Abstract
Background
When asphyxiated neonates require additional cardiovascular support to moderate doses of dopamine infusion, controversy exists on differential hemodynamic effects of two approaches (adding a second inotrope vs. increasing dopamine dosage). We hypothesized that high-dose dopamine (HD) would be detrimental on systemic and regional perfusion when compared with dopamine and epinephrine (D+E) combination therapy using a swine model of neonatal hypoxia-reoxygenation (H-R).
Methods
Twenty-seven piglets (1–4 days, 1.5–2.5kg) were used for continuous monitoring of systemic (MAP) and pulmonary (PAP) arterial pressures, cardiac output (CI) and carotid (CAFI), superior mesenteric (SMAFI) and renal arterial flows. H-R piglets underwent 2h of hypoxia followed by 2h of reoxygenation prior to drug infusion (2h).
Results
The hemodynamics of H-R piglets deteriorated gradually after reoxygenation. HD and D+E infusions improved CI similarly (both groups vs. control; p<0.05). Both regimens increased MAP (p<0.05) but not PAP, with decreased PAP/MAP ratio in D+E piglets. Both regimens improved CAFI and SMAFI with decreased mesenteric vascular resistance in HD-treated piglets. No significant effect on renal perfusion was observed.
Conclusion
In H-R newborn piglets treated with a moderate dose of dopamine, adding epinephrine or further increasing dopamine improved systemic hemodynamics similarly, and have differential effects on the pulmonary and mesenteric circulations.
INTRODUCTION
The World Health Organization attributes 23% of the 4 million neonatal deaths each year to asphyxia (1). Neonatal survival is challenged by asphyxia with variable effects on different organ systems. When 72 asphyxiated neonates were followed prospectively, evidence of multi-organ dysfunction was appreciated with abnormalities of the central nervous, cardiovascular, gastrointestinal and renal systems (2).
Following asphyxia, neonates develop shock with reduced cardiac output, systemic hypotension and pulmonary hypertension (3–5). Subsequent regional hypoperfusion compromises cerebral, gastrointestinal and renal function. Vasoactive medications – inotropes and vasopressors – have long been used to treat shock in neonates despite a paucity of evidence on their effects, particularly in regard to organ blood flow and tissue perfusion (6,7).
Dopamine, the most frequently used vasoactive agent in neonates, exerts dose-dependent α and β-adrenergic and dopaminergic effects (8). Other than dopamine, epinephrine is commonly used in neonatal intensive care units. A significant improvement of cardiac function has been observed after administration of epinephrine in various asphyxiated newborn porcine models (9,10). Clinically, epinephrine has been shown as effective as dopamine for the treatment of hypotension in low-birth-weight infants (11). Because it may exert significant adrenergic effect at low doses in the developing immature cardiovascular system, epinephrine has often been considered as an additional agent in treating neonatal hypotension refractory to initial dopamine infusion in lieu of increasing dopamine doses (12). Indeed, we previously showed that in asphyxiated newborn piglets, low-dose epinephrine (0.2 mcg/kg/min) added to dopamine (10 mcg/kg/min) enhanced cardiac output and blood pressure to a similar extent as high-dose epinephrine (1 mcg/kg/min) alone (13). Furthermore, in the hypotensive low-birth-weight preterm population, Seri and Evans demonstrated normalized blood pressure and improved urine output with the addition of epinephrine to dopamine infusion (Seri and Evans unpublished data, 1998, doi:10.1203/00006450-199804001-01152).
Treatment with high doses of dopamine (≥20 mcg/kg/min) is often avoided given potentially excessive α-adrenergic stimulation with vasoconstriction and increased afterload (7,14). However, we previously demonstrated an improved mesenteric perfusion at high-dose dopamine (HD) infusion in asphyxiated newborn piglets (9). Thus, monotherapy with HD may be an alternative approach to combination therapy of adding a second vasoactive/inotropic agent at low dose to dopamine infusion for neonates with severe cardiovascular dysfunction (13,15,16). Little information is available regarding direct comparison of these two treatment strategies on the systemic and regional hemodynamics.
Using an established swine model of neonatal hypoxia-reoxygenation (H-R), we primarily compared the efficacy of dopamine and low-dose epinephrine (D+E) combination treatment to HD alone on cardiac recovery after asphyxia. Other than the cardiac output, we also examined their effects on (a) carotid, mesenteric and renal perfusion, (b) oxygen transport, and (c) degree of histologic injury after H-R. We hypothesized that monotherapy with HD would have similar effects on cardiac output but differential effects on regional circulation when compared with D+E combination therapy in neonatal H-R.
RESULTS
Of 29 piglets instrumented, 2 were excluded for complications related to surgery (1) and hypoxia (1), leaving 27 piglets for analysis. Animals were aged 2.2±0.2 days and weighed 1.9±0.1 kg. There was no significant difference in gender, hemodynamic and physiologic parameters recorded after stabilization among experimental groups.
Hypoxia
Following 2h of hypoxia (PaO2 40±1 mmHg)(Table 1), all H-R piglets were in cardiogenic shock exhibiting significant hypotension (MAP: 28±1 mmHg), pulmonary hypertension (PAP: 32±2 mmHg) and reduced CI (36±2% of normoxic baseline) in comparison with sham (all p<0.05) (Table 2). Corresponding reductions in systemic oxygen delivery and consumption were also found (data not shown). H-R piglets showed significant decreases in common carotid, superior mesenteric and renal oxygen perfusion (22±2%, 15±1% and 21±2% of respective normoxic baselines; p<0.05 vs. sham). Severe metabolic acidosis was present with significantly elevated plasma lactate levels in H-R piglets (Table 1). No differences were found between H-R groups regarding hemodynamic and biochemical parameters at the end of hypoxia.
Table 1.
