Abstract
Background
Myeloperoxidase (MPO) anti-neutrophil cytoplasm autoantibody (ANCA)-associated vasculitis commonly causes life-threatening pulmonary alveolar hemorrhage or fibrosis. Only a limited number of candidate gene variants have been explored, but hitherto, are not widely confirmed. In the present study, we investigated the importance of energy homeostasis associated gene (Enho) mutations and adropin deficiency in the development of MPO-ANCA associated lung injury.
Methods
We analyzed the peripheral blood mononuclear cells from 152 unrelated patients and 220 population-matched healthy individuals for genetic variations in Enho. Functional studies with adropin knockout (AdrKO) on C57BL/6J mice were also performed.
Findings
Sequencing revealed six patients with p.Ser43Thr and that five patients shared Cys56Trp amino acid substitution in Enho. Serum concentration of adropin was significantly lower in patients than that of the healthy subjects (P < 0.0001), especially those with Enho mutations. In vivo, homo- and heterozygous carriers of the null adropin allele exhibited MPO-ANCA associated pulmonary alveolar hemorrhage as compared to wild-type mice. AdrKO mice exhibit reduced eNOS (Ser1177) and Akt1 (Ser473) phosphorylation and loss of Treg cells.
Interpretation
Our findings indicate that the presence of Enho mutations or adropin-deficiency is a probable molecular basis for the initial events triggered in MPO-ANCA associated lung injury.
Abbreviations: AAV, ANCA-associated vasculitis; ANA, anti-nuclear antibodies; ANCA, anti-neutrophil cytoplasmic antibody; ELISA, enzyme-linked immunosorbent assay; MPO, myeloperoxidase; PR3 or PRTN3, proteinase 3; MHC, major histocompatibility complex; SERPINA1, serpin A1 gene; Enho, energy homeostasis associated; AdrKO, adropin knockout mice; eNOS, endothelial nitric oxide synthase; RNA-seq, Transcriptome deep sequencing; CRISPR, clustered regularly interspaced short palindromic repeats; EC, endothelial cells; CRP, C-reactive protein; TNF-α, tumor necrosis factor alpha; AECA, anti-endothelial cell antibody; OPN, osteopontin; ET-1, endothelin-1; AdrHET, Heterozygous males and females mice; DAPI, 4′, 6-diamidine-2-phenylindole dihydrochloride; VCAM-1, vascular cell adhesion molecule-1; COPA, Coatomer subunit alpha; STING, stimulator of interferon genes
Keywords: Enho mutations, Adropin, MPO-ANCA associated lung injury, eNOS
Graphical Abstract
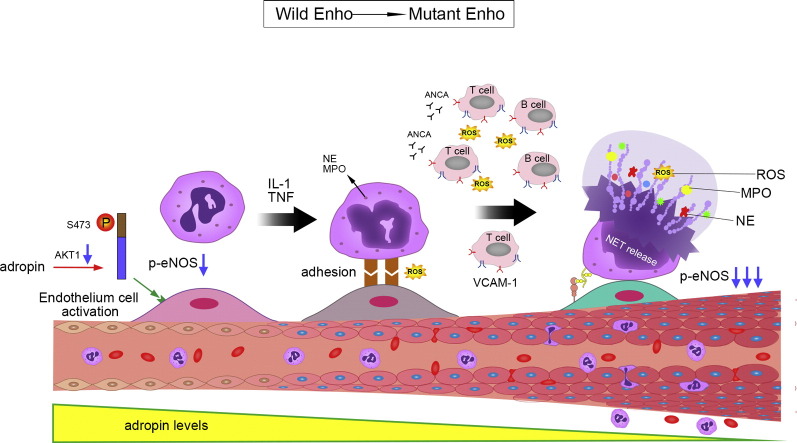
Adropin-deficiency causes ANCA vasculitis.
Adropin-deficiency plays a critical role in activating endothelial cells during neutrophil recruitment and neutrophil-endothelium cell interactions under IL-1 and TNF-α-induced vascular inflammation. Neutrophil extracellular trap (NET) formation, also termed as NETosis, has been linked to develop autoantibodies against endothelial cell, such as VCAM-1. Then, VCAM-1 mediates the adhesion of lymphocytes and monocytes to vascular endothelium.
Highlights
-
•
Enho mutations result in adropin deficiency.
-
•
Adropin deficiency cause MPO-ANCA-related pulmonary hemorrhage or lung fibrosis.
-
•
Adropin knockout (AdrKO) mice exhibit reduced eNOS (Ser1177) and Akt1 (Ser473) phosphorylation and loss of Treg cells in lung tissue.
-
•
PR3-AAV and MPO-AAV may be the two independent disease subtypes and have their different susceptibility genes.
1. Introduction
Anti-neutrophil cytoplasm autoantibody (ANCA)-associated diseases are autoimmune conditions characterized by necrotizing inflammation of small blood vessels with significantly higher mortality rates than other autoimmune diseases (Jones et al., 2010, Nakaya et al., 2013). In ANCA-associated vasculitis (AAV), particularly in myeloperoxidase (MPO)-specific ANCA-positive cases, the clinical studies have been mainly focused on renal lesions (Jennette and Falk, 2014). However, it has become clear that pulmonary lesions such as alveolar hemorrhage or fibrosis appear concurrently to renal lesions (Zhang et al., 2014). Furthermore, there is in vitro as well as in vivo evidence to suggest a potential pathogenic role of ANCA in pulmonary vasculitis (Falk et al., 1990, Holguin et al., 2008), but the pathomechanisms are yet unidentified. Additionally, a debate has ensued about whether it is an autoimmune syndrome of a single disease entity or distinct between proteinase 3 (PR3)-AAV and MPO-AAV (Lyons et al., 2012, Hogan et al., 2006).
Several studies provide evidence of potential genetic contribution towards AAV (Knight et al., 2008, Monach and Merkel, 2010). The most convincing association has been with the major histocompatibility complex (MHC), especially the locus HLA DPB1*0401 (Wieczorek et al., 2010, Jagiello et al., 2004). The other has been suggested between AAV and the rare Z (or null) allele of the serpin A1 gene (SERPINA1) that encodes α1-antitrypsin, a serine proteinase inhibitor for PR3 (Monach and Merkel, 2010, Morris et al., 2011). Both, the HLA-DP and SERPINA1 associations are observed unambiguously in granulomatosis patients with polyangiitis, also positive for PR3-ANCA, but not for MPO-AAV. Thus, the genetic etiology leading to MPO-AAV or MPO-ANCA associated lung injury has remained elusive.
Adropin, a product of the energy homeostasis associated gene (Enho), is a recently identified protein that has been implicated in insulin resistance (Aydin et al., 2013, Kumar et al., 2008) and as a novel regulator of endothelial function (Goetze and Albrethsen, 2014, Lovren et al., 2010). It has been reported that adropin exhibits a potential role to protect endothelium by upregulating endothelial nitric oxide synthase (eNOS) expression through the VEGFR2-PI3K-Akt pathways (Lovren et al., 2010, Ganesh Kumar et al., 2012, Kessenbrock et al., 2009). Our preliminary study found, human MPO-ANCA-related pulmonary hemorrhage displaying adropin-deficiency and target genes mutations. Therefore, we hypothesized that adropin may also exert direct effects on the endothelium in AAV and MPO-ANCA associated lung injury.
2. Materials and Methods
2.1. Study Population
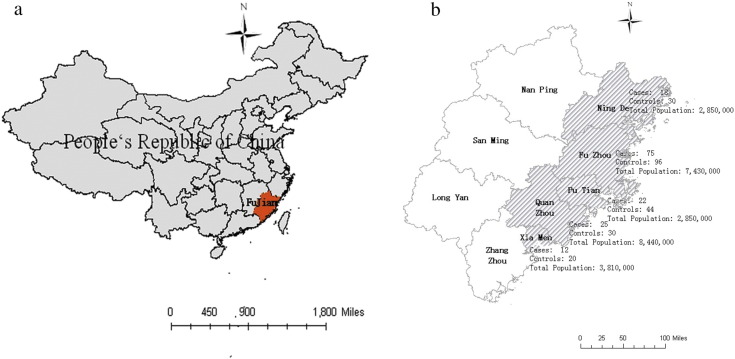
We conducted a chart review for patients who were diagnosed with MPO-ANCA-related vasculitis during hospitalization from February 2013 through November 2015 at the 1st Affiliated Hospital, 2nd Affiliated Hospital, Union Hospital, Mindong Affiliated Hospital, and Quanzhou 1st Affiliated Hospital, Fujian Medical University; Xiamen Hospital of T.C.M; Affiliated Hospital (Group) of Putian University; China (Supplementary Fig. 1). MPO-ANCA-related pulmonary vasculitis cases were further defined by the following inclusion criteria: positive MPO-ANCA serology, open or thoracoscopic lung biopsy, evidence of vasculitis in the histology, and negative serum glomerular basement membrane antibodies. All patients were evaluated for the presence of autoimmune antibodies including anti-nuclear antibody (ANA), MPO-ANCA, and PR3-ANCA. According to the Japanese guideline for idiopathic interstitial pneumonias, basically all interstitial pneumonia cases are recommended to examine serum ANA, MPO-ANCA, PR3-ANCA and other autoimmune antibody as a routine screening test to exclude the collagen vascular disease from idiopathic interstitial pneumonias. In the guideline, the serum MPO-ANCA level of > 20 EU is suggested to be positive. There were 152 patients included, with female predominance (female: male ratio approximately 3:2), age from 39 to 72 years, and the median age was 52.5 years old. What's more, 30 cases of pulmonary bulla (aged from 25 to 60 years old, median age 37.6 years), 34 cases of pathologically confirmed lung cancer (age from 42 to 73, median age 57.5 years), 40 cases with pneumonia (age from 36 to 70, median age 55.3 years) were included, and 220 healthy controls (aged from 35 to 60 years old, with a median age of 47.6 years) were serving as controls. This research project was approved by the Ethics Committee of the Fujian Medical University, which supervised the study.
Supplementary Fig. 1.
Information of patients and controls.
Patients were mainly from the eastern coast of China.
2.2. Analysis of Gene Mutations
Informed consent was obtained from patients and healthy donor controls. Blood was collected and DNA extracted using a Tiangen Genomic extraction kit (Beijing, China). Full-length Enho was amplified, purified, and sequenced.
2.3. Gene Targeting in AdrKO Mice
AdrKO mice were generated by clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 by the Shanghai Biomodel Organism Science & Technology Development Co., Ltd. on the C57BL/6J background. Heterozygous males and females (AdrHET) were then mated to produce homozygous carriers of the null Enho allele (AdrKO). All animal experimental procedures were approved by the Committee, use of Live Animals for Teaching and Research at Fujian Medical University and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals. AdrKO mice and wild-type littermates (WT) were housed in a 12 h light or dark cycle room under controlled temperatures (23 ± 1 °C) with free access to water and standard chow (20% kcal protein, 10% kcal fat, and 70% kcal carbohydrates).
2.4. Reference Multi-testing Algorithm
Serum levels of adropin, C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), anti-endothelial cell antibody (AECA), osteopontin (OPN), endothelin-1 (ET-1), and MPO from AdrKO, AdrHET, and WT mice were measured using a specific enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's protocols.
2.5. RNA-seq
Transcriptome deep sequencing (RNA-seq) was performed using total RNA isolated from lung tissue of three AdrKO and three age-matched littermates (male F2 intercross mice). Three individuals from each genotypic group were randomly selected. Total RNA was extracted from frozen tissue using the SV Total RNA Isolation System (Promega Corporation, Madison, WI) according to the manufacturer's instructions. The quantity and quality of RNA samples were assessed by Nanodrop 1000 (Thermo Fisher Scientific Inc., Wilmington, DE, USA). Total RNA samples were sent to DRIGEN Co., Ltd. for RNA-seq library preparation using the TruSeq SBS Kit (75 Cycles) and single end sequencing by means of an Illumina NextSeq 500 machine (Illumina). RNA-seq reads were quality filtered using SolexaQA packages with default parameters and a filter for the requisite length greater than 70 bp for both ends of each read pair. Sequencing data have been submitted to the NCBI Sequence Read Archive.
Quality filtered RNA-seq reads were mapped to the mouse reference genome, mm10, with TopHat v2.1.0. The comparisons between treated and normal mice were made using custom Perl scripts. Genes that showed significant (P < 0.05) difference in transcript levels were termed as differentially expressed (DE) genes.
2.6. Histology and Immunohistochemistry
Lungs tissue were fixed in 4% formalin overnight, embedded in paraffin, sectioned at 4 mm and stained with hematoxylin and eosin (H&E) for pathology. The following antibodies were used: OPN (ABclonal, A1361), CD3 (ABclonal, A1753), CD20 (ABclonal, A1793), CD38 (ABclonal, A1680), Ki-67 (ABclonal, A18522), CD31 (ABclonal, A31811), and VCAM-1 (ABclonal, A0279).
2.7. Western Blot Analyses
Proteins from lungs of AdrKO and age-matched littermates were separated on 4 to 12% Tris-glycine gels and transferred to nitrocellulose membranes. Membranes were probed with antibodies directed against (phospho-) eNOS (ABclonal, A1548), total eNOS (ABclonal, A1796), (phospho-T450) Akt1 (ABclonal, AP0004), (phospho-S473) Akt1 (ABclonal, AP0140), Mapk1 (ABclonal, A0229), and Gapdh (ABclonal, AP5809).
2.8. Localization of VEGFR2/PI3K/Akt1 and CD4/FOXP3
Immunofluorescence confocal microscopy was also undertaken to determine the correlation of VEGFR2, PI3K, and Akt1. VEGFR2 was detected with rabbit anti-human antibody (Sangon, Shanghai, China) and labelled with a goat anti-rabbit secondary antibody conjugated either to Cy3 or FITC. PI3K was detected with a rabbit anti-human antibody (Wanlei, Shenyang, China) and labelled with a goat anti-rabbit secondary antibody conjugated to Cy3. Akt1 was detected with a mouse anti-human antibody (Sanying, Wuhan, China) and labelled with a goat anti-mouse secondary antibody conjugated to FITC. Nuclei were costained using 4′, 6-diamidine-2-phenylidole dihydrochloride (DAPI).
EC was identified by staining using antiCD31 which was detected with a rabbit antihuman antibody (Santa, USA) and labelled with a goat anti-rabbit secondary antibody conjugated to Cy3. VCAM-1 was detected with a mouse anti-human antibody (Santa, USA) and labelled with a goat anti-mouse secondary antibody conjugated to FITC. And nuclei were costained using DAPI.
Tregs was identified by staining using antiCD4 and antiFoxp3. CD4 was detected with a rabbit antihuman antibody (Sanying, Wuhan, China) and labelled with a goat anti-rabbit secondary antibody conjugated to Cy3. Foxp3 was detected with a mouse anti-human antibody (Santa, USA) and labelled with a goat anti-mouse secondary antibody conjugated to FITC. Nuclei were costained using DAPI.
2.9. Statistics
Statistical differences between groups were assessed by the nonparametric Mann-Whitney U-test for two groups and Kruskal-Wallis test for more than two groups. Bonferroni-Dunn's correction method was applied for post hoc multiple pair-wise comparisons. Spearman's rank correlation coefficient estimated the degree of association between two variables. Significance was calculated at P < 0.05 by GraphPad Prism 5 (La Jolla, CA).
3. Results
3.1. Clinical Characteristics
We identified all of the 152 patients showing higher serum levels of MPO-ANCA (26.90–420.96 EU). Data of other autoimmune antibodies available at the time of biopsy were positive for RF (12/152), ANA (6/152), SS-A (12/152), Jo-1 (0/152), RNP (0/152), Scl-70 (0/152), and SS-B (13/152). The serum levels of hyaluronic acid, type IV collagen, and laminin were significantly increased in the patients with MPO-ANCA associated lung injury, which suggests that the patients develop into fibrosis. Baseline characteristics in the different genotype showed in Table 1.
Table 1.
The clinical data of the MPO-ANCA associated lung injury with- and without-Enho mutations.
|
Enho mutations |
Without Enho mutations | ||||
|---|---|---|---|---|---|
| p.Ser43Thr | p.Cys56Trp | p.Tyr72Tyr | Intron | ||
| Men/women | 3/3 | 4/1 | 1/0 | 8/7 | 45/80 |
| BVAS activity | 18(10–36) | 20 (15–39) | 25 | 21(12–28) | 23 (14–38) |
| Involvement of other organs | |||||
| Kidney | 6(100%) | 5(100%) | 1(100%) | 12(80%) | 96(76.8%) |
| Lung and kidney | 6(100%) | 5(100%) | 1(100%) | 5(33.3%) | 86(68.8%) |
| Digestive system | 2(33.3%) | 2(40%) | 0(0%) | 2(13.3%) | 13(10.4%) |
| Lung involvement type | |||||
| Cavitating infiltrates | 4(66.7%) | 2(40%) | 0(0%) | 6(40%) | 56(44.8%) |
| Interstitial fibrosis | 5(100%) | 5(100%) | 1(100%) | 12(80%) | 78(62.4%) |
| Laboratory parameters | |||||
| MPO-ANCA (< 20 EU) | 186 (86–228) | 163 (81–225) | 206 | 159 (55–289) | 172 (36–356) |
| Adropin (pg/mL) | 33.21 (15.84–55.29) |
59.83 (18.34–68.30) |
36.13 | 98.95 (69.33–172.34) |
71.79 (16.01–160.56) |
| Serum creatinine (58–110) mmol/L | 259.3 ± 96.8 | 219.6 ± 83.6 | 105.8 | 234.6 ± 110.3 | 226.3 ± 75.9 |
| CRP level (< 5) mg/L | 95.6 ± 45.2 | 71.3 ± 55.2 | 36.5 | 106.8 ± 55.7 | 85.2 ± 42.6 |
| Hyaluronic acid (0 − 120) ng/mL | 289(166–362) | 201(93–320) | 169 | 223 (105–332) | 165(90–350) |
| Laminin (0 − 130) ng/mL | 205(106–285) | 152(88–205) | 173 | 186 (102–285) | 182 (155–356) |
| Procollagen type III (< 12) ng/mL | 45 (15–113) | 39 (22–80) | 23 | 19 (12–83) | 29 (16–58) |
| Collagen type IV (0–140 ) ng/mL | 255 (118–368) | 216 (105–338) | 165 | 236 (182–309) | 176 (136–388) |
Values are mean (± SD), median (range) or number (%) of patients.
3.2. Identification of Heterozygous Mutations in Enho
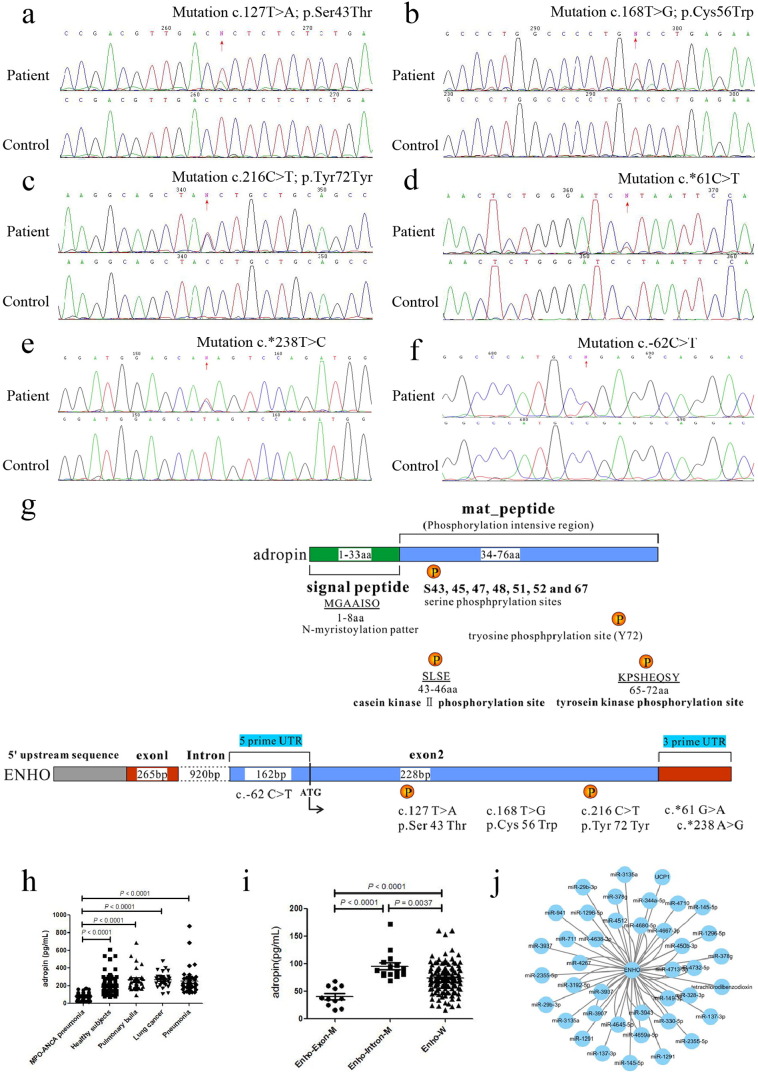
We identified two heterozygous protein-altering variants, that is, 6 patients with c.127T > A (ENSP00000382675.2: p.Ser43Thr) (Fig. 1a) and 5 patients with c.168T > G (p.Cys56Trp) amino acid substitution (Fig. 1b). Moreover, c.216C > T heterozygous mutation (ENST00000399775.2: p.Tyr72Tyr) (Fig. 1c) was found in one patient, which was located at the predicted tyrosine phosphorylation site (Wang et al., 2015, Huttlin et al., 2010, Blom et al., 1999). Another 13 patients harbored mutations located at the 3′ UTR of Enho, i.e., 3 patients with c.*61T > C (Fig. 1d) and 10 patients with c.*238T > C (Fig. 1e). 2 patients were identified with c.-62C > T located at the 5′ UTR of Enho (Fig. 1f). However, none of the mutations were found in the control participants.
Fig. 1.
Mutations of Enho in MPO-ANCA associated lung injury.
(a) c.127T > A (p.Ser43Thr) mutation. (b) c.168T > G mutation result in p.Cys56Trp amino acid substitution. (c) c.216C > T (p.Tyr72Tyr) mutation. (d) c.*61T > C mutation. (e) c.*238T > C mutation. (f) c.-62C > T mutation. (g) Top panel shows scheme of the adropin domain structure. Adropin contains a signal peptide domain (1-33aa, green), and a mat_peptide domain (34-76aa, light blue) which containing predicted serine phosphorylation sites (S43, 45, 47, 48, 51, 52 and 67) and tyrosine phosphorylation site (Y72). Using the Motif Scan program, there are an N-myristoylation patter (MGAAISQ), a casein kinase II phosphorylation site (positions 43–46, SLSE), and a tyrosine kinase phosphorylation site (positions 65–72, KPSHEQSY). Bottom panel shows scheme of the location of Enho mutations causing MPO-ANCA-related pneumonia. (h) The serum concentration of adropin in patients with MPO-ANCA-related pneumonia (MPO-ANCA associated lung injury), healthy subjects, pulmonary bulla, lung cancer, and pneumonia. (i) Serum concentration of adropin in MPO-ANCA associated lung injury patients with different Enho genotypes. (j) MicroRNA-mRNA interaction network of Enho.
The median levels of serum adropin before therapy were significantly lower in the patients with MPO-ANCA associated lung injury than that of the healthy subjects (n = 152, 72.94 pg/mL and n = 220, 173.08 pg/mL, respectively; P < 0.0001). In addition, it was also lower than the patients with pulmonary bulla (n = 30, 231.10 pg/mL; P < 0.0001), lung cancer (n = 33, 272.21 pg/mL; P < 0.0001), and pneumonia (n = 40, 213.05 pg/mL; P < 0.0001) (Fig. 1h).
MPO-ANCA associated lung injury patients were divided into three groups according to Enho genotypes, patients with Enho exon mutations (Ser43Thr, Cys56Trp, and Tyr72Tyr) (Enho-Exon-M, n = 12), patients with 5′ UTR or 3′ UTR mutations of Enho (Enho-Intron-M, n = 15), and patients with wild-type of Enho (Enho-W, n = 125). It was found that serum adropin of the patients with Enho exon mutations was significantly lower as compared to the patients with wild-type Enho (36.91 pg/mL and 71.79 pg/mL, respectively, P < 0.0001) (Fig. 1i).
Furthermore, a microRNA-mRNA interaction network was constructed and the disturbed biological pathways were identified following adropin knockout in order to explore the pathogenesis and occurrence of adropin-deficiency associated with MPO-ANCA associated lung injury (http://www.ingenuity.com/products/ipa). It showed that Enho was mainly regulated by miRNAs, along with the only gene, mitochondrial uncoupling protein 1 (UCP1), which is responsible for the nonshivering thermogenesis in brown adipose tissue (BAT) (Fig. 1j) (Fedorenko et al., 2012).
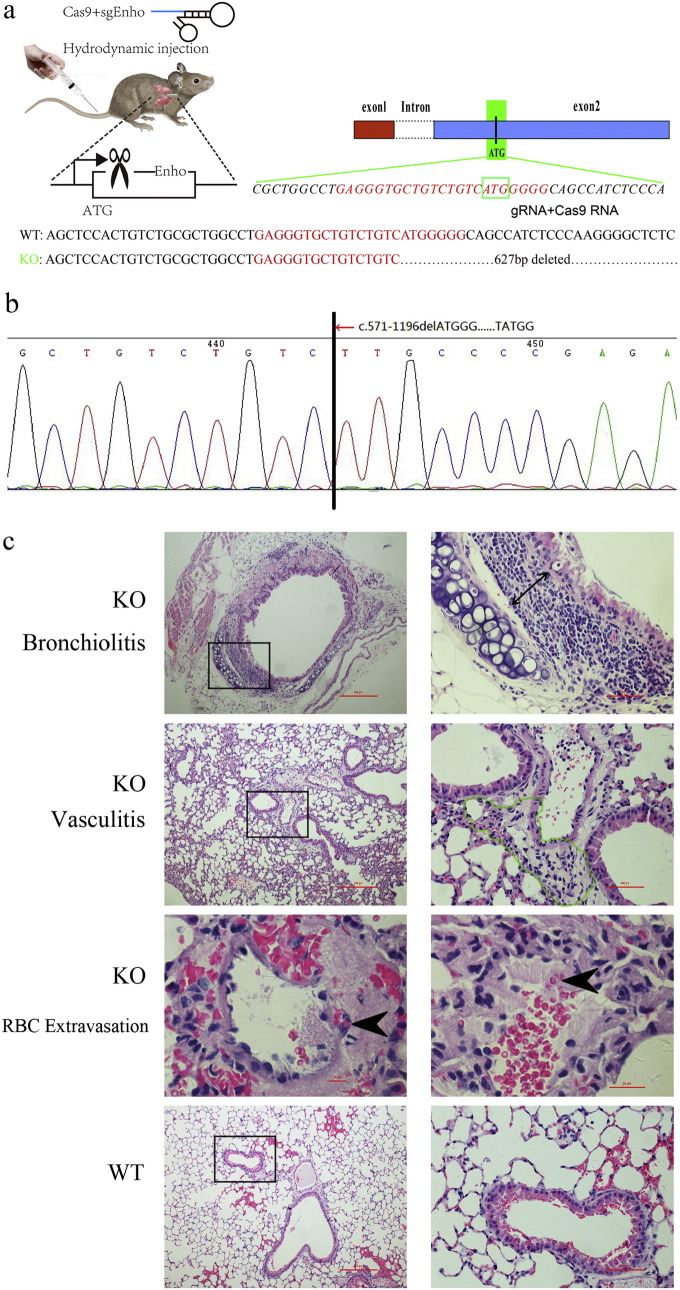
3.3. Pathogenesis of MPO-ANCA Associated Lung Vasculitis in AdrKO Mice
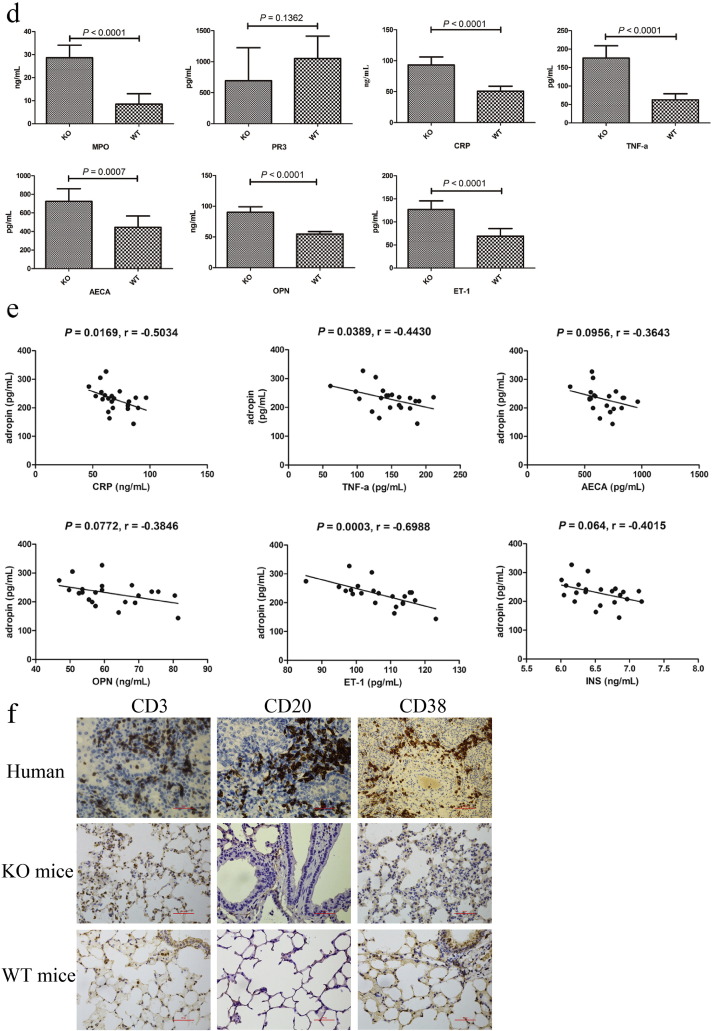
Adropin cDNA from human, mouse, rat, and pig are similar (Goetze and Albrethsen, 2014). To investigate the possibility that adropin serves as an endogenous vasoprotective substance, we used AdrKO mice (Fig. 2a) for assessing the effect of adropin-deficiency on the formation of neointima or vasculitis. F2 intercrossed mice were genotyped by Sanger sequencing (Fig. 2b). Hematoxylin and eosin (H&E) staining of the biopsy from AdrKO mice revealed the typical histopathological features of vasculitis and pneumonia, including red-blood-cell extravasation, a dense perivascular, predominantly neutrophilic infiltrate in the involved blood vessel walls or bronchus of the lung tissue (Fig. 2c), which is a common histopathologic feature found in human MPO-ANCA-related pulmonary hemorrhage. Serum levels of MPO, CRP, TNF-α, AECA, OPN, and ET-1 increased by the age of 8 weeks indicating tissue damage, but serum PR3 was not significantly different between AdrKO and WT mice (P = 0.1362) (Fig. 2d), to some extent, it may show the distinct genetic background for PR3-AAV and MPO-AAV. In AdrHET mice, serum adropin levels were inversely associated with CRP (r = − 0.5034, P = 0.0169), TNF-α (r = − 0.4430, P = 0.0389), AECA (r = − 0.3643, P = 0.0956), OPN (r = − 0.3846, P = 0.0772), ET-1 (r = − 0.6988, P = 0.0003), and insulin (INS) (r = − 0.4015, P = 0.0640), respectively (Fig. 2e). A drastic increase in the immune cell populations, CD3, CD20, and CD38 positive cells in AdrKO lung tissue was defined by histological evaluations. In contrast, the wild-type displayed almost no detectable inflammatory cells (Fig. 2f).
Fig. 2.
AdrKO mice exhibit MPO-ANCA associated lung injury.
(a) Enho single-guide (sg) RNA and target site. The sgRNA target site is located at exon 2, which encodes amino acids upstream of the adropin. (b) Genotyping of F2 intercrossed mice. (c) Development of pneumonia and vasculitis visualized by H&E staining from AdrKO mice. Pneumonia which depicted by double headed arrow (Original magnification: 400 ×), vasculitis depicted with green outline (Original magnification: 400 ×), and red-blood-cell extravasation with black arrowheads (Original magnification: 1000 ×). Scale bar, 100 μm. (d) Serum MPO, CRP, TNF-α, AECA, OPN, and ET-1 were increased in AdrKO mice compared with wild type. n = 8 mice per group. (e) In AdrHET, serum adropin levels were inversely associated with CRP, TNF-α, AECA, OPN, and ET-1. n = 43. (f) Immune cell populations in patient with p.Ser43Thr mutation and AdrKO mice. CD3-positive T-lymphocytes, CD20 immunostaining demonstrates multiple B-cell aggregates, and CD38 demonstrates plasmacytic aggregates are present within the lesion in patient and AdrKO mice. Scale bar, 100 μm.
3.4. Expression Profiling of Lung Tissue Isolates by RNA-seq
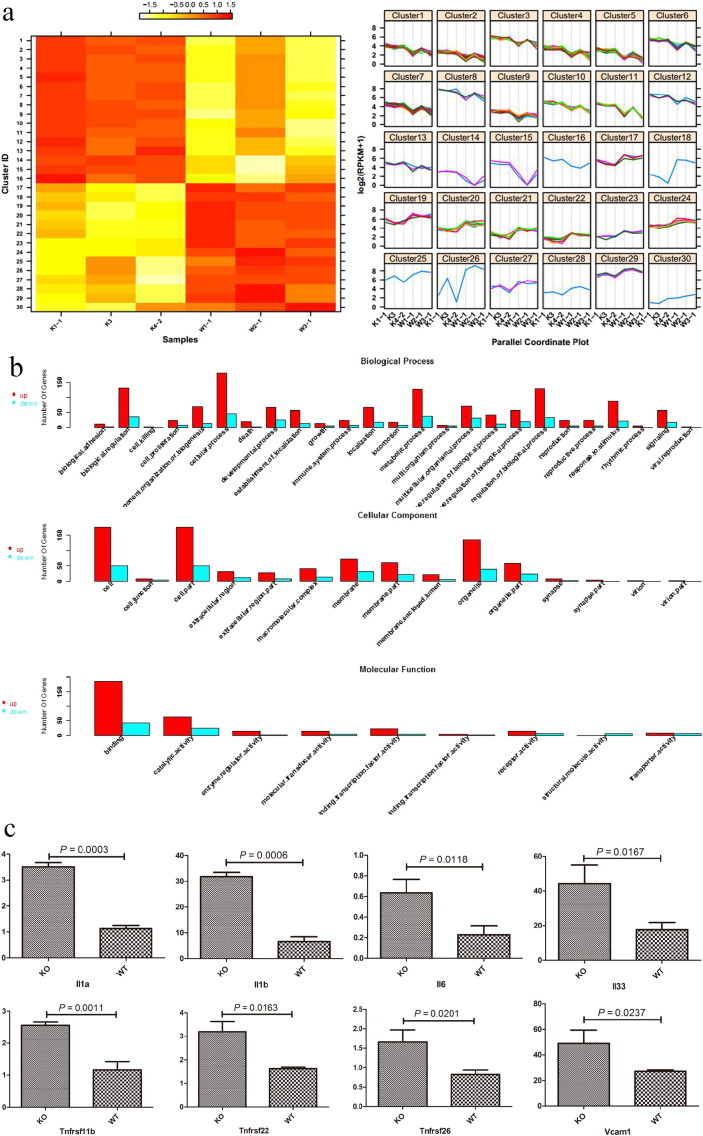
We observed a strong transcriptional interferon response gene signature and decreased levels of adropin and other interferon-induced cytokines in the lung tissues. The profiling also identified a series of genes whose expression level differed significantly between the two groups (KO vs. WT), but not between the samples within the same group (Fig. 3a). The up-regulated genes were mainly observed in enriched iron ion binding, cilia, and radioactive stimulation of the gene ontology in AdrKO mice. The down-regulated genes are mainly enriched in the function of myocardial development and blood circulation (Fig. 3b).
Fig. 3.
Identification of the differentially expressed genes of lungs tissue from AdrKO mice and negative littermates.
(a) Differential gene expression pattern cluster analysis. Color depth in the heatmap represents the different expression levels (heatmap) (left). Levels of differentially expressed genes among samples (right). n = 3 mice per group. AdrKO: K1-1, K3, K4-2. Wild type (negative littermates): W1-1, W2-1, W3-1. (b) Gene Ontology (GO) enrichment of differentially expressed genes. (c) The upregulated angiogenesis-associated genes (The number of label above the bar is fold change obtained from RNA-seq data). (d) MPO was increased in AdrKO mice (The number of label above the bar is fold change obtained from RNA-seq data).
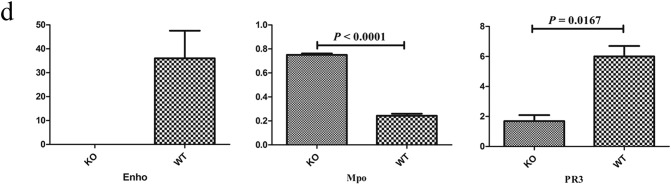
Moreover, increased expression of adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), inflammatory cytokines such as interleukins-1α, -1β, -6, -33, and TNF-α was detected by RNA-seq (Fig. 3c). Similar to human MPO-AAV that is presented as chronic lung hemorrhage, MPO was also significantly increased in the lungs tissue of AdrKO mice (P < 0.0001), while PR3 was significantly lower in AdrKO than that of WT mice (P = 0.0167) (Fig. 3d).
3.5. AdrKO Mice Exhibit Reduced eNOS (Ser1177) and Akt1 (Ser473) Phosphorylation
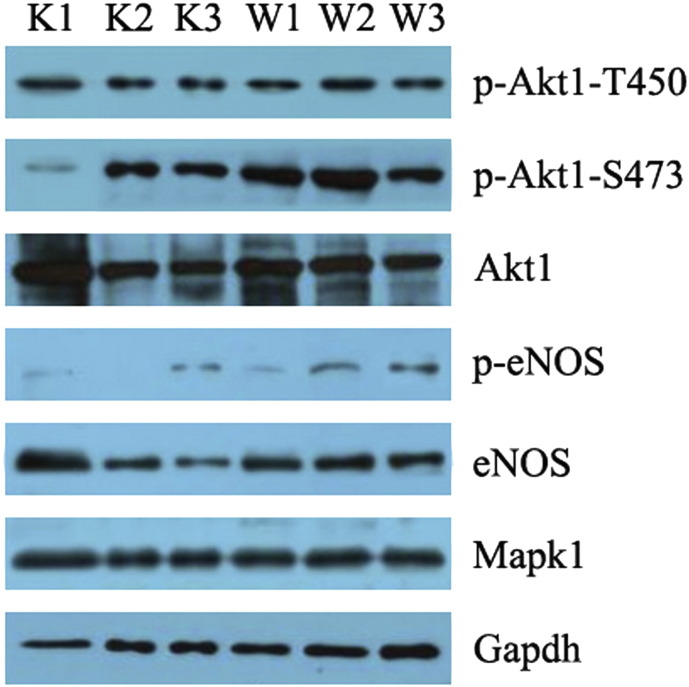
Representative Western blots showed that pAkt1 (T450), total Akt1, total eNOS, and Mapk1 levels were unaffected by adropin, but AdrKO mice exhibited reduced Akt1 (Ser473) phosphorylation. Furthermore, adropin-elicited eNOS phosphorylation at Ser1177 was significantly lower in the lung tissues from AdrKO mice than that of the negative littermates (Fig. 4).
