group at baseline, and the effect of heat acclimation only became apparent after insult: (i) GluA2 protein levels in the C group dropped in both areas, with a statistically significant difference in the frontal cortex (a decrease of 12 ± 8%; P < 0.05); (ii) the GluA2 levels in the HyAc30d group rose in both the frontal cortex and hippocampus (Δ18 ± 7% and Δ9 ± 7% respectively; P < 0.05).
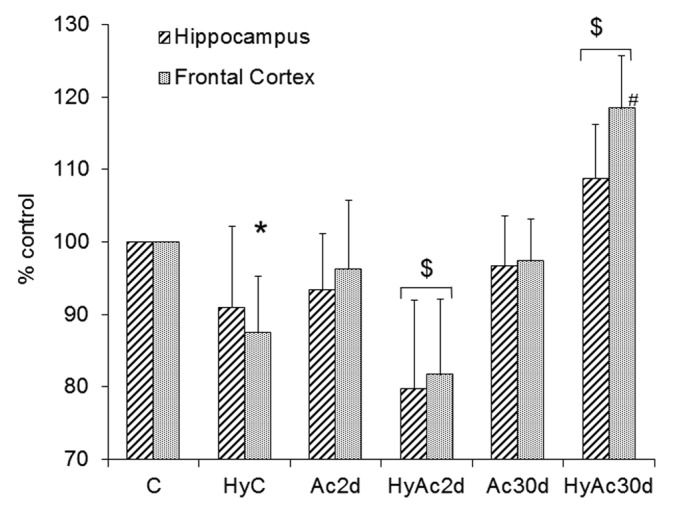
Figure 6. GluA2 - Hippocampus Vs. Frontal Cortex. Changes in the GluA2 subunit in enriched membranal lysates of the hippocampus and frontal cortex compared with the C group. Each bar represents M ± SE, n = 8. * P < 0.05 frontal cortex of HyC vs. C # P < 0.05 HyC vs. HyAc30d (for both areas); $ P < 0.05 both areas vs. C group. For abbreviations see legend of Figure 2.
Ac2d GluA2 levels were slightly lower than controls. As in the Ac30d group, the effect of acclimation was readily apparent after hypoxia (HyAc2d), the GluA2 levels of the frontal cortex decreased to 82 ± 10%, and those of the hippocampus to 80 ± 12% (P < 0.05 for both results).
mRNA Expression
In order to assess the contribution of genomic translational processes to the phenotypic changes observed, mRNA levels were measured.
GluN1 mRNA
In the acclimated groups, GluN1 mRNA levels were 2.5–3 times greater than the non-acclimated groups (Fig. 7A; P < 0.05). Similarly to the GluN1 protein profile, there were no differences between mRNA levels in the frontal cortex and the hippocampus throughout the experiment. In both the frontal cortex and hippocampus (Fig. 7A), there was a significant rise in mRNA expression 4 h after the stress (Δ23 ± 9% and Δ19 ± 6% in the frontal cortex and the hippocampus, respectively; P < 0.05), peaking at 8h. Notably, protein levels where highest 4 h post injury (Figs. 7A vs. 3A) in the non-acclimated rats. No differences were detected in heat acclimated GluN1 mRNA in the frontal cortex and hippocampus following hypoxia (Fig. 7B). Collectively, we can conclude that there was a rapid significant increase in the GluN1 mRNA in the C group after hypoxia, whereas the acclimated group showed a small decline in transcription after the insult.
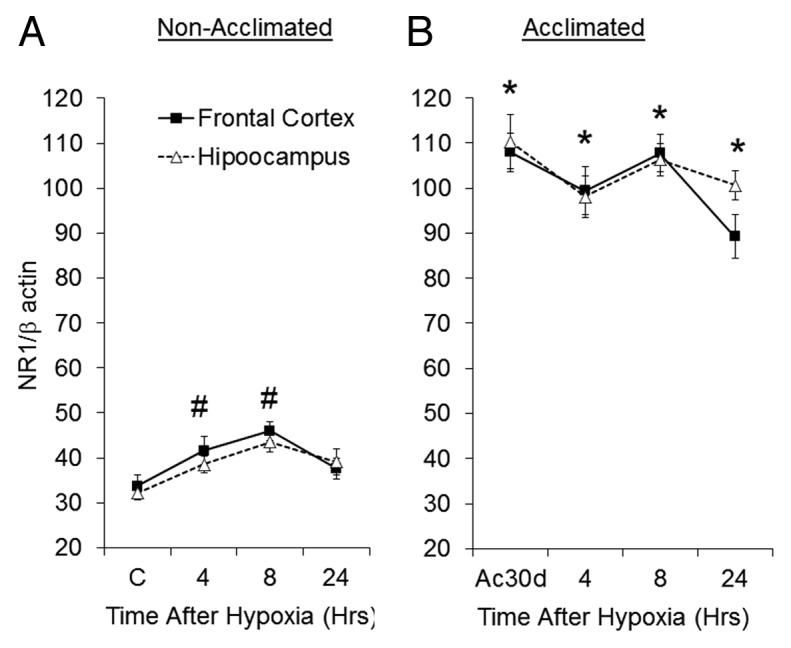
Figure 7. Changes in GluN1 mRNA following hypoxic stress in (A) non-acclimated and (B) acclimated groups. Each data point represents M ± SE, n = 8. # P < 0.05 frontal cortex and the hippocampus vs. C group. * P < 0.05 C vs. Ac30d (for both areas).
