Abstract
Alcoholic liver disease (ALD) is a major public health problem worldwide and is the leading cause of end-stage liver disease. While the ultimate control of ALD will require the prevention of alcohol abuse, better understanding of the mechanisms of alcohol-induced liver injury may lead to treatments of fatty liver, alcoholic hepatitis, and prevention or delay of occurrence of cirrhosis. The elucidation and the discovery of several new concepts in ALD pathogenesis have raised our understanding on the complex mechanisms and the potential in developing the new strategies for therapeutic benefits. In this review, we provide the most up-to-date information on the basic molecular mechanisms focusing on the role of fat-specific protein 27/CIDEC in the pathogenesis of ALD.
INTRODUCTION
Alcoholic liver disease (ALD) represents a spectrum of liver disorders with clinical and pathological changes in individuals after chronic excessive alcohol consumption.1,2 Patients may have minimal abnormalities from steatosis or may develop more severe signs and symptoms of liver disease seen in alcoholic hepatitis (AH) or cirrhosis.2,3 While the ultimate control of ALD will require the prevention of alcohol abuse, better understanding of the mechanisms/pathogenesis may lead to treatments of ALD.
Fatty liver, the accumulation of triglyceride droplets in the liver, is the most common and earliest response of the liver to excessive alcohol use.4 Synthesis of fatty acids and triglyceride in excess of the capacity to oxidize it or export it in very low-density lipoprotein particles results in hepatic steatosis.5,6 The effect of ethanol was initially attributed to the changes in the redox state, generated from alcohol metabolism by alcohol and aldehyde dehydrogenase; however, recent evidence suggested a complex molecular regulation of ALD. Its pathogenesis involves the dysregulation of transcription factors and metabolic regulators, protein adduct formation, activation of inflammatory cytokines and Kupffer cells, elevation of lipopolysaccharide, and endoplasmic reticulum (ER) stress response (see review by Gao and Bataller2). In this report, we will focus on an emerging new mechanism on the role of fat-specific protein 27 (FSP27)/cell death-inducing DFF45-like effector C (CIDEC) in the pathogenesis of ALD.
FSP27/CIDEC
The mouse Fsp27 gene is the human homolog of CIDEC, belonging to the CIDE family of proteins. Three CIDEs have been reported in mouse (Cidea, Cideb, and FSP27/Cidec) and human (CIDEA, CIDEB, and CIDEC).7,8 FSP27/CIDEC proteins play an important role in the development of metabolic disorders as well as regulation of cell apoptosis.7,9–11 The Fsp27 gene has two isoforms, Fsp27α and Fsp27β.12 Fsp27α is highly expressed in white adipose tissues, the major organ for triacylglycerol (TAG) storage, whereas Fsp27β is highly expressed in brown adipose tissue and fatty liver.12 FSP27/CIDEC is a lipid droplet (LD) protein that plays an important role in droplet formation.13
LDs, intracellular organelles, are composed of a core of neutral lipids (TAG) covered by a monolayer of phospholipids, free cholesterol and specific proteins.14,15 The ability to store neutral lipids in the form of LDs is evolutionarily conserved across species.14 LDs in the adipose tissues serve as the reservoirs for fatty acids (in the form of TAG), which can be released during starvation14,16 and can be used for energetic substrates to high-demand tissues such as liver and muscle.17 There are several proteins, in addition to FSP27/CIDEC, which are involved in LD formation, notably the PAT (perilipin, adipophilin, and the tail-interacting protein of 47 kDa) family proteins, perilipin (PLIN 1–5).14,18,19
In the adipose tissues, FSP27/CIDEC stimulates formation of TAG droplets and inhibits β-oxidation of non-esterified fatty acids.13 It is significantly upregulated10,16,20–22,23 and is important for expansion of LD size during adipogenesis.13 Recent studies found that FSP27/CIDEC plays an important role in lipolysis through its interaction with adipose tissue triglyceride lipase (ATGL) and regulates insulin sensitivity in human adipocytes.17,18 FSP27/CIDEC facilitates the inhibitory effect of early growth response protein 1 (Erg1) on the transcription of ATGL,18 leading to reduced lipolysis, and enhancing lipid storage capacity in the adipocytes.17 The optimal fat storage is important in maintaining the overall metabolic phenotypes in the adipose tissues.17,24 High levels of free fatty acid can inhibit protein kinase B (AKT) phosphorylation and impair insulin sensitivity.25 It is interesting that insulin-stimulated AKT activation is inhibited by siRNA-mediated FSP27 silencing. Using the gain of function approach, FSP27 overexpression protects the adipocytes from free fatty acid (FFA)-induced insulin resistance. These data suggest that FSP27 might protect adipocytes from the deleterious effects of FFAs via suppression of ATGL-mediated lipolysis.17
In mouse liver, Fsp27 expression is significantly induced during early fasting and in the presence of hepatic steatosis.9,26,27 Knockdown of Fsp27 ameliorates hepatic steatosis,9 while overexpression of Fsp27 induces the accumulation of LDs and TAG contents.16 The regulation of hepatic Fsp27 expression is complex. Fasting-induced Fsp27 expression was completely obliterated in cyclic AMP-responsive element binding protein H (CREBH) knockout mice.12 Interestingly, the expression of other LD proteins in the PAT family was largely unaffected by the loss of CREBH,12 suggesting the role of CREBH in regulating Fsp27 expression. Overexpression of the constitutively active CREBH strongly induced Fsp27β in mouse hepatocytes and promoted LD enlargement and TAG accumulation in the liver, while the loss of CREBH decreased hepatic Fsp27β expression in fasted mice.12
In summary, FSP27/CIDEC, a lipid protein, plays an important role in lipolysis, insulin sensitivity, and TAG accumulation in steatotic liver.