Arterial blood gas and acid-base status
| Time | Baseline 0min | End-Hypoxia 120min | Pre-Drug 240min | End-Drug 360min |
|---|---|---|---|---|
| pH | ||||
| Sham | 7.47±0.04 | 7.44±0.02 | 7.46±0.01 | 7.45±0.02 |
| Control | 7.45±0.02 | 7.02±0.02* | 7.38±0.02 | 7.41±0.02 |
| D+E | 7.44±0.02 | 7.04±0.03* | 7.40±0.01 | 7.36±0.02* |
| HD | 7.47±0.04 | 7.06±0.03* | 7.39±0.04 | 7.37±0.03* |
|
| ||||
| HCO3 (mmol/L) | ||||
| Sham | 27.1±1.9 | 27.2±1.5 | 28.3±1.0 | 27.4±1.2 |
| Control | 26.7±1.1 | 9.2±0.4* | 22.5±0.9* | 25.0±1.4 |
| D+E | 26.4±4.4 | 9.7±0.7* | 21.5±1.2* | 22.1±1.0* |
| HD | 26.7±1.9 | 10.5±0.9* | 22.0±1.5* | 22.6±1.3* |
|
| ||||
| PaO2 (mm Hg) | ||||
| Sham | 87.1±5.8 | 71.5±3.0 | 70.5±2.9 | 67.1±1.8 |
| Control | 83.6±3.3 | 38.3±2.2* | 65.2±1.1 | 70.1±2.2 |
| D+E | 79.6±7.7 | 42.0±4.6* | 68.3±6.4 | 78.7±5.7 |
| HD | 89.1±6.9 | 39.9±4.4* | 70.5±4.1 | 70.1±1.3 |
|
| ||||
| Lactate (mmol/L) | ||||
| Sham | 3.7±0.4 | 2.6±0.3 | 1.8±0.3 | 1.7±0.2 |
| Control | 2.8±0.2 | 13.6±1.1* | 4.1±0.4* | 2.0±0.1 |
| D+E | 3.2±0.4 | 14.0±1.0* | 4.7±0.8* | 3.2±0.6 |
| HD | 3.0±0.2 | 13.0±0.8* | 3.4±0.3* | 1.8±0.3 |
p<0.05 vs. Sham
Hypoxic-reoxygenated piglets treated with combination dopamine and epinephrine (D+E, n=7), high-dose dopamine (HD, n=7) or saline placebo (control, n=7). Sham-operated piglet had no hypoxia-reoxygenation (n=6).
Table 2.
Systemic hemodynamic parameters
| Time | Baseline 0min | End-Hypoxia 120min | Pre-Drug 240min | End-Drug 360min |
|---|---|---|---|---|
| Heart Rate (beats/min) | ||||
| Sham | 173±11 | 171±8 | 195±11 | 207±11 |
| Control | 176±6 | 208±11* | 228±14 | 206±6 |
| D+E | 181±12 | 208±14* | 237±9* | 269±12*† |
| HD | 174±12 | 233±7* | 219±11 | 247±20*† |
|
| ||||
| Mean Arterial Pressure (MAP) (mmHg) | ||||
| Sham | 77±3 | 63±4 | 54±2 | 48±2 |
| Control | 77±5 | 29±1* | 48±4 | 41±2 |
| D+E | 74±4 | 26±1* | 38±3*† | 43±4 |
| HD | 75±4 | 29±1* | 42±2* | 44±1 |
|
| ||||
| Pulmonary Arterial Pressure (PAP) (mmHg) | ||||
| Sham | 24±1 | 24±1 | 26±1 | 29±2 |
| Control | 25±2 | 30±2* | 26±1 | 26±1 |
| D+E | 25±1 | 32±4* | 26±1 | 29±2 |
| HD | 23±1 | 34±4* | 24±1 | 26±2 |
|
| ||||
| PAP/MAP Ratio | ||||
| Sham | 0.32±0.04 | 0.38±0.03 | 0.50±0.04 | 0.62±0.06 |
| Control | 0.33±0.03 | 1.03±0.03* | 0.56±0.02 | 0.65±0.04 |
| D+E | 0.34±0.02 | 1.20±0.10* | 0.70±0.08* | 0.69±0.09 |
| HD | 0.31±0.02 | 1.18±0.09* | 0.57±0.02 | 0.59±0.03 |
|
| ||||
| Cardiac Index (ml/kg/min) | ||||
| Sham | 224±30 | 189±30 | 199±26 | 176±21 |
| Control | 229±15 | 78±5* | 178±14 | 176±12 |
| D+E | 205±28 | 75±11* | 137±22* | 176±24 |
| HD | 188±15 | 70±10* | 136±14* | 177±15 |
p<0.05 vs. Sham
p<0.05 vs. Control
Hypoxic-reoxygenated piglets treated with combination dopamine and epinephrine (D+E, n=7), high-dose dopamine (HD, n=7) or saline placebo (control, n=7). Sham-operated piglet had no hypoxia-reoxygenation (n=6).
Reoxygenation
After 10 min of 100% oxygen resuscitation (PaO2 351±15 mmHg), hemodynamic parameters improved significantly (data not shown) but then deteriorated over the following 2h. In H-R piglets, MAP was 43±2 mmHg and CI 73±4% of normoxic baseline (Table 2). Common carotid, superior mesenteric and renal oxygen perfusion was 56±4%, 86±7% and 79±8% of respective normoxic baselines after 2h of reoxygenation and prior to medication delivery. All H-R piglets had reduced serum bicarbonate and elevated plasma lactate (Table 1) and troponin I levels (p<0.05 vs. sham). Of note, MAP of D+E piglets was significantly lower than that of control piglets (p<0.05)(Table 2).