Fig. 4.
AdrKO mice exhibit reduced eNOS (Ser1177) and Akt1 (Ser473) phosphorylation.
Phosphorylation of Akt1 on serine 473 (pAkt1, S473), threonine 450 (pAkt1, T450), and total Akt1 protein. Phosphorylation of eNOS Ser1177 (peNOS, Ser1177) and total eNOS protein. Gapdh levels were assessed as a control for loading. AdrKO mice: K1, K2, and K3. Negative littermates: W1, W2, and W3. n = 3 mice per group.
3.6. Evaluation of Endothelial Cells (ECs) in AdrKO Mice
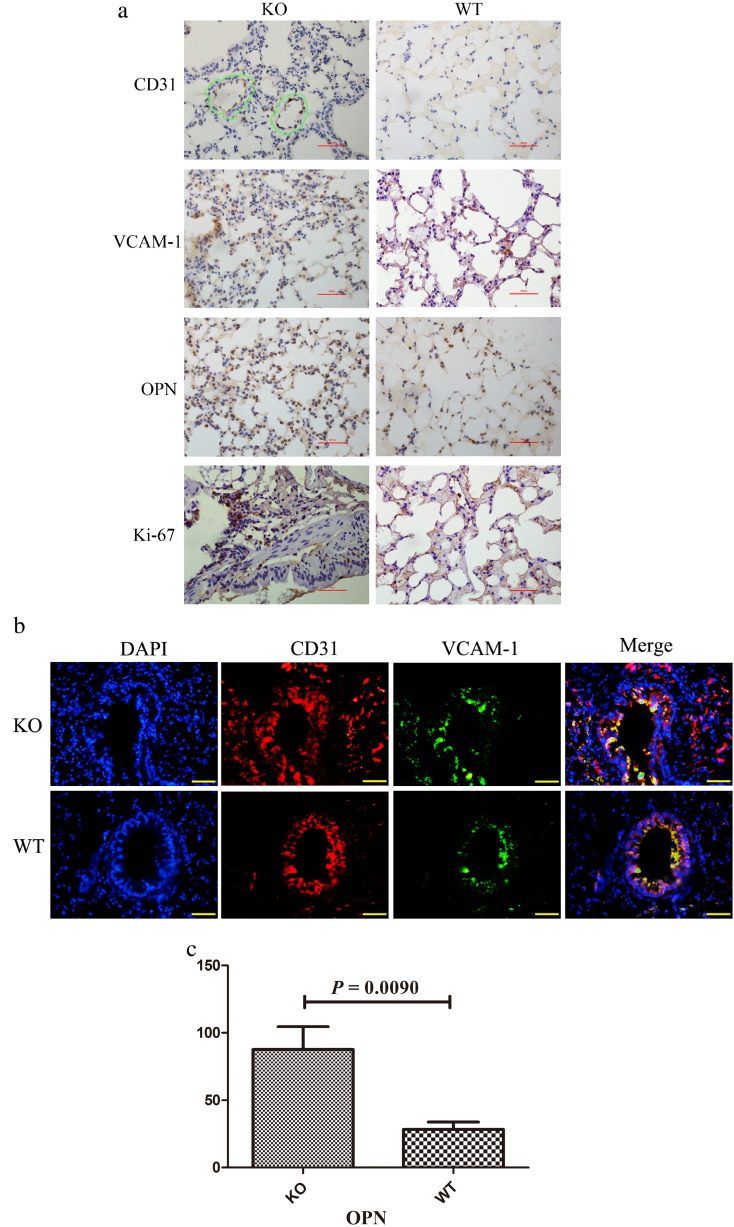
Because early re-endothelialization after vascular injury is known to attenuate neointimal formation, we evaluated the EC marker CD31 by immunostaining (Fig. 5a) and immunofluorescence (Fig. 5b), and observed that CD31 is significantly expressed in AdrKO than WT mice, indicating endothelial cells higher proliferative and vascular injury in AdrKO mice. We next investigated whether AdrKO mice developed autoantibodies against endothelial cell by immunohistochemical staining. VCAM-1 was significantly increased in the 8-week-old AdrKO mice (Fig. 5a). Furthermore, tissue immunofluorescence analysis showed CD31 overlap with VCAM-1 in the endothelial and smooth muscle layers, which confirmed that AdrKO mice developed autoantibodies against endothelial cell and more than the WT mice (Fig. 5a, b). Enho−/− epithelial cell expression of osteopontin (OPN) was significantly increased and the result was reflected as such by RNA-seq (P = 0.0090) (Fig. 5c) and immunohistochemical staining (Fig. 5a). We also estimated the level of cells proliferative activity in vivo by immunostaining for Ki-67 in the endothelial and smooth muscle layers. The number of Ki-67-positive cells was significantly higher in neointimal lesions from AdrKO than that of the WT mice (Fig. 5a), indicating high cell proliferative capacity in adropin-decreased mice.
Fig. 5.
Adropin-deficiency influences the proliferation of VSMCs and ECs.
(a) Immunohistochemical staining of CD31, VCAM-1, OPN, and Ki-67. CD31 positive in the endothelial and smooth muscle layers depicted with green outline. Scale bar, 100 μm. Original magnification: 400 ×. (b) Tissue immunofluorescence analysis showed the CD31 overlap with VCAM-1. A blue DAPI counterstain was used. Original magnification: 400 ×. (c) OPN express in lungs tissue from AdrKO and WT mice by RNA-seq.