GluN2A and GluN2B mRNA
Following hypoxic stress in the non-acclimated group, mRNA levels of both subunits increased significantly at 4 and 8h, returning to basal levels at 24 h (Table 1). At 4h, mRNA levels peaked in both subunits in both the frontal cortex and the hippocampus (P < 0.05), gradually returning to baseline levels at 24h. Acclimated animals (Table 1) exhibited a similar gradual increase at 4h, peaking 8 h post insult (P < 0.05). Similarly to non-acclimated animals, (i) 24 h after the insult the mRNA levels of the subunits in the heat acclimated group returned to baseline; and (ii) no significant differences were found between the two subunits throughout the experiment (data not shown).
Table 1. GluN2A and GluN2B mRNA in non-acclimated and heat acclimated rats.
| Time | Hippocampus GluN2A GluN2B |
Frontal cortex GluN2A GluN2B |
||
|---|---|---|---|---|
| Basal Non-acclimated Heat acclimated |
64.4 ± 5.7 71.0 ± 12.8 |
61.1 ± 3.5 83.5 ± 8.5 # |
55.7 ± 10.4 74.1 ± 1.8# |
55.1 ± 9.7 88.6 ± 4.1# |
| 4 h Non-acclimated Heat acclimated |
84.3 ± 0.8* 91.2 ± 10.9 |
69.7 ± 2.0* 86.8 ± 9.9# |
95.1 ± 11.1* 77.1 ± 8.0 |
77.0 ± 9.9* 89.9 ± 13.9 |
| 8 h Non-acclimated Heat acclimated |
85.0 ± 3.6* 97.3 ± 2.8*# |
80.8 ± 3.8* 92.0 ± 9.3# |
66.2 ± 11.1* 120.0 ± 8.0*# |
58.9 ± 7.9 109.4 ± 14.5*# |
| 24 h Non-acclimated Heat acclimated |
57.8 ± 5.6 75.7 ± 6.3# |
55.4 ± 7.0 78.4 ± 6# |
55.7 ± 3.1 77.4 ± 6.2 # |
49.3 ± 1.9 75.0 ± 2.6 # |
P < 0.05 vs. matched subunit levels under basal-non hypoxic conditions ; #P < 0.05 vs. non-acclimated subunit levels following the same treatment ; No significant differences were found between frontal cortex and hippocampus for the same subunit.
Discussion
This study demonstrates that long-term, but not short-term heat acclimation enhances neuroprotection from hypoxic insult. This protection is demonstrated by the results of a behavioral sensory-motor test. The neroprotected phenotype had a profound elevation in basal levels of the NMDA-R transcript and a decrease in receptor density. Following hypoxia an increase in the GluN2B/GluN2A subunit ratio, accompanied by an upregulation of the AMPA-R subunit GluA2, promote the assumption that the hypoxic-neuroprotection in the acclimated phenotype is associated with changes in the NMDA receptor subunits, perhaps affecting Ca2+ turnover. The differential post hypoxia response between the mRNA and protein levels of the receptors supports the hypothesis that post translational modifications in receptor trafficking play a role in cross-tolerance.
Cross-tolerance between Heat Acclimation and Hypoxia - Behavioral Evidence
As a first step in our study, the protective effect of heat acclimation from hypoxic damage was demonstrated behaviorally. The Ac30 rats performed significantly better in the behavioral sensory-motor test, showing a superior ability to cope with hypoxia than non-acclimated rats. This finding matched our hypothesis that was based on heat acclimation mediated cross-tolerance mechanisms for traumatic brain injury33-35 and cardiac ischemia/reperfusion insults.36 Unlike long-term heat acclimation, exposure to the acclimating conditions for only 2 d (Ac2d) does not achieve acclimatory homeostasis or cross-tolerance. During short-term acclimation, the superimposition of an additional insult aggravates the stressed state that characterizes the commencement of acclimation. These results are congruent with our findings regarding cardioprotection.37,38
NMDA-R and Hypoxia
Structural changes in glutamate receptors affect their calcium permeability. During hypoxic episodes there is a massive release of glutamate into the synaptic space activating glutamate-gated ion channels39; accelerating calcium entrance into cells; activating proteases and phospholipases that cause mitochondrial damage and free radical production that destroys the cell membrane. The culmination of these processes is cell death.40 Therefore, changes in the level or structure of the NMDA-R in the most hypoxia sensitive tissues, the frontal cortex and hippocampus, probably have a significant influence on the ability of these cells to survive hypoxic stress. Measurements of Ca2+ were beyond the scope of the current study. There were, however, significant changes in the GluN1, GluN2B/GluN2A ratio and AMPA-R GluA2 subunits. The findings support the hypothesis that at least part of the neuroprotection noted behaviorally in the long-term acclimated phenotype is due to Ca2+ mediated events. In another brain injury model, Nadler et al. 41 used 45Ca and demonstrated that attenuation of Ca2+ fluxes through the NMDA receptor-mediated calcium channels is protective. Below we will discuss a possible protective pathway as emerged from the current study.
