Abstract
Red blood cell (RBC) transfusions are essential for patients with hematological disorders and bone marrow failure syndromes. Despite ABO matching, RBC transfusions can lead to production of alloantibodies against “minor” blood group antigens. Non-ABO alloimmunization is a leading cause of transfusion-associated mortality in the U.S. Despite its clinical importance, little is known about the immunological factors that promote alloimmunization. Prior studies indicate that inflammatory conditions place patients at higher risk for alloimmunization. Additionally, co-exposure to pro-inflammatory pathogen associated molecular patterns (PAMPs) promotes alloimmunization in animal models, suggesting that RBC alloimmunization depends on innate immune cell activation. However, the specific innate immune stimuli and sensors that induce a T cell-dependent alloantibody response to transfused RBCs have not been identified. The NLRP3 inflammasome senses chemically diverse PAMPs and damage associated molecular patterns (DAMPs), including extracellular ATP and iron-containing heme. We hypothesized that activation of the NLRP3 inflammasome by endogenous DAMPs from RBCs promotes the alloimmune response to a sterile RBC transfusion. Using genetically modified mice lacking either NLRP3 or multiple downstream inflammasome response elements, we ruled out a role for the NLRP3 inflammasome or any Caspase-1 or -11 dependent inflammasome in regulating RBC alloantibody production to a model antigen.
Keywords: NLRP3, Inflammasome, Alloimmunization, Conventional dendritic cells (cDCs), Red blood cell (RBC) storage
Highlights
-
•
Transfusion of stored red blood cells (RBCs) induces proinflammatory cytokine production and alloimmunization to an RBC antigen in mice.
-
•
Transfusion of stored RBCs, regardless of alloantigen expression, activates conventional dendritic cells in the spleen.
-
•
NOD-like receptor (NLR) inflammasomes, including NLRP3, do not regulate inflammation and alloimmunization induced by stored RBCs.
Following a blood transfusion, the immune system may produce antibodies that have detrimental effects. To understand how the immune system recognizes factors in transfused blood, we examined the immune response of mice lacking important inflammatory molecules, called inflammasomes. The results demonstrate that inflammasomes do not affect the production of potentially harmful antibodies that recognize transfused red blood cells.
1. Introduction
With 15 million units transfused per year, red blood cell (RBC) transfusion is the most common procedure performed in United States hospitals (Pfuntner et al., 2013). Exposure to numerous foreign non-ABO RBC antigens during transfusion can induce production of antibodies to “minor” blood group antigens. As a result, approximately 3% of hospitalized patients (Fluit et al., 1990) and as many as 30–50% of patients with sickle cell anemia (Vichinsky et al., 1990), develop alloantibodies against these RBC antigens. Subsequent exposure to the offending RBC antigen may result in potentially fatal hemolytic transfusion reactions, which is a leading cause of transfusion-associated mortality (FDA, 2014). Although such reactions can be avoided by providing antigen-negative RBC units, patients with rare or multiple RBC alloantibodies may experience clinically significant delays in transfusion, as laboratories search for rare compatible blood products (Ryder et al., 2014). To mitigate these adverse effects of alloimmunization, there is heightened interest in identifying genetic and environmental factors that influence the likelihood of alloantibody formation.
Multiple factors place patients at higher risk of alloimmunization. These include genetic predisposition (e.g., HLA type), transfusion burden, and co-incident inflammation (Higgins and Sloan, 2008, Telen et al., 2015, Tatari-Calderone et al., 2009). Recipient inflammatory status significantly influences the frequency of alloimmunization; an increased incidence of alloimmunization was reported in patients with inflammatory bowel disease, autoimmune disease, febrile transfusion reactions, and sickle cell-mediated acute chest syndrome (Ramsey and Smietana, 1995, Fasano et al., 2015, Papay et al., 2012, Yazer et al., 2009). In murine RBC transfusion models, exposure to pro-inflammatory pathogen-associated molecular patterns (PAMPs) promotes RBC alloimmune responses (Bao et al., 2009, Hendrickson et al., 2006, Hendrickson et al., 2007, Elayeb et al., 2016). This suggests that RBC alloimmunization, like most adaptive immune responses, depends on innate immune cell activation to initiate antigen presentation and the requisite T cell priming signals (Calabro et al., 2016, Krishnaswamy et al., 2013). However, the specific innate immune stimuli provided by transfused RBCs that regulate a T cell-dependent alloantibody response are not yet known. Recently, the species mismatch of CD47 on transfused xenogeneic sheep RBCs was shown to induce significant inflammation in murine recipients (Yi et al., 2015). However, in allogeneic RBC murine models, such a mismatch does not exist and CD47 expression does not significantly decrease on stored murine RBCs (Gilson et al., 2009). Nonetheless, other alloantigen-independent properties of donor RBCs, including length of refrigerated storage, can influence post-transfusion inflammation and immunogenicity (Ryder et al., 2014, Desai et al., 2015). During prolonged storage, RBCs undergo morphologic transformations, metabolic changes and oxidative damage, leading to increased post-transfusion clearance and decreased RBC viability (Bennett-Guerrero et al., 2007, Tinmouth et al., 2006). However, the cellular and molecular mechanisms, including the recognition of stored RBC factors, underlying these observations are poorly understood.
To examine the effect of RBC storage on inflammatory responses and alloimmunization, we previously generated a mouse model of RBC storage that approximates human RBC storage (Gilson et al., 2009). Our studies demonstrated that transfusion of stored RBCs expressing a chimeric protein containing hen egg lysozyme, ovalbumin, and the human Duffy antigen (HOD), leads to accelerated RBC clearance, increased inflammatory cytokine production, and enhanced alloantibody production, compared to freshly collected RBCs (Hod et al., 2010, Hendrickson et al., 2010). Transfusion of stored RBCs also resulted in elevated levels of tissue iron in the spleen and liver and production of circulating non-transferrin bound iron (NTBI) in plasma. The inflammatory response was attributed to phagocytosis of intact damaged stored RBCs (i.e., extravascular hemolysis) (Hod et al., 2010). Although storage of mouse and human RBCs may have differential effects, a prospective study with human volunteers also demonstrated that transfusion of stored RBCs led to elevated NTBI and extravascular hemolysis (Hod et al., 2011). However, the mechanism by which innate immune cells sensed iron or other RBC products was not investigated.
Innate non-immune and immune cells, including macrophages and dendritic cells, utilize pattern recognition receptors (PRRs) to detect PAMPs (Takeuchi and Akira, 2010). In addition, they can recognize damage-associated molecular patterns (DAMPs), which derive from endogenous molecules modified or released following cellular stress or damage (Krishnaswamy et al., 2013). Many PRRs exist, including Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), each sensing discrete types of stimuli. For example, TLR4 recognizes the Gram-negative bacterial cell wall component lipopolysaccharide (LPS) whereas NLR containing pyrin domain 3 (NLRP3) is activated by chemically diverse PAMPs and DAMPs including protein aggregates, insoluble crystals, and bacterial pore-forming toxins. Thus, identifying PRRs that promote storage-induced alloimmunization can inform the identity of potential RBC DAMPs.
Many NLRs, including NLRP3, form a multi-molecular complex, termed an ‘inflammasome’, that results in NLR oligomerization, caspase-1 activation and enzymatic release of IL-1β and IL-18, which contribute to T cell activity (Latz et al., 2013) (Liu et al., 2013). NLRP3 is activated by a wide array of DAMPs, including extracellular ATP (Mariathasan et al., 2006), which is released from stored RBCs (Dern et al., 1967). Hemozoin, a byproduct of heme catabolism in malarial infection was also shown to activate NLRP3 (Shio et al., 2009). More recently, Dutra et al. demonstrated that inflammation caused by iron-containing heme is critically regulated by NLRP3 and downstream inflammasome components (Dutra et al., 2014). Therefore, we hypothesized that activation of NLRP3 or other NLR inflammasomes by RBC-associated DAMPs promotes the alloimmune response to sterile RBC transfusion. Using genetically modified mice lacking either Nlrp3 or multiple downstream common inflammasome response elements, we tested whether the NLRP3 inflammasome was a sensor of stored RBCs. Our data unambiguously excludes a critical role for the NLRP3 inflammasome or any caspase-1 or -11 dependent inflammasome in alloimmunization to stored HOD RBCs.
2. Materials & Methods
2.1. Mice
C57BL/6 and UBC-GFP mice were purchased from Charles River and Jackson Laboratory, respectively. HOD mice on the FVB background were generated as previously described (Hendrickson et al., 2011, Desmarets et al., 2009). Il1r−/−, Il18−/−, Casp1x11−/−, and Nlrp3−/− mice were previously described (Sutterwala et al., 2006, Kuida et al., 1995) (Labow et al., 1997). Il1r−/−, Il18−/− and Zbtb46-DTr mice (Meredith et al., 2012) were purchased from The Jackson Laboratory. All protocols used in this study were approved by the Yale Institutional Animal Care and Use Committee.
2.2. RBC Transfusion
RBCs were collected from HOD and UBC-GFP transgenic or WT C57BL/6 mice in 12% Citrate Phosphate Dextrose Adenine (CPDA-1) anticoagulant (Desmarets et al., 2009) and leuko-reduced using a murine-adapted Pall Acrodisc PSF 25 mm WBC filter or a Pall neonatal filter with Leukosorb Media prior to 4 °C storage for 7 or 14 days. Fresh RBCs were not stored. Before transfusion, RBCs were washed with PBS. Following centrifugation, packed RBCs were diluted 1:2 with sterile PBS. Diluted RBCs (200 μL, the human equivalent of 1–2 RBC units) were transfused i.v. into recipient mice. For inflammation-induced alloimmunization, 100 μg of polyinosinic:polycytidylic acid (poly(I:C), Amersham) were injected i.p. 4 h prior to transfusion of fresh RBCs.
2.3. Detection of Alloantibodies
Serum was collected three weeks after RBC transfusion. Levels of alloantibodies were measured by flow cytometric cross-match or an anti-HEL specific ELISA. For cross-match, serum was incubated with HOD+ RBCs or FVB RBCs, lacking the HOD antigen, for 30 min. RBCs were then washed and stained with goat polyclonal anti-mouse Ig (BD Pharmingen) for 30 min. Stained samples were washed and RBCs were analyzed for the presence of anti-Ig by flow cytometry. Anti-RBC antibodies in figures indicate the level of anti-HOD antibodies, calculated by subtracting the mean fluorescence intensity (MFI) of a serum sample incubated with FVB RBCs from the MFI of a paired sample incubated with HOD+ RBCs. For ELISA, anti-HEL specific IgG1 antibodies were detected in sera (starting dilution 1:50) as described previously (Hendrickson et al., 2007). Anti-IgG1 (clone A85-1) served as the detection antibody and HEL-specific IgG1 (clone 4B7) was used as the reference standard.
2.4. Inflammatory Cytokine Detection
Levels of inflammatory cytokines were measured as previously described (Hod et al., 2010). Briefly, serum cytokines, including interleukin-6 (IL-6), tumor necrosis factor-α (TNF- α), monocyte chemoattractant protein-1 (MCP-1), and keratinocyte-derived chemokine (KC), were quantified using a Cytometric Bead Array Mouse Flex Kit (BD Biosciences). Data were analyzed using FlowJo software (Tree Star).
2.5. IL-1β Measurement
Thioglycollate-elicited peritoneal macrophages were primed with 50 ng/mL LPS from E.coli serotype 0111:B4 (Invivogen) for 16–18 h prior to stimulation with either 500 mg/mL Imject aluminum hydroxide (Pierce) or 5 mM ATP for 8 h. For ATP-stimulated cells, the media was changed at 20 min and all stimulants were replaced. IL-1β released into culture supernatants was measured by ELISA. Antibody pairs for ELISA were purchased from R&D Systems.
2.6. Flow Cytometry
Single cell suspensions of splenocytes were acquired with a MACSQuant (Miltenyi) flow cytometer and analyzed using FlowJo software (Tree Star). The following antibodies were used for quantifying cDCs and measuring cDC activation: TCRβ (H57-597) and CD11c (N418) from eBiosciences; CD19 (RA3-6B2), MHC II (M5/114.15.2), and CD86 (GL-1) from Biolegend. Zombie-NIR (Biolegend) was used for exclusion of dead cells. For evaluating cDC activation, splenocytes were processed 4 h following transfusion or i.v. injection of LPS.
2.7. Deletion of Conventional Dendritic Cells
To generate Zbtb46-DTr BM chimeric mice, wild type recipients were irradiated with 2 doses of 650 rad 3 h apart. Two hours after the second irradiation, 1 × 106 bone marrow cells from Zbtb46-DTr mice were adoptively transferred via i.v. injection into recipient mice. For depletion of cDCs, chimeric mice were treated with diphtheria toxin (DT, Sigma-Aldrich), 7–10 weeks after bone marrow transplant. 60 ng of DT/g of body weight was injected i.p. on Day 0 followed by a second dose of 40 ng DT/g on Day 2. For alloimmunization experiments, HOD RBCs were transfused on Day 3. DT dosage was titrated in Zbtb46-DTr BM chimeric mice.
2.8. Statistical Analysis
Statistical significance between two groups was determined by unpaired t-test or Mann Whitney U test for parametric and nonparametric data, respectively. Significance among multiple groups was determined by one-way ANOVA with Tukey post-test or Kruskal-Wallis with Dunns post-test for parametric and nonparametric data, respectively. Normality was determined by the Kolmogorov-Smirnov test. Data were analyzed with GraphPad Prism software.
3. Results
3.1. Storage of HOD RBCs Promotes Inflammation and Alloimmunization
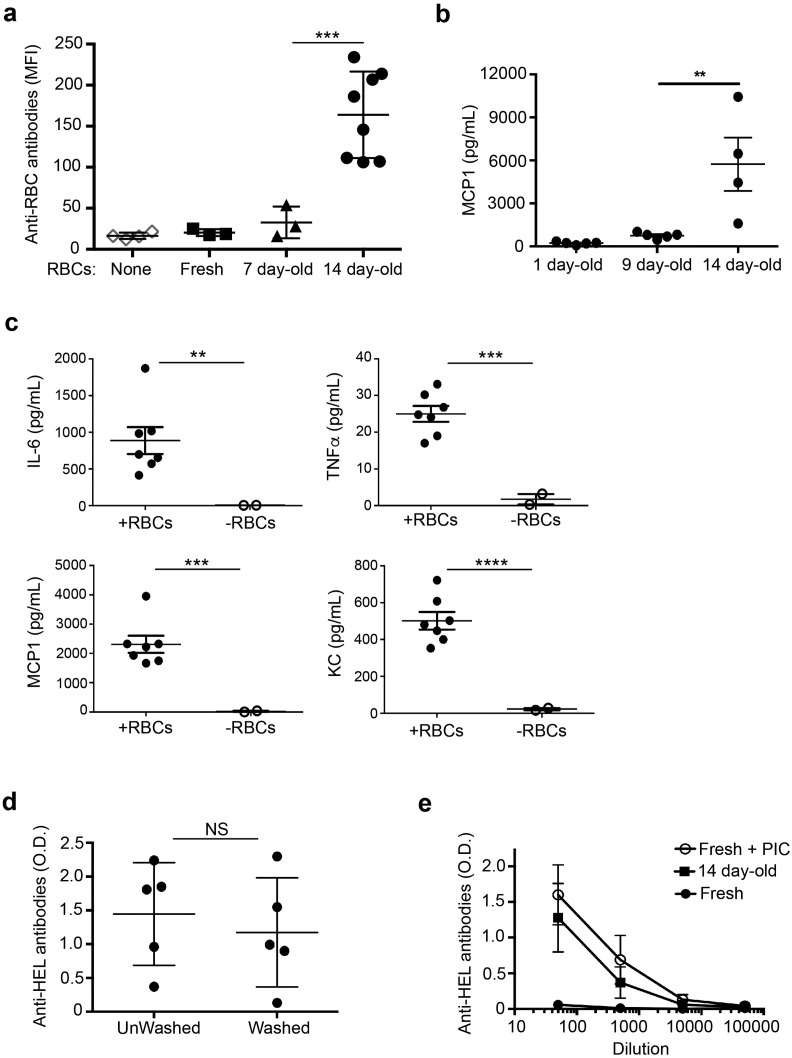
To examine the effect of RBC storage on post-transfusion inflammation and alloantibody responses, we transfused C57BL/6 wildtype (WT) mice with leuko-reduced HOD RBCs that were freshly collected or stored for 7 to 14 days in blood bank grade citrate-phosphate dextrose adenine solution (CPDA-1) at 4 °C. As previously reported (Hendrickson et al., 2010), transfusion of 14-day stored, but not freshly collected RBCs, resulted in a robust alloantibody response to the HOD antigen (Fig. 1a). In accordance with our prior study (Hod et al., 2010), transfusion of RBCs stored for 14 days also induced inflammatory cytokine and chemokine production (Fig. 1b, c). Storage for at least one week was required to promote alloimmunization and inflammation, as RBC storage for 7 and 9 days, respectively, led to minimal responses (Fig. 1a, b). In our prior study, infusion of RBC storage supernatant, RBC lysate, or RBC ghosts did not induce inflammatory cytokine production. Accordingly, we examined whether transfusion of stored RBCs in the absence of accompanying supernatant was also sufficient to induce alloimmunization. Transfusion of either washed or un-washed RBCs, stored for 14 days, led to similar levels of alloantibody production as measured by HEL-specific ELISA (Fig. 1d), suggesting that the pro-inflammatory factor(s) in stored RBCs is contained within the intact RBC. Thus, washed 14 day stored HOD RBCs were used for subsequent experiments.
Fig. 1.
Storage of HOD RBCs profoundly enhances transfusion-induced alloimmunization. WT C57BL/6 mice were transfused i.v. with stored or freshly collected HOD RBCs. a) Flow cytometric cross-match of anti-HOD RBC antibodies measured in sera of naïve untransfused mice and mice transfused with RBCs stored for 7 or 14 days, or freshly collected. Anti-RBC antibodies were calculated by subtracting the MFI of a serum sample incubated with RBCs lacking the HOD antigen from the MFI of a paired sample incubated with HOD + RBCs. n = 3–8 mice/group; results are representative of 3 independent experiments. b) Monocyte chemoattractant protein-1 (MCP-1) in serum 2–4 h following transfusion of RBCs stored for indicated times prior to transfusion measured by cytometric bead array. c) Inflammatory cytokines in serum as in (b) from mice transfused with RBCs stored for 14 days (+ RBCs) or not transfused (− RBCs); Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), MCP-1, and keratinocyte-derived chemokine (KC). n = 2–7 mice/group; results are representative of 4 independent experiments. d) Anti-HEL antibodies, quantified by ELISA, in sera diluted 1:50 from mice transfused with RBCs stored for 14 days and either washed with PBS or unwashed prior to transfusion. Unwashed samples contain storage supernatant. n = 5 mice/group; results are representative of 2 independent experiments. NS, not significant. e) Serum anti-HEL antibodies in mice that received stored (14 day-old), freshly collected (Fresh), or Fresh RBCs 4 h following i.p. administration of 100 μg poly(I:C) (Fresh + PIC). Error bars indicate standard deviation. n = 5 mice/group; results are representative of 2–3 independent experiments. **p < 0.01; ***p < 0.001; ****p < 0.0001.
Given that pre-treatment of recipient mice with pro-inflammatory stimuli, including PAMPs, promotes alloimmune responses to transfused RBCs (Bao et al., 2009, Hendrickson et al., 2006, Elayeb et al., 2016), we compared the level of alloantibodies in mice transfused with stored HOD RBCs to that induced by fresh RBCs in mice treated with a model PAMP, polyinosinic:polycytidylic acid (poly(I:C)), 4 h prior to transfusion. Both groups produced comparable levels of alloantibodies (Fig. 1e), indicating that alterations that occur during RBC storage make the cells pro-inflammatory and immunogenic. Given that innate inflammatory responses can promote APC activation and adaptive immune responses (Iwasaki and Medzhitov, 2010), we next asked whether stored RBCs promoted alloimmunization by activating innate immune receptors on antigen presenting cells (APCs).
3.2. Stored HOD RBCs Activate cDCs Required for Alloimmunization
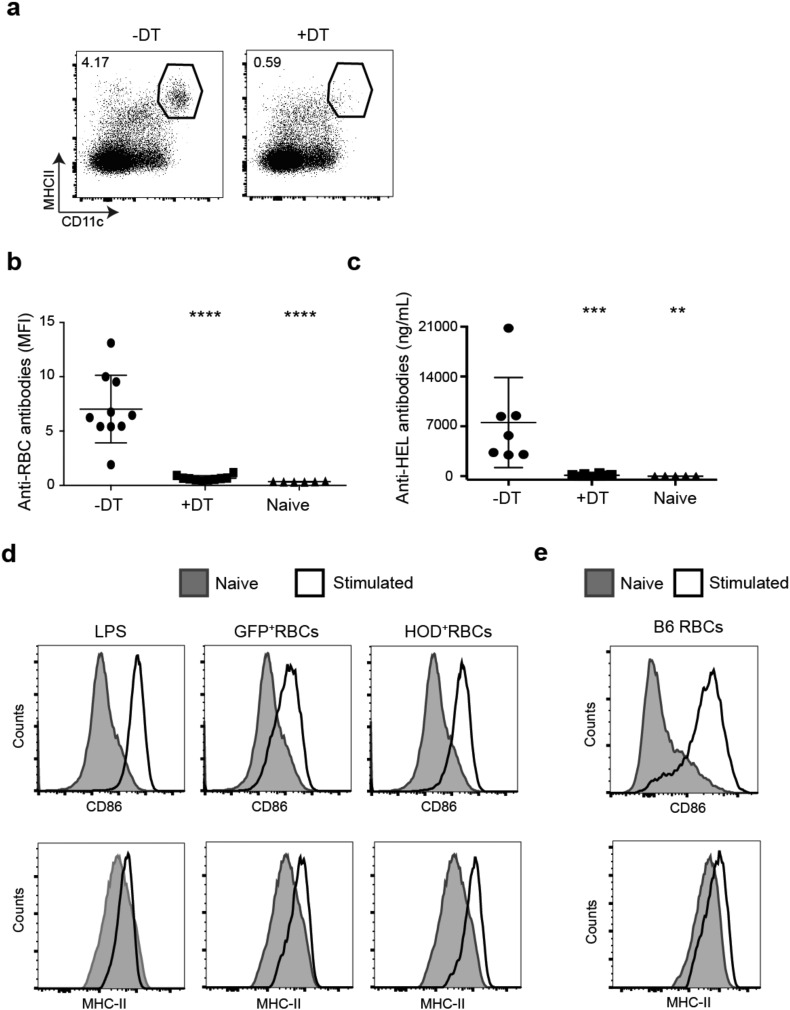
We previously demonstrated that inflammatory stimuli increase RBC consumption by splenic dendritic cells (DCs) following transfusion (Hendrickson et al., 2007). Conventional DCs (cDCs) are the primary APCs that activate naïve CD4 + T cells (Meredith et al., 2012, Satpathy et al., 2012). Thus, we examined the role of cDCs in the alloimmune response to transfusion of stored HOD RBCs. By generating chimeric mice with bone marrow from Zbtb46-DTR mice, which specifically express the human diphtheria toxin receptor (DTR) under control of the zinc finger transcription factor Zbtb46, cDCs can be selectively depleted (Meredith et al., 2012). Administration of diphtheria toxin (DT) led to a nearly complete depletion of cDCs in the spleen (Fig. 2a). Using this system, we recently demonstrated (Calabro et al., 2016) that cDCs are required for T cell-dependent alloantibody production in response to transfusion of stored HOD RBCs (Fig. 2b). Using sensitive ELISA-based methods, we confirmed that cDC depletion by DT treatment prevented alloantibody production to transfused stored HOD RBCs (Fig. 2c). Thus, cDCs, which are activated by innate immune stimuli, are required for alloimmunization to stored HOD RBCs. To determine if transfusion of stored RBCs leads to cDC activation in vivo, we measured splenic DC surface expression of MHC-II and CD86, which are upregulated during DC maturation. Four hours after transfusion of stored HOD RBCs, both activation markers on cDCs were upregulated to levels comparable to LPS-treated mice, which served as a positive control. Further, transfusion of stored GFP-expressing RBCs and syngeneic C57BL/6 RBCs, which lack the HOD antigen, also led to DC activation, indicating that the effect of RBC storage on DC activation is independent of the RBC alloantigen (Fig. 2d-e). However, the stored RBC-derived factors that activate cDCs have not been identified.
Fig. 2.
Stored RBCs activate splenic DCs. (a–c) Chimeric mice were generated with bone marrow from Zbtb46-DTR mice, as described in the Methods, and treated with diphtheria toxin prior to transfusion with HOD RBCs stored for 14 days. a) Representative flow cytometric analysis of CD11c+ MHC-II+ cDCs in spleens of mice treated with (+) or without (−) diphtheria toxin (DT). Dot plots were gated on live TCRβ− CD19− non-lymphocytes. Numbers on plots indicate the percentage of gated cells within the indicated gate. b, c) Anti-RBC antibodies in sera of mice treated with or without DT prior to stored HOD RBC transfusion, quantified by flow cytometric cross-match (b) or ELISA (c) as in Fig. 1. n = 6–10 mice/group; results are representative of 5 independent experiments. ****p < 0.0001; **p < 0.01. d–e) Expression of activation markers, CD86 and MHC-II, on spleen cDCs (gated as in a) from naïve mice (shaded) or mice injected i.v. 4 h prior (open histogram) with (d) LPS, stored HOD RBCs, stored GFP-expressing RBCs or (e) stored WT C57BL/6 RBCs. 1 representative mouse from 2 mice/group from 3 independent experiments.
3.3. The NLRP3 Inflammasome is not Required for Stored RBC-Induced Inflammation and Alloimmunization
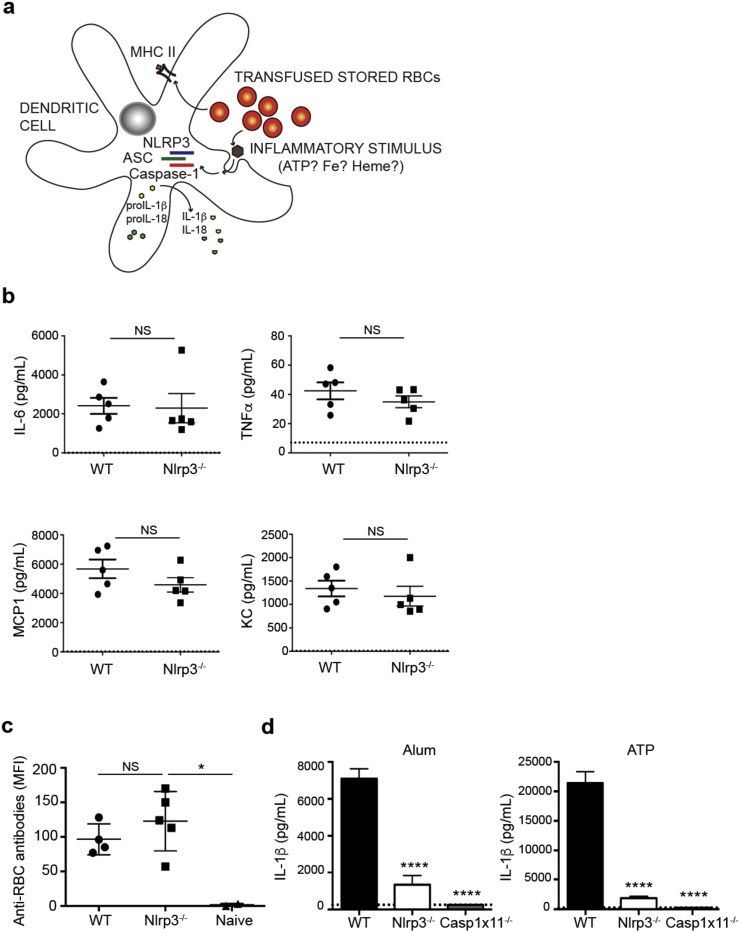
DAMPs formed during RBC storage may activate cDCs by engaging pattern recognition receptors, including NLRs. NLRP3, the best characterized inflammasome-forming NLR, is activated by a wide variety of DAMPs, including ATP (Mariathasan et al., 2006), which is released from stored RBCs (Dern et al., 1967). Previous studies have shown that transfusion of stored HOD RBCs results in elevated levels of intracellular iron in APCs and NTBI in plasma (Hod et al., 2010, Hod et al., 2011). Heme-containing iron and other factors produced by stored RBCs have been shown to activate the NLRP3 inflammasome (Mariathasan et al., 2006, Dutra et al., 2014). As shown in Fig. 3a, following recognition of discrete inflammatory stimuli, NLRP3 oligomerizes and recruits the adapter, apoptosis-associated speck-like protein containing a CARD (ASC). This complex activates caspase-1, which leads to activation and secretion of IL-1β and IL-18 cytokines. To determine whether the NLRP3 inflammasome is required for stored RBC-induced inflammation and alloimmunization, we assessed post-transfusion production of multiple inflammatory cytokines and chemokines (as in Fig. 1) and anti-HOD RBC alloantibodies in Nlrp3−/− and WT mice. There were no significant differences in levels of IL-6, TNFα, MCP1 or KC produced by Nlrp3−/− as compared to WT mice (Fig. 3b). Additionally, Nlrp3 deficiency did not impair HOD-specific alloantibody production (Fig. 3c). Yet primary macrophages from Nlrp3−/− and Casp1x11−/− mice failed to respond, as measured by IL-1β secretion, to known Nlrp3 inflammasome activators including aluminum hydroxide and ATP (Fig. 3d) (Mariathasan et al., 2006, Eisenbarth et al., 2008, Li et al., 2008). Thus, NLRP3 does not critically regulate the inflammatory or alloantibody response to transfused stored HOD RBCs.
Fig. 3.