3.7. Adropin via VEGFR2-PI3K-AKT1 Pathways and Resulting in Autoimmune Inflammation
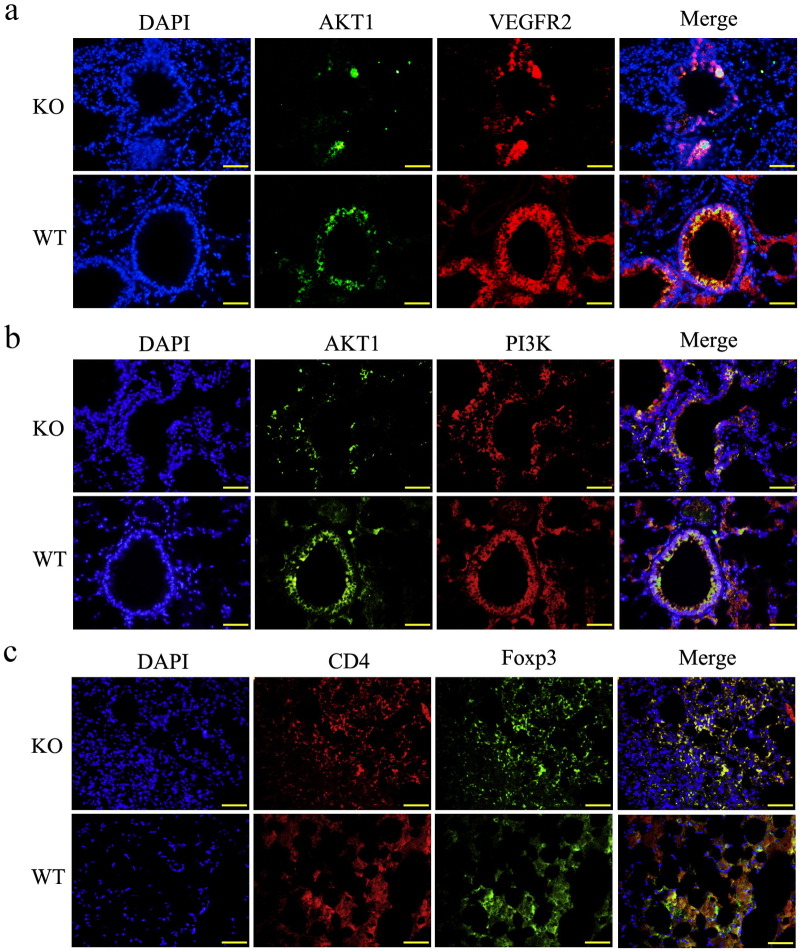
It has been reported that adropin-induced Akt1 phosphorylation is dependent on PI3K. Furthermore, it is also suggested that VEGFR2 is a target of adropin with resultant downstream effects on PI3K-mediated eNOS activation (Lovren et al., 2010). Thus, we investigated the co-localization of the three proteins in the endothelial and smooth muscle layers. It showed the lung tissues from AdrKO expressed lower Akt1 than that of the WT mice (Fig. 6a, b). For cellular localization of proteins, tissue immunofluorescence analysis for staining in the endothelial and smooth muscle layers, showed that the overlap of Akt1 and VEGFR2, and Akt1 and PI3K (yellow staining in the merged image) was also lower in AdrKO mice (Fig. 6a, b) indicating that adropin-deficiency reduced Akt1.
Fig. 6.
In vivo evidence for co-localization of VEGFR2-PI3K-Akt1 and loss of Treg cells in AdrKO mice.
(a) Confocal immunofluorescence analysis showing diminutive areas of colocalized DNA in blue, VEGFR2 in red and Akt1 in green, indicates reduced Akt1 formation in AdrKO mice. (b) Confocal immunofluorescence analysis showing small areas of co-localized DNA in blue, PI3K in red and Akt1 in green, in the endothelial and smooth muscle layers, indicates decreased Akt1 and PI3K activity in AdrKO mice. (c) Colocalization of DNA (blue), CD4 (red) and Foxp3 (green) indicates Treg cells formation. Original magnification: 400 ×.
The production of isotype-switched autoantibodies in AAV suggests the involvement of CD4+ helper T cells in the autoimmune process (Free et al., 2013). In this study, we found that the proportion and an absolute number of CD4+ Foxp3+ (Treg) cells were significantly decreased in lung tissues of AdrKO mice when compared with the matched Enho+/+ littermates which further suggested that adropin-deficiency is associated with inhibition of Treg cells (Fig. 6c).
4. Discussion
ANCA-associated vasculitis (AVV) is a life-threatening condition and patients typically sustain renal and pulmonary injury (Holguin et al., 2008, Lally and Spiera, 2015, Cid, 2012). It has further confirmed that the impaired endothelial function contributes to the development and progression of diverse vascular, inflammatory, and pulmonary hemorrhage (Verma et al., 2003, Dzau et al., 2002). Moreover, endothelium and inflammatory cell infiltration of the neointima is intimately related to oxidative stress (Duckers et al., 2001). In the present study, we showed Enho mutations/adropin-deficiency results in the activation of endothelial cells during neutrophil recruitment and neutrophil-endothelial cell interactions under TNF-α-induced vascular inflammation to be associated with MPO-ANCA associated lung injury.
We identified the serum concentration of adropin was significantly lower in the patients, and we bring hypothesis whether it is associated with Enho mutations. To our surprise, there were not all patients carried Enho mutations. In order to explore the pathogenesis and occurrence of adropin associated with MPO-ANCA-related pulmonary hemorrhage or fibrosis. A microRNA-mRNA interaction network was constructed and it showed that Enho is mainly regulated by miRNAs which revealed that adropin low expression in wild-type patients may be related to the regulation of micro-RNA. To gain more insight into the genotype-phenotype correlation in adropin-deficiency, we used AdrKO mice to investigate the genotype-proteotype-phenotype correlation in patients with adropin-deficiency.
Enho−/− mice showed MPO-ANCA-related pulmonary hemorrhage typical of vasculitis, including red-blood-cell extravasation, a dense perivascular, and predominantly neutrophilic infiltrate in the vascular or bronchus, developed autoantibodies against endothelial cell (VCAM-1 elevated), and autoimmunity pulmonary injury (CD3+, CD20+, and CD38+ cell infiltrate). Elevated serum levels of MPO, CRP, TNF-α, AECA, ET-1, and OPN were detected in AdrKO mice. Furthermore, our study describes the RNA-seq profiling of the pneumonia from AdrKO mice. 507 genes were identified as vascular or autoimmune target candidates. Some examples are cyclin E and mitogen- and stress-activated protein kinases 1 (MSK1) which confirmed cell cycle G1/S progression, monocyte chemoattractant protein-1 gene (MCP-1) for phagocyte recruitment or inflammation, and atrial natriuretic peptide for vascular tone. As compared to the lean mice, the deficiency of adropin decreased Akt1 (Ser473) phosphorylation and downregulated peNOS expression in the lungs of AdrKO mice. It has been reported that Treg cells expressing Foxp3 control the onset and the development of autoimmune disease (Sakaguchi et al., 1995). So, we hypothesized that adropin-deficiency maybe the driver of Treg cells impairment in patients with MPO-ANCA-related lung disease. In vivo, it showed that the proportion and an absolute number of CD4+ Foxp3+ cells were significantly decreased in AdrKO mice and these results provide further support for the hypothesis that adropin is a peptide hormone involved in regulating vascular inflammation and autoimmunity.
Adropin may be linked to insulin resistance and the pathogenesis of various diseases through altered immune responses (Ganesh Kumar et al., 2012, Hotamisligil, 2006, Bapat et al., 2015). In this study, we have demonstrated that VCAM-1 and CD31 were significantly increased in AdrKO mice and the two proteins were co-localized in the endothelial and smooth muscle layers which confirmed that antibody (VCAM-1) against endothelial cell (Kaplanski et al., 2005). This may be another manifestation or characteristic of ANCA associated vasculitis. Furthermore, adropin-deficiency leads to the activation of vascular endothelium expressing adhesion molecules which may accelerate and localize foci to the inflammatory processes (Graphical abstract).
Although there were other genes mutations detected in autoimmune-mediated lung disease and led to the production of anti-ANCA antibodies, their clinical manifestations may be significantly different. Enho mutations cause MPO-ANCA-related pulmonary hemorrhage, who presented with acute renal or respiratory failure, or both. However, its greatest feature is that almost all patients with arthritis in coatomer subunit alpha (COPA) mutations (Watkin et al., 2015) and stimulator of interferon genes (STING)-associated vasculopathy presented in early infancy with systemic inflammation and violaceous, scaling lesions of fingers, toes, nose, cheeks, and ears and did not respond to hormone therapy (Liu et al., 2014). Half of the patients had severe interstitial lung disease and autoantibodies (ANCAs) that are seen in vasculitis and the antiphospholipid syndrome, but these antibodies disappeared over time (Liu et al., 2014). The role of genetic variation in the immune mechanism remains to be further studied.
In conclusion, Enho mutations or adropin-deficiency plays a critical role in activating endothelial cells during neutrophil recruitment and neutrophil-endothelium cell interactions under IL-1 and TNF-α-induced vascular inflammation and increases susceptibility to MPO-ANCA associated lung injury.
The following are the supplementary data related to this article.
