Abstract
Bacteria in humans play an important role in health and disease. Considerable emphasis has been placed in understanding the role of bacteria in host-microbiome interkingdom communication. Here we show that serotonin, responsible for mood in the brain and motility in the gut, can also act as a bacterial signaling molecule for pathogenic bacteria. Specifically, we found that serotonin acts as an interkingdom signaling molecule via quorum sensing and that it stimulates the production of bacterial virulence factors and increases biofilm formation in vitro and in vivo in a novel mouse infection model. This discovery points out at roles of serotonin both in bacteria and humans, and at phenotypic implications not only manifested in mood behavior but also in infection processes in the host. Thus, regulating serotonin concentrations in the gut may provide with paradigm shifting therapeutic approaches.
Keywords: Host-microbiome interactions, Interkingdom signaling molecule, Las pathway, Pseudomonas aeruginosa, N-acyl homoserine lactone, Quorum sensing, Serotonin
Highlights
-
•
Serotonin can act as a bacterial quorum sensing molecule through the LasR quorum sensing pathway.
-
•
Serotonin stimulates quorum sensing-dependent virulence factor and biofilm production in Pseudomonas aeruginosa.
-
•
Serotonin exacerbates Pseudomonas aeruginosa infection in vivo.
Bacteria play many roles in human health and disease, thus investigating host-microbiome communication is of great importance. To this end, our research demonstrates that serotonin, a neurotransmitter responsible for mood in the brain and motility in the gut, can act as a bacterial communication molecule for pathogenic bacteria, specifically Pseudomonas aeruginosa. Serotonin increases Pseudomonas virulence factor and biofilm production in vitro and enhances virulence in infected mice. These findings are beneficial in understanding the impact of the microbiome on human health and may open the door for novel treatments for human disease.
1. Introduction
There is compelling evidence demonstrating a gut-microbiome-brain connection linking the enteric microbiome to modulations in human health and behavior, as well as regulation of gut motility, nutrient absorption, immune system, and fat distribution (Cryan and Dinan, 2012, Backhed et al., 2005, Lee and Mazmanian, 2010). Further, the microbiome plays a role in several disorders including autism, anxiety, and depression (Cryan and Dinan, 2012, Bravo et al., 2011). Studies in mice elucidated a few of these roles: improved behavior (Hsiao et al., 2013), reduction in levels of hormones linked to stress (Desbonnet et al., 2010, Bravo et al., 2011), increased cognitive performance (Backhed et al., 2005), and modulation of brain control of emotion and sensation (Tillisch et al., 2013). These studies point out to the gut microbiota being a virtual endocrine organ, manipulating and producing hormones and neurotransmitters that influence the host's brain and behavior (Clarke et al., 2014).
Key to unlocking the role of the gut microbiome is to understand the interactions with its environment. More than 90% of the body's monoamine serotonin is synthesized by gut enterochromaffin cells (Berger et al., 2009). However, the molecular mechanism that dictates the levels of serotonin produced and its metabolism is not fully elucidated. This is of utmost importance given that gut-derived serotonin is responsible for regulation of functions such as bone development, immune responses, gut motility, and platelet aggregation (Berger et al., 2009). Perhaps more interesting is the role that dysregulation of serotonin plays in the pathogenesis of certain intestinal diseases such as irritable bowel syndrome (IBS). IBS has been shown to have a microbial etiology (Knights et al., 2013, Jeffery et al., 2012) and as such, the link between the microbiome and serotonin is of interest. Two hypotheses have been formulated regarding the microbiome's contribution to serotonin levels in the host. The first relates to the fact that some species of bacteria, such as Escherichia coli and Streptococcus species, are capable of de novo serotonin synthesis (Lyte, 2013). The second hypothesis states that the microbiome influences the biosynthesis of serotonin by the host (Yano et al., 2015). Indigenous gut bacteria are able to regulate peripheral host serotonin biosynthesis by interactions with the intestinal enterochromaffin cells (Yano et al., 2015). Further, the microbiota plays a role in the regulation of central nervous system serotonergic neurotransmission profiles in a sex-dependent manner (Clarke et al., 2013). It has been also demonstrated that germ free mice synthesize lower levels of serotonin and its metabolites (Marcobal et al., 2013), indicating that the microbiome is an important factor for the synthesis of serotonin.
Despite all these observations linking bacteria to intrinsic serotonin synthesis, very little is understood regarding why, how, and what are the consequences of the microbiome's influence on the host's neurotransmitter levels and its manipulation, and vice versa. Most studies focus on the influence of the microbiome on changes to the host's production of these molecules, while very few examine the effects these molecules have on bacteria. It was observed that serotonin was able to stimulate growth of specific bacteria in culture (Oleskin et al., 1998), and some virulent bacteria can use neurotransmitters such as epinephrine and norepinephrine to activate virulence genes (Clarke et al., 2006). Both of these phenomena are linked to bacterial quorum sensing (QS), which is, in bacteria, a phenomenon when populations reach a specific threshold they communicate with organisms in their surroundings by releasing small diffusible quorum sensing molecules (QSMs). The QSMs then bind to regulatory proteins, causing a conformational change and allowing the protein to bind to DNA and initiate the transcription of virulence factors (Fig. 1). Herein, we hypothesized that bacteria are able to interact with host serotonin molecules and exploit them as a bacterial QSMs. Specifically, through experiments conducted in vitro and in vivo in animal studies, we demonstrate for the first time that serotonin acts as a signaling molecule for the las regulatory QS system of Pseudomonas aeruginosa inducing, among other effects, serious pathogenicity in the host. P. aeruginosa has a well-studied QS network that relies on multiple QS pathways critical in activating Pseudomonas virulence including the las and rhl systems. This work helps explains how high levels of serotonin found in the gut, produced endogenously or by bacteria, can be linked to the host's health.