Animal models for ALD
Currently, the most widely used model for alcohol-induced liver injury is ad libitum feeding with the Lieber-DeCarli liquid diet containing ethanol for 4–6 weeks;28 however, this model, without additional secondary insult, only induces mild steatosis, slight elevation of serum alanine transaminase (ALT) and little or no inflammation.28 It is not an ideal mouse model to study the mechanism of ALD beyond the hepatic steatosis stage.
AH, a severe form of ALD, can occur in patients with ALD, especially in those with recent excessive alcohol consumption.2,3 In addition to the presence of steatosis, the typical findings of AH demonstrate neutrophilic infiltration, hepatocyte ballooning, and hyaline inclusions.2,3 Slow progress in the field of ALD has resulted partly from a lack of experimental models of advanced ALD and AH. A model of short-term (10-day) plus binge ethanol feeding in mice (the National Institute on Alcohol Abuse and Alcoholism (NIAAA) model) was developed, which showed significant elevations of transaminases (AST and ALT), mild steatosis, and neutrophil infiltration, yet no fibrosis.28 When chronic ethanol feeding is extended to a period to 8 weeks, followed by gavage administration of single or multiple doses of ethanol, mice developed the phenotypic features of severe alcoholic steatohepatitis (ASH) and mild fibrosis.7 Alteration in gene expression profiles, determined by microarray analyses, in this model was found to be similar to those in human AH, suggesting that this is a very useful model to study the mechanism of AH.7
FSP27/CIDEC promotes development of ASH in mice
Among the most highly upregulated genes based on the array data, the Fsp27 gene was 13-fold upregulated in mice chronically fed with ethanol for 8 weeks followed by 1 binge (E8W+1B).7 The array data were confirmed by realtime PCR (RT-PCR) analysis of the liver tissues which showed that hepatic expression of Fsp27 was upregulated by 10-fold in mice after E8w+1B feeding.7
The gene as well as protein expression of both isoforms of Fsp27 gene were highly upregulated in mice fed with E8w+1B, when compared with controls.7 However, it is important to note that ethanol does not directly upregulate hepatic FSP27 protein expression, as the expression of this protein was not observed when primary hepatocytes were treated with ethanol (100 mM) in vitro.7 Further studies suggest that the upregulation of Fsp27 is, in fact, secondary to the activation of ER by ethanol.7
The role of Fsp27 in the pathogenesis of AH was also examined by using the ‘loss of function’ approach with Ad-Fsp27 short hairpin RNA (shRNA) and hepatocyte-specific Fsp27 deletion (Fsp27Hep−/−). Interestingly, the levels of serum transaminases (aspartate aminotransferase (AST) and ALT), hepatic steatosis, and degree of hepatic apoptosis (as measured by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay) were reduced in Ad-Fsp27 shRNA-treated mice as well in Fsp27Hep−/− mice compared with those in pair-fed controls.7 Further, the hepatic levels of malondialdehyde and 4-hydroxynonenal (oxidative stress/lipid peroxidation markers), which were highly elevated in E8w+1B mice, were significantly reduced after treatment with Ad-Fsp27 shRNA, suggesting that FSP27 promotes ethanol-induced hepatic oxidative injury.7
FSP27 protein is found in the cytoplasm of adipocytes. Recent studies demonstrated that FSP27 protein is present in the cytoplasm and mitochondria from steatotic hepatocytes from ethanol-fed mice.7 Overexpression of FSP27 protein via the injection of Ad-FSP27 exacerbated the elevation of serum ALT and AST levels and decreased mitochondrial contents and mitochondrial complex I activity in E8W+1B treated mice.7 The combination of FSP27 overexpression and ethanol exposure synergistically increased mitochondrial reactive oxygen species (ROS) generation in hepatocytes in vitro.7 Finally, FSP27 overexpression also induces hepatocyte death in the presence of ethanol by induction of Bax translocation and cytochrome C release, the two important early events in apoptotic pathway.7
Role of FSP27/CIDEC in human ASH
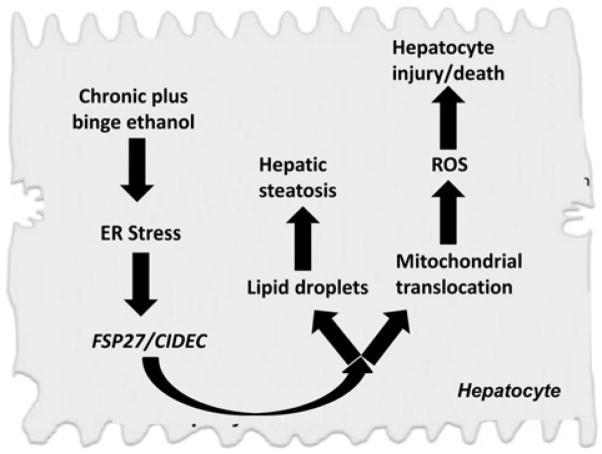