Author Contributions
Q.-C.L., F.G., and X.-H.L. planned the project. F.-L. C., Q.-C. L., F.G., and J.F. conceived of and designed the study. F.G., C.-D.W., J.-C.Z., S.Z., X.-T.L., and Q.-L.H. performed the sample collection. S.C., S.-H.W., F.G., and S.Z. performed immunohistochemistry. Q.-C.L., F.G., and S.C. participated in the in vivo procedures. Q.-C.L., F.G., and X.-H.L. performed the expression analysis. Q.-C.L., F.G., S.-H.W., and X.-T.L. analyzed the data and drafted the manuscript. All authors reviewed the manuscript and approved the final version.
Conflicts of Interest
The authors have no competing interests to declare.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81571613, no. 81572442, no. 81201590, and no. 21275028) and by the National key Technology R&D Program of China (no. 2012AA022604). These funding sources played key supportive role for sample collection, molecular analysis of patient samples, and bioinformatics analysis. The authors also thank Dr. Qingquan Chen, Fujian Medical University, China, for his help with formatting the manuscript.
References
- Aydin S., Kuloglu T., Aydin S., Eren M.N., Yilmaz M., Kalayci M. Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol. Cell. Biochem. 2013;380(1–2):73–81. doi: 10.1007/s11010-013-1660-4. [DOI] [PubMed] [Google Scholar]
- Bapat S.P., Myoung Suh J., Fang S., Liu S., Zhang Y., Cheng A. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528(7580):137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom N., Gammeltoft S., Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999;294(5):1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- Cid M.C. The search for genetic links in ANCA-associated vasculitis and its variants. N. Engl. J. Med. 2012;367(3):271–273. doi: 10.1056/NEJMe1203592. [DOI] [PubMed] [Google Scholar]
- Duckers H.J., Boehm M., True A.L., Yet S.F., San H., Park J.L. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat. Med. 2001;7(6):693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- Dzau V.J., Braun-Dullaeus R.C., Sedding D.G. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat. Med. 2002;8(11):1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- Falk R.J., Terrell R.S., Charles L.A., Jennette J.C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl. Acad. Sci. U. S. A. 1990;87(11):4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko A., Lishko P.V., Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151(2):400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free M.E., Bunch D.O., McGregor J.A., Jones B.E., Berg E.A., Hogan S.L. Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheum. 2013;65(7):1922–1933. doi: 10.1002/art.37959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh Kumar K., Zhang J., Gao S., Rossi J., McGuinness O.P., Halem H.H. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring) 2012;20(7):1394–1402. doi: 10.1038/oby.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetze J.P., Albrethsen J. Adropin: a new regulatory peptide in cardiovascular endocrinology. Regul. Pept. 2014;190–191:41–42. doi: 10.1016/j.regpep.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Hogan S.L., Falk R.J., Nachman P.H., Jennette J.C. Various forms of life in antineutrophil cytoplasmic antibody-associated vasculitis. Ann. Intern. Med. 2006;144(5):377–378. doi: 10.7326/0003-4819-144-5-200603070-00019. (author reply 378-379) [DOI] [PubMed] [Google Scholar]
- Holguin F., Ramadan B., Gal A.A., Roman J. Prognostic factors for hospital mortality and ICU admission in patients with ANCA-related pulmonary vasculitis. Am. J. Med. Sci. 2008;336(4):321–326. doi: 10.1097/MAJ.0b013e31816805fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Huttlin E.L., Jedrychowski M.P., Elias J.E., Goswami T., Rad R., Beausoleil S.A. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143(7):1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagiello P., Gencik M., Arning L., Wieczorek S., Kunstmann E., Csernok E. New genomic region for Wegener's granulomatosis as revealed by an extended association screen with 202 apoptosis-related genes. Hum. Genet. 2004;114(5):468–477. doi: 10.1007/s00439-004-1092-z. [DOI] [PubMed] [Google Scholar]
- Jennette J.C., Falk R.J. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat. Rev. Rheumatol. 2014;10(8):463–473. doi: 10.1038/nrrheum.2014.103. [DOI] [PubMed] [Google Scholar]
- Jones R.B., Tervaert J.W., Hauser T., Luqmani R., Morgan M.D., Peh C.A. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N. Engl. J. Med. 2010;363(3):211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- Kaplanski G., Maisonobe T., Marin V., Grès S., Robitail S., Farnarier C. Vascular cell adhesion molecule-1 (VCAM-1) plays a central role in the pathogenesis of severe forms of vasculitis due to hepatitis C-associated mixed cryoglobulinemia. J. Hepatol. 2005;42(3):334–340. doi: 10.1016/j.jhep.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Krumbholz M., Schonermarck U., Back W., Gross W.L., Werb Z. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009;15(6):623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A., Sandin S., Askling J. Risks and relative risks of Wegener's granulomatosis among close relatives of patients with the disease. Arthritis Rheum. 2008;58(1):302–307. doi: 10.1002/art.23157. [DOI] [PubMed] [Google Scholar]
- Kumar K.G., Trevaskis J.L., Lam D.D., Sutton G.M., Koza R.A., Chouljenko V.N. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8(6):468–481. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally L., Spiera R.F. Pulmonary vasculitis. Rheum. Dis. Clin. N. Am. 2015;41(2):315–331. doi: 10.1016/j.rdc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Liu Y., Jesus A.A., Marrero B., Yang D., Ramsey S.E., Montealegre Sanchez G.A. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 2014;371(6):507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovren F., Pan Y., Quan A., Singh K.K., Shukla P.C., Gupta M. Adropin is a novel regulator of endothelial function. Circulation. 2010;122(11 Suppl):S185–S192. doi: 10.1161/CIRCULATIONAHA.109.931782. [DOI] [PubMed] [Google Scholar]
- Lyons P.A., Rayner T.F., Trivedi S., Holle J.U., Watts R.A., Jayne D.R. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 2012;367(3):214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monach P.A., Merkel P.A. Genetics of vasculitis. Curr. Opin. Rheumatol. 2010;22(2):157–163. doi: 10.1097/BOR.0b013e32833654a8. [DOI] [PubMed] [Google Scholar]
- Morris H., Morgan M.D., Wood A.M., Smith S.W., Ekeowa U.I., Herrmann K. ANCA-associated vasculitis is linked to carriage of the Z allele of alpha(1) antitrypsin and its polymers. Ann. Rheum. Dis. 2011;70(10):1851–1856. doi: 10.1136/ard.2011.153569. [DOI] [PubMed] [Google Scholar]
- Nakaya I., Yahata M., Takahashi S., Sasajima T., Sakuma T., Shibagaki Y. Long-term outcome and efficacy of cyclophosphamide therapy in Japanese patients with ANCA-associated microscopic polyangiitis: a retrospective study. Intern. Med. 2013;52(22):2503–2509. doi: 10.2169/internalmedicine.52.0199. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155(7):1151–1164. [PubMed] [Google Scholar]
- Verma S., Buchanan M.R., Anderson T.J. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108(17):2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- Wang S.P., Gao Y.L., Liu G., Deng D., Chen R.J., Zhang Y.Z. Molecular cloning, characterization and expression of the energy homeostasis-associated gene in piglet. J. Zhejiang Univ. Sci. B. 2015;16(6):524–532. doi: 10.1631/jzus.B1400260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkin L.B., Jessen B., Wiszniewski W., Vece T.J., Jan M., Sha Y. COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat. Genet. 2015;47(6):654–660. doi: 10.1038/ng.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek S., Holle J.U., Epplen J.T. Recent progress in the genetics of Wegener's granulomatosis and Churg-Strauss syndrome. Curr. Opin. Rheumatol. 2010;22(1):8–14. doi: 10.1097/BOR.0b013e3283331151. [DOI] [PubMed] [Google Scholar]
- Zhang S., Shu X., Tian X., Chen F., Lu X., Wang G. Enhanced formation and impaired degradation of neutrophil extracellular traps in dermatomyositis and polymyositis: a potential contributor to interstitial lung disease complications. Clin. Exp. Immunol. 2014;177(1):134–141. doi: 10.1111/cei.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]