Systemic/Pulmonary Hemodynamic Effects of Medications
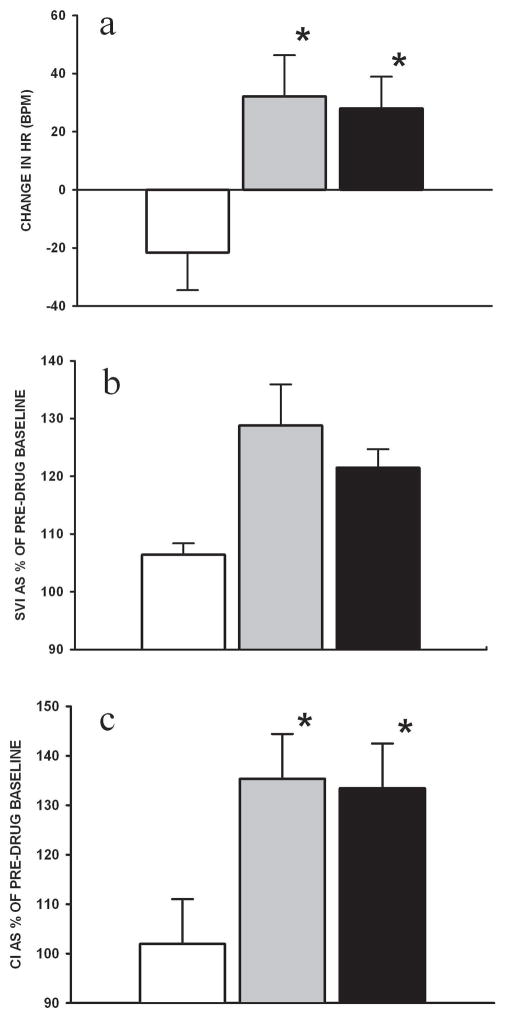
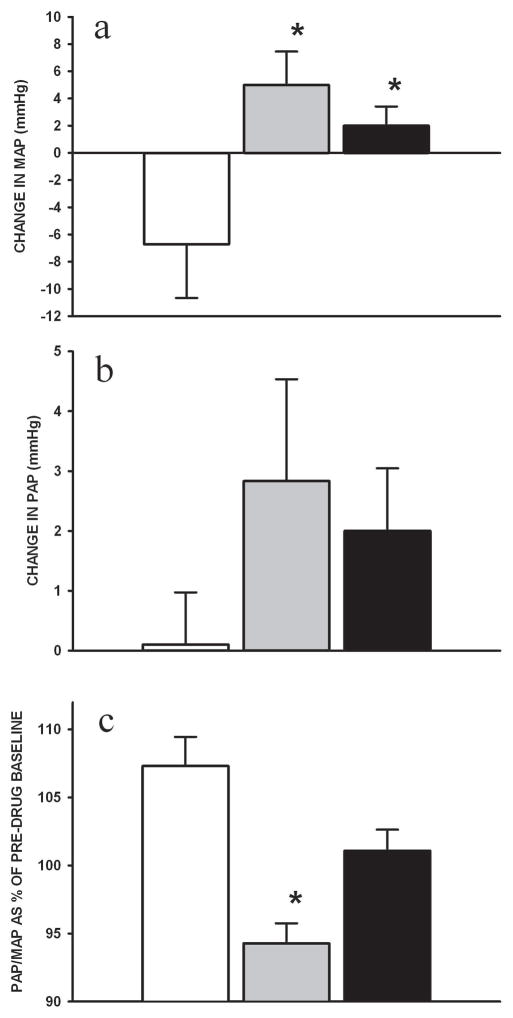
Both HD and D+E groups increased heart rate over 2h of infusion (vs. control, p<0.05) (Figure 1A and Table 2). The effect of these agents on stroke volume index was modest with transient increases seen early during infusion (Figure 1B). The combined effect offered an increase in CI over the duration of infusion in D+E and HD piglets (vs. control, p<0.05, Figure 1C). MAP decreased further in control piglets, whereas it increased in D+E and HD piglets (vs. control, p<0.05) (Figure 2A). There was no change in systemic vascular resistance during drug infusion (data not shown). PAP was unchanged during drug infusion (Figure 2B). D+E treated piglets decreased PAP/MAP ratio during infusion, relative to controls which increased in 2h (p<0.05) (Figure 2C).
Figure 1.
Changes in (A) heart rate (HR), (B) stroke volume index (SVI) and (C) cardiac index (CI) during the 2h infusion of combination dopamine and epinephrine (grey) and high-dose dopamine (black) in H-R piglets in comparison with controls (white) (n=7/group). Stroke volume index and cardiac index are expressed as mean percentage change over duration of infusion from pre-drug baseline. * p<0.05 vs. control (2-way repeated measures ANOVA).
Figure 2.
Changes in (A) mean arterial pressure (MAP), (B) pulmonary artery pressure (PAP) and (C) PAP/MAP ratio during the 2h infusion of combination dopamine and epinephrine (grey) and high-dose dopamine (black) in H-R piglets in comparison with controls (white) (n=7/group). PAP/MAP ratio is expressed as mean percentage change over duration of infusion from pre-drug baseline. * p<0.05 vs. control (2-way repeated measures ANOVA).
Regional Hemodynamic Effects of Medication Infusion
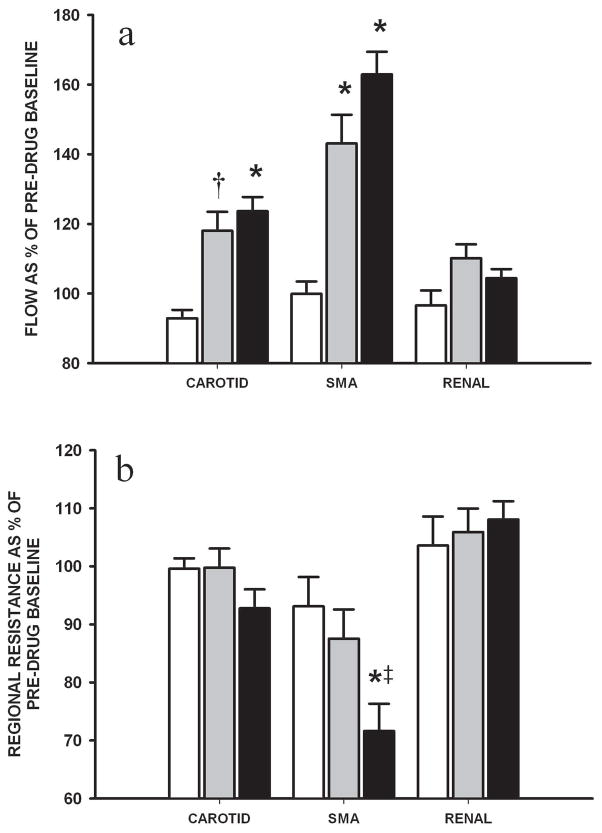
Both HD and D+E groups improved CAFI (p<0.05 and p=0.05 vs. control, respectively) and SMAFI (both p<0.05) (Figure 3A). There were no associated changes in common carotid vascular resistance. However, mesenteric vascular resistance decreased significantly in the HD group (p<0.05 vs. control; p=0.07 vs. D+E) (Figure 3B). There were no differences in RAFI or renal vascular resistance among H-R groups during drug infusion.
Figure 3.