Recent evidence also suggests an important role for FSP27 in the pathogenesis of human ASH. First, microarray data revealed that CIDEC, the human homolog of Fsp27, was upregulated by fivefold in human liver samples from patients with AH compared with healthy controls.7 RT-PCR analyses demonstrated that the expression of CIDEC mRNA was more than 40-fold increase in these samples. Second, the upregulation of hepatic CIDEC was closely associated with the severity of hepatic steatosis as well as the prognostic models for AH such as model for end-stage liver disease and age, serum bilirubin, international normalized ratio, and serum creatinine (ABIC) scores.7 It is also positively correlated with hepatic venous pressure gradient and is an independent predictor for 90-day mortality in patients with AH.7 The schematic diagram on the mechanism of FSP27/CIDEC and ASH is shown in figure 1.
Figure 1.
Schematic diagram on the potential role of FSP27/CIDEC in the pathogenesis of AH (modified from Xu et al7). AH, alcoholic hepatitis; CIDEC, cell death-inducing DFF45-like effector C; ER, endoplasmic reticulum; FSP27, fat-specific protein 27; ROS, reactive oxygen species.
FSP27/CIDEC contributes to the synergistic effect of obesity and acute ethanol-induced ASH
Obesity and alcohol consumption often coexist and synergistically promote the development and progression of liver injury, fibrosis, and hepatocellular carcinoma in patients.29,30 The studies from several animal models also revealed that alcohol feeding and high-fat diet (HFD) feeding synergistically promote steatohepatitis in rodents.31 Interestingly, a simple model of mixed steatohepatitis by feeding mice an HFD followed by gavage with a single dose of ethanol was recently developed.32 The most striking finding from this model was that feeding mice an HFD for as little as 3 days, which has been shown to impair hepatic insulin sensitivity,33 significantly aggravated the acute ethanol binge-induced neutrophilia, hepatic neutrophil infiltration, and liver injury.32 Long-term (3 months) HFD feeding plus gavage of a single dose of ethanol caused severe steatohepatitis with severe steatosis, massive neutrophil infiltration, and marked elevation of serum ALT and AST. Mechanistic studies revealed that hepatic expression of chemokine (C-X-C motif) ligand 1 (CXCL1) was highly upregulated (up to 20-fold and 30-fold) in the liver after 3-day HFD+ethanol and 3-month HFD+ethanol feeding, respectively.32 Genetic deletion of the Cxcl1 or blocking CXCL1 with a neutralizing antibody ameliorated HFD+acute ethanol-induced liver inflammation and injury. In addition, it is known that hepatic Fsp27 mRNA is highly elevated after HFD feeding34 and that overexpression of FSP27 increases the sensitivity of hepatocytes to ethanol-induced ROS production and injury.7 Thus, it is plausible to speculate that upregulated hepatic FSP27 expression is another important mechanism by which HFD-fed mice are very sensitive to acute alcohol-induced acute ASH.
CONCLUSION
The use of chronic ethanol feeding for 8 weeks followed by gavage administration of a single dose of ethanol can induce hepatic histology mimicking ASH. This model, therefore, will be useful for the future study to identify and investigate other important mediators that may contribute to the pathogenesis of ASH. Using this mouse model, the researchers have found the important role of hepatic FSP27/CIDEC in promoting ASH in mice as well as in patients with ASH. However, further studies investigating the role of FSP27/CIDEC notably in the adipose tissues and the cross talk between adipose tissue and liver on the pathogenesis of hepatic steatosis and ASH in mouse model of obesity and alcohol feeding will be needed.
Acknowledgments
Synopsis from the symposia entitled ‘Emerging New Mechanism in Alcoholic Liver Disease’, which was presented at the Experimental Biology 2016 meeting in San Diego, California, USA. The meeting is supported by the American Federation for Medical Research (AFMR).
Funding This work was supported by the intramural program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health (to BG), VA Merit Award 1I01CX000361, NIH U01AA021840, US DOD W81XWH-12-1-0497(to SL).
Footnotes
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
References
- 1.Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30:378–90. doi: 10.1055/s-0030-1267538. [DOI] [PubMed] [Google Scholar]
- 2.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandrekar P, Bataller R, Tsukamoto H, et al. Alcoholic hepatitis: