Abstract
Aims
We aimed to determine whether the levels of total serum IgM and IgG, together with specific antibodies against malondialdehyde-conjugated low-density lipoprotein (MDA-LDL), can improve cardiovascular risk discrimination.
Methods and Results
The Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) randomized 9098 patients in the UK and Ireland into the Blood Pressure-Lowering Arm. 485 patients that had cardiovascular (CV) events over 5.5 years were age and sex matched with 1367 controls. Higher baseline total serum IgG, and to a lesser extent IgM, were associated with decreased risk of CV events (IgG odds ratio (OR) per one standard deviation (SD) 0.80 [95% confidence interval, CI 0.72,0.89], p < 0.0001; IgM 0.83[0.75,0.93], p = 0.001), and particularly events due to coronary heart disease (CHD) (IgG OR 0.66 (0.57,0.76); p < 0.0001, IgM OR 0.81 (0.71,0.93); p = 0.002). The association persisted after adjustment for a basic model with variables in the Framingham Risk Score (FRS) as well as following inclusion of C-reactive protein (CRP) and N-terminal pro-B-type natriuretic peptide (NtProBNP). IgG and IgM antibodies against MDA-LDL were also associated with CV events but their significance was lost following adjustment for total serum IgG and IgM respectively. The area under the receiver operator curve for CV events was improved from the basic risk model when adding in total serum IgG, and there was improvement in continuous and categorical net reclassification (17.6% and 7.5% respectively) as well as in the integrated discrimination index.
Conclusion
High total serum IgG levels are an independent predictor of freedom from adverse cardiovascular events, particularly those attributed to CHD, in patients with hypertension.
Keywords: Immunoglobulins, Anti-oxidized-LDL antibodies, Cardiovascular risk stratification
Highlights
-
•
We studied whether immunoglobulins predict cardiovascular events in hypertension.
-
•
Total serum IgG was strongly associated with freedom from adverse events.
-
•
The predictive power of total IgG was superior to antibodies against MDA-LDL.
-
•
Total serum IgG significantly improved cardiovascular risk reclassification.
-
•
Further work is needed to determine whether these observations extend to other populations.
Current clinical tools for stratifying cardiovascular risk remain relatively imprecise. In view of interests in the immune system in atherosclerosis, our study explored whether measuring IgG and IgM immunoglobulin levels provides any improvement in determining risk in hypertensive patients. We found that high levels of total serum IgG, and to a lesser extent IgM, were strongly associated with freedom from coronary events. We also found that antibodies against one type of oxidized LDL conferred protection, but the effect was weaker than with total immunoglobulins. Our results support a link between robust humoral immunity and cardiac health.
1. Introduction
Efforts to improve the predictive capacity of existing cardiovascular risk-scoring models have been intense and have included the study of both novel blood and imaging biomarkers (Hoefer et al., 2015). Despite this, better and affordable tools to predict cardiovascular risk are still needed to avoid misclassification of “high risk” patients and resultant over- or under-treatment (Yeboah et al., 2015).
Atherosclerosis, the main pathological entity that leads to cardiovascular events, is a chronic inflammatory disease in which the innate and adaptive immune systems can play pathological or protective roles, depending on context (Hansson and Libby, 2006). Although total serum immunoglobulins (Igs) are not normally considered as relevant to clinical CV disease, there is abundant evidence in the preclinical literature to suggest links with atherosclerosis. Our group established that mice deficient in IgM develop marked acceleration of atherosclerosis (Lewis et al., 2009). Moreover, passive immunization of mice with polyclonal IgM or IgG, or adoptive transfer of IgM-secreting B-1 cells, retards atherosclerosis progression (Nicoletti et al., 1998, Yuan et al., 2003, Kyaw et al., 2011, Cesena et al., 2012, Rosenfeld et al., 2015). On the other hand, depletion of IgG-secreting B cells reduces atherosclerosis and their adoptive transfer accelerates it (Ait-Oufella et al., 2010, Kyaw et al., 2010). Whilst the weight of evidence favors IgM being protective, there is still uncertainty as to how IgG antibodies influence atherosclerosis, in view of the pathogenic potential of IgG Fc-mediated pro-inflammatory effector functions.
A previous study found that serum IgG but not IgM was associated with a higher risk of myocardial infarction in dyslipidemic patients (Kovanen et al., 1998), whilst another failed to show any association between IgG or IgM and risk of myocardial infarction in a general population (Muscari et al., 1995). However, the roles of specific antibodies as biomarkers in atherosclerosis have been more extensively studied. These include antibodies directed against epitopes induced by oxidative modifications of low-density lipoprotein (LDL), in particular antibodies reacting with phosphorylcholine or adducted malondialdehyde (MDA), as well as many less well-defined epitopes (Leibundgut et al., 2013, Tsiantoulas et al., 2014). In the Bruneck study, IgG antibodies against copper-oxidized LDL (heavily-oxidized LDL that includes MDA-LDL epitopes) were associated with higher risk of CV events, whilst IgM antibodies were associated with lower risk (Rosenfeld et al., 1990, Tsimikas et al., 2012). In contrast, the EPIC Norfolk study implicated IgG and IgM anti-MDA-LDL antibodies as possible modifiers of the risk associated with oxidative biomarkers, rather than independent predictors of coronary artery disease events (Ravandi et al., 2011). Recently, a study from Sweden has reported that individuals with low levels of antibodies against MDA-adducted LDL peptides are associated with significantly higher coronary acute event rate (Bjorkbacka et al., 2016).
The Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) was an independent, investigator-led, multicenter, randomized trial designed to compare two anti-hypertensive treatment strategies for the prevention of CV events in more than 19,000 hypertensive patients without a clinical history of CHD. Using a nested case-control substudy of ASCOT, we set out to test the hypothesis that total serum IgM and IgG levels, as well as antibodies to MDA-LDL, are negative risk factors for cardiovascular events in a hypertensive population. Our analyses incorporated adjustments for levels of C-reactive protein (CRP) and N-terminal pro-B-type natriuretic peptide (NtProBNP), which have previously been studied in the ASCOT population (Sever et al., 2012, Welsh et al., 2014).
2. Material and Methods
2.1. Study Design and Subjects
ASCOT was an independent, investigator-led, multicenter, randomized trial designed to compare two anti-hypertensive treatment strategies for the prevention of CV events in more than 19,000 hypertensive patients without a clinical history of CHD (9098 were randomized in the UK or Ireland). Hypertensive patients with three or more other risk factors for CV disease were eligible for inclusion in ASCOT. The detailed ASCOT protocol has been published previously and further information is available at http://www.ascotstudy.org (Sever et al., 2001). Informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the participating institutions research ethics committees. In the main cohort, the ASCOT-Blood Pressure-Lowering Arm (BPLA), subjects received a beta-blocker with a thiazide diuretic as required or a dihydropyridine calcium channel blocker (CCB) with an angiotensin-converting enzyme inhibitor as required (Sever et al., 2006). In addition to randomization into ASCOT-BPLA, those with a fasting total cholesterol of ≤ 6.5 mmol/L (250 mg/dL) were further randomized, using a factorial design, to either 10 mg atorvastatin daily or matching placebo (ASCOT-LLA) (Sever et al., 2003, Dahlof et al., 2005). Baseline characteristics of participants and primary outcomes of each arm of the trial have been reported previously (Sever et al., 2001, Dahlof et al., 2005).