Changes in (A) carotid, superior mesenteric (SMA) and renal arterial flows and (B) respective vascular resistance during the 2h infusion of combination dopamine and epinephrine (grey) and high-dose dopamine (black) in H-R piglets in comparison with controls (white) (n=7/group). Data are expressed as mean percentage change over duration of infusion from pre-drug baseline. * p<0.05 and † p=0.05 vs. control; ‡ p=0.07 vs. combination dopamine and epinephrine group (2-way repeated measures ANOVA).
Oxygen Delivery and Metabolism Effects of Medication Infusion
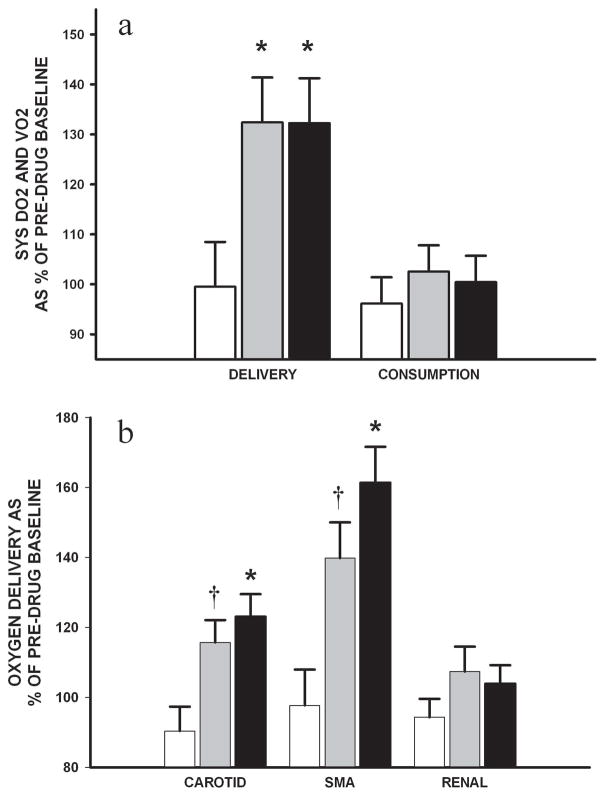
H-R piglets in HD and D+E groups had higher systemic oxygen delivery than that of controls (p<0.05), with no effect on systemic oxygen consumption (Figure 4A). Common carotid and mesenteric oxygen delivery declined and remained unchanged, respectively, in control piglets. However, corresponding to the improvements in regional flow, HD piglets improved common carotid and superior mesenteric oxygen delivery (p<0.05 vs. control), whereas D+E group had modest respective improvements (p<0.1 vs. control) (Figure 4B). There was no effect found on renal oxygen delivery. Plasma lactate levels of H-R groups improved with no difference among groups over the 2h infusion.
Figure 4.
Changes in (A) systemic (SYS) oxygen delivery (DO2) and consumption (VO2) and (B) carotid, superior mesenteric and renal DO2 during the 2h infusion of combination dopamine and epinephrine (grey) and high-dose dopamine (black) in H-R piglets in comparison with controls (white) (n=7/group). Data are expressed as mean percentage change over duration of infusion from pre-drug baseline. * p<0.05 and † p<0.1 vs. control (2-way repeated measures ANOVA).
During the course of experimentation, plasma lactate levels correlated modestly with CI in all H-R piglets (r=−0.4, p<0.05). Interestingly, the correlation was not significant in the control or HD groups, whereas significant correlation was found in the D+E group (r=−0.6, p<0.01). Systemic oxygen delivery correlated with plasma lactate (r=−0.5, p<0.01), particularly in the D+E and HD groups (r=−0.6, p<0.01 and r=−0.5, p<0.01, respectively) but not in controls.
Tissue Oxidative Stress Markers, Plasma Troponin Levels and Histologic Scores of H-R Injury
Ventricular lactate correlated with systemic oxygen delivery (r=−0.6, p<0.01) in the H-R groups. Intestinal lactate correlated with SMAFI and mesenteric oxygen delivery following 2h of infusion (r=−0.4, p=0.05 and r=−0.5, p=0.02, respectively). Myocardial and intestinal tissue lactate, glutathione and malondialdehyde levels were not different among groups. Ischaemic-looking colon was found in 2 cases each in the control and HD groups and 3 in the D+E group at the time of necropsy. Despite these findings, there were no significant differences in histopathologic findings of H-R injury in the left ventricle and gut (data not shown). However, the degree of left ventricle histologic injury did correlate modestly with plasma lactate levels at the end of experimentation (r=0.4, p<0.05).
DISCUSSION
Following asphyxia of the neonate, shock with hypotension is often treated with vasoactive and/or inotropic medications (17,18). Based on the findings in this swine model of neonatal H-R, monotherapy with HD and D+E combination therapy have differential hemodynamic benefits in mesenteric and pulmonary circulations which need to be considered when asphyxiated neonates are the risk of compromised mesenteric perfusion and increased unbalanced pulmonary-systemic arterial pressure (PAP/MAP) ratio, respectively.
The treatment of piglets after H-R with either HD alone or D+E combination improved myocardial function with chronotropic and transient inotropic effects. The improvement in CI with HD was not appreciated in previous studies using a similar model (9), nor was the predominant chronotropic effects of both regimens (Repetto et al., unpublished data, 1999, doi:10.1203/00006450-199904020-01314). This may be secondary to a significant protocol change with the use of only 30 minutes of pure oxygen resuscitation in lieu of a full hour. There were no differences in effect on systemic vascular resistance, further substantiating arguments against the detrimental vasoconstrictive effects of HD in neonatal H-R (19). Similar to the findings of the only term neonate trial studying dopamine, MAP was improved in both treatment groups (20). Interestingly, at the doses studied, CI was improved without significant increase in afterload so as to worsen plasma lactate. Therefore, either regimen, at least in the short period of therapy, does not adversely cause vasoconstrictive effects and overall tissue perfusion. Further studies need to be conducted, however, to determine if this effect holds with prolonged infusion.