Nlrp3 is not required for RBC alloimmunization. a) Theoretical model of NLRP3 inflammasome activation by stored RBC DAMPs in a dendritic cell. Following recognition of possible inflammatory stimuli, such as ATP, iron or heme, NLRP3 oligomerizes, recruits ASC and caspase-1, which enzymatically cleaves pro-cytokines, resulting in secretion of active IL-18 and IL-1β. MHC-II is upregulated following activation and presents RBC antigens to antigen-specific T cells. ASC, apoptosis-associated speck-like protein containing a CARD. b,c) Inflammatory cytokines (b) and anti-RBC alloantibodies (c) in sera of indicated mice following transfusion with stored HOD RBCs, quantified as in Fig. 1. n = 3–5 mice/group. Dotted line in (b) indicates level of serum cytokines in naïve mice from the same experiment. Data are representative of 5 independent experiments. d) IL-1β, measured by ELISA, in supernatants of ex vivo cultured thioglycollate-elicited peritoneal macrophages from indicated mice primed with 50 ng/mL LPS for 16–18 h prior to stimulation with either 500 ng/mL alum or 5 mM ATP for 8 h. Dotted line indicates level of detection of ELISA. Mean with s.d. from triplicate wells. Representative data from 3 independent experiments. NS, not significant; *p < 0.05; ****p < 0.0001.
3.4. NLR Inflammasomes Do Not Regulate Inflammation and Alloimmunization Induced by Stored RBCs
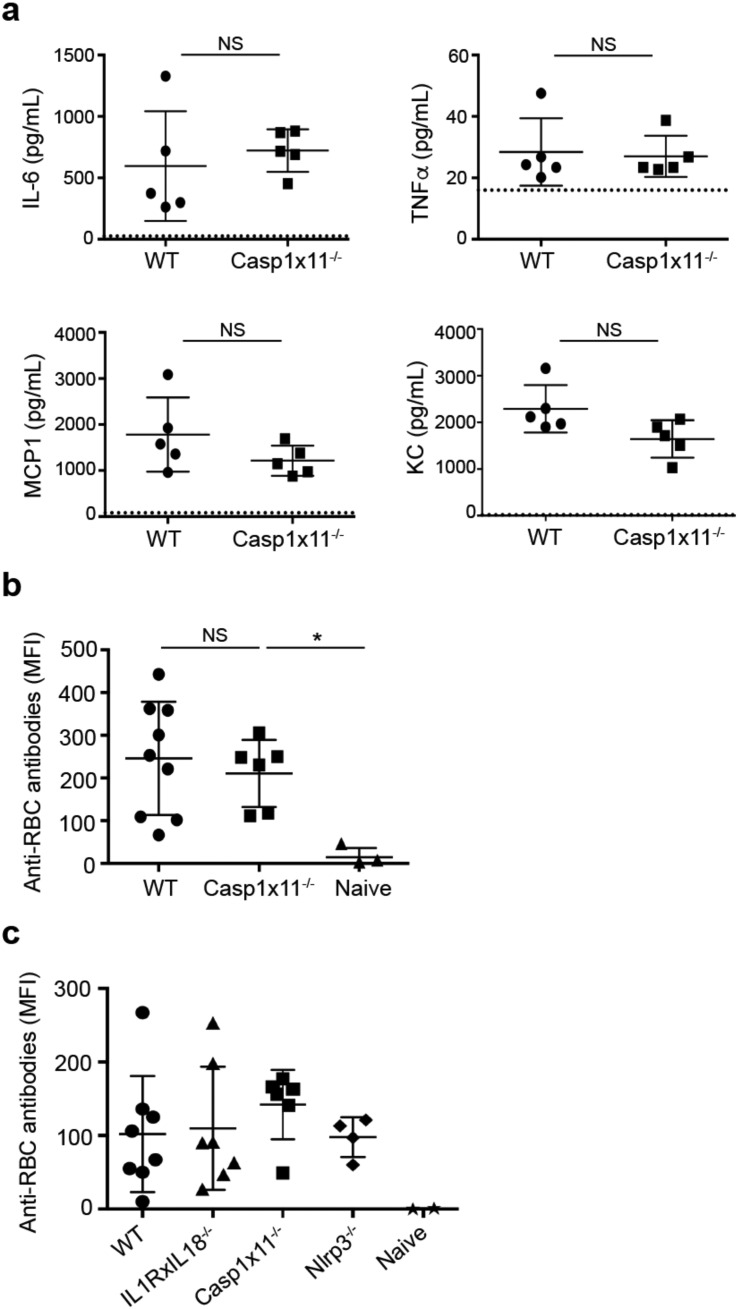
Although NLRP3 is the best characterized inflammasome-forming NLR, numerous other NLRs, including NLRC4, NLRP1 and NLRP6, also form inflammasomes that activate caspase-1 and, under certain circumstances, also caspase-11 (Liu et al., 2013) (Afonina et al., 2015). Thus, to determine the potential role of other inflammasome-forming NLRs in storage-induced inflammation and alloimmunization, we utilized caspase-1 and caspase-11 double deficient mice (Casp1x11−/−) and mice unable to respond with inflammasome-dependent cytokines, Il-1r−/− and Il-18−/− mice (Il1rxIl18−/−). These latter double deficient mice lack both IL-18 production and signaling via the IL-1 receptor. As both IL-1α and IL-1β signal via IL-1R, the IL-1R−/− mice abrogate signaling to both cytokines. First, Casp1x11−/− mice were transfused with stored HOD RBCs and serum pro-inflammatory cytokine/chemokine and alloantibody levels were assessed. As shown in Fig. 4a and b, loss of caspase-1 and caspase-11 did not significantly affect IL-6, TNFα, MCP1 or KC serum levels or alloantibody production as compared to WT mice. Second, Il1rxIl18−/− mice were transfused with stored HOD RBCs and alloantibodies were measured to assess the potential impact of loss of inflammasome-dependent cytokines on the alloimmune response. Compared to WT controls, Il1rxIl18−/− mice, as well as Nlrp3−/− and Casp1x11−/− mice, produced equivalent levels of alloantibodies to stored HOD RBCs after transfusion (Fig. 4c). Thus, NLR-inflammasomes are not required for the acute inflammatory response or the adaptive alloimmune response to transfused stored RBCs.
Fig. 4.
Inflammasomes are not required for RBC alloimmunization. a) Inflammatory cytokines in sera of WT and Caspase-1xCaspase-11 (Casp1x11−/−) double deficient mice quantified by cytometric bead array 2 h after transfusion with HOD RBCs stored for 14 days. Dotted line indicates level of serum cytokines in naïve mice from the same experiment. b) Anti-HOD RBC alloantibodies in sera were quantified by cytometric cross-match, as in Fig. 1, from WT and Casp1x11−/− mice 21 days after HOD RBC transfusion. Naïve mice did not receive RBC transfusion. n = 3–9 mice/group; results are representative of 6 independent experiments. c) Anti-HOD RBC alloantibodies in sera from WT, Nlrp3−/−, Casp1x11−/− and IL1R and IL-18 double deficient mice 21 days after transfusion with stored HOD RBCs. No significant differences determined by one-way ANOVA. n = 2–8 mice/group; results are representative of 3 independent experiments. *p < 0.05; NS, not significant.
4. Discussion
Identifying patients with an elevated risk of forming alloantibodies to non-ABO RBC antigens could lead to directed preventative measures, including antigen matching, or therapeutic interventions that mitigate the alloimmune response (Hendrickson et al., 2014). In light of the clinical significance of RBC alloimmunization, progress in this area could substantially decrease associated adverse events, including hemolytic transfusion reactions and general morbidity and mortality (Nickel et al., 2016). However, to develop criteria for biomarkers or cytokine profiles that have predictive value, mechanisms underlying the alloantibody-forming adaptive immune responses must be determined.
Using the mouse transfusion model of HOD RBCs, we and others have shown that refrigerated RBC storage increases the inflammatory (Hod et al., 2010) and alloantibody (Hendrickson et al., 2010) responses of recipients. In parallel, a clinical study has shown that the age of transfused RBCs is associated with alloimmunization in patients with sickle cell disease (Desai et al., 2015). The pronounced increase in alloantibodies in our studies occurred following 7–14 days of murine RBC storage. Significant differences have been noted in the risk of alloantibody formation between 7 and 35 days of human RBC storage (Desai et al., 2015). In addition, a recent study by Veale et al. found that the susceptibility of stored human RBCs to in vitro erythrophagocytosis increased significantly with storage duration (Veale et al., 2014). We conclude that DAMPs are formed and contained within intact murine RBCs during the second week of storage, and hypothesize that DAMPs may also be formed in human RBCs the week prior to outdate. This finding is supported by a prior study demonstrating that intact HOD RBCs, but not RBC lysate or RBC ghosts generated from stored cells, induce inflammatory cytokine production following transfusion (Hod et al., 2010). Further studies addressing metabolic changes within intact stored RBCs could potentially identify immunogenic DAMPs.
Treatment of recipients with the pro-inflammatory PAMP, poly(I:C), prior to fresh HOD RBC transfusion resulted in alloantibody production comparable to the response to stored RBCs (Fig. 1e). It should be noted that this study did not directly address whether the demonstrated pro-inflammatory cytokine response to transfused stored HOD RBCs was necessary for alloimmunization. However, multiple studies in mice and humans have reported associations between inflammatory states and alloimmunization (Ramsey and Smietana, 1995, Fasano et al., 2015, Papay et al., 2012, Yazer et al., 2009, Hendrickson et al., 2006, Ryder et al., 2015). Additionally, it is firmly accepted that innate inflammatory responses can promote APC activation and adaptive immune responses (Iwasaki and Medzhitov, 2010). Although many APC cell types consume transfused RBCs in the spleen (Richards et al., 2016), we have demonstrated that splenic cDCs critically regulate the alloantibody response to stored HOD RBCs in a T cell-dependent manner (Calabro et al., 2016). We also observed that stored RBCs induce cDC activation, independent of HOD expression. However, it is not clear whether stored RBC DAMPs are directly recognized by cDCs. PRR activation in CD11b+ Ly6C+ F4/80+ macrophages, shown to consume stored RBCs (Calabro et al., 2016), may instead cause production of pro-inflammatory cytokines that activate cDCs and promote cDC phagocytic activity. Such an indirect mechanism is supported by a prior study reporting that clodronate-induced macrophage depletion abrogates MCP-1 and KC production following transfusion of stored RBCs (Wojczyk et al., 2014). Regardless, these findings suggest that cDC activation may critically regulate the alloimmune response. Yet, the initiation process for cDC activation has not been defined.
To address this challenge, we took advantage of the discrete nature of PRR:DAMP interactions. Thus, identifying a PRR pathway that regulates alloimmunization will greatly narrow the search for relevant immunomodulatory DAMPs in stored RBCs. It also has the potential to identify downstream cytokine profiles associated with alloimmunization. Although numerous PRRs could be tested, NLRP3 and other inflammasome-forming NLRs were excellent candidates. NLRP3 is activated by numerous DAMPs, including protein aggregates, insoluble crystals, and products of cell death (Krishnaswamy et al., 2013) (Guo et al., 2015, Storek and Monack, 2015). With regards to potential RBC DAMPs, NLRP3 inflammasomes are activated by ATP (Mariathasan et al., 2006), which is released from damaged RBCs (Dern et al., 1967), and iron-containing heme (Dutra et al., 2014), which can be produced by intracellular catabolism of phagocytosed RBCs (Hod et al., 2010). In addition, reactive oxygen species, which prime NLRP3 activation (Juliana et al., 2012), were implicated in stored HOD RBC-induced inflammation, which was reduced by anti-oxidant treatment (Hod et al., 2010). Nonetheless, the lack of NLRP3 or associated caspase-1 or caspase-11 had no significant effect on stored RBC-induced inflammatory and alloimmune responses. This finding was supported by alloimmune responses of Il1rxIl18−/− mice that were comparable to WT responses. We cannot exclude the possibility that NLR-forming inflammasomes could perform more significant roles in response to storage of RBCs expressing other antigens, not tested in this study. We believe this possibility is unlikely given our findings that the nature of the alloantigen on the stored RBCs did not impact DC activation.
Although not examined in this study, other PRRs, including TLRs and Rig-I-like receptors (RLRs), may regulate responses to stored RBCs. Even though TLRs and RLRs primarily recognize foreign PAMPs, they can also recognize certain DAMPs, including self-nucleic acids and intracellular molecules released during cell death (Piccinini and Midwood, 2010). Red blood cells are not nucleated, yet the washed leuko-reduced RBC preparations contain reticulocytes, platelets, and a low level of leukocytes (Ryder et al., 2014) that may release TLR ligands during storage. Future studies examining inflammatory and adaptive immune responses of various PRR-deficient mice to different RBC preparations may lead to progress in identifying DAMPs associated with alloimmunization. Indeed, identification of a potential DAMP endogenous to RBCs themselves or to the RBC product preparation could redefine transfusion practices.
Of note, it is not known whether the findings observed in the HOD model will translate to murine RBCs expressing other antigens, or to humans. Human studies investigating the role of RBC storage duration on alloimmunization and other adverse effects have reported varied outcomes (Dinardo et al., 2015, Yazer and Triulzi, 2010, Zalpuri et al., 2013, Desai et al., 2015, Koch et al., 2008, Offner et al., 2002, Purdy et al., 1997, Vandromme et al., 2009, Weinberg et al., 2008, Zallen et al., 1999, Fergusson et al., 2012, Lacroix et al., 2015, Steiner et al., 2015) Future studies are needed in animals and humans, to determine which immune pathways are common or divergent.
5. Conclusion
In conclusion, this study utilized a mouse model of stored HOD RBC transfusion to investigate underlying mechanisms of inflammation-induced alloimmunization. Transfusion of stored RBCs induced pro-inflammatory cytokine production and pronounced alloantibody responses. In addition, storage of RBCs resulted in activation of cDCs, which are required to promote the alloimmune response. Finally, although inflammasome-forming NLRs were excellent candidates for responding to DAMPs formed by stored RBCs, this study rules out a critical role for NLR inflammasomes in regulating the inflammatory and alloimmune responses to stored HOD RBCs.
Author Contributions
The study was designed by S.E. and E.H. Data collection and analysis were performed by D.G., S.C., D.L., J.H., E.H., and S.E. RBC transgenic mice were made by J.Z. All authors contributed to data interpretation and editing the written report, written by D.G, E.H., and S.E.
Acknowledgments
We would like to thank M. Firla for technical assistance. This work was supported by the National Blood Foundation (S.C.E) and the American Society of Hematology (S.C.E). Funders had no role in study design, data collection, data analysis, interpretation or writing of the report.
Contributor Information
Eldad A. Hod, Email: eh2217@cumc.columbia.edu.
Stephanie C. Eisenbarth, Email: stephanie.eisenbarth@yale.edu.