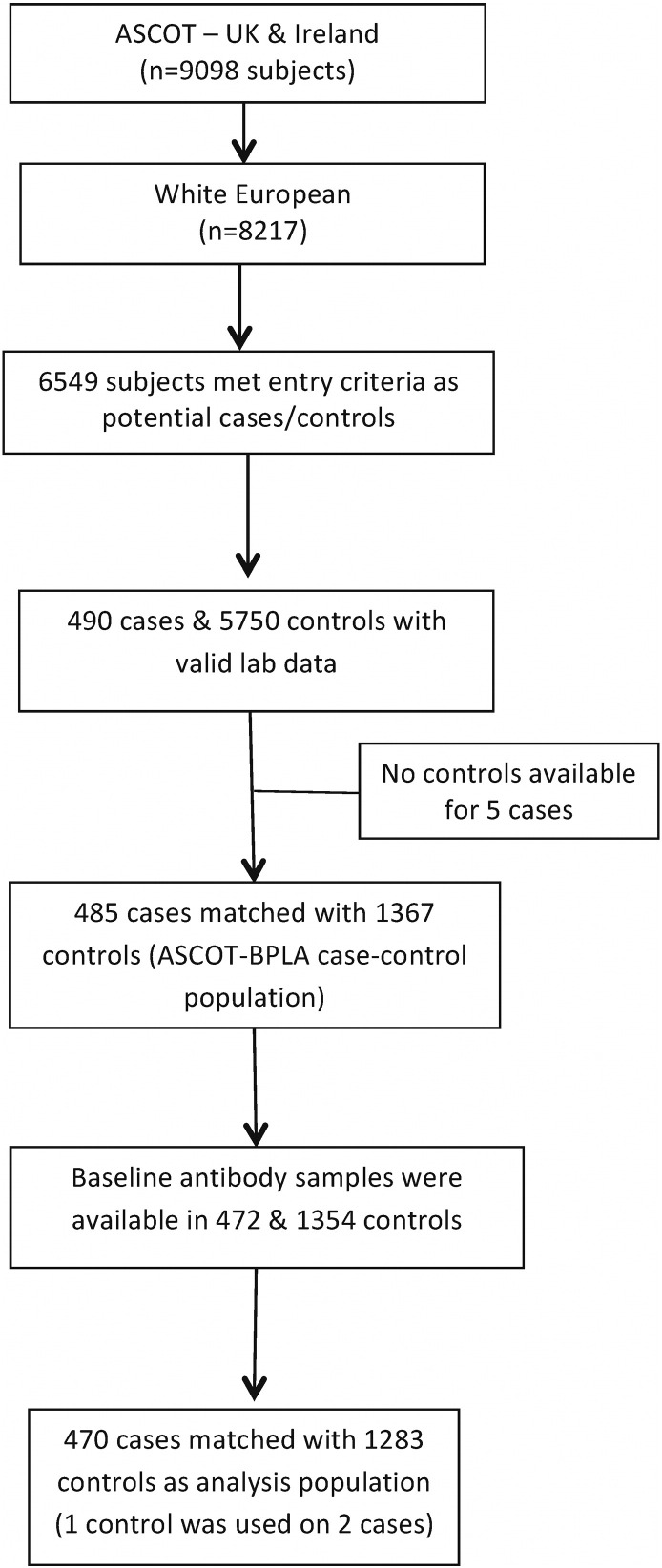
For the purposes of this case-control study (CCS), all events of fatal coronary heart disease (CHD), symptomatic non-fatal myocardial infarction (MI), coronary revascularization, and fatal and non-fatal stroke occurring in the UK and Ireland during the ASCOT-BPLA study period between February 1998 and October 2005 were identified as cases. Subjects with established cardiovascular disease (CVD) at baseline (including peripheral vascular disease and previous history of stroke, transient ischemic attack and recorded other relevant CVD) were excluded from this CCS. Hence, the present report is based on a nested CCS drawn from ASCOT participants in the UK and Ireland, 485 of whom developed CV events (355 CHD and 130 strokes) during a median follow-up period of 5.5 years. Further details of the selection process into the study are shown in Fig. 1.
Fig. 1.
ASCOT immunoglobulin nested CCS profile.
Subjects who were alive and free from CV events during the study period were selected from the same study population as controls. Up to three controls were matched to each case by age (± 1 year), sex and study entry time (± 90 days), with one control being used with two different cases to facilitate matching. The study population was restricted to those self-defined as of white European ethnicity.
2.2. Endpoint Adjudication
All cases suspected of fulfilling criteria for the fatal and non-fatal events were classified by the Endpoint Committee. This included endpoints notified by investigators and potential silent MIs identified by the core ECG laboratory. All the documentation for each potential endpoint was reviewed independently by two of the Endpoint Committee members. In the event of disagreement, a third member the Endpoint Committee adjudicated. Final classification required agreement between two members of the Endpoint Committee. The Endpoint Committee met to resolve such disagreements and “grey cases.”
2.3. Measurements
All major cardiovascular disease (CVD) risk factors incorporated in the FRS were determined at baseline (Sever et al., 2001). Sera were prepared and aliquoted at entry to the study, and stored at − 70 °C prior to analysis. Serum IgM and IgG, and anti-MDA-LDL antibodies, were measured by enzyme-linked immunosorbent assay (ELISA) (see below). Other laboratory tests (including CRP and NtProBNP) were performed as previously described (www.ascotstudy.org) (Sever et al., 2012, Welsh et al., 2014).
2.4. Immunoglobulin and Anti-MDA-LDL Antibody Level Measurements by ELISA
All ELISAs were conducted blindly with both cases and controls mixed according to random laboratory sample number. Personnel conducting assays were blind to patient-specific data and not aware of the statistical analysis plan. The intra-plate variability for all assays was below 3% and inter-plate variability below 13%, with assay ranges that span well above and below expected normal ranges for total IgG and IgM and well below and above the study range for IgG and IgM anti-MDA-LDL (Supplementary Methods Table 1).
Total IgG and IgM antibody levels were measured using capture ELISA. Briefly, either goat anti-human IgG or mouse anti-human IgM (Southern Biotech, Birmingham AL) was used to capture either IgG or IgM respectively. Detection antibodies used were either biotinylated goat F(ab')² anti-human IgG or biotinylated mouse anti-human IgM (both Southern Biotech). Detection was with HRP-conjugated streptavidin (R&D Systems, Minneapolis, MN) followed by 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma Aldrich, UK), and the reaction was stopped with 0.5 M H2SO4. Optical densities were measured with a Synergy HT microplate reader (BioTek, USA) at wavelength 450 nm. Once background was subtracted, results were expressed in Units (U). Cut off and tertile as well as interquartile values in mg/mL for total IgG and IgM were interpolated via standard curve fit using standard ELISA methodology in PRISM 6 (GraphPad, La Jolla, Ca).
IgG and IgM anti-MDA-LDL antibody levels were estimated by ELISA against solid phase MDA-LDL. Briefly, MDA-LDL was prepared as described (Palinski et al., 1990). The extent of modification was determined by the relative electrophoretic mobility of the LDL using pre-cast 0.6% agarose, 1.0% barbital buffer gels (Beckman Coulter, Fullerton, Ca) and with a carbonyl assay (Chang et al., 2012). IgG and IgM antibody binding to solid phase antigens was identified by mouse anti-human IgG (Cambridge Bioscience, Cambridge, UK) or biotinylated mouse anti-human IgM (Cambridge Bioscience) followed by horse radish peroxidase (HRP) conjugated rabbit anti-mouse Ig (Dako, Cambridgeshire, UK) or as above with HRP-conjugated streptavidin. The plates were read as above at an optical density of 450 nm (OD450), after which the background was subtracted. All serum anti-MDA-LDL sample values were corrected to a reference serum with a standard curve used on all plates and results expressed in Units (U).
All assays were optimized for serum concentration, secondary antibody concentration and timing of TMB reactions using representative sera. Standard curves were established for each of the assays on several representative sera and selected serum dilution was at the steepest part of the curve to achieve the highest sensitivity. Furthermore, standard curves were included for a control serum on each ELISA plate for quality control. The lower and higher limit of assay sensitivity for all assays are shown in Supplementary Methods Table 1. The intra-plate and inter-plate coefficients of variation (CoV) for all assays are in Supplementary Methods Table 2.
2.5. Statistical Methods
Continuous variables are presented as means ± standard deviation (SD), and non-normally distributed variables as medians with interquartile range (IQR). Crude comparisons between cases and controls with regard to baseline characteristics were explored by t-tests, Mann-Whitney U or χ2 tests. Age- and sex-adjusted correlations between continuous variables were assessed using the Spearman rank-correlation coefficient (rho). The association between each of the antibodies and the risk of CV events was reported as an odds ratio (OR) obtained from a conditional logistic regression model: first by treating each log-transformed baseline antibody as a continuous variable giving an odds with 95% confidence intervals (CI) of having an event per 1 SD change; and, secondly, by categorizing variables into tertiles with the low tertile as reference. To assess the predictive role of Igs and specific antibodies beyond conventional risk factors, we established five statistical models. Model 1 was matched for age and sex but was otherwise unadjusted; model 2 was adjusted for traditional CV risk factors based on an extended Framingham risk model (smoking, baseline systolic blood pressure (SBP), high-density lipoprotein (HDL), total cholesterol (TC), body mass index (BMI), creatinine), as well as randomized anti-hypertensive and statin treatment; model 3 included paired antibody levels and serum Ig (i.e. IgM anti-MDA-LDL with IgM and IgG anti-MDA-LDL with IgG) to assess the effect of adjustment for total serum Ig; model 4 adjusted for log CRP and log NtProBNP in addition to the covariates in model 2. Collinearity was tested using the variance inflation factor (VIF), and model goodness-of-fit and calibration was assessed using a likelihood ratio test and the Hosmer Lemeshow statistic. To examine the extent to which baseline log-transformed variable improved discrimination and reclassified participants over risk thresholds, model 2 plus further adjustment for age and sex was used in an unconditional logistic model. This reference model was compared with models including each log-transformed variable using area under receiver operator curves (AUROC), net reclassification index (NRI) and integrated discrimination index (IDI) (Pencina et al., 2011).
All statistics were conducted independently by a statistician that is blinded to the laboratory techniques, using SAS version 9.3 (SAS Institute Inc., Cary, NC) and Stata release 12 (Stata Corp, College Station, TX). A two-tailed p value < 0.05 was considered to indicate statistical significance.
Discrimination of the different risk models was assessed by the areas under the receiver operating characteristic curves (AUROC) using the nonparametric approach of DeLong et al. (DeLong et al., 1988). Likelihood ratio tests were performed to evaluate whether the global model fit improved after the addition of each of the antibody in turn. Model calibration was assessed with the Hosmer–Lemeshow goodness-of-fit test. We also computed the NRI as a continuous and category-based NRI and an integrated discrimination improvement (IDI) analysis. IDI is defined as the difference in the mean predicted probability of being a case and being a control in the model with the addition of antibody minus the difference in the mean predicted probability of being a case and being a control in the model without the antibody level included. We also investigated whether the use of combinations of total serum Igs and anti-MDA-LDL antibodies would improve risk prediction. In these analyses, each of these study antibodies was treated as a continuous variable. Further NRI analyses for the most significant independent markers was undertaken to test reclassification within FRS with categories (< 10%, 10–20% and > 20% risk) were selected to define as low, intermediate and high risk of CVD.
3. Results
3.1. Baseline Characteristics
1753 subjects (mean age 65.3 ± 7.62 years; 1486, 84.8% male) were included in this nested case-control analysis (Fig. 1 and Table 1) and had a median follow-up of 5.5 years (interquartile range, 5.0–6.0). Of the 470 cases that were analyzed (Fig. 1), there were 343 with CHD and 127 who had a stroke (see breakdown in Table 1 footnote). Those with a CV event had a worse cardiovascular profile, including traditional risk factors (SBP, diastolic blood pressure [DBP] and LDL), as well as higher CRP and NtProBNP as previously shown (Table 1) (Sever et al., 2012, Welsh et al., 2014). Significantly higher total serum IgG and IgM levels were found in controls compared to cases (Table 2). Levels of anti-MDA-LDL antibodies were also higher in controls, although this was not as pronounced as with total serum Igs, and indeed was only significant at p < 0.05 for IgG anti-MDA-LDL antibodies (Table 2).
Table 1.
Characteristics of the study population.
| Controls (n = 1283) |
Cases (n = 470) |
p values | |
|---|---|---|---|
| Male | 1089 (84.9%) | 397 (84.5%) | 0.83 |
| Age (years) | 65.30 (7.57) | 65.34 (7.80) | 0.93 |
| Current smokers | 272 (21.2%) | 121 (25.7%) | 0.043 |
| Alcohol: never | 279 (21.7%) | 121 (25.7%) | 0.21⁎ |
| Alcohol: ≤ 14 (F)/21(M) units/week | 753 (58.7%) | 260 (55.3%) | |
| Alcohol: > 14 (F)/21(M) units/week | 251 (19.5%) | 89 (18.9%) | |
| Completed education age (years): | 0.004⁎ | ||
| ≤ 12 | 425 (33.2%) | 176 (37.4%) | |
| ≤ 15 | 590 (46.0%) | 227 (48.3%) | |
| ≤ 18 | 144 (11.2%) | 45 (9.6%) | |
| > 18 | 123 (9.6%) | 22 (4.7%) | |
| SBP (mm Hg) | 161.82 (17.39) | 164.63 (17.75) | 0.003 |
| DBP (mm Hg) | 92.12 (9.72) | 92.99 (10.41) | 0.10 |
| Heart rate (bpm) | 70.27 (11.78) | 69.74 (12.75) | 0.42 |
| BMI (kg/m2) | 28.97 (4.51) | 28.70 (4.03) | 0.25 |
| Total cholesterol (mmol/L) | 5.91 (1.07) | 5.99 (1.07) | 0.15 |
| LDL cholesterol (mmol/L) | 3.79 (0.95) | 3.92 (0.97) | 0.012 |
| HDL cholesterol (mmol/L) | 1.30 (0.35) | 1.25 (0.32) | 0.011 |
| Triglyceride (mmol/L) | 1.60 (1.20–2.30) | 1.70 (1.20–2.20) | 0.35ǂ |
| Glucose (mmol/L) | 5.60 (5.10–6.50) | 5.60 (5.10–6.80) | 0.17ǂ |
| Creatinine (μmol/L) | 99.20 (16.13) | 102.63 (19.45) | 0.0002 |
| CRP (mg/L) | 2.53 (1.27–4.97) | 3.04 (1.55–5.53) | 0.001ǂ |
| NtProBNP (pg/mL) | 84.50 (44.0–166.0) | 111.00 (52.0–241.0) | < 0.0001ǂ |
| Diabetes | 337 (26.3%) | 145 (30.9%) | 0.06 |
| Family history of coronary disease | 218 (17.0%) | 81 (17.2%) | 0.90 |
| Amlodipine | 646 (50.4%) | 220 (46.8%) | 0.18 |
| BP treatment at baseline | 1161 (90.5%) | 429 (91.3%) | 0.62 |
Values are mean (SD) or n (%). Missing: Triglyceride: 75, LDL-cholesterol, creatinine: 42, glucose: 79, CRP and NtProBNP: 4 each. Details of the 470 CV event cases: 343 CHD cases (108 non-fatal MI, 85 fatal CHD, 134 coronary revascularization and 16 had both revascularization and non-fatal MI); 127 stroke cases (100 ischemic strokes, 21 hemorrhagic strokes and 6 unspecified strokes).
Values are presented in median (interquartile range), p values by Wilcoxon–Mann–Whitney test.
p values for alcohol and education are χ2 statistics.
Table 2.
Median and interquartile range values of total serum IgM, total serum IgG and anti-MDA-LDL antibodies at baseline.
| Controls (n = 1283) |
Cases (n = 470) |
p value | |
|---|---|---|---|
| Total serum IgG (U) (g/L)⁎ |
0.42 (0.37–0.47) 15.88 (12.18–20.95) |
0.40 (0.35–0.45) 14.35 (10.96–18.71) |
< 0.0001 |
| Total serum IgM (U) (g/L)⁎ |
0.44 (0.31–0.60) 1.31 (0.84–2.06) |
0.40 (0.29–0.56) 1.16 (0.78–1.86) |
0.003 |
| IgG anti-MDA-LDL (U) | 0.44 (0.33–0.58) | 0.42 (0.31–0.55) | 0.02 |
| IgM anti-MDA-LDL (U) | 0.89 (0.59–1.20) | 0.86 (0.56–1.13) | 0.06 |
Expressed as g/L as interpolated from standard curves. p values obtained by using Wilcoxon–Mann–Whitney test.