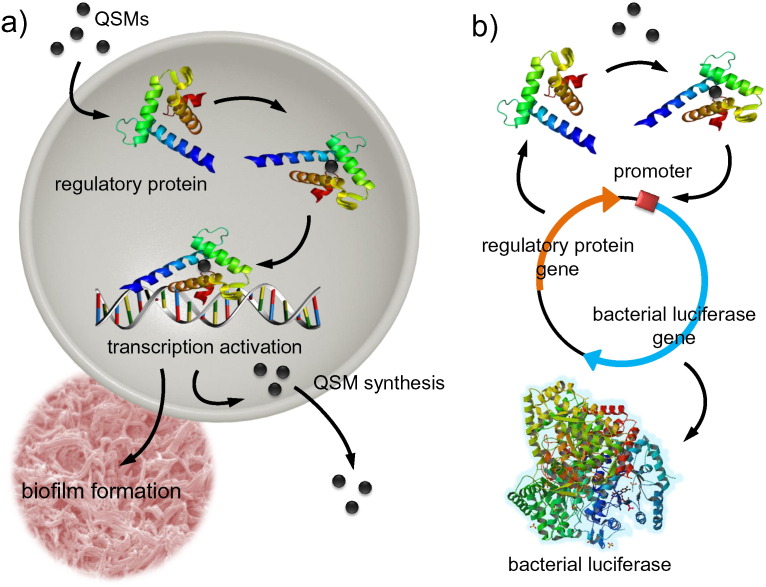
Fig. 1.
Bacterial quorum sensing and quorum sensing molecule detection. (a) Quorum sensing molecules (QSMs) enter the bacterial cell and bind to specific regulatory proteins. The QSM-regulatory protein complex activates transcription of virulence factors, such as biofilm formation, as well as production of more QSMs. (b) QSMs can be detected by employing the native regulatory proteins and corresponding promoter regions in a bacterial plasmid based system. The plasmid consists of a regulatory protein that is constitutively expressed and the corresponding promoter region that is fused to a reporter protein, such as bacterial luciferase. This allows for dose-dependent generation of signal when QSMs are present. The plasmid is then expressed in a strain of bacteria that does not generate the QSM of interest.
2. Materials and Methods
2.1. Plasmids and Bacterial Strains
For the whole-cell bioassays, E. coli DH5α cells harboring pSB1075 and pSB904, for LasR and RhlR production, respectively, were used. Native P. aeruginosa strain PAO-1 and the lasI and rhlI double mutant, JP2, were supplied by Dr. Johanna Schwingel.
2.2. Dose-response curves for LasR and serotonin
Dose-response curves to determine response to analyte and competitive assays were generated as previously described (Kumari et al., 2006).
2.3. Elastase Studies
Elastase production was measured using a previously established protocol with minor adjustments (Smith et al., 2003). P. aeruginosa PAO1 cells and JP2 cells were grown overnight in LB broth at 37 °C and 250 rpm. The cells were then diluted to an OD600 of 0.05 in fresh PTSB media and incubated in the presence of either N-3-oxo-C12-HSL or serotonin for 16 h at 37 °C and 250 rpm. After incubation, the OD600 of the cells was measured and they were centrifuged to separate the cells from the supernatant. The supernatant was then added to tubes containing 25 mg of Elastin congo red and a 20 mM Tris, 1 mM CaCl2 buffer and incubated overnight at 37 °C and 250 rpm. The reaction was then quenched with EDTA and the tubes were then centrifuged to remove unreacted Congo red-elastin and the absorbance of the supernatant was measured at 495 nm. All absorbance values were normalized to the OD600 values in order to correct for the variations of cell growth.
2.4. Protease Studies
To measure proteolytic activity, 3 mL of supernatant from an overnight culture of PAO1 or JP2 cells were added to 10 mM Tris HCl buffer, pH 7.5 and incubated with 15 mg of Hide powder at 37 ° C for 1 h at 250 rpm. Undissolved substrate was removed by centrifugation at 3000g for 10 min and the absorbance of the supernatant was measured at 595 nm.
2.5. Biofilm Formation
P. aeruginosa strain PAO1 and JP2 were grown overnight in LB broth at 37 °C and 250 rpm. The culture was then diluted 100-fold in M9 minimal media supplemented with 0.5% (weight per volume) casamino acids, 11.1 mM glucose, and 1 mM MgSO4. Next, 1.0 mL solutions of cells containing either the analytes or the appropriate blanks were prepared according to table S1. Then, 200 μL of each solution was placed on poly-l-lysine coated microscope slides which was then placed in 100 mm petri dishes. A humidity chamber was created by placing a 35 mm petri dish filled with 5 mL of water in the larger dish and sealing with parafilm. The dishes were then incubated at 37 °C for 16 h without shaking. After incubation, the slides were gently washed with double distilled deionized water and imaged using a scanning electron microscope.
2.6. Crystal Violet Biofilm Studies
P. aeruginosa strain PAO-1 and JP2 were grown overnight in LB broth at 37 °C and 250 rpm. The culture was then diluted 100-fold in M9 minimal media supplemented with 0.5% (weight per volume) casamino acids, 11.1 mM glucose, and 1 mM MgSO4. Next, 125 μL of each dilution was added in triplicate to the wells of a 96-well flat-bottomed, white microtiter plate. The plate was then covered and incubated overnight at 37 °C in a humidity chamber. After incubation, the plates were inverted to remove any non-adhering cells. The plate was then washed three times by submerging the whole plate into container filled with double distilled, deionized water and inverting after each wash. Then, a 0.1% aqueous solution of crystal violet was added to each well and incubated for 15 min at room temperature. The plates were then washed three times, as described above, to remove any excess crystal violet. After drying overnight in open air at room temperature, 125 μL of a 30% acetic acid solution was added to each well and incubated 15 min to dissolve the crystal violet. The resultant solution was transferred to a clear, flat-bottomed microtiter plate and the absorbance was measured at 550 nm using an absorbance reader (PolarStar Optima). All values were blank subtracted using 30% acetic acid as the blank.
2.7. Establishment of Mouse Model of PA Induced Intestinal Infection
Pregnant (E15) C57BL/6 mice were purchased from Charles Rivers Laboratory and were housed according to NIH guidelines, with one pregnant female per cage and corncob bedding. Enrichment of nestlets and sunflower seeds were provided when the animals were received to help acclimate them to the facility. Animals were kept in an Animal Biosafety Level 2 (ABSL) facility. The entire litter from each pregnant female (~ 6 mouse pups) was used for the experimental condition, with experimental conditions assigned at random. Samples collected were then blinded before being sent for subsequent analysis. The Institutional Animal Care and Use Committee (IACUC) of the University of Miami approved all animal procedures (protocol 15-177). Infections, intraperitoneal (IP) injections, and sacrificing animals were performed in the mornings at approximately 10:00 am within the ABSL2 facility. Three days post birth, the pups were orally inoculated with 103, 104, or 105 CFUs of PAO1 or PBS. Blood was taken from a facial vein every other day for three days, at which time the pups were sacrificed and their intestines were harvested. Intestinal homogenates were plated on cetrimide agar plates and incubated at 37 °C. After over-night incubation, the colony forming units (CFUs) were counted and normalized to the mass of the intestines.
2.8. Serotonin Effects In Vivo
Serotonin (20 mg/kg) or PBS was administered via intraperitoneal injection (IP) 1 day post infection. On the third day, the mice were sacrificed and the intestines were removed and plated on cetrimide agar plates to determine colony counts and cytokine levels.
2.9. Histopathological Examination
The intestinal tissues harvested from mice were fixed in formalin, cut and stained with H&E (Mittal et al., 2009). Intestinal sections were graded microscopically by a pathologist blinded to groups from grade 0 (normal) to grade 4 (severe) based on pathological manifestations including submucosal edema, villus core edema, epithelial sloughing and obliteration, immune cell infiltration, intestinal perforation, and necrosis (Mittal et al., 2009).
2.10. Scanning Electron Microscopy (SEM)
Intestines harvested from the mice were washed in PBS and then fixed in 2% glutaraldehyde in PBS buffer followed by three changes of PBS buffer for 10 min each. The samples were then post–fixed in 1% osmium tetroxide in PBS buffer for 45 min and rinsed in three changes of PBS buffer for 10 min each. The samples were dehydrated in a graded series of ethanol, dried in hexamethyldisilazane (HMDS) and mounted on carbon adhesive tabs fixed to metal stubs. The samples were coated with palladium in a plasma sputter coater and viewed in a scanning electron microscope (FEI, ESEM-FEG XL-30).
2.11. Determination of Myeloperoxidase (MPO), Lactate Dehydrogenase (LDH), Malonaldehyde (MDA), Gluthathione (GSH) and Catalase
The levels of MPO, LDH, MDA, GSH and catalase were determined in the intestinal homogenates using commercially available kits from Biovision and Cayman Chemical as per manufacturer's instructions.
2.12. Cytokine Determination
The levels of TNF-α, IL-1β, IL-6 and IL-12 were measured in intestinal homogenates using antibody pair kits from Life Technologies (Carlsbad, CA) according to the manufacturer's instructions.
2.13. Serotonin Determination
The levels of serotonin was measured in intestinal homogenates and stool samples via ELISA from Enzo Life Sciences, Inc. (Farmingdale, NY) according to the manufacturer's instructions.
3. Results
3.1. Serotonin Enhances Virulence of P. aeruginosa in Mice
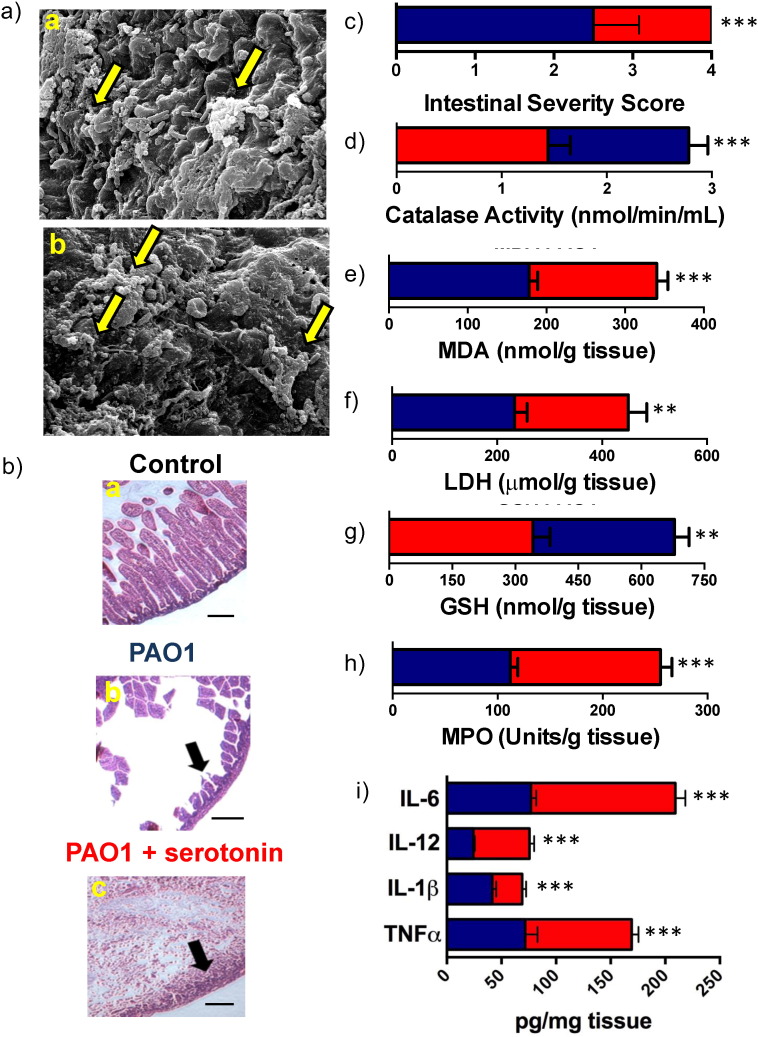
To understand the effect of serotonin on bacterial infection in vivo, we established a P. aeruginosa intestinal infection mouse model by feeding three-day old C57BL/6 mice with wild-type PAO1 cells. Three-day old mice were employed in this study because they are naturally susceptible to Pseudomonas infection without the use of immunocomprimising compounds that could interfere with the study. Mice were orally inoculated with 103, 104, or 105 CFUs of wild-type P. aeruginosa (PAO1) or PBS, were sacrificed three days post infection, and the intestines were harvested, homogenized, and plated on cetrimide agar to determine bacterial load (Fig. S1A). 104 CFUs was selected for use in our mouse model due to it establishing infection 24 h post oral inoculation (Fig. S1B) and limited mortality (Fig. S1C). Exogenous serotonin was then administered via intraperitoneal injection to the infected mice revealing an increase in intestinal bacterial load and mortality compared to untreated animals (Fig. S1C–D). This was confirmed with SEM imaging of the mouse intestines, showing an increase in biofilm formation when serotonin was administered (Fig. 2A). Histopathological examination of the intestinal tissue showed that in uninfected mice, the intestinal villi were elongated with no indication of damage, both in the presence and absence of serotonin (Fig. 2B(a)). PAO1 infection resulted in intestinal epithelial damage and villi destruction (Fig. 2B(b)) and the administration of serotonin exacerbated these effects, demonstrating complete villi destruction, severe epithelial damage, increased intraepithelial inflammatory cells, and degenerative changes to the base of the crypts (Fig. 2B(c)). The intestinal sections were then scored via a blinded pathology review, which confirmed severe epithelial injury, mucosal sloughing and villi destruction in mice administered serotonin as compared to the other groups (Mittal et al., 2009) (Fig. 2C). Serotonin levels in the homogenized intestine and intestinal content were assessed via ELISA, which revealed peak serotonin levels 8 h post IP injection (Fig. S1E—F). To further confirm our findings, we assessed the effect of markers of tissue damage (lactate dehydrogenase, LDH, and malondialdehyde, MDA) and oxidative stress (catalase and glutathione, GSH) in intestinal tissue homogenates. The groups that were administered serotonin showed significantly increased levels of LDH and MDA, while the levels of catalase and GSH were decreased (Fig. 2D–G), thus suggesting exacerbation of oxidative stress and tissue damage (Odobasic et al., 2007) in the presence of serotonin. There was significantly higher recruitment of immune cells in infections where serotonin was present indicated by myeloperoxidase (MPO) levels, suggesting heightened inflammation (Fig. 2H).
Fig. 2.
Serotonin enhances virulence of P. aeruginosa in mice. (A) Three-day old mice were fed orally with 104 CFU's of PAO1. SEM images of mice intestines inoculated with (a) PAO1 cells and PBS, and (b) PAO1 cells and serotonin. Arrows indicate biofilm formation on intestinal tissues. Scale bars represent 10 μm. (B) Intestines were harvested and subjected to histopathological examination after staining with H&E. Uninfected control mice fed with (a) PBS had no changes to the intestinal tissue. PAO1 infected mice treated with (b) PBS showed loss of villi and some epithelial damage as indicated by the arrows. This intestinal tissue damage was further exacerbated in (c) animals infected with PAO1cells and treated with serotonin showing complete destruction of villi and severe epithelial damage. (C) Intestinal sections were scored for disease severity by a pathologist blinded to specimen. Significant tissue damage was observed in mice infected with bacteria and treated with serotonin (red bar) compared to mice infected with only bacteria (blue bar). Tissue damage and oxidative stress markers (D) catalase, (E) MDA, (F) LDH, and (G) GSH were determined in intestinal tissue homogenates both in the absence (blue bar) and presence (red bar) of serotonin. (H) Recruitment of immune cells was examined by MPO activity. (I) The levels of TNFα, IL-1β, IL-12, and IL-6 were determined in intestinal tissues of infected and uninfected (control) mice by ELISA. The levels of cytokines were significantly higher in serotonin treated animals (red bars) compared to untreated mice (blue bars). In all cases, data represents mean ± SD. Results are representative of 2 independent experiments with a minimum of 4 mice per group. **P < 0.001 or ***P < 0.0001 by ANOVA. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The aforementioned observations demonstrate that serotonin affects PAO1 infectivity on a macroscopic scale. To investigate the virulence on a biochemical scale, the levels of TNFα, IL-1β, IL-12, and IL-6 in intestinal tissues of sacrificed mice were evaluated: these cytokines have been shown to play an important role in P. aeruginosa infections (Zhang et al., 2005). The concentrations of all these cytokines were significantly elevated in infected mice when serotonin was administered (Fig. 2I), correlating with the aforementioned intestinal damage.