References
- (FDA) Fatalities reported to the FDA following blood collection and transfusion: annual summary for fiscal year 2014. 2014. http://www.fda.gov/downloads/biologicsbloodvaccines/safetyavailability/reportaproblem/transfusiondonationfatalities/ucm459461.pdf
- Afonina I.S., Muller C., Martin S.J., Beyaert R. Proteolytic processing of interleukin-1 family cytokines: variations on a common theme. Immunity. 2015;42:991–1004. doi: 10.1016/j.immuni.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Bao W., Yu J., Heck S., Yazdanbakhsh K. Regulatory T-cell status in red cell alloimmunized responder and nonresponder mice. Blood. 2009;113:5624–5627. doi: 10.1182/blood-2008-12-193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Guerrero E., Veldman T.H., Doctor A., Telen M.J., Ortel T.L., Reid T.S., Mulherin M.A., Zhu H., Buck R.D., Califf R.M., McMahon T.J. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro S., Gallman A., Gowthaman U., Liu D., Chen P., Liu J., Krishnaswamy J.K., Nascimento M.S., Xu L., Patel S.R., Williams A., Tormey C.A., Hod E.A., Spitalnik S.L., Zimring J.C., Hendrickson J.E., Stowell S.R., Eisenbarth S.C. Bridging channel dendritic cells induce immunity to transfused red blood cells. J. Exp. Med. 2016;213:887–896. doi: 10.1084/jem.20151720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dern R.J., Brewer G.J., Wiorkowski J.J. Studies on the preservation of human blood. II. The relationship of erythrocyte adenosine triphosphate levels and other in vitro measures to red cell storageability. J. Lab. Clin. Med. 1967;69:968–978. [PubMed] [Google Scholar]
- Desai P.C., Deal A.M., Pfaff E.R., Qaqish B., Hebden L.M., Park Y.A., Ataga K.I. Alloimmunization is associated with older age of transfused red blood cells in sickle cell disease. Am. J. Hematol. 2015;90:691–695. doi: 10.1002/ajh.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarets M., Cadwell C.M., Peterson K.R., Neades R., Zimring J.C. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114:2315–2322. doi: 10.1182/blood-2009-04-214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinardo C.L., Fernandes F.L., Sampaio L.R., Sabino E.C., Mendrone A., Jr. Transfusion of older red blood cell units, cytokine burst and alloimmunization: a case-control study. Rev. Bras. Hematol. Hemoter. 2015;37:320–323. doi: 10.1016/j.bjhh.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra F.F., Alves L.S., Rodrigues D., Fernandez P.L., de Oliveira R.B., Golenbock D.T., Zamboni D.S., Bozza M.T. Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4110–E4118. doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth S.C., Colegio O.R., O'Connor W., Sutterwala F.S., Flavell R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elayeb R., Tamagne M., Bierling P., Noizat-Pirenne F., Vingert B. Red blood cell alloimmunization is influenced by the delay between toll-like receptor agonist injection and transfusion. Haematologica. 2016;101:209–218. doi: 10.3324/haematol.2015.134171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano R.M., Booth G.S., Miles M., Du L., Koyama T., Meier E.R., Luban N.L. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br. J. Haematol. 2015;168:291–300. doi: 10.1111/bjh.13123. [DOI] [PubMed] [Google Scholar]
- Fergusson D.A., Hebert P., Hogan D.L., LeBel L., Rouvinez-Bouali N., Smyth J.A., Sankaran K., Tinmouth A., Blajchman M.A., Kovacs L., Lachance C., Lee S., Walker C.R., Hutton B., Ducharme R., Balchin K., Ramsay T., Ford J.C., Kakadekar A., Ramesh K., Shapiro S. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA, J. Am. Med. Assoc. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- Fluit C.R., Kunst V.A., Drenthe-Schonk A.M. Incidence of red cell antibodies after multiple blood transfusion. Transfusion. 1990;30:532–535. doi: 10.1046/j.1537-2995.1990.30690333485.x. [DOI] [PubMed] [Google Scholar]
- Gilson C.R., Kraus T.S., Hod E.A., Hendrickson J.E., Spitalnik S.L., Hillyer C.D., Shaz B.H., Zimring J.C. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson J.E., Desmarets M., Deshpande S.S., Chadwick T.E., Hillyer C.D., Roback J.D., Zimring J.C. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- Hendrickson J.E., Chadwick T.E., Roback J.D., Hillyer C.D., Zimring J.C. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736–2743. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- Hendrickson J.E., Hod E.A., Spitalnik S.L., Hillyer C.D., Zimring J.C. Storage of murine red blood cells enhances alloantibody responses to an erythroid-specific model antigen. Transfusion. 2010;50:642–648. doi: 10.1111/j.1537-2995.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson J.E., Hod E.A., Cadwell C.M., Eisenbarth S.C., Spiegel D.A., Tormey C.A., Spitalnik S.L., Zimring J.C. Rapid clearance of transfused murine red blood cells is associated with recipient cytokine storm and enhanced alloimmunogenicity. Transfusion. 2011;51:2445–2454. doi: 10.1111/j.1537-2995.2011.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson J.E., Tormey C.A., Shaz B.H. Red blood cell alloimmunization mitigation strategies. Transfus. Med. Rev. 2014;28:137–144. doi: 10.1016/j.tmrv.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Higgins J.M., Sloan S.R. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–2553. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- Hod E.A., Zhang N., Sokol S.A., Wojczyk B.S., Francis R.O., Ansaldi D., Francis K.P., Della-Latta P., Whittier S., Sheth S., Hendrickson J.E., Zimring J.C., Brittenham G.M., Spitalnik S.L. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hod E.A., Brittenham G.M., Billote G.B., Francis R.O., Ginzburg Y.Z., Hendrickson J.E., Jhang J., Schwartz J., Sharma S., Sheth S., Sireci A.N., Stephens H.L., Stotler B.A., Wojczyk B.S., Zimring J.C., Spitalnik S.L. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliana C., Fernandes-Alnemri T., Kang S., Farias A., Qin F., Alnemri E.S. Non-transcriptional Priming and Deubiquitination Regulate NLRP3 Inflammasome Activation. J. Biol. Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C.G., Li L., Sessler D.I., Figueroa P., Hoeltge G.A., Mihaljevic T., Blackstone E.H. Duration of red-cell storage and complications after cardiac surgery.[see comment] N. Engl. J. Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy J.K., Chu T.C., Eisenbarth S.C. Beyond pattern recognition: NOD-like receptors in dendritic cells. Trends Immunol. 2013;34:224–233. doi: 10.1016/j.it.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K., Lippke J.A., Ku G., Harding M.W., Livingston D.J., Su M.S., Flavell R.A. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Labow M., Shuster D., Zetterstrom M., Nunes P., Terry R., Cullinan E.B., Bartfai T., Solorzano C., Moldawer L.L., Chizzonite R., McIntyre K.W. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J. Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- Lacroix J., Hebert P.C., Fergusson D.A., Tinmouth A., Cook D.J., Marshall J.C., Clayton L., McIntyre L., Callum J., Turgeon A.F., Blajchman M.A., Walsh T.S., Stanworth S.J., Campbell H., Capellier G., Tiberghien P., Bardiaux L., van de Watering L., van der Meer N.J., Sabri E., Vo D. Investigators, A. & Canadian critical care trials, G. Age of transfused blood in critically ill adults. N. Engl. J. Med. 2015;372:1410–1418. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- Latz E., Xiao T.S., Stutz A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Willingham S.B., Ting J.P.-Y., Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J. Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Rhebergen A.M., Eisenbarth S.C. Licensing adaptive immunity by NOD-like receptors. Front. Immunol. 2013;4:486. doi: 10.3389/fimmu.2013.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S., Weiss D.S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W.P., Weinrauch Y., Monack D.M., Dixit V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Meredith M.M., Liu K., Darrasse-Jeze G., Kamphorst A.O., Schreiber H.A., Guermonprez P., Idoyaga J., Cheong C., Yao K.H., Niec R.E., Nussenzweig M.C. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J. Exp. Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel R.S., Hendrickson J.E., Fasano R.M., Meyer E.K., Winkler A.M., Yee M.M., Lane P.A., Jones Y.A., Pashankar F.D., New T., Josephson C.D., Stowell S.R. Impact of red blood cell alloimmunization on sickle cell disease mortality: a case series. Transfusion. 2016;56:107–114. doi: 10.1111/trf.13379. [DOI] [PubMed] [Google Scholar]
- Offner P.J., Moore E.E., Biffl W.L., Johnson J.L., Silliman C.C. Increased rate of infection associated with transfusion of old blood after severe injury. Arch. Surg. 2002;137:711–716. doi: 10.1001/archsurg.137.6.711. (discussion 716–717) [DOI] [PubMed] [Google Scholar]
- Papay P., Hackner K., Vogelsang H., Novacek G., Primas C., Reinisch W., Eser A., Mikulits A., Mayr W.R., Kormoczi G.F. High risk of transfusion-induced alloimmunization of patients with inflammatory bowel disease. Am. J. Med. 2012;125(717):e711–e718. doi: 10.1016/j.amjmed.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Pfuntner A., Wier L.M., Stocks C. 2013. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. [Google Scholar]
- Piccinini A.M., Midwood K.S. DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010;2010 doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy F.R., Tweeddale M.G., Merrick P.M. Association of mortality with age of blood transfused in septic ICU patients. Can. J. Anaesth. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- Ramsey G., Smietana S.J. Multiple or uncommon red cell alloantibodies in women: association with autoimmune disease. Transfusion. 1995;35:582–586. doi: 10.1046/j.1537-2995.1995.35795357881.x. [DOI] [PubMed] [Google Scholar]
- Richards A.L., Hendrickson J.E., Zimring J.C., Hudson K.E. Erythrophagocytosis by plasmacytoid dendritic cells and monocytes is enhanced during inflammation. Transfusion. 2016;56:905–916. doi: 10.1111/trf.13497. [DOI] [PubMed] [Google Scholar]
- Ryder A.B., Zimring J.C., Hendrickson J.E. Factors Influencing RBC Alloimmunization: lessons learned from murine models. Transfus. Med. Hemother. 2014;41:406–419. doi: 10.1159/000368995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder A.B., Hendrickson J.E., Tormey C.A. Chronic inflammatory autoimmune disorders are a risk factor for red blood cell alloimmunization. Br. J. Haematol. 2015 doi: 10.1111/bjh.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy A.T., Kc W., Albring J.C., Edelson B.T., Kretzer N.M., Bhattacharya D., Murphy T.L., Murphy K.M. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J. Exp. Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shio M.T., Eisenbarth S.C., Savaria M., Vinet A.F., Bellemare M.J., Harder K.W., Sutterwala F.S., Bohle D.S., Descoteaux A., Flavell R.A., Olivier M. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M.E., Ness P.M., Assmann S.F., Triulzi D.J., Sloan S.R., Delaney M., Granger S., Bennett-Guerrero E., Blajchman M.A., Scavo V., Carson J.L., Levy J.H., Whitman G., D'Andrea P., Pulkrabek S., Ortel T.L., Bornikova L., Raife T., Puca K.E., Kaufman R.M., Nuttall G.A., Young P.P., Youssef S., Engelman R., Greilich P.E., Miles R., Josephson C.D., Bracey A., Cooke R., McCullough J., Hunsaker R., Uhl L., McFarland J.G., Park Y., Cushing M.M., Klodell C.T., Karanam R., Roberts P.R., Dyke C., Hod E.A., Stowell C.P. Effects of red-cell storage duration on patients undergoing cardiac surgery. N. Engl. J. Med. 2015;372:1419–1429. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storek K.M., Monack D.M. Bacterial recognition pathways that lead to inflammasome activation. Immunol. Rev. 2015;265:112–129. doi: 10.1111/imr.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala F.S., Ogura Y., Szczepanik M., Lara-Tejero M., Lichtenberger G.S., Grant E.P., Bertin J., Coyle A.J., Galán J.E., Askenase P.W., Flavell R.A. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tatari-Calderone Z., Minniti C.P., Kratovil T., Stojakovic M., Vollmer A., Barjaktarevic I., Zhang E., Hoang A., Luban N.L., Vukmanovic S. rs660 polymorphism in Ro52 (SSA1; TRIM21) is a marker for age-dependent tolerance induction and efficiency of alloimmunization in sickle cell disease. Mol. Immunol. 2009;47:64–70. doi: 10.1016/j.molimm.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Telen M.J., Afenyi-Annan A., Garrett M.E., Combs M.R., Orringer E.P., Ashley-Koch A.E. Alloimmunization in sickle cell disease: changing antibody specificities and association with chronic pain and decreased survival. Transfusion. 2015;55:1378–1387. doi: 10.1111/trf.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinmouth A., Fergusson D., Yee I.C., Hebert P.C. Investigators, A. & Canadian critical care trials, G. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- Vandromme M.J., McGwin G., Jr., Marques M.B., Kerby J.D., Rue L.W., III, Weinberg J.A. Transfusion and pneumonia in the trauma intensive care unit: an examination of the temporal relationship. J. Trauma. 2009;67:97–101. doi: 10.1097/TA.0b013e3181a5a8f9. [DOI] [PubMed] [Google Scholar]
- Veale M.F., Healey G., Sparrow R.L. Longer storage of red blood cells is associated with increased in vitro erythrophagocytosis. Vox Sang. 2014;106:219–226. doi: 10.1111/vox.12095. [DOI] [PubMed] [Google Scholar]
- Vichinsky E.P., Earles A., Johnson R.A., Hoag M.S., Williams A., Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N. Engl. J. Med. 1990;322:1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- Weinberg J.A., McGwin G., Jr., Griffin R.L., Huynh V.Q., Cherry S.A., III, Marques M.B., Reiff D.A., Kerby J.D., Rue L.W., III Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J. Trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. (discussion 282–274) [DOI] [PubMed] [Google Scholar]
- Wojczyk B.S., Kim N., Bandyopadhyay S., Francis R.O., Zimring J.C., Hod E.A., Spitalnik S.L. Macrophages clear refrigerator storage-damaged red blood cells and subsequently secrete cytokines in vivo, but not in vitro, in a murine model. Transfusion. 2014;54:3186–3197. doi: 10.1111/trf.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazer M.H., Triulzi D.J. Receipt of older RBCs does not predispose D-negative recipients to anti-D alloimmunization. Am. J. Clin. Pathol. 2010;134:443–447. doi: 10.1309/AJCP2J8SVWOXRLRB. [DOI] [PubMed] [Google Scholar]
- Yazer M.H., Triulzi D.J., Shaz B., Kraus T., Zimring J.C. Does a febrile reaction to platelets predispose recipients to red blood cell alloimmunization? Transfusion. 2009;49:1070–1075. doi: 10.1111/j.1537-2995.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- Yi T., Li J., Chen H., Wu J., An J., Xu Y., Hu Y., Lowell C.A., Cyster J.G. Splenic dendritic cells survey red blood cells for missing self-CD47 to trigger adaptive immune responses. Immunity. 2015 doi: 10.1016/j.immuni.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen G., Offner P.J., Moore E.E., Blackwell J., Ciesla D.J., Gabriel J., Denny C., Silliman C.C. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am. J. Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- Zalpuri S., Schonewille H., Middelburg R., van de Watering L., de Vooght K., Zimring J., van der Bom J.G., Zwaginga J.J. Effect of storage of red blood cells on alloimmunization. Transfusion. 2013;53:2795–2800. doi: 10.1111/trf.12156. [DOI] [PubMed] [Google Scholar]