The failure to show differences in plasma lactate levels as well as histopathological changes may be secondary to the limited 2h time frame of study. The trend for increased lactate levels in the combination treatment piglets may indeed be proven valid if observation were carried further or higher doses of epinephrine infusion (13). Increased plasma lactate, which is related to the epinephrine-induced increase in glycogenolysis and the associated increase in lactate production, has been observed in preterm neonates (11) and newborn piglets (21). Please consider. Further, safety from myocardial injury may need further investigation. The analysis of plasma troponin levels was complicated by non-significant but large inter-group differences prior to administration of the blinded medications. The measurement of brain natriuretic peptide levels in plasma may be useful as this parameter is clinically often used to assess myocardial performance after hypoxia and/or pulmonary hypertension. Nonetheless, with 2h infusion of either HD or D+E combination, there were no significant differences in myocardial lactate, glutathione or malondialdehyde levels among groups.
Given that blood pressure and tissue perfusion are not synonymous, assessment of other parameters is required to determine the status of asphyxiated neonates (22). In this study, we observed significant improvements in systemic oxygen delivery with either treatment, with no differences in oxygen consumption relative to controls. End-organ perfusion similarly improved with benefits to carotid and mesenteric perfusion in both groups. Indeed, we demonstrated that improvements in mesenteric perfusion were associated with reduction of intestinal tissue lactate, a marker of anaerobic metabolism. However, treatment with HD alone significantly reduced mesenteric vascular resistance relative to controls and modestly when compared with combination treatment. Previously, we found an increased mesenteric hemodynamics with similar dose of dopamine in asphyxiated newborn piglets (9). Similar to our observations, a marked increase in mesenteric blood flow has been reported in normotensive neonatal piglets after dopamine infusion (21). Although the relationship between mesenteric perfusion and intestinal injury has not been clearly defined, ischemia is one of the pathophysiological factors for necrotizing enterocolitis-like intestinal injury in asphyxiated term neonates (23,24). Any improvement of mesenteric perfusion would be beneficial to neonates who are at risk for ischemic intestinal injury. The hemodynamic effect of HD treatment in mesenteric circulation should be studied in appropriately designed prospective trials in human neonates so that it can be considered when mesenteric perfusion and intestinal injury are of concern in neonates at risk for developing ischemic enterocolitis or intestinal vasculopathy.
The net effect observed on PAP/MAP ratio was different between HD and D+E combination group, the former stabilizing the ratio relative to placebo controls which continued to increase. The addition of epinephrine, reduced the ratio at the specified doses. This apparent “protective” effect of epinephrine in pulmonary-systemic hemodynamics is consistent with our previous observations of its differential effects on systemic and pulmonary vasculature in monotherapy experiments (25). These results may be relevant when we consider the combination of agents in managing neonates with persistent pulmonary hypertension or those at risk for developing pulmonary hypertension, given preferable effects on the risk for shunting of deoxygenated blood to the systemic circulation. However, appropriately designed prospective trials in neonates are required before the clinical relevance of these findings can safely be established.
In this study, we did not observe any significant effect on renal perfusion with HD or D+E administration in H-R piglets. Neither was there a preferential vasodilatory increase in CAFI relative to CI although dopaminergic receptors are found in the vessels of head and neck. In hemodynamically stable, normoxic newborn piglets, Nachar et al (21) recently reported increases in blood flow in the renal, mesenteric and common carotid circulations following escalating dosages of dopamine administration. Apart from differences in the drug administration protocol, the functionality of dopamine and adrenergic receptors after H-R may at least explain the lack of improved renal perfusion or preferentially increased CAFI.
The study is limited by the lack of improvement in systemic oxygen consumption following improvement in oxygen delivery in the drug treatment groups. One may infer that the piglets were not adequately stressed given that oxygen metabolism was at maximal levels prior to drug infusion, offering no room for further improvement. This is different from prior studies using a similar H-R model (9,13,26) and may be related to the aforementioned differences in the reoxygenation protocol. Further, this study uses a neonatal model to draw inferences regarding perinatal care and thus has significant limitations. As in other experimental studies, we used infusions of drug at fixed doses. Clinically, these agents tend to be titrated to effect. Indeed, despite of the lack of correlation between blood pressure and perfusion, it is common to use blood pressure as the target parameter of cardiovascular supportive therapy for critically ill neonates. Nonetheless, clinical practitioners are increasingly staying away to solely rely on MAP in the management of critically ill neonates with shock. Further, upward titration of drug doses in pursuit of “normal” blood pressures may pose a concern given the potential for detrimental excessive vasoconstriction and impairment of tissue perfusion (18). Interestingly, we did not observe this phenomenon in H-R piglets treated with HD. The logistical implications with blinding and the relatively short duration of treatment will also limit the time for variable doses to effect a response, which was not possible in our short-term study. Furthermore, although the current drug protocol does not address if there is any priming effect by possible selective stimulation of dopaminergic and adrenergic receptors, we believe that our findings remain significant with good translational value regarding to the hemodynamic effects of HD vs. D+E administration. Indeed, the additional inotropic support is not uncommonly needed soon after starting dopamine at 5–10 mcg/kg/min. Further, the in vivo nature of experiments limits a direct examination of possible vasoconstrictive action of these drugs. Vascular resistance is indicative of vascular resistance per sec, although it has often been interpreted as a surrogate of vascular state.
The model is limited by differences between species, anesthetic effects, surgical instrumentation, clinical and experimental asphyxia and resuscitation protocols. For example, although asphyxia is commonly associated with hypercapnia, severely hypoxic conditions of various cardiopulmonary pathologies are not uncommon in critically ill neonates and have similar clinical features as asphyxia with acidosis and hemodynamic compromise. Further hypotension in asphyxiated neonates is related to a combination of cardiogenic and vasoplegic factors. Based on our previous observations in this model (26), the treatment with dobutamine does not provide significant vasoconstrictive effect. There is no clinical guideline in choosing the second inotrope. Nonetheless, studying the effects of dopamine and dobutamine combination will be interesting if different systemic hemodynamic effects would be found with direct and specific β-adrenergic stimulation. There are possible differences in adrenergic receptor functions between piglets and term human neonates. Differences in adrenoreceptor expression over the early life of the neonate as well as the functionality after H-R may complicate application of results in the perinatal setting of asphyxia. Therefore, it would be interesting albeit challenging to perform experiments on asphyxiated-reoxygenated animals in cardiogenic shock under the influence of adrenoceptor antagonists in order to distinguish mechanistically the systemic and regional hemodynamic effects.
In summary, in H-R newborn piglets with severe cardiogenic shock and hypotension despite of a moderate dose of dopamine infusion, adding low-dose epinephrine or further increasing dopamine has similar improvements in systemic hemodynamics (blood pressure and cardiac performance). However, the two regimens have differential effects on the pulmonary and mesenteric circulations. Further studies are needed to examine the peripheral circulations of brain or other organs and to investigate the appropriate therapeutic approach in correcting the systemic and regional perfusion deficits in asphyxiated neonates after resuscitation.
METHODS
Animals
Mixed breed (Duroc/Large White) 1–4 day-old piglets, weighing 1.5–2.5 kg, were obtained from the university swine research unit on the morning of experimentation. The following protocol was approved by the University Animal Care and Use Committee and adheres to the Canadian Council on Animal Care guidelines.
Anesthesia
Isoflurane (5%) was used to induce anesthesia, thereafter, titrated between 1 and 3% for maintenance. Following definitive airway placement and vascular access, inhalational anesthesia was discontinued. Mechanical ventilation was started with pressure control of 20/4 cm H2O and rates of 18–20 breaths/min (Sechrist Infant Ventilator Model IV-100, Sechrist Industries Inc., Anaheim, CA) with fractionated inspired oxygen concentrations (FiO2) of 0.21–0.25. Oxygen saturation was maintained at 88–100% (Nellcor, Hayward, CA). FiO2 was measured using a MiniOx III oxygen monitor (Catalyst Research, Owings Mills, MD). Throughout experimentation, piglets were maintained at a temperature of 38.5 to 40°C using both a heating underpad and overhead warmer. Anesthesia was maintained with a combination of intravenous midazolam (0.2–1 mg/kg/h) for sedation and fentanyl (5–20 mcg/kg/h) for analgesia. Pancuronium (0.05–0.1 mg/kg/h) was administered for paralysis during surgery. Boluses of fentanyl (10 mcg/kg) and acepromazine (0.25 mg/kg) were used as needed. 5% dextrose (20 ml/h) and 0.9% normal saline (4 ml/h) were infused with boluses of lactated Ringer’s solution given as indicated.
Surgical Instrumentation
The right femoral artery and vein were exposed through a groin incision. The femoral vein was cannulated with a 5-Fr dual-lumen catheter (Sherwood Medical Co., St. Louis, MO) advanced 13–15 cm to the right atrium to administer fluid and medications, and measure central venous pressure. A 5-Fr single-lumen catheter was then inserted in the femoral artery and advanced 5 cm to the infrarenal aorta for mean arterial pressure (MAP) monitoring. Next, via a transverse neck incision, a 3.5 mm inner diameter endotracheal tube was inserted through a tracheotomy to allow mechanical ventilation.
The left common carotid artery was encircled with a calibrated 2-mm flow probe (2SS, Transonic Systems Inc., Ithaca, NY). A left subcostal incision was used for exposure of the retroperitoneum where the left kidney was reflected anteriorly and superior mesenteric artery (SMA) exposed. Here, a 3-mm flow probe (3SB) was placed and the left renal artery was then encircled with a similar 2-mm (2SB) flow probe. Following a left anterior thoracotomy, the pericardium was opened and ductus arteriosus ligated. The pulmonary artery was then cannulated using a 20-gauge angiocatheter (Insyte-W, Becton Dickinson Infusion Therapy Systems Inc., Sandy, UT) for pulmonary artery pressure (PAP) monitoring. A 6-mm flow probe (6SB) was then placed around the main pulmonary artery to measure blood flow as a surrogate for cardiac output.
Stabilization and Monitoring
After instrumentation, piglets received a bolus of 10 ml/kg lactated Ringer’s solution and were allowed to recover for 60 min. Arterial PCO2 of 35–45 mmHg was maintained throughout experimentation. Heart rate and blood pressures were monitored continuously using a Hewlett Packard 78834A monitor (Hewlett Packard Co., Palo Alto, CA). Hemodynamic readings were digitized at a sampling rate of 24/s and recorded in a personal computer equipped with custom Asyst programming software (Data Translation, ON, Canada).
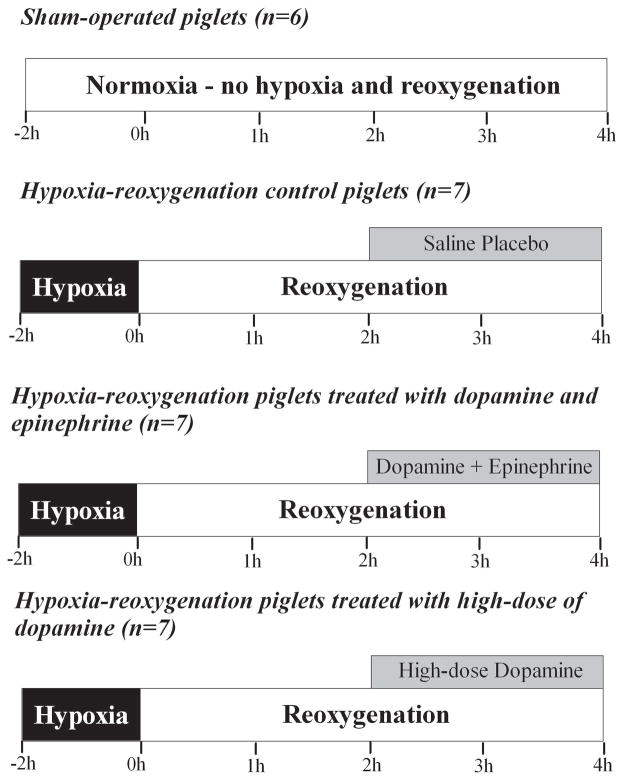
Hypoxia-Reoxygenation (H-R) Protocol (Figure 5)
Figure 5.
Experimental protocol.
Piglets were block randomized to either a surgical sham group or one of three H-R groups. Sham-operated piglets (sham, n=6) were maintained at FiO2 of 0.21–0.25 for the duration of experimentation (6h). H-R piglets underwent normocapnic alveolar hypoxia, through addition of inhaled nitrogen, reducing FiO2 to 0.10–0.15 for 2h. PaO2 was maintained at 20–40 mmHg during hypoxia to produce cardiac dysfunction and hypotension as previously described (26–28). Subsequently, piglets were resuscitated with 100% oxygen for 30 min and kept at FiO2 0.21–0.25 for the remainder of experimentation (3.5h) to maintain normoxia in these sick animals. A 10 ml/kg bolus of lactated Ringer’s solution was administered during reoxygenation prior to drug infusion.