3.2. Serotonin Acts as a Quorum Sensing Molecule
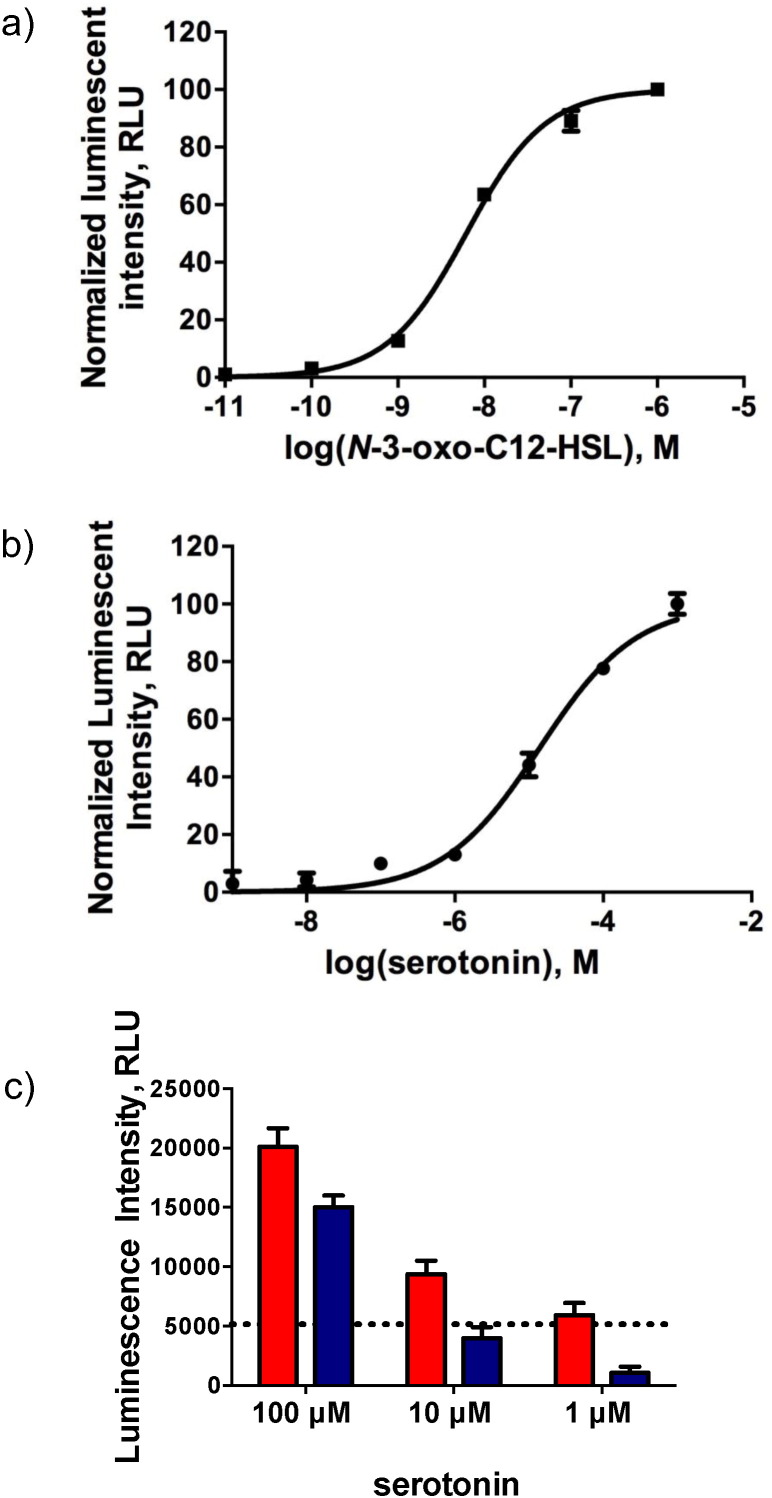
To elucidate serotonin's role in bacterial communication, we employed a cellular system that, upon activation by exogenous bacterial QSMs, elicits a quantifiable production of bioluminescence (Fig. 3a). Due to the complex nature of P. aeruginosa's QS pathways, an E. coli system was chosen to perform these studies to better isolate which specific protein serotonin was able to interact with and produce the virulence phenotypes noted in the above studies in the native organism. Specifically, serotonin's effect on two bacterial communication pathways in P. aeruginosa, the las and rhl pathway, was assessed. Serotonin elicited a response from the las QS cellular system at μM concentrations (Fig. 3b). No response was noted in the rhl cellular system, indicating specificity for the las QS pathway (data not shown). Additionally, we examined the effects of serotonin on the las QS cellular system in the presence of las activating QSMs. Serotonin had an additive response when present with QSMs as compared to serotonin alone (Fig. 3c).
Fig. 3.
Serotonin acts as a QSM. (a) Dose-response curve for N-3-oxo-C12-HSL of E. coli cells harboring pSB1075. (b) Dose-response curve for serotonin using E. coli cells harboring pSB1075. Serotonin activates the LasR regulatory protein at μM concentrations. (c) Response of E. coli cells harboring pSB1075 when incubated with QSM at a constant concentration of 0.1 nM and varied serotonin concentrations (red) and serotonin alone (blue). The dotted line represents the signal from the QSM alone. In all cases, data represents mean ± SD. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Serotonin is biosynthesized by the metabolism of the essential amino acid tryptophan (O'mahony et al., 2015). Due to subtle structural modifications of molecules during this process, it is important to evaluate the efficacy of precursor molecules in serotonin's synthesis as QSM. Employing the same cellular system, it was found that these precursor molecules in the tryptophan metabolic pathway were unable to cause a response comparable to serotonin (Fig. S2), demonstrating a selectivity of the LasR protein for serotonin.
3.3. Serotonin Enhances Virulence Phenotypes in P. aeruginosa
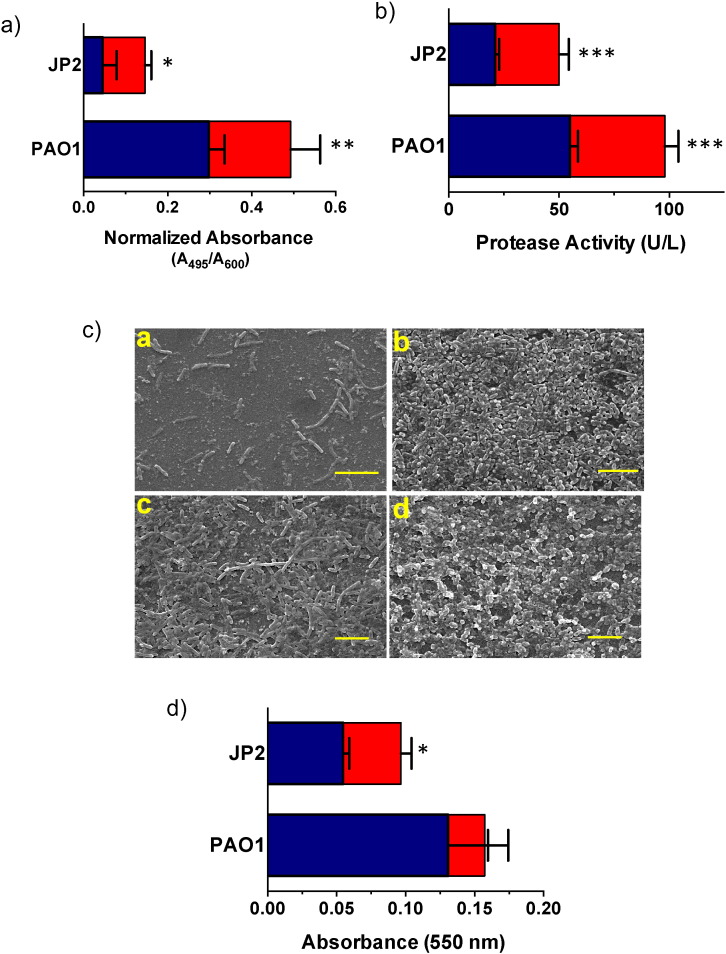
While the data indicate that serotonin does interact with the LasR regulatory protein, it is still important to determine if the presence of serotonin enhances virulence phenotypes in P. aeruginosa. To this end, the effects of serotonin on the native organism were evaluated by monitoring production of elastase, protease, and biofilm formation, all of which depend on the las QS circuit (Pearson et al., 1997, Davies et al., 1998). Furthermore, to ensure that the effects were due to interactions with QS, a lasI mutant strain of P. aeruginosa, JP2, was employed: this strain is a virulent and lacks the synthase for QSMs (De Kievit et al., 2001). Thus, JP2 cells are able to re-establish virulence only in the presence of exogenous molecules that can bind to and activate the LasR protein. In the presence of serotonin, there were significant increases in the production of elastase and protease in both PAO1 and JP2 cells (Fig. 4A–B). Biofilm formation, the mediator of on-set of pathogenicity in the hosts, was also evaluated. We observed that JP2 cells only formed biofilms when serotonin, QSM or both were present (Fig. 4C(a–b)). PAO1 cells also demonstrated increased biofilm formation in the presence of serotonin (Fig. 4C(c–d)). Crystal violet assays further confirmed these results showing higher biofilm formation in the presence of serotonin for both strains (Fig. 4D).
Fig. 4.
Serotonin enhances virulence phenotypes in P. aeruginosa. (A) JP2 cells were employed to evaluate serotonin's ability to activate QS gene regulation in the native organism. The presence of serotonin (red bars) was able to significantly enhance elastase synthesis in PAO1 cells and activate elastase synthesis in JP2 cells as opposed to the bacteria alone (blue bars) (averages (n = 3) ± one standard deviation). Serotonin (red bars) increased production of (B) protease in both PAO1 and JP2 cells. (C) Biofilm development in the presence and absence of serotonin. (a) JP2 cells only formed biofilms in (b) serotonin's presence while (c) PAO1 cells biofilm formation increased with the addition of (d) serotonin. Scale bars represent 10 μm. (D) Crystal violet assay confirmed biofilm formation in PAO1 cells and JP2 cells in the absence (blue bars) and presence (red bars) of serotonin. In all cases, data represents mean ± SD and is representative of five individual experiments carried out in triplicate. *P < 0.05 or **P < 0.001 or ***P < 0.0001 by ANOVA. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We also examined two P. aeruginosa phenotypes non-dependent on the las pathway, namely, swarming motility and lipopolysaccharide (LPS) production (De Kievit et al., 2001, Beatson et al., 2002). There was no increase in either upon addition of serotonin (data not shown).
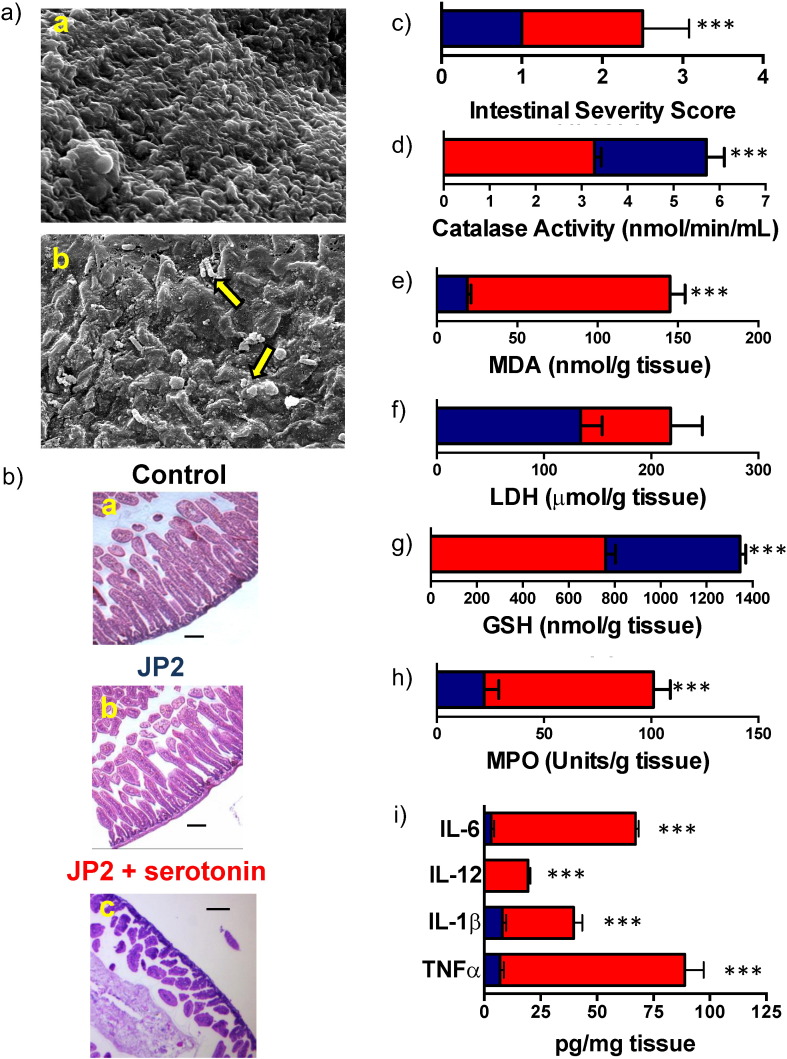
3.4. Serotonin Restores Virulence In Vivo in P. aeruginosa QSM Mutant
After establishing that serotonin can mimic QSMs in vitro in Pseudomonas, we wanted to elucidate the effects serotonin would have on the JP2 strain in vivo. The administration of a 104 CFU bolus of the avirulent JP2 strain caused no mortality of the mice after the three-day period (Fig. S3A). When serotonin was administered, there was only a 50% (4/8) survival after three days. This correlates with an increase in CFUs of JP2 in the presence of serotonin (Fig. S3B). This increase in colonization was confirmed via SEM imaging of the intestinal tissue (Fig. 5A). Histopathological examination showed that tissue from mice infected with JP2 was similar to the negative control (Fig. S1H) while tissue from mice infected with JP2 and dosed with serotonin showed villi destruction and epithelial injury (Fig. 5B) very similar to that observed of PAO1 infected mice (Fig. 2B). Additionally, tissue from JP2 infection in the presence of exogenous serotonin was scored similarly to PAO1 infection in the absence of exogenous serotonin (Fig. 5C), indicating that serotonin is able to restore the pathogenicity of JP2 cells. We also assessed markers of tissue damage (lactate dehydrogenase, LDH and malondialdehyde, MDA) and oxidative stress (catalase and glutathione, GSH) in intestinal tissue homogenates. The groups that were administered serotonin showed significantly increased levels of MDA, while the levels of catalase and GSH were significantly decreased (Fig. 5D–G), thus suggesting exacerbation of oxidative stress and tissue damage (Odobasic et al., 2007) in the presence of serotonin. There was significantly higher recruitment of immune cells in infections where serotonin was present indicated by myeloperoxidase (MPO) levels, suggesting heightened inflammation (Fig. 5H). Further, in the presence of serotonin, JP2 infection significantly enhanced the production of the cytokines TNFα, IL-1β, IL-12, and IL-6 (Fig. 5I).