3.2. Correlations and Interactions Between Variables
IgM anti-MDA-LDL antibody levels correlated strongly with total serum IgM (rho = 0.78, p < 0.0001), and there was a weaker but still highly significant correlation for IgG anti-MDA-LDL with total serum IgG (rho = 0.36, p < 0.0001) (Table 3). Though statistically significant, correlations between serum IgM and IgG and between IgM anti-MDA-LDL and IgG anti-MDA-LDL were modest (Table 3). Total IgG and IgG anti-MDA-LDL antibodies correlated weakly with CRP (rho = 0.13 and rho = 0.09 respectively). There was also a weak inverse correlation of IgG anti-MDA-LDL antibodies with LDL cholesterol (rho = − 0.085, p < 0.001).
Table 3.
Spearman Partial Correlation (rho) between total serum IgM and IgG, IgM and IgG anti-MDA-LDL antibodies, CRP and NtProBNP.
| IgG anti-MDA-LDL | IgM anti-MDA-LDL | Total IgG | Total IgM | LDL-c | CRP | NtProBNP | |
|---|---|---|---|---|---|---|---|
| IgG anti-MDA-LDL | 1.000 | ||||||
| IgM anti-MDA-LDL | 0.233⁎⁎⁎ | 1.000 | |||||
| Total serum IgG | 0.363⁎⁎⁎ | 0.151⁎⁎⁎ | 1.000 | ||||
| Total serum IgM | 0.149⁎⁎⁎ | 0.779⁎⁎⁎ | 0.185⁎⁎⁎ | 1.000 | |||
| LDL-c | − 0.085⁎⁎ | 0.002 | − 0.033 | 0.010 | 1.000 | ||
| CRP | 0.129⁎⁎⁎ | 0.031 | 0.089⁎⁎ | 0.028 | 0.094⁎⁎ | 1.000 | |
| NtProBNP | 0.005 | − 0.073⁎ | 0.023 | − 0.070⁎ | − 0.008 | 0.046 | 1.000 |
All data apart from LDL cholesterol (LDL-c) were log-transformed before correlation. Adjusted for age and sex.
p < 0.05.
p < 0.001.
p < 0.0001.
Serum Ig and anti-MDA-LDL antibodies among the controls did not strongly correlate with age, SBP, DBP or mean arterial pressure (MAP) (Supplementary Table S1). Interestingly, there was a clear correlation with sex, with total serum IgM and IgG, as well as IgM anti-MDA-LDL antibodies, being higher in women than in men (p < 0.05 for each) (Supplementary Table S2).
3.3. Total Serum IgG and IgM Are Inversely Associated With CV Events
Unadjusted, all Ig and specific antibody levels measured were inversely associated with risk of CV events (Table 4, model 1). In the highest third compared with the lowest, total serum IgG levels had the strongest association with CV events (OR = 0.62 [95% confidence interval, CI 0.47, 0.81]; p = 0.0004), and the weakest association was seen with IgM anti-MDA-LDL (OR 0.76 [0.58, 0.98]; p = 0.037). Trends were similar when the data were evaluated as continuous variables per 1 SD. Associations between IgM anti-MDA-LDL antibodies and CV events were modestly attenuated by adjustment for the basic risk variables and were no longer statistically significant, but associations with total serum IgG and IgM, or with IgG anti-MDA-LDL, were little affected and retained significance (Table 4, model 2). Thus, for total serum IgG, there was an OR of 0.62 (0.46, 0.82) comparing highest and lowest total IgG levels (p = 0.0007) or an OR of 0.81 (0.72, 0.91) per 1 SD increase in total IgG levels (p = 0.0003). Associations between IgG and IgM anti-MDA-LDL antibody levels and CV events were abolished after adjustment for total serum IgG and IgM levels respectively (Table 4, model 3). The significant inverse associations of total serum Ig levels with events remained even after adjustment for the basic risk variables plus log-transformed CRP and NtProBNP (Table 4, model 4). Total serum IgG remained the strongest predictor of protection after full adjustment in model 4 (OR 0.81 [0.72, 0.91] per one increase in SD, p = 0.003; OR 0.61 [0.46, 0.82] comparing upper and lower thirds, p = 0.0008).
Table 4.
Odds ratios of cardiovascular events (coronary heart disease or stroke) in relation to baseline total serum Igs and anti-MDA-LDL antibodies (per SD increase in log-transformed antibodies and in antibody tertiles).
| Cases/ controls |
Model 1 |
Model 2 |
Model 3 |
Model 4 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |||
| Per 1 SD increase in loge total IgG | 0.80 (0.72,0.89) | < 0.0001 | 0.81 (0.72,0.91) | 0.0003 | 0.81 (0.72,0.91) | 0.0003 | ||||
| Total serum IgG | Low | 180/406 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| Mid | 162/420 | 0.86 (0.67,1.11) | 0.26 | 0.92 (0.70,1.20) | 0.52 | 0.97 (0.74,1.28) | 0.83 | |||
| High | 128/458 | 0.62 (0.47,0.81) | 0.0004 | 0.62 (0.46,0.82) | 0.0007 | 0.61 (0.46,0.82) | 0.0008 | |||
| Trend | p = 0.0004 | p = 0.0008 | p = 0.0009 | |||||||
| Per 1 SD increase in loge total IgM | 0.83 (0.75,0.93) | 0.001 | 0.84 (0.75,0.94) | 0.003 | 0.85 (0.76,0.96) | 0.006 | ||||
| Total serum IgM | Low | 177/404 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| Mid | 160/429 | 0.82 (0.63,1.06) | 0.13 | 0.82 (0.62,1.07) | 0.15 | 0.85 (0.65,1.13) | 0.26 | |||
| High | 133/451 | 0.65 (0.49,0.85) | 0.002 | 0.67 (0.51,0.89) | 0.005 | 0.70 (0.53,0.93) | 0.014 | |||
| Trend | p = 0.0016 | p = 0.005 | p = 0.014 | |||||||
| Per 1 SD increase in loge IgG anti-MDA-LDL | 0.88 (0.79,0.98) | 0.016 | 0.89 (0.79,0.99) | 0.040 | 0.96 (0.85,1.08) | 0.46 | 0.87 (0.78,0.98) | 0.021 | ||
| IgG anti-MDA- LDL |
Low | 179/405 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Mid | 147/438 | 0.75 (0.58,0.97) | 0.030 | 0.74 (0.57,0.97) | 0.030 | 0.81 (0.61,1.07) | 0.13 | 0.74 (0.57,0.98) | 0.034 | |
| High | 144/441 | 0.74 (0.57,0.96) | 0.022 | 0.76 (0.58,1.00) | 0.048 | 0.90 (0.67,1.20) | 0.46 | 0.74 (0.56,0.97) | 0.030 | |
| Trend | p = 0.02 | p = 0.044 | p = 0.43 | p = 0.027 | ||||||
| Per 1 SD increase in loge IgM anti-MDA-LDL | 0.88 (0.79,0.98) | 0.023 | 0.90 (0.80,1.00) | 0.059 | 1.09 (0.90,1.32) | 0.38 | 0.91 (0.81,1.02) | 0.10 | ||
| IgM anti-MDA-LDL | Low | 166/418 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Mid | 163/422 | 0.95 (0.73,1.23) | 0.69 | 0.93 (0.71,1.21) | 0.58 | 1.16 (0.84,1.59) | 0.37 | 0.94 (0.71,1.24) | 0.66 | |
| High | 141/444 | 0.76 (0.58,0.98) | 0.037 | 0.81 (0.61,1.06) | 0.13 | 1.18 (0.80,1.75) | 0.40 | 0.84 (0.64,1.11) | 0.22 | |
| Trend | p = 0.038 | p = 0.10 | p = 0.41 | p = 0.22 | ||||||
Model 1: unadjusted but matched for age and sex; model 2: adjusted for smoking status, diabetic status, baseline SBP, baseline total cholesterol, HDL, baseline creatinine, BMI, family history of coronary disease, randomized BP and statin treatments; model 3: adjusted as in model 2 plus either total IgG or IgM; model 4: adjusted in model 2 plus loge CRP and NtProBNP. Immunoglobulin and specific antibody tertile levels in Units (U) as measured by ELISA and (in g/L as interpolated per standard curves for total Ig levels) were: total IgG: Low < 0.384 (< 13.11 g/L), Mid 0.3845–0.4465 (13.12–18.40 g/L), High > 0.4465 (18.4 g/L); total IgM Low < 0.3440 (< 0.95 g/L), Mid 0.3445–0.5305 (0.96–1.71 g/L), High > 0.5305 (> 1.71 g/L); IgG anti-MDA-LDL Low < 0.3626, Mid 0.3637–0.5205, High > 0.05205; IgM anti-MDA-LDL: Low < 0.6825, Mid 0.6835–1.0628, High > 1.0628.
When exploring the ability of Igs and specific antibodies to predict events linked to either CHD or stroke, it became clear that the relationships described above for total serum Ig levels are only significant for CHD (Table 5) and not stroke (Supplementary Table S3). The unadjusted OR for the highest tertiles in predicting CHD was 0.42 for total IgG (p < 0.0001) and 0.57 for total IgM (p = 0.001) (Table 5). Further adjustments in models 2, 3 and 4 demonstrated that again anti-MDA-LDL antibodies were dependent on total IgG and IgM, and that total serum Ig levels are independent of basic risk variables, CRP and NtProBNP as predictors. A further analysis of all CV events excluding hemorrhagic stroke did not appreciably alter the results shown in Table 4 (Supplementary Table S4).
Table 5.
Odds ratios of events due to coronary heart disease in relation to baseline total serum Igs and anti-MDA-LDL antibodies (per SD increase in log-transformed antibodies and in antibody tertiles).
| Cases/controls | Model 1 |
Model 2 |
Model 3 |
Model 4 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |||
| Per 1 SD increase in loge total IgG | 343/939 | 0.66 (0.57,0.76) | < 0.0001 | 0.68 (0.58,0.79) | < 0.0001 | 0.68 (0.58,0.79) | < 0.0001 | |||
| Total serum IgG | Low | 153/309 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| Mid | 120/301 | 0.79 (0.59,1.06) | 0.12 | 0.83 (0.61,1.13) | 0.23 | 0.89 (0.64,1.22) | 0.45 | |||
| High | 70/329 | 0.42 (0.30,0.59) | < 0.0001 | 0.43 (0.30,0.61) | < 0.0001 | 0.43 (0.30,0.62) | < 0.0001 | |||
| Trend | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||||||
| Per 1 SD increase in loge total IgM | 343/939 | 0.81 (0.71,0.93) | 0.002 | 0.82 (0.71,0.94) | 0.005 | 0.83 (0.72,0.95) | 0.008 | |||
| Total serum IgM | Low | 134/296 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| Mid | 124/326 | 0.82 (0.61,1.11) | 0.19 | 0.84 (0.61,1.15) | 0.28 | 0.88 (0.64,1.22) | 0.44 | |||
| High | 85/317 | 0.57 (0.41,0.80) | 0.001 | 0.59 (0.42,0.83) | 0.003 | 0.62 (0.43,0.87) | 0.007 | |||
| Trend | p = 0.001 | p = 0.003 | p = 0.007 | |||||||
| Per 1 SD increase in loge IgG anti-MDA-LDL | 343/939 | 0.83 (0.73,0.94) | 0.003 | 0.85 (0.74,0.98) | 0.020 | 0.98 (0.84,1.13) | 0.77 | 0.83 (0.72,0.95) | 0.008 | |
| IgG anti-MDA-LDL | Low | 142/302 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Mid | 106/320 | 0.70 (0.52,0.94) | 0.018 | 0.67 (0.49,0.91) | 0.012 | 0.81 (0.59,1.13) | 0.22 | 0.67 (0.49,0.92) | 0.015 | |
| High | 95/317 | 0.64 (0.47,0.87) | 0.005 | 0.70 (0.50,0.97) | 0.031 | 0.95 (0.66,1.35) | 0.76 | 0.66 (0.47,0.93) | 0.016 | |
| Trend | p = 0.004 | p = 0.023 | p = 0.71 | p = 0.012 | ||||||
| Per 1 SD increase in loge IgM anti-MDA-LDL | 343/939 | 0.88 (0.77,0.99) | 0.038 | 0.89 (0.78,1.02) | 0.09 | 1.12 (0.89,1.40) | 0.33 | 0.90 (0.78,1.03) | 0.12 | |
| IgM anti-MDA-LDL | Low | 123/308 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Mid | 123/310 | 0.97 (0.72,1.31) | 0.86 | 0.96 (0.70,1.32) | 0.80 | 1.20 (0.83,1.73) | 0.33 | 0.96 (0.69,1.33) | 0.80 | |
| High | 97/321 | 0.72 (0.53,0.99) | 0.043 | 0.78 (0.56,1.09) | 0.14 | 1.18 (0.74,1.86) | 0.49 | 0.81 (0.58,1.13) | 0.22 | |
| Trend | p = 0.046 | p = 0.15 | p = 0.49 | p = 0.22 | ||||||
Model 1: unadjusted but matched for age and sex; model 2: adjusted for smoking status, diabetic status, baseline SBP, baseline total cholesterol, HDL, baseline creatinine, BMI, family history of coronary disease, randomized BP and statin treatments; model 3: adjusted as in model 2 plus either total IgG or IgM; model 4: adjusted in model 2 plus loge CRP and NtProBNP. Immunoglobulin and specific antibody tertile levels in Units (U) as measured by ELISA and (in g/L as interpolated per standard curves for total Ig levels) were: total IgG: Low < 0.384 (< 13.11 g/L), Mid 0.3845–0.4465 (13.12–18.40 g/L), High > 0.4465 (18.4 g/L); total IgM Low < 0.3440 (< 0.95 g/L), Mid 0.3445–0.5305 (0.96–1.71 g/L), High > 0.5305 (> 1.71 g/L); IgG anti-MDA-LDL Low < 0.3626, Mid 0.3637–0.5205, High > 0.05205; IgM anti-MDA-LDL: Low < 0.6825, Mid 0.6835–1.0628, High > 1.0628.
3.4. Total Serum IgG Significantly Improves Prediction of CV Risk
Addition of each of total serum IgG, total serum IgM and IgG anti-MDA-LDL antibodies significantly improved model fit (Table 6). The AUROC was significantly improved by total serum IgG (0.6391 vs. 0.6213, p = 0.009), whereas IgG anti-MDA, IgM anti-MDA or total serum IgM did not significantly increase AUROC. Addition of all four measures to the basic model improved model fit and AUROC (0.6421 vs. 0.6213, p = 0.011), but there was no difference in AUROCs between the basic model including just total serum IgG and the basic model containing all four measures (Table 6).
Table 6.
Measures of model fit and reclassification by adding each variable in turn to the basic CV events model using unconditional logistic regression model.
| Basicǂ | Total serum IgG | Total serum IgM | IgG anti-MDA-LDL | IgM anti-MDA-LDL | All 4§ | |
|---|---|---|---|---|---|---|
| Goodness of fit | ||||||
| LR χ2 (df) | 65.77 (13) | 79.57 (14) | 75.17 (14) | 70.12 (14) | 68.97 (14) | 87.78 (17) |
| p value | Ref | 0.0002 | 0.002 | 0.04 | 0.07 | 0.0002 |
| Calibration | ||||||
| Hosmer-Lemeshow | 8.17 | 7.07 | 3.98 | 7.7 | 8.74 | 3.96 |
| χ2, deciles | p = 0.42 | 0.53 | 0.86 | 0.46 | 0.36 | 0.86 |
| Discrimination | ||||||
| AUROC | 0.6213 (0.5921,0.6505) |
0.6391 (0.6096,0.6686) |
0.6293 (0.6000,0.6586) |
0.6270 (0.5978,0.6563) |
0.6241 (0.5948,0.6533) |
0.6421 (0.6127,0.6715) |
| p (difference) | Ref | 0.009 | 0.18 | 0.16 | 0.45 | 0.011 |
| p (total IgG vs. all 4) | Ref | 0.54 | ||||
| Reclassification | ||||||
| IDI | Ref | 0.90% p < 0.0001 | 0.59% p = 0.003 | 0.27% p = 0.03 | 0.20% p = 0.066 | 1.38% p < 0.0001 |
| Continuous NRI | Ref | 17.59% p = 0.001 | 16.67% p = 0.002 | 8.65% p = 0.11 | 6.05% p = 0.26 | 17.40% p = 0.001 |
| Categorical NRI | Ref | 7.54% p < 0.0001 | 1.18% p = 0.47 | 4.52% p < 0.0001 | 1.14% p = 0.32 | 8.27% p < 0.0001 |
Adjusted for age, sex, smoking status, diabetic status, baseline SBP, baseline total cholesterol, HDL, baseline creatinine values, BMI, family history of CHD, randomized BP and statin treatments.
Basic model plus IgG anti-MDA-LDL, IgM anti-MDA-LDL, total serum IgG and total serum IgM.
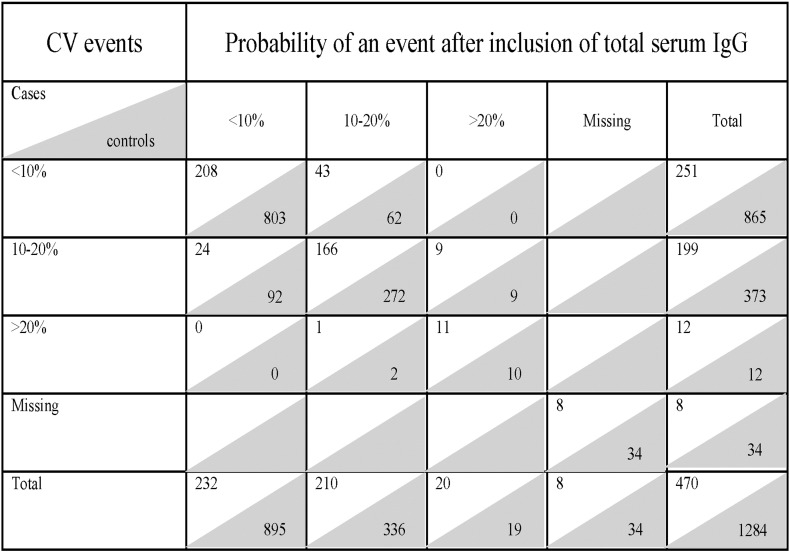
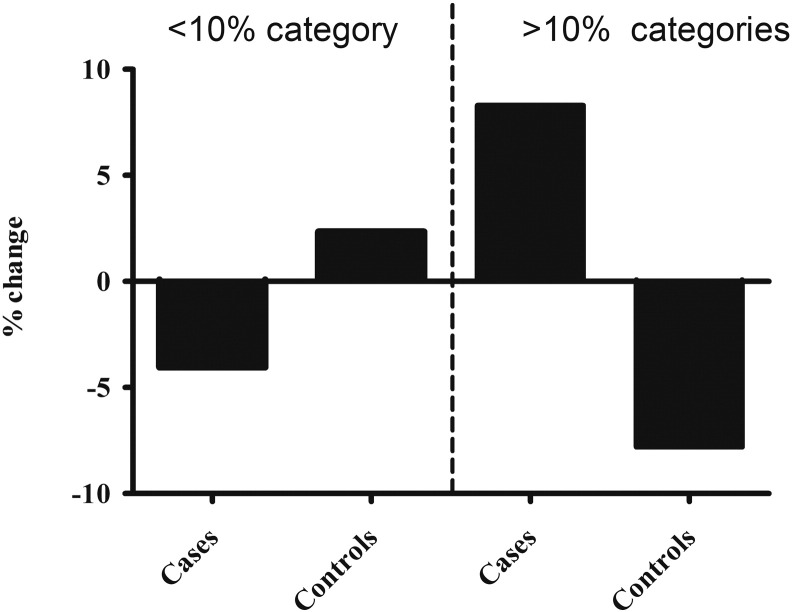
The addition of total serum IgG to the basic model resulted in an absolute IDI of 0.9 (p < 0.0001), and the highest NRI (17.6% and 7.5% for continuous and category-based NRI, respectively) (Table 6). Total serum IgG also led to the greatest absolute number of subjects with CV events correctly reclassified: 52 (11.1%) to a higher risk category and 25 (5.3%) to a lower risk category (Fig. 2). Of the 1284 controls, 71 (5.5%) were correctly reclassified using total serum IgG to a higher risk category and 94 (7.3%) to a lower risk category (Fig. 2). Fig. 3 demonstrates the clinically relevant reclassification into and out of the < 10% risk category for CVD, with a clear advantage of adding in total serum IgG to the model. Total serum IgG also performed best for CV event risk reclassification (18.6 and 8.3%, for continuous and category-based NRI, respectively) in the extended basic model (basic model + CRP and NtProBNP) (Supplementary Table S5).
Fig. 2.
The number of subjects in different CV event risk categories with reclassification after inclusion of total serum IgG. The figure shows estimates of probabilities using the basic risk factor model without (vertical axis) and with (horizontal axis) inclusion of total serum IgG. Each cell includes the number of cases (clear background) and controls (shaded background). The basic risk factor model included age, sex, smoking status, diabetic status, baseline SBP, baseline TC, HDL, baseline creatinine values, BMI, family history of CHD, randomized BP and statin treatment.
Fig. 3.
Percentage change in cardiovascular event cases and controls within the < 10% risk and > 10% CVD risk categories when total serum IgG added to the basic risk model.
There was a much more pronounced improvement in model performance when studying CHD alone. The most significant increase in AUROC, and highest NRI and IDI were after the addition of total IgG to either the basic model or the extended model (Supplementary Tables S6 and S7). The finding that total serum IgG results in a continuous NRI of a 29.6% (p < 0.0001) despite adjustment for the basic model, log CRP and log NtProBNP puts this measurement in the forefront of risk prediction of CHD in this hypertensive population (Supplementary Table S7). Basic models with addition of the other immunoglobulins did not perform better than the basic model plus only total IgG (Supplementary Table S6).
4. Discussion
The main novel finding in this paper is that in patients with hypertension the total IgG serum level, and to a lesser extent the total serum IgM level, is an independent predictor of freedom from CV events in general and from CHD in particular. Furthermore, total serum Ig levels significantly improved risk classification for CV events. High IgG and IgM levels were not associated with freedom from stroke, probably reflecting its more heterogeneous underlying pathology.
Although IgG and IgM anti-MDA-LDL antibodies were also significantly associated with freedom from CV events, these correlated significantly with their respective total serum Ig levels. Indeed, total serum IgM and IgG were much stronger indicators of freedom from events, and the univariate significance of anti-MDA-LDL antibodies was lost after adjusting for total serum levels of the respective Ig class. It is clear however that our comparison of specific antibodies with total IgG and IgM levels was restricted to those against just one form of oxidized LDL (i.e. MDA-LDL) and it remains possible that other specific autoantibodies against modified LDL or other antigens could have greater negative or positive predictive ability. Nevertheless, our results indicate that adjustment for total Ig levels will need to form part of further analyses investigating specific antibodies as prognostic markers of CVD.
We postulate that the greater predictive power of total Ig levels reflects a broad role of the polyreactive antibody repertoire in cardiovascular protection. On the one hand, the composite antibody repertoire can be expected to contribute to the safe disposal of a variety of other endogenous atherogenic autoantigens besides modified LDL, including apoptotic cells and microparticles (Chang et al., 1999, Chou et al., 2009, Tsiantoulas et al., 2015). On the other hand, the conventional role of antibodies in preventing bacterial infection may also be important for reducing the rate of atherosclerosis progression and reducing infection-triggered acute cardiac events (Smeeth et al., 2004). In this light, the greater predictive capacity of the total IgG level compared to IgM argues for the importance of a robust adaptive immune response. Certainly the relative roles of genetic and environmental influences in setting Ig levels merits further study (Chen et al., 2011, Paavola et al., 2012), and in this light it is interesting that serum IgG and IgM levels in our study were significantly higher in women, consistent with previous observations (Gonzalez-Quintela et al., 2008).
The strong negative association between IgG and IgM levels and CHD events needs to be considered in the context of other biomarkers that have been evaluated as adjuncts to traditional risk assessments. Although the ASCOT-CRP substudy concluded that measuring CRP had little utility in refining CV risk assessment beyond classical risk factors in the study population (Sever et al., 2012), a meta-analysis concluded that adding CRP to the risk assessment led to predicting one additional event over a period of 10 years for every 400–500 people screened (Kaptoge et al., 2012). Not only did IgG levels remain highly significant in predicting protection from events after adjustment for CRP, they were also independent from NtProBNP which was previously shown to be a predictor of events in ASCOT (Welsh et al., 2014).
A particularly important outcome of the study was the reclassification of more cases and less controls into the clinically relevant > 10% risk categories and more controls with less cases in the < 10% risk category by incorporating total serum IgG in the analysis. If confirmed in further cohorts, this has the potential to be used for more robust risk stratification in hypertension, thus redirecting primary prevention therapies towards those who would benefit the most.
Our findings are at odds with those of Kovanen et al. who showed that serum IgG levels (as well as IgA and IgE), but not IgM, were predictive of MI (Kovanen et al., 1998). However the two studies differ in entrance requirements, with that of Kovanen et al. being selected for dyslipidemia whilst ours was based on hypertension. It is possible that IgG levels in our study population might have been affected by hypertension, although we did not detect any correlations with SBP, DBP or MAP (Chan et al., 2014). It therefore remains to be seen to what degree our findings on the ASCOT population generalize to wider populations. In particular, raised Ig levels are probably not associated with freedom from CV events in patients with autoimmune conditions such rheumatoid arthritis and systemic lupus erythematosus in which there is a disease-related increase in risk of CV events and in which a polyclonal expansion of Ig levels signifies disease activity. Furthermore, our study has focused on the prediction of first adverse cardiovascular incidents in a high risk population, and does not address the relevance of immunoglobulins or specific antibodies during and after acute coronary events.
5. Conclusion
In conclusion, our study suggests total serum IgG levels are strongly and independently associated with lower risk of CHD events in individuals with hypertension. Furthermore, IgG levels appear to significantly improve prediction beyond established risk predictors, even when including CRP and NtProBNP. As the measurement of total serum IgG is readily available to clinicians and relatively cheap, our findings have high potential for future clinical translation.
Author Contributions
RYK, JM, ADH and DOH designed the current study, wrote the protocol and the analysis plan, supervised the analyses, interpreted the results and wrote the report. RYK supervised the laboratory work and the statistical analysis. M C-A undertook the technical laboratory work with contribution from CK. CLC conducted statistical analyses for the study. MJ, JJB, NS, PW and PS reviewed the protocol and commented upon the manuscript. PS designed and coordinated the original ASCOT study.
Conflict of Interest
None to declare.
Acknowledgements
The work was funded by a grant from the National Institute for Health Research Comprehensive Biomedical Research Centre (RDB02_P46385) at Imperial College Healthcare NHS Trust. The parent ASCOT study was an independent, investigator-initiated, investigator-designed, and investigator-led study funded by a grant program from Pfizer UK. DH receives BHF Professorial Support. CK is supported by Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad. RYK was funded as a Wellcome Trust Clinical Research Fellow. DOH has professorial support from the British Heart Foundation. JJB acknowledges the British Heart Foundation SCRF03 for his financial support. The original ASCOT study was supported by an investigational grant from Pfizer International, New York, NY, USA. The principal funding source for ASCOT was Pfizer, New York, NY, USA. Servier Research Group, Paris, France, and Leo Laboratories, Copenhagen, Denmark provided additional funding. The investigators acknowledge the invaluable support of the clinical trial doctors, nurses, and support staff for their important contributions. We thank Dr. John Morris (Imperial College Healthcare NHS Trust) contribution to standard sera measurements, as well as the ASCOT study team, collaborators and co-investigators. In addition, we thank all study participants.
Footnotes
The data in this paper were presented as a poster at the American College of Cardiology annual meeting in Chicago on 2nd April 2016, and published as an abstract in the meeting proceedings (Journal of the American College of Cardiology 67 (13_S):1912; doi: 10.1016/S0735-1097(16)31913-1).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.06.012.
Appendix A. Supplementary data
Supplementary tables.
References
- Ait-Oufella H., Herbin O., Bouaziz J.D., Binder C.J., Uyttenhove C., Laurans L., Taleb S., Van Vre E., Esposito B., Vilar J., Sirvent J., Van Snick J., Tedgui A., Tedder T.F., Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010;207(8):1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkbacka H., Alm R., Persson M., Hedblad B., Nilsson J., Fredrikson G.N. Low levels of apolipoprotein B-100 autoantibodies are associated with increased risk of coronary events. Arterioscler. Thromb. Vasc. Biol. 2016;36(4):765–771. doi: 10.1161/ATVBAHA.115.306938. [DOI] [PubMed] [Google Scholar]
- Cesena F.H., Dimayuga P.C., Yano J., Zhao X., Kirzner J., Zhou J., Chan L.F., Lio W.M., Cercek B., Shah P.K., Chyu K.Y. Immune-modulation by polyclonal IgM treatment reduces atherosclerosis in hypercholesterolemic apoE −/− mice. Atherosclerosis. 2012;220(1):59–65. doi: 10.1016/j.atherosclerosis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Chan C.T., Lieu M., Toh B.H., Kyaw T.S., Bobik A., Sobey C.G., Drummond G.R. Antibodies in the pathogenesis of hypertension. Biomed Res. Int. 2014;2014:504045. doi: 10.1155/2014/504045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M.K., Bergmark C., Laurila A., Horkko S., Han K.H., Friedman P., Dennis E.A., Witztum J.L. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. U. S. A. 1999;96(11):6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.H., Johns M., Boyle J.J., McConnell E., Kirkham P.A., Bicknell C., Zahoor-ul-Hassan Dogar M., Edwards R.J., Gale-Grant O., Khamis R., Ramkhelawon K.V., Haskard D.O. Model IgG monoclonal autoantibody-anti-idiotype pair for dissecting the humoral immune response to oxidized low density lipoprotein. Hybridoma. 2012;31(2):87–98. doi: 10.1089/hyb.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Reis S.E., Kammerer C., Craig W., McNamara D.M., Holubkov R., Sharaf B.L., Sopko G., Pauly D.F., Merz C.N., Kamboh M.I. Association of anti-oxidized LDL and candidate genes with severity of coronary stenosis in the Women's Ischemia Syndrome Evaluation study. J. Lipid Res. 2011;52(4):801–807. doi: 10.1194/jlr.M012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou M.Y., Fogelstrand L., Hartvigsen K., Hansen L.F., Woelkers D., Shaw P.X., Choi J., Perkmann T., Backhed F., Miller Y.I., Horkko S., Corr M., Witztum J.L., Binder C.J. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 2009;119(5):1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlof B., Sever P.S., Poulter N.R., Wedel H., Beevers D.G., Caulfield M., Collins R., Kjeldsen S.E., Kristinsson A., McInnes G.T., Mehlsen J., Nieminen M., O'Brien E., Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- Gonzalez-Quintela A., Alende R., Gude F., Campos J., Rey J., Meijide L.M., Fernandez-Merino C., Vidal C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 2008;151(1):42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G.K., Libby P. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 2006;6(7):508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- Hoefer I.E., Steffens S., Ala-Korpela M., Back M., Badimon L., Bochaton-Piallat M.L., Boulanger C.M., Caligiuri G., Dimmeler S., Egido J., Evans P.C., Guzik T., Kwak B.R., Landmesser U., Mayr M., Monaco C., Pasterkamp G., Tunon J., Weber C. Novel methodologies for biomarker discovery in atherosclerosis. Eur. Heart J. 2015;36(39):2635–2642. doi: 10.1093/eurheartj/ehv236. [DOI] [PubMed] [Google Scholar]