Changes in GluN1 - a Quantitative Marker of NMDA-R on the Cell Surface
The GluN1 subunit was used as a quantitative marker of surface NMDA-Rs. The long-term heat acclimated group had less NMDA-Rs than the control group before and after the hypoxic stress (Figs. 2, 3). Likewise, the response of the long-term heat acclimated group to hypoxia, in the hippocampus and the frontal cortex, was reciprocal to that of the non-acclimated rats (Fig. 2) as were the changes over time (Fig. 3). There were only mild fluctuations in HyAc30 receptor levels in the acclimated group, whereas the non-acclimated group showed continuous upregulation peaking at 4h post hypoxia. The latter profile resembles that reported by Zhang et al.23 We hypothesize that for a similar glutamate surge (caused by the hypoxic insult) there will be less calcium influx into the acclimated cells, thereby improving survival. The decrease in protein levels following heat acclimation is unique to NMDA-Rs, all other proteins studied in our laboratory with respect to cross-tolerance, both in the heart and the brain (e.g., HSP72, HIF 1α, erythropoietin, Glut135,42-44 Levi and Horowitz, unpublished) were upregulated by acclimation and by the stress conditions.
The response of the short-term heat acclimated group to hypoxia is complex (HyAc2d, Figure 2). Receptor levels on the cell membrane before hypoxia were the same as the non-acclimated group after the insult, indicating that the tissue was already experiencing stress. This agrees with Schwimmer et al.34 where upregulation of genes encoding calcium channels was found in short-term heat acclimated rat brains (hypothalamus). Following the hypoxic insult there was a significant reduction in surface GluN1 levels in the 2d acclimated rats. Considering the poor results in the behavioral tests in this group and the pre-hypoxia GluN1 state, it is reasonable to suggest that a reduction in receptor levels alone does not improve function or enhance cytoprotection. Along this line, Assayag et al., 2010, 201237,38 substantiated that despite membrane adaptations found following short-term acclimation, the upregulation of many proteins following long-term acclimation seems to be essential for heat acclimation mediated cross-tolerance.
Changes in GluN2B/GluN2A Ratio affect Channel Properties
To further understand the remodeling mechanisms involved in forming the heat acclimated phenotype, we examined changes in levels of the GluN2A and GluN2B subunits. Changes in the GluN2B/GluN2A subunit ratio enable us to understand the impact of each treatment. When the GluN2A subunit is present the channel is more permeable to calcium, has a higher opening probability and a faster recovery from desensitization.12 The acclimated cells had an increased GluN2B/GluN2A ratio in response to hypoxia whereas the non-acclimated showed a profound drop (Fig. 4). This beneficial effect was more evident in the frontal cortex, with GluN2B/GluN2A peaking 4h post hypoxic insult, and remained elevated for 24 h post insult. Inability of non-acclimated rats to maintain higher GluN2B levels suggests a continuing difficulty of these rats to cope. The HyAC30 GluN2B/GluN2A ratio profile implies reduced calcium permeability and thus provides an additional explanation of heat acclimation mediated neuroprotection following hypoxic stress. This finding is in congruence with our knowledge regarding enhanced cytoprotection and decreased calcium sensitivity in the heat acclimated cardio-phenotype.45
AMPA-R GluA2 Subunit
The GluA2 subunit determines the calcium permeability of AMPA-R11 and plays a role in delaying cell death following hypoxic-ischemic insult.31 Cultures of cortical neurons subjected to hypoxic stress showed increased calcium permeability via NMDA-Rs and AMPA-Rs that led to free radical production and cell damage.30 In this investigation, hypoxia induced a significant decrease in GluA2 levels in the frontal cortex in non-acclimated rats. In contrast, the long-term-acclimated group responded to the insult with a significant increase in receptor protein levels. Given Gorter et al.,30 our results may mean that there is a rapid increase in inter-cellular calcium concentration, causing damage and promoting delayed cell death in the C animals. These results also support the positive influence of long-term heat acclimation on the ability of cells within the hippocampus and frontal cortex to reduce the calcium permeability of glutamate receptors in response to hypoxia. The changes in GluA2 in response to hypoxic stress in the hippocampus showed similar profile to that of the frontal cortex but were less intense. This is comparable with our findings regarding the GluN2B/GluN2A ratio, where the frontal cortex was also more affected than the hippocampus. These results correspond to the enhanced protection from hypoxia in the frontal cortex.
Basal GluA2 of short-term acclimated rats resembled that of C group. However, after the hypoxic insult this group had the greatest reduction in GluA2. This may highlight a greater sensitivity to hypoxic stress in short-term acclimated rats that may be caused by the increase in AMPA-R calcium permeability. These findings support our previous data,37,38 regarding the inability of short-term heat acclimated cells to survive following the superimposition of another stress.
Comparison between Changes in mRNA and Protein Levels
To assess whether molecular processes play a role in cross-tolerance mRNA levels of the NMDA-R subunits were measured. Our data show that changes in NMDA-R transcript developed during the acclimation, leading to 2.5–3 time higher basal GluN1 levels at all acclimatory phases (Fig. 7) but with no significant transcriptional changes post hypoxia. In contrast, non-acclimated rats showed a rapid increased transcription; which matched the significant increase in GluN1 protein levels. The continued rise in mRNA transcript levels in the latter may be evidence of cell damage and imminent cell death.30 The mRNA levels of GluN1 returned to basal levels 24 h after hypoxia, in agreement with Zhang et al.23
In the acclimated animals, the changes in the transcriptome-proteome NMDA-R profile suggest that the sensitivity of the translational machinery has been modified. Given that GluNR1 receptors of HyAc30 rats are upregulated, it is likely that GluNR1 trafficking to the cell surface takes place. A similar mechanism of receptor upregulation was observed for angiotensin AT1 receptors in the hypothalamus of long-term heat acclimated rats.34
Both GluN2A and GluN2B mRNA responded in the same manner during the 24 h post ischemia (Table 1). Since major changes were found in the protein levels (Fig. 5A), it is likely that post-translational processes were taking place. Zhang et al. 23 studied GluN2A and GluN2B mRNA levels in the hippocampus for 24 h after transient ischemic stress and showed that GluN2B mRNA was significantly elevated 3 h after stress and then returned to basal levels. Regarding GluN2A mRNA, Zhang el al. findings differ from our study - there was almost no response 3 h after the stress. This apparent contradiction is probably due to differences between the insults and cell preparations. Similarly to GluN1 mRNA, the basal levels of acclimated GluN2A and GluN2B mRNA (Table 1) were significantly higher than the non-acclimated group, but showed a delayed increased (vs. non-acclimated) after stress, suggesting two acclimatory response patterns - quantitative and qualitative.
Conclusions and future perspectives
This study demonstrates that long- but not short-term heat acclimation confers hypoxia cross-tolerance. It is likely that transcriptional and translational processes leading to acclimatory homeostasis are required for the developed of this process. Since glutamate receptors are known to be involved in coping with hypoxia, we investigated the kinetics of their change in the most sensitive areas of the brain, the frontal cortex and hippocampus. These channels underwent extensive qualitative and quantitative changes during the acclimation, at both mRNA and protein levels, implying that a switch, potentially leading to decreased channel calcium permeability, contributes to the protective arsenal endowed by heat acclimation. However, while NMDA-R acclimatory level has established during the acclimation and was not affected profoundly by hypoxia both GluN2B/GluN2A subunits ratio and GluA2 of the long-term acclimated rats demonstrated beneficial abrupt post hypoxic beneficial response.
Can we bridge between the basic science reported here and applied sciences: One practical physiological approach is translation of heat acclimation cross-tolerance into training protocols that will lead to improved systemic functions for human benefits under challenging new environments. Translation of cross-adaptation [heat and exercise training36] was previously proved to be very successful. Along this line, White et al.,46 for example have suggested heat acclimation as a possible alternative for individuals as a means to maintain or improve performance during high altitude exposures. An intriguing translational approach is also the utilization of cross-tolerance to develop new therapeutic strategies in which concert of processes takes place; as in the case of the heat acclimated phenotype.
Materials and Methods
All experimental protocols were approved by the Ethics Committee for Animal Experimentation of The Hebrew University, Jerusalem, Israel. Male 3 wk old Rattus norvegicus (Sabra strain, albino var), initially weighing 80–90 g, were fed Ambar laboratory chow with food and water ad libitum. The animals were randomly assigned to heat acclimation and control normothermic groups. The normothermic group was subdivided into groups that received no treatment (C group), and those that were only subjected to hypoxia (HyC). Previous heat acclimation studies substantiated that the adaptive process biphasic.1 Hence, the heat acclimated rats were divided into short- and long-term acclimation groups: exposed to heat for 2 d (Ac2d) and 30 d (Ac30d), respectively. The acclimated rats either received no additional treatment or were subjected to hypoxic stress (HyAc2d and HyAc30d, respectively).
Experimental Conditions
The C group was held at an ambient temperature of 24 ± 1 °C; heat acclimation was attained by continuous exposure to 34 ± 1 °C and 30- 40% relative humidity in a light-dark cycled room (12h: 12h) for 30 d (long-term acclimation), as previously described.44 For characterization of the effects of hypoxia on C and heat acclimated rats transient hypoxia was applied: each animal was placed in a hypoxic chamber for 15 min, where a continuously flowing mixture of air and nitrogen was adjusted to achieve 4.5 ± 0.5% oxygen. The oxygen concentration was monitored online, using the Oxygen Analyzer S-3A system. All exposures were conducted at the same time of the day, and the chamber was maintained at a normothermic temperature. Upon termination of treatment, the animals were allowed to recover for 0, 4, 8 and 24 h at room temperature and then sacrificed by cervical dislocation.33,47 The hippocampus and frontal cortex were quickly removed and stored at -70 °C until analysis.