At 2h of reoxygenation, H-R piglets received infusions of either placebo (0.9% saline solution – control) or study drug at a constant rate in a blinded, randomized fashion (n=7/group). Study drug groups included: HD group- dopamine (20 mcg/kg/min; Baxter Corp., Toronto, ON, Canada), or D+E combination group - dopamine (10 mcg/kg/min) and epinephrine (0.1 mcg/kg/min; Erfa Canada Inc., Westmount, QC, Canada). A laboratory technician prepared drug solutions into standard volume syringes for blinding purposes. The dosages were derived in part from previous studies in a similar swine model of neonatal H-R and the epinephrine dosage was decided in consideration of its concomitant administration with dopamine (9,13).
Hemodynamic and Oxygen Measurements
Heart rate, MAP, PAP and central venous pressure were recorded continuously and analyzed at set intervals from normoxic baseline through hypoxia and reoxygenation and at every 30 min of placebo or drug treatment. The data were averaged as means over a 2 min recording at each time point. Also, systemic and pulmonary arterial blood was sampled every 15 min during hypoxia and at 10, 30, 60, 120, 150, 180, 210 and 240 min after reoxygenation for blood gas analysis using ABL 700 blood gas analyzer and OSM3 Hemoximeter (Radiometer, Copenhagen, Denmark).
Biochemical Analysis and Histopathology
Arterial samples were analyzed for lactate using the ABL 700 analyzer and plasma was stored at −80°C. Plasma levels of porcine cardiac-specific troponin-I at baseline, 2h of reoxygenation and 2h of drug treatment were analyzed using enzyme-linked immunosorbent assay (#2010-4, Life Diagnostics, West Chester, PA).
At the end of experimentation, piglets were euthanized using intravenous pentobarbital (100 mg/kg). Necropsy was performed immediately for retrieval of left ventricular tissue and terminal ileum. A small sample was stored in 10% formalin solution and the remaining tissue was snap frozen in liquid nitrogen and stored at −80°C. The formalin-preserved specimen was then processed for hematoxylin and eosin staining. Histologic findings were interpreted without knowledge of treatment allocation using previously described scoring systems for H-R injury (29,30).
For measurement of tissue lactate, frozen myocardial and intestinal tissue was crushed and homogenized in 6% perchloric acid (PCA)/0.5mM EGTA on ice. Samples were centrifuged and supernatant collected. 5M potassium carbonate was then added slowly in a 1μL:10μL of supernatant ratio. Following precipitation over 30 min, samples were again centrifuged and supernatant collected for NAD enzyme-coupled colorimetric microplate assay. After the addition of glycylglycine buffer, NAD, double-distilled water, glutamate-pyruvate transaminase and lactate dehydrogenase, absorbance was read at 340 nm using a microplate spectrophotometer (Spectramax 190; Molecular Devices, Sunnyvale, CA).
Measurement of tissue glutathione (GSH) was performed using a commercially available glutathione assay kit (Cayman Chemical, Ann Arbor, MI). Frozen tissue was crushed and homogenized in buffer containing 0.2M 2-(N-morpholino)ethanesulphonic acid, 50mM phosphate and 1mM EDTA at pH 6–7. Following centrifugation, supernatant was deproteinated with 10% metaphosphoric acid and 4M triethanolamine. Colorimetric microplate assay was then performed after the addition of glutathione reductase, glucose-6-phosphate dehydrogenase, NADP+, 5,5′-dithiobis-2-nitrobenzoic acid. Absorbance was read at 405 nm after 25 minutes using the aforementioned spectrophotometer. To measure oxidized GSH (GSSG), reduced GSH was derivatized to GSSG using 2-vinylpyridine. The assay was then carried out using this sample. Oxidative status of GSH was determined through interpretation of the GSSG/GSH ratio.
For measurement of tissue malondialdehyde, frozen tissues were homogenized 1:10 in phosphate-buffered saline and fluorescence assays were compared with standard concentrations of malondialdehyde as described by Ohkawa (31).
Statistics
Data were analyzed using SigmaPlot v11 software (Systat Software Inc., San Jose, CA). Analysis of variance (one-way and two-way repeated measures) was used where appropriate for parametric data, with post hoc pairwise analysis by Student-Newman-Keuls test. For nonparametric data, Kruskal-Wallis test was performed with Dunn’s method for post hoc intergroup comparison. For practical interpretation of drug effect after infusion, percentage change respective to pre-drug baseline was used to analyze data during the drug infusion phase. Correlations between variables were performed with Pearson Moment or Spearman test as appropriate. Significance was defined as p<0.05. Results are expressed as mean±standard error of mean.
Acknowledgments
STATEMENT OF FINANCIAL SUPPORT: The project was funded by operating grant (MOP53116) from the Canadian Institutes of Health Research. NM received support from the Clinician Investigator Program of the Royal College of Physicians and Surgeons of Canada. PYC was an investigator of the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research.
References
- 1.The World Health Report 2005: Make every mother and child count. Geneva, Switzerland: WHO Press, World Health Organization; 2005. Newborns: no longer going unnoticed; pp. 79–101. [Google Scholar]
- 2.Martín-Ancel A, García-Alix A, Gayá F, Cabañas F, Burgueros M, Quero J. Multiple organ involvement in asphyxia. J Pediatr. 1995;127:786–93. doi: 10.1016/s0022-3476(95)70174-5. [DOI] [PubMed] [Google Scholar]
- 3.Evans N. Which inotrope for which baby? Arch Dis Child Fetal Neonatal Ed. 2006;91:F213–20. doi: 10.1136/adc.2005.071829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso-Spilsbury M, Mota-Rojas D, Villanueva-García D, et al. Perinatal asphyxia pathophysiology in pig and human: A review. Anim Reprod Sci. 2005;90:1–30. doi: 10.1016/j.anireprosci.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Cheung PY, Johnson ST, Obaid L, Chan GS, Bigam DL. The systemic, pulmonary and regional hemodynamic recovery of asphyxiated newborn piglets resuscitated with 18%, 21% and 100% oxygen. Resuscitation. 2008;76:457–64. doi: 10.1016/j.resuscitation.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Fanaroff JM, Fanaroff AA. Blood pressure disorders and the neonate: Hypotension and hypertension. Sem Fetal Neonatal Med. 2006;11:174–81. doi: 10.1016/j.siny.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Subhedar NV. Treatment of hypotension in newborns. Sem Neonatol. 2003;8:413–23. doi: 10.1016/S1084-2756(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 8.Seri I. Systemic and pulmonary effects of vasopressors and inotropes in the neonate. Biol Neonate. 2006;89:340–2. doi: 10.1159/000092872. [DOI] [PubMed] [Google Scholar]
- 9.Obaid L, Johnson ST, Emara M, Bigam DL, Cheung PY. Epinephrine versus dopamine to treat shock in hypoxic newborn pigs resuscitated with 100% oxygen. Shock. 2008;29:262–8. doi: 10.1097/shk.0b013e31811ff509. [DOI] [PubMed] [Google Scholar]
- 10.Voelckel WG, Lurie KG, McKnite S, et al. Comparison of epinephrine and vasopressin in pediatric porcine model of asphyxial cardiac arrest. Crit Care Med. 2000;28:3777–83. doi: 10.1097/00003246-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Valverde E, Pellicer A, Madero R, Elorza D, Quero J, Cabañas F. Dopamine versus epinephrine for cardiovascular support in low birth weight infants: Analysis of systemic effects and neonatal clinical outcomes. Pediatrics. 2006;117:e1213–22. doi: 10.1542/peds.2005-2108. [DOI] [PubMed] [Google Scholar]
- 12.Heckmann M, Trotter A, Pohlandt F, Lindner W. Epinephrine treatment of hypotension in very low birth weight infants. Acta Pediatr. 2002;91:566–70. doi: 10.1080/080352502753711704. [DOI] [PubMed] [Google Scholar]
- 13.Cheung PY, Abozaid S, Al-Salam Z, Johnson S, Li Y, Bigam D. Systemic and regional hemodynamic effects of high-dose epinephrine infusion in hypoxic piglets resuscitated with 100% oxygen. Shock. 2007;28:491–7. doi: 10.1097/shk.0b013e31804f77b8. [DOI] [PubMed] [Google Scholar]
- 14.Seri I. Cardiovascular, renal and endocrine actions of dopamine in neonates and children. J Pediatr. 1995;126:333–44. doi: 10.1016/s0022-3476(95)70445-0. [DOI] [PubMed] [Google Scholar]
- 15.Paradisis M, Evans N, Kluckow M, Osborn D. Randomized trial of milrinone versus placebo for prevention of low systemic blood flow in very preterm infants. J Pediatr. 2009;154:189–95. doi: 10.1016/j.jpeds.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 16.Pellicer A, Valverde E, Elorza MD, et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: A randomized, blinded, clinical trial. Pediatrics. 2005;115:1501–12. doi: 10.1542/peds.2004-1396. [DOI] [PubMed] [Google Scholar]
- 17.Piazza AJ. Postasphyxial management of the newborn. Clin Perinatol. 1999;26:749–65. [PubMed] [Google Scholar]
- 18.Seri I. Inotrope, lusitrope, and pressor use in neonates. J Perinatol. 2005;25:s28–30. doi: 10.1038/sj.jp.7211316. [DOI] [PubMed] [Google Scholar]
- 19.Seri I, Evans J. Controversies in the diagnosis and management of hypotension in the newborn infant. Curr Opin Pediatr. 2001;13:116–23. doi: 10.1097/00008480-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 20.DiSessa TG, Leitner M, Ti CC, Gluck L, Coen R, Friedman WF. The cardiovascular effects of dopamine in the severely asphyxiated neonate. J Pediatr. 1981;99:772–6. doi: 10.1016/s0022-3476(81)80409-x. [DOI] [PubMed] [Google Scholar]
- 21.Nachar RA, Booth EA, Friedlich P, et al. Dose-dependent hemodynamic and metabolic effects of vasoactive medications in normotensive, anesthetized neonatal piglets. Pediatr Res. 2011;70:473–9. doi: 10.1203/PDR.0b013e31822e178e. [DOI] [PubMed] [Google Scholar]
- 22.Seri I, Barrington K. Cardiovascular support in the preterm: Treatments in search of indications. J Pediatr. 2007:e31–3. doi: 10.1016/j.jpeds.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Doelle SM, Clark JA, Halpern MD, McCuskey RS, Dvorak B. Intestinal microcirculatory dysfunction during the development of experimental necrotizing enterocolitis. Pediatr Res. 2007;61:180–4. doi: 10.1203/pdr.0b013e31802d77db. [DOI] [PubMed] [Google Scholar]
- 24.Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Semin Perinatol. 2008;32:83–91. doi: 10.1053/j.semperi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Cheung PY, Barrington KJ. The effects of dopamine and epinephrine on hemodynamics and oxygen metabolism in hypoxic anesthetized piglets. Crit Care. 2001;5:158–66. doi: 10.1186/cc1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Salam Z, Johnson S, Abozaid S, Bigam D, Cheung PY. The hemodynamic effects of dobutamine during reoxygenation after hypoxia: a dose-response study in newborn pigs. Shock. 2007;28:317–25. doi: 10.1097/shk.0b013e318048554a. [DOI] [PubMed] [Google Scholar]
- 27.Johnson ST, Bigam DL, Emara M, et al. N-acetylcysteine improves the hemodynamics and oxidative stress in hypoxic newborn pigs reoxygenated with 100% oxygen. Shock. 2007;28:484–90. doi: 10.1097/shk.0b013e31804f775d. [DOI] [PubMed] [Google Scholar]
- 28.Joynt C, Bigam DL, Charrois G, Jewell LD, Korbutt G, Cheung PY. Dose-response effects of milrinone on hemodynamics of newborn pigs with hypoxia-reoxygenation. Intensive Care Med. 2008;34:1321–9. doi: 10.1007/s00134-008-1060-5. [DOI] [PubMed] [Google Scholar]
- 29.Rose AG, Opie LH, Bricknell OL. Early experimental myocardial infarction. Evaluation of histologic criteria and comparison with biochemical and electrocardiographic measurements. Arch Pathol Lab Med. 1976;100:516–21. [PubMed] [Google Scholar]
- 30.Park PO, Haglund U, Bulkley GB, Fält K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107:574–80. [PubMed] [Google Scholar]
- 31.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]