- Kaptoge S., Di Angelantonio E., Pennells L., Wood A.M., White I.R., Gao P., Walker M., Thompson A., Sarwar N., Caslake M., Butterworth A.S., Amouyel P., Assmann G., Bakker S.J., Barr E.L., Barrett-Connor E., Benjamin E.J., Bjorkelund C., Brenner H., Brunner E., Clarke R., Cooper J.A., Cremer P., Cushman M., Dagenais G.R., D'Agostino R.B., Sr., Dankner R., Davey-Smith G., Deeg D., Dekker J.M., Engstrom G., Folsom A.R., Fowkes F.G., Gallacher J., Gaziano J.M., Giampaoli S., Gillum R.F., Hofman A., Howard B.V., Ingelsson E., Iso H., Jorgensen T., Kiechl S., Kitamura A., Kiyohara Y., Koenig W., Kromhout D., Kuller L.H., Lawlor D.A., Meade T.W., Nissinen A., Nordestgaard B.G., Onat A., Panagiotakos D.B., Psaty B.M., Rodriguez B., Rosengren A., Salomaa V., Kauhanen J., Salonen J.T., Shaffer J.A., Shea S., Ford I., Stehouwer C.D., Strandberg T.E., Tipping R.W., Tosetto A., Wassertheil-Smoller S., Wennberg P., Westendorp R.G., Whincup P.H., Wilhelmsen L., Woodward M., Lowe G.D., Wareham N.J., Khaw K.T., Sattar N., Packard C.J., Gudnason V., Ridker P.M., Pepys M.B., Thompson S.G., Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 2012;367(14):1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen P.T., Manttari M., Palosuo T., Manninen V., Aho K. Prediction of myocardial infarction in dyslipidemic men by elevated levels of immunoglobulin classes A, E, and G, but not M. Arch. Intern. Med. 1998;158(13):1434–1439. doi: 10.1001/archinte.158.13.1434. [DOI] [PubMed] [Google Scholar]
- Kyaw T., Tay C., Khan A., Dumouchel V., Cao A., K T., Kehry M., Dunn R., Agrotis A., Tipping P., Bobik A., Toh B.H. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 2010;185(7):4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- Kyaw T., Tay C., Krishnamurthi S., Kanellakis P., Agrotis A., Tipping P., Bobik A., Toh B.H. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 2011;109(8):830–840. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- Leibundgut G., Scipione C., Yin H., Schneider M., Boffa M.B., Green S., Yang X., Dennis E., Witztum J.L., Koschinsky M.L., Tsimikas S. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J. Lipid Res. 2013;54(10):2815–2830. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.J., Malik T.H., Ehrenstein M.R., Boyle J.J., Botto M., Haskard D.O. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120(5):417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscari A., Bozzoli C., Puddu G.M., Sangiorgi Z., Dormi A., Rovinetti C., Descovich G.C., Puddu P. Association of serum C3 levels with the risk of myocardial infarction. Am. J. Med. 1995;98(4):357–364. doi: 10.1016/S0002-9343(99)80314-3. [DOI] [PubMed] [Google Scholar]
- Nicoletti A., Kaveri S., Caligiuri G., Bariety J., Hansson G.K. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. J. Clin. Invest. 1998;102(5):910–918. doi: 10.1172/JCI119892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola T., Kangas-Kontio T., Salonurmi T., Kuusisto S., Huusko T., Savolainen M.J., Kakko S. Plasma levels of antibodies against oxidized LDL are inherited but not associated with HDL-cholesterol level in families with early coronary heart disease. Atherosclerosis. 2012;224(1):123–128. doi: 10.1016/j.atherosclerosis.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Palinski W., Yla-Herttuala S., Rosenfeld M.E., Butler S.W., Socher S.A., Parthasarathy S., Curtiss L.K., Witztum J.L. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10(3):325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- Pencina M.J., D'Agostino R.B., Sr., Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravandi A., Boekholdt S.M., Mallat Z., Talmud P.J., Kastelein J.J., Wareham N.J., Miller E.R., Benessiano J., Tedgui A., Witztum J.L., Khaw K.T., Tsimikas S. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J. Lipid Res. 2011;52(10):1829–1836. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M.E., Palinski W., Yla-Herttuala S., Butler S., Witztum J.L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S.M., Perry H.M., Gonen A., Prohaska T.A., Srikakulapu P., Grewal S., Das D., McSkimming C., Taylor A.M., Tsimikas S., Bender T.P., Witztum J.L., McNamara C.A. B-1b cells secrete atheroprotective IgM and attenuate atherosclerosis. Circ. Res. 2015;117(3):e28–e39. doi: 10.1161/CIRCRESAHA.117.306044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever P.S., Dahlof B., Poulter N.R., Wedel H., Beevers G., Caulfield M., Collins R., Kjeldsen S.E., McInnes G.T., Mehlsen J., Nieminen M., O'Brien E., Ostergren J. Rationale, design, methods and baseline demography of participants of the Anglo-Scandinavian Cardiac Outcomes Trial. ASCOT investigators. J. Hypertens. 2001;19(6):1139–1147. doi: 10.1097/00004872-200106000-00020. [DOI] [PubMed] [Google Scholar]
- Sever P.S., Dahlof B., Poulter N.R., Wedel H., Beevers G., Caulfield M., Collins R., Kjeldsen S.E., Kristinsson A., McInnes G.T., Mehlsen J., Nieminen M., O'Brien E., Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- Sever P., Dahlof B., Poulter N., Wedel H., Beevers G., Caulfield M., Collins R., Kjeldsen S., Kristinsson A., McInnes G., Mehlsen J., Nieminem M., O'Brien E., Ostergren J. Potential synergy between lipid-lowering and blood-pressure-lowering in the Anglo-Scandinavian Cardiac Outcomes Trial. Eur. Heart J. 2006;27(24):2982–2988. doi: 10.1093/eurheartj/ehl403. [DOI] [PubMed] [Google Scholar]
- Sever P.S., Poulter N.R., Chang C.L., Hingorani A., Thom S.A., Hughes A.D., Welsh P., Sattar N., Investigators A.S.C.O.T. Evaluation of C-reactive protein prior to and on-treatment as a predictor of benefit from atorvastatin: observations from the Anglo-Scandinavian Cardiac Outcomes Trial. Eur. Heart J. 2012;33(4):486–494. doi: 10.1093/eurheartj/ehr262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- Tsiantoulas D., Diehl C.J., Witztum J.L., Binder C.J. B cells and humoral immunity in atherosclerosis. Circ. Res. 2014;114(11):1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantoulas D., Perkmann T., Afonyushkin T., Mangold A., Prohaska T.A., Papac-Milicevic N., Millischer V., Bartel C., Horkko S., Boulanger C.M., Tsimikas S., Fischer M.B., Witztum J.L., Lang I.M., Binder C.J. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 2015;56(2):440–448. doi: 10.1194/jlr.P054569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimikas S., Willeit P., Willeit J., Santer P., Mayr M., Xu Q., Mayr A., Witztum J.L., Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J. Am. Coll. Cardiol. 2012;60(21):2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- Welsh P., Poulter N.R., Chang C.L., Sever P.S., Sattar N. The value of N-terminal pro-B-type natriuretic peptide in determining antihypertensive benefit: observations from the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Hypertension. 2014;63(3):507–513. doi: 10.1161/HYPERTENSIONAHA.113.02204. [DOI] [PubMed] [Google Scholar]
- Yeboah J., Polonsky T.S., Young R., McClelland R.L., Delaney J.C., Dawood F., Blaha M.J., Miedema M.D., Sibley C.T., Carr J.J., Burke G.L., Goff D.C., Jr., Psaty B.M., Greenland P., Herrington D.M. Utility of nontraditional risk markers in individuals ineligible for statin therapy according to the 2013 American College of Cardiology/American Heart Association Cholesterol Guidelines. Circulation. 2015;132(10):916–922. doi: 10.1161/CIRCULATIONAHA.115.016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., Kishimoto C., Sano H., Shioji K., Xu Y., Yokode M. Immunoglobulin treatment suppresses atherosclerosis in apolipoprotein E-deficient mice via the Fc portion. Am. J. Physiol. Heart Circ. Physiol. 2003;285(2):H899–H906. doi: 10.1152/ajpheart.00926.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.