Behavioral Assessment of the Impact of Transient Hypoxia
To assess the impact of the hypoxic stress on the various groups, a motor functional test used for ischemia33,47,48 was modified and applied. This test evaluates deficits in a battery of sensory-motor reflexes reflecting damage in the cortex and the hippocampus and are considered sensitive enough to detect recovery (spontaneous, as well as from medications.33 The sensory-motor deficit was evaluated within one minute of the hypoxic insult and neurological status was rated for:37 grasping reflex of the forepaws - 1 point for each forepaw;38 postural reflex 2 points for symmetrical and strong forelimb extension when held by the tail while reaching for a distant object48 walking - 2 points for symmetric and stable walking;11 thorax twisting - 1 point for trying to get free itself when suspended by the tail;29 lateral push - 1 point for a steady and symmetrical response; and31 hanging - 2 points for a strong firm grasp on a horizontal bar.49 The sum of these points is the neurological score out of 10, and a score of 9 or greater indicates that there is no functional deficit.
Cellular Fractionation
To prepare enriched membrane lysates, the hippocampus and frontal cortex were homogenized in a buffered solution of 250mM Sucrose, 50mM Tris, 1mM PMSF, and protease inhibitor 1:100, titrated to a pH of 7.4.
The homogenate was centrifuged for 15 min at 3,500 g, 4 °C. The supernatant went through a second centrifugation at 48,000 g, 4 °C for 15 min, yielding an enriched membrane precipitate. Protein concentration was quantified using Bradford reagent (Bio-Rad Laboratories, Richmond, CA, USA).
Western Blot Analysis
Total protein (50μg per lane) was fractionated by electrophoresis on 9% or 12% polyacrylamide gels under denaturing conditions,43,44 transferred onto nitrocellulose membranes, blocked for 2h in phosphate-buffered saline (PBS) containing 5% dried skimmed milk powder, and then probed overnight at 4 °C with primary the antibody, diluted 1:1000. After repeated washings, the membranes were incubated at room temperature for 1h with horseradish peroxidase-conjugated goat anti-rabbit
IgG (Sigma) diluted 1:10,000. Each sample was run 3 times and the control sample was present in all runs and therefore used to normalize protein levels44). Polyclonal, (Chemicon) affinity purified to the specific subunit- GluN1, GluN2A, GluN2B or GluA2 primary antibodies were used. Specific antibody binding was detected using enhanced chemiluminescence (Amersham) and visualized by exposing an X-ray film to the membrane.44,50,51
The density of the scanned protein bands was calculated using Tina software (Raytest, Straubenhardt, Germany).
mRNA Detection
Changes in mRNA transcripts were detected using semi-quantitative RT-PCR performed as previously described.18 Briefly, total RNA was extracted from the brain tissue, using TRI-REAGENT (Molecular Research Center, Inc., OH, USA). Total RNA (10μg) was reverse transcribed in a 50μl reaction mixture containing 0.5μg of oligo(dT)15 as primer, together with 400 U of MMLV reverse transcriptase, according to the manufacturer’s instructions (USB, United States Biochemical, Cleveland, OH, USA). For the PCR, 5μl of the cDNA mixture was added to 50μl of a master mix containing 200μM of dNTP, 100pM of primer, and 1.5 units of Vent polymerase (USB). We synthesized DNA oligonucleotide primers for GluN1, GluN2A and GluN2B subunits of NMDA-R. The oligonucleotide sequences were taken from the published literature49 and Clontech Laboratory, Palo Alto, Ca, USA. Sense and antisence sequences, respectively were: NR1: GGCACAGGCAGTTCACGAACTCC; TAGATGCCCACTTGCACCAGCTTG; NR2A:CCTCCCGGAGCATAAGCCTCAAGG; GTGTTTGTAAGGGTCCGAGGGAC NR2B:AGCTCCATTGATGGGCTCTATGACTG; GTTGCCCTCGATGTTCCCATAGGTG. β actin was used as a housekeeping gene as our protocols do not change its levels43,52. β ACTIN - sense: GAGACCTTCAACAACCCAGCC, antisense: GGCCATCTCTTGCTCGAAGTC. The PCR products were resolved on 1.5% agarose gel, stained with ethidium bromide, and visualized under UV light. Band density was analyzed using TINA software (Raytest, Straubenhardt).
Statistical Analysis
For statistical analysis, one- and two-way ANOVA followed by post hoc (Tukey; Dunn’s) tests were used with commercially available computer software (Sigmastat, SPPS Inc.). Treatments were taken as the fixed effects, and the individual tissues were assumed to be random samples from the population. The data are expressed as means ± SEM; values of P < 0.05 were considered statistically significant.
Glossary
Abbreviations:
- Ac2d
heat acclimation for 2 days
- Ac30d
heat acclimation for 30d
- C
control
- AMPA-R
α-amino-3-hydroxy-5-methyl-4-isoazole-propionic acid receptor
- GluA2
subunit of the AMPA-R
- NMDA-R
N-methyl-D-aspartate receptor
- GluN1
subunit of the NMDA-R
- GluN2A
subunit of the NMDA-R
- GluN2B
subunit of the NMDA-R
- Hy
hypoxia
Disclosure
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgment
This study was supported by the Research Committee of the Faculty of Dentistry of The Hebrew University.
References
- 1.Horowitz M, Kaspler P, Marmary Y, Oron Y. . Evidence for contribution of effector organ cellular responses to the biphasic dynamics of heat acclimation. J Appl Physiol (1985) 1996; 80:77 - 85; PMID: 8847335 [DOI] [PubMed] [Google Scholar]
- 2.Horowitz M, Meiri U. . Thermoregulatory activity in the rat: effects of hypohydration, hypovolemia and hypertonicity and their interaction with short-term heat acclimation. Comp Biochem Physiol A Comp Physiol 1985; 82:577 - 82; http://dx.doi.org/ 10.1016/0300-9629(85)90436-0; PMID: 2866878 [DOI] [PubMed] [Google Scholar]
- 3.Levy E, Hasin Y, Navon G, Horowitz M. . Chronic heat improves mechanical and metabolic response of trained rat heart on ischemia and reperfusion. Am J Physiol 1997; 272:H2085 - 94; PMID: 9176273 [DOI] [PubMed] [Google Scholar]
- 4.Horowitz M, Eli-Berchoer L, Wapinski I, Friedman N, Kodesh E. . Stress-related genomic responses during the course of heat acclimation and its association with ischemic-reperfusion cross-tolerance. J Appl Physiol (1985) 2004; 97:1496 - 507; http://dx.doi.org/ 10.1152/japplphysiol.00306.2004; PMID: 15155711 [DOI] [PubMed] [Google Scholar]
- 5.Horowitz M.. . Heat acclimation and cross-tolerance against novel stressors: genomic-physiological linkage. Prog Brain Res 2007; 162:373 - 92; http://dx.doi.org/ 10.1016/j.humpath.2007.01.001; PMID: 17376512 [DOI] [PubMed] [Google Scholar]
- 6.Kirino T, Tamura A, Sano K. . Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol 1984; 64:139 - 47; http://dx.doi.org/ 10.1007/BF00695577; PMID: 6475501 [DOI] [PubMed] [Google Scholar]
- 7.Choi DW. . Excitotoxic cell death. J Neurobiol 1992; 23:1261 - 76; http://dx.doi.org/ 10.1002/neu.480230915; PMID: 1361523 [DOI] [PubMed] [Google Scholar]
- 8.Doble A. . The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther 1999; 81:163 - 221; http://dx.doi.org/ 10.1016/S0163-7258(98)00042-4; PMID: 10334661 [DOI] [PubMed] [Google Scholar]
- 9.Gozal E, Roussel AL, Holt GA, Gozal L, Gozal YM, Torres JE, Gozal D. . Protein kinase C modulation of ventilatory response to hypoxia in nucleus tractus solitarii of conscious rats. J Appl Physiol (1985) 1998; 84:1982 - 90; PMID: 9609793 [DOI] [PubMed] [Google Scholar]
- 10.Lau A, Tymianski M. . Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 2010; 460:525 - 42; http://dx.doi.org/ 10.1007/s00424-010-0809-1; PMID: 20229265 [DOI] [PubMed] [Google Scholar]
- 11.Bochet P, Audinat E, Lambolez B, Crépel F, Rossier J, Iino M, Tsuzuki K, Ozawa S. . Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron 1994; 12:383 - 8; http://dx.doi.org/ 10.1016/0896-6273(94)90279-8; PMID: 7509161 [DOI] [PubMed] [Google Scholar]
- 12.Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. . Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 1994; 12:1281 - 9; http://dx.doi.org/ 10.1016/0896-6273(94)90444-8; PMID: 8011338 [DOI] [PubMed] [Google Scholar]
- 13.Osuga H, Hakim AM. . Relevance of interstitial glutamate to selective vulnerability in focal cerebral ischemia. J Cereb Blood Flow Metab 1994; 14:343 - 7; http://dx.doi.org/ 10.1038/jcbfm.1994.42; PMID: 7906692 [DOI] [PubMed] [Google Scholar]
- 14.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. . Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 1992; 256:1217 - 21; http://dx.doi.org/ 10.1126/science.256.5060.1217; PMID: 1350383 [DOI] [PubMed] [Google Scholar]
- 15.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, et al. . Molecular diversity of the NMDA receptor channel. Nature 1992; 358:36 - 41; http://dx.doi.org/ 10.1038/358036a0; PMID: 1377365 [DOI] [PubMed] [Google Scholar]
- 16.Laurie DJ, Bartke I, Schoepfer R, Naujoks K, Seeburg PH. . Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Res Mol Brain Res 1997; 51:23 - 32; http://dx.doi.org/ 10.1016/S0169-328X(97)00206-4; PMID: 9427503 [DOI] [PubMed] [Google Scholar]
- 17.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. . Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994; 12:529 - 40; http://dx.doi.org/ 10.1016/0896-6273(94)90210-0; PMID: 7512349 [DOI] [PubMed] [Google Scholar]
- 18.Dingledine R, Borges K, Bowie D, Traynelis SF. . The glutamate receptor ion channels. Pharmacol Rev 1999; 51:7 - 61; PMID: 10049997 [PubMed] [Google Scholar]