Fig. 5.
serotonin restores virulence in vivo in P. aeruginosa QSM mutant. (A) SEM images of mice intestines inoculated with (a) JP2 cells and PBS, and (b) JP2 cells and serotonin. Arrows indicate biofilm formation on intestinal tissues. Scale bars represent 10 μm. (B) Intestines were harvested and subjected to histopathological examination after staining with H&E. Uninfected control mice fed with (a) PBS and mice infected with (b) JP2 and fed PBS had no changes to the intestinal tissue. JP2 infected mice treated with (c) serotonin showed loss of villi and some epithelial damage as indicated by the arrows. (C) Intestinal sections were scored for disease severity by a pathologist blinded to specimen. Significant tissue damage was observed in mice infected with bacteria and treated with serotonin (red bars) as compared to mice infected only with the bacteria (blue bars). Tissue damage and oxidative stress markers (D) catalase, (E) MDA, (F) LDH, and (G) GSH were determined in intestinal tissue homogenates both in the absence (blue bars) and presence (red bars) of serotonin. (H) Recruitment of immune cells was examined by MPO activity. (I) The levels of TNFα, IL-1β, IL-12, and IL-6 were determined in intestinal tissues of infected mice in the absence (blue bars) and presence (red bars) of serotonin by ELISA. In all cases, data represents mean ± SD. Results are representative of 2 independent experiments with a minimum of 4 mice per group. **P < 0.001 or ***P < 0.0001 by ANOVA. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This study establishes serotonin's role as a bacterial quorum sensing molecule. Our cellular system data show that serotonin can activate the LasR QS pathway at μM concentrations (Fig. 3b). This finding is of great interest as physiological levels of serotonin in the digestive tract of healthy and diseased individuals is 10 μM (Erspamer, 1966) and ~ 100 μM (Miwa et al., 2001) respectively. The raised levels of serotonin in patients with Inflammatory Bowel Disease (IBD), combined with the insight that serotonin can activate QS pathways, may allow for better patient care and therapeutics. Additionally, the additive effects of serotonin combined with QSM (Fig. 3c) are relevant as bacteria exist naturally in the gut, thus it is highly likely that serotonin will co-exist with bacterial QSMs.
Previous reports have shown that virulence factor production, such as biofilm formation and protease production, are quorum-dependent (Waters et al., 2008, Valiente et al., 2007), which supports the increase in these factors seen when serotonin was administered in both our in vitro (Fig. 4) and in vivo (Fig. 2, Fig. 5) experiments as serotonin activating the LasR QS system. In vitro, our results show that serotonin mimics the effects of exogenous QSM addition in JP2 cells' biofilm formation (Fig. 4C–D), indicating that serotonin acts as a bacterial signaling molecule that is capable of activating QS-regulated phenotypes. Our developed Pseudomonas infection model demonstrated that serotonin was able to activate Pseudomonas virulence in vivo, within the intestines of the mice (Fig. 2, Fig. 5). Within these experiments, there are two points of particular interest: in the absence of serotonin the JP2 mutant that cannot generate its own QSMs was not able to establish an infection, and the effects of PAO1 infection alone were similar to the effects of JP2 with serotonin. When administered the same CFU of JP2 cells compared to PAO1 cells, the JP2 mutant Pseudomonas did not establish any detectable colonies (Fig. S3B). This lack of infection is supported by the SEM images (Fig. 5A) and histology data (Fig. 5B–C). As the JP2 mutant is only lacking in QSM synthesis, this inability to establish an infection both supports the current literature that Pseudomonas virulence is QS dependent and allows for the assessment of restoration of function, specifically the ability to establish an infection. Thus when we restore the infectivity of JP2 by administering serotonin (Fig. S3B), it indicates that serotonin is fulfilling the role of a QSM. The infection with PAO1 alone and JP2 with serotonin resulted in similar levels of CFUs harvested from the intestines (Fig. S1D, S3B), which further supports serotonin's role as a QSM. This similarity between PAO1 alone and JP2 with serotonin is also noted in the histology data (Figs. 2B–C, 5B–C), with both exhibiting intestinal epithelial damage and villi destruction. These data further demonstrate serotonin's ability to restore function to the JP2 mutant lacking QSMs, providing greater evidence for its role as a QSM.
Our experiments demonstrate that serotonin activates the las quorum sensing pathway, which leads to greater infectivity of P. aeruginosa, however continued experimentation with Pseudomonas infection and serotonin can help to fully elucidate this complex interplay. Our mouse model was limited due to its focus on three day old mouse pups based upon their natural susceptibility to Pseudomonas infection, however, it may be possible to exploit serotonin's enhancement of Pseudomonas virulence to investigate Pseudomonas infections in adult mice. Additionally, there are limitations to current biochemical assays for QSMs, such as a lack of a full crystal structure for LasR. Advancements in this field will allow for binding studies of serotonin with LasR, which would further enhance our findings.
While it is uncertain why Pseudomonas utilizes another organism's molecules to enhance their QS networks, it is not a novel phenomenon. Social cheating by bacteria, where the bacteria exploit sensing molecules produced by other organisms in their vicinity to activate their QS systems, has been observed in many circumstances. Thus serotonin may be a way the opportunistic bacteria P. aeruginosa participates in social cheating to enhance its virulence. While our knowledge of the human microbiome is in its nascent stage, the observed Janus-type behavior of serotonin, acting as both mammalian neurotransmitter and bacterial communication molecule, undoubtedly will contribute to further our knowledge of the complex relationship between the host and its' microbiome. Specifically, these findings could lead to a better understanding of the host regulation of the microbiome, especially during infection, potentially leading to a paradigm shift in the management of intestinal bacterial-related illnesses or disorders of the digestive system that are triggered by bacteria.