- 19.Chen N, Luo T, Raymond LA. . Subtype-dependence of NMDA receptor channel open probability. J Neurosci 1999; 19:6844 - 54; PMID: 10436042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. . Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol 2005; 563:345 - 58; http://dx.doi.org/ 10.1113/jphysiol.2004.080028; PMID: 15649985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huh KH, Wenthold RJ. . Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem 1999; 274:151 - 7; http://dx.doi.org/ 10.1074/jbc.274.1.151; PMID: 9867823 [DOI] [PubMed] [Google Scholar]
- 22.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. . Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci 2004; 24:7821 - 8; http://dx.doi.org/ 10.1523/JNEUROSCI.1697-04.2004; PMID: 15356193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Hsu JC, Takagi N, Gurd JW, Wallace MC, Eubanks JH. . Transient global ischemia alters NMDA receptor expression in rat hippocampus: correlation with decreased immunoreactive protein levels of the NR2A/2B subunits, and an altered NMDA receptor functionality. J Neurochem 1997; 69:1983 - 94; http://dx.doi.org/ 10.1046/j.1471-4159.1997.69051983.x; PMID: 9349543 [DOI] [PubMed] [Google Scholar]
- 24.Esteban JA. . AMPA receptor trafficking: a road map for synaptic plasticity. Mol Interv 2003; 3:375 - 85; http://dx.doi.org/ 10.1124/mi.3.7.375; PMID: 14993459 [DOI] [PubMed] [Google Scholar]
- 25.Pollard H, Héron A, Moreau J, Ben-Ari Y, Khrestchatisky M. . Alterations of the GluR-B AMPA receptor subunit flip/flop expression in kainate-induced epilepsy and ischemia. Neuroscience 1993; 57:545 - 54; http://dx.doi.org/ 10.1016/0306-4522(93)90004-Y; PMID: 8309523 [DOI] [PubMed] [Google Scholar]
- 26.Hollmann M, Heinemann S. . Cloned glutamate receptors. Annu Rev Neurosci 1994; 17:31 - 108; http://dx.doi.org/ 10.1146/annurev.ne.17.030194.000335; PMID: 8210177 [DOI] [PubMed] [Google Scholar]
- 27.Hollmann M, Hartley M, Heinemann S. . Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science 1991; 252:851 - 3; http://dx.doi.org/ 10.1126/science.1709304; PMID: 1709304 [DOI] [PubMed] [Google Scholar]
- 28.Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. . Structural determinants of ion flow through recombinant glutamate receptor channels. Science 1991; 252:1715 - 8; http://dx.doi.org/ 10.1126/science.1710829; PMID: 1710829 [DOI] [PubMed] [Google Scholar]
- 29.Buchan AM, Li H, Cho S, Pulsinelli WA. . Blockade of the AMPA receptor prevents CA1 hippocampal injury following severe but transient forebrain ischemia in adult rats. Neurosci Lett 1991; 132:255 - 8; http://dx.doi.org/ 10.1016/0304-3940(91)90314-J; PMID: 1664505 [DOI] [PubMed] [Google Scholar]
- 30.Gorter JA, Petrozzino JJ, Aronica EM, Rosenbaum DM, Opitz T, Bennett MV, Connor JA, Zukin RS. . Global ischemia induces downregulation of Glur2 mRNA and increases AMPA receptor-mediated Ca2+ influx in hippocampal CA1 neurons of gerbil. J Neurosci 1997; 17:6179 - 88; PMID: 9236229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carriedo SG, Yin HZ, Sensi SL, Weiss JH. . Rapid Ca2+ entry through Ca2+-permeable AMPA/Kainate channels triggers marked intracellular Ca2+ rises and consequent oxygen radical production. J Neurosci 1998; 18:7727 - 38; PMID: 9742143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opitz T, Grooms SY, Bennett MV, Zukin RS. . Remodeling of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit composition in hippocampal neurons after global ischemia. Proc Natl Acad Sci U S A 2000; 97:13360 - 5; http://dx.doi.org/ 10.1073/pnas.97.24.13360; PMID: 11087875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roof RL, Schielke GP, Ren X, Hall ED. . A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke 2001; 32:2648 - 57; http://dx.doi.org/ 10.1161/hs1101.097397; PMID: 11692030 [DOI] [PubMed] [Google Scholar]
- 34.Schwimmer H, Eli-Berchoer L, Horowitz M. . Acclimatory-phase specificity of gene expression during the course of heat acclimation and superimposed hypohydration in the rat hypothalamus. J Appl Physiol (1985) 2006; 100:1992 - 2003; http://dx.doi.org/ 10.1152/japplphysiol.00850.2005; PMID: 16469936 [DOI] [PubMed] [Google Scholar]
- 35.Shein NA, Horowitz M, Alexandrovich AG, Tsenter J, Shohami E. . Heat acclimation increases hypoxia-inducible factor 1alpha and erythropoietin receptor expression: implication for neuroprotection after closed head injury in mice. J Cereb Blood Flow Metab 2005; 25:1456 - 65; http://dx.doi.org/ 10.1038/sj.jcbfm.9600142; PMID: 15902197 [DOI] [PubMed] [Google Scholar]
- 36.Horowitz M. . Heat acclimation, epigenetics, and cytoprotection memory. Compr Physiol 2014; 4:199 - 230; http://dx.doi.org/ 10.1002/cphy.c130025; PMID: 24692139 [DOI] [PubMed] [Google Scholar]
- 37.Assayag M, Gerstenblith G, Stern MD, Horowitz M. . Long- but not short-term heat acclimation produces an apoptosis-resistant cardiac phenotype: a lesson from heat stress and ischemic/reperfusion insults. Cell Stress Chaperones 2010; 15:651 - 64; http://dx.doi.org/ 10.1007/s12192-010-0178-x; PMID: 20221856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Assayag M, Saada A, Gerstenblith G, Canaana H, Shlomai R, Horowitz M. . Mitochondrial performance in heat acclimation--a lesson from ischemia/reperfusion and calcium overload insults in the heart. Am J Physiol Regul Integr Comp Physiol 2012; 303:R870 - 81; http://dx.doi.org/ 10.1152/ajpregu.00155.2012; PMID: 22895744 [DOI] [PubMed] [Google Scholar]
- 39.Choi DW. . Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci 1995; 18:58 - 60; http://dx.doi.org/ 10.1016/0166-2236(95)80018-W; PMID: 7537408 [DOI] [PubMed] [Google Scholar]
- 40.Contractor A, Heinemann SF. . Glutamate receptor trafficking in synaptic plasticity. Sci STKE 2002; 2002:re14; PMID: 12407224 [DOI] [PubMed] [Google Scholar]
- 41.Nadler V, Biegon A, Beit-Yannai E, Adamchik J, Shohami E. . 45Ca accumulation in rat brain after closed head injury; attenuation by the novel neuroprotective agent HU-211. Brain Res 1995; 685:1 - 11; http://dx.doi.org/ 10.1016/0006-8993(95)00367-Y; PMID: 7583233 [DOI] [PubMed] [Google Scholar]
- 42.Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. . HIF-1alpha-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics 2005; 23:79 - 88; http://dx.doi.org/ 10.1152/physiolgenomics.00279.2004; PMID: 16046617 [DOI] [PubMed] [Google Scholar]
- 43.Maloyan A, Horowitz M. . beta-Adrenergic signaling and thyroid hormones affect HSP72 expression during heat acclimation. J Appl Physiol (1985) 2002; 93:107 - 15; PMID: 12070193 [DOI] [PubMed] [Google Scholar]
- 44.Maloyan A, Palmon A, Horowitz M. . Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am J Physiol 1999; 276:R1506 - 15; PMID: 10233045 [DOI] [PubMed] [Google Scholar]
- 45.Cohen O, Kanana H, Zoizner R, Gross C, Meiri U, Stern MD, Gerstenblith G, Horowitz M. . Altered Ca2+ handling and myofilament desensitization underlie cardiomyocyte performance in normothermic and hyperthermic heat-acclimated rat hearts. J Appl Physiol (1985) 2007; 103:266 - 75; http://dx.doi.org/ 10.1152/japplphysiol.01351.2006; PMID: 17395755 [DOI] [PubMed] [Google Scholar]
- 46.White AC, Salgado RM, Schneider S, Loeppky JA, Astorino TA, Mermier CM. . Does Heat Acclimation Improve Exercise Capacity at Altitude? A Cross-tolerance Model. Int J Sports Med 2014; In press PMID: 24816886 [DOI] [PubMed] [Google Scholar]
- 47.Wahl F, Allix M, Plotkine M, Boulu RG. . Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke 1992; 23:267 - 72; http://dx.doi.org/ 10.1161/01.STR.23.2.267; PMID: 1561657 [DOI] [PubMed] [Google Scholar]
- 48.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. . Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 1986; 17:472 - 6; http://dx.doi.org/ 10.1161/01.STR.17.3.472; PMID: 3715945 [DOI] [PubMed] [Google Scholar]
- 49.Pantos CI, Malliopoulou VA, Mourouzis IS, Karamanoli EP, Tzeis SM, Carageorgiou HC, Varonos DD, Cokkinos DV. . Long-term thyroxine administration increases heat stress protein-70 mRNA expression and attenuates p38 MAP kinase activity in response to ischaemia. J Endocrinol 2001; 170:207 - 15; http://dx.doi.org/ 10.1677/joe.0.1700207; PMID: 11431153 [DOI] [PubMed] [Google Scholar]
- 50.Chomczynski P, Sacchi N. . Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162:156 - 9; http://dx.doi.org/ 10.1016/0003-2697(87)90021-2; PMID: 2440339 [DOI] [PubMed] [Google Scholar]
- 51.Sullivan WP, Beito TG, Proper J, Krco CJ, Toft DO. . Preparation of monoclonal antibodies to the avian progesterone receptor. Endocrinology 1986; 119:1549 - 57; http://dx.doi.org/ 10.1210/endo-119-4-1549; PMID: 2428599 [DOI] [PubMed] [Google Scholar]
- 52.Hiestand WA, Stemler FW, Jasper RL. . Increased anoxic resistance resulting from short period heat adaptation. Proc Soc Exp Biol Med 1955; 88:94 - 5; http://dx.doi.org/ 10.3181/00379727-88-21501; PMID: 14357352 [DOI] [PubMed] [Google Scholar]