Conflict of Interest
The authors declare no conflicts of interest.
Author Contributions
L.D.K. assisted in the study design, collected in vitro and in vivo experimental data, and wrote the initial draft of the manuscript. G.O. assisted in the experimental design, collected data in the in vivo experiment, and assisted in finalizing the manuscript. R.M. designed and performed the in vivo studies, and facilitated SEM imaging. X.L., P.D., P.P, S.K.D, and S.D. collaborated in the design of the study and analyzed the data. SD postulated the hypothesis of the work, was the initial creator of the study, edited, and finalized the manuscript. All authors discussed the results and had input on the manuscript writing process.
Acknowledgments
S.D. would like to thank the National Science Foundation (ECC-08017788 and CHE-1506740), the Department of Defense Peer Reviewed Medical Research Program (PRMRP) Discover Award (W1XWH-13-1-0343) and the National Institutes of Health (R01GM047915) for funding this work. S.D. thanks the Miller School of Medicine of the University of Miami for the Lucille P. Markey Chair in Biochemistry and Molecular Biology. L.D.K. would like to thank the National Science Foundation Integrative Graduate Education Research Traineeship (DGE-0653710). The research work in Dr. Liu's laboratory is supported by the National Institutes of Health grants R01DC005575, R01DC012546, and R01DC012115. We would like to thank Dr. Pascal J. Goldschmidt and Dr. Michal Toborek for stimulating and helpful discussions as well as Dr. Patricia Blackwelder for helping in scanning electron microscopy experiments. The authors thank Dr. Peter Greenberg for supplying the E. coli strain harboring plasmids pJN105Q and pJL101 and Dr. Johanna Schwingel for providing P. aeruginosa strains PAO1 and JP2.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.05.037.
Appendix A. Supplementary data
Supplementary figures.
References
- Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Beatson S.A., Whitchurch C.B., Sargent J.L., Levesque R.C., Mattick J.S. Differential regulation of twitching motility and elastase production by Vfr in Pseudomonas aeruginosa. J. Bacteriol. 2002;184:3605–3613. doi: 10.1128/JB.184.13.3605-3613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Gray J.A., Roth B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.B., Hughes D.T., Zhu C., Boedeker E.C., Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. PNAS. 2006;103:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Clarke G., Stilling R.M., Kennedy P.J., Stanton C., Cryan J.F., Dinan T.G. Minireview: gut microbiota: the neglected endocrine organ. Mol. Endocrinol. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Davies D.G., Parsek M.R., Pearson J.P., Iglewski B.H., Costerton J.W., Greenberg E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- De Kievit T.R., Gillis R., Marx S., Brown C., Iglewski B.H. Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl. Environ. Microbiol. 2001;67:1865–1873. doi: 10.1128/AEM.67.4.1865-1873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L., Garrett L., Clarke G., Kiely B., Cryan J.F., Dinan T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Erspamer V. 1966. Occurence of Indolealkylamines in Nature: Springer-Verlag) [Google Scholar]
- Hsiao E.Y., Mcbride S.W., Hsien S., Sharon G., Hyde E.R., Mccue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., Patterson P.H., Mazmanian S.K. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery I.B., O'toole P.W., Ohman L., Claesson M.J., Deane J., Quigley E.M., Simren M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- Knights D., Lassen K.G., Xavier R.J. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505–1510. doi: 10.1136/gutjnl-2012-303954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A., Pasini P., Deo S.K., Flomenhoft D., Shashidhar H., Daunert S. Biosensing systems for the detection of bacterial quorum signaling molecules. Anal. Chem. 2006;78:7603–7609. doi: 10.1021/ac061421n. [DOI] [PubMed] [Google Scholar]
- Lee Y.K., Mazmanian S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal A., Kashyap P.C., Nelson T.A., Aronov P.A., Donia M.S., Spormann A., Fischbach M.A., Sonnenburg J.L. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013;7:1933–1943. doi: 10.1038/ismej.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R., Wang Y., Hunter C.J., Gonzalez-Gomez I., Prasadarao N.V. Brain damage in newborn rat model of meningitis by Enterobacter sakazakii: a role for outer membrane protein A. Lab. Investig. 2009;89:263–277. doi: 10.1038/labinvest.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa J., Echizen H., Matsueda K., Umeda N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–194. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- Odobasic D., Kitching A.R., Semple T.J., Holdsworth S.R. Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2007;18:760–770. doi: 10.1681/ASN.2006040375. [DOI] [PubMed] [Google Scholar]
- Oleskin A.V., Kirovskaia T.A., Botvinko I.V., Lysak L.V. Effect of serotonin (5-hydroxytryptamine) on the growth and differentiation of microorganisms. Mikrobiologiia. 1998;67:305–312. [PubMed] [Google Scholar]
- O'mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Pearson J.P., Pesci E.C., Iglewski B.H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.M., Bu Y., Suga H. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 2003;10:81–89. doi: 10.1016/s1074-5521(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Tillisch K., Labus J., Kilpatrick L., Jiang Z., Stains J., Ebrat B., Guyonnet D., Legrain-Raspaud S., Trotin B., Naliboff B., Mayer E.A. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. doi: 10.1053/j.gastro.2013.02.043. (1401 e1-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente E., Lee C., Hor L., Fouz B., Amaro C. Role of the metalloprotease Vvp and the virulence plasmid pR99 of Vibrio vulnificus serovar E in surface colonization and fish virulence. Environ. Microbiol. 2007;10:328–338. doi: 10.1111/j.1462-2920.2007.01454.x. [DOI] [PubMed] [Google Scholar]
- Waters C.M., Lu W., Rabinowitz J.D., Bassler B.L. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 2008;190:2527–2536. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wu X.Y., Yu F.S. Inflammatory responses of corneal epithelial cells to Pseudomonas aeruginosa infection. Curr. Eye Res. 2005;30:527–534. doi: 10.1080/02713680590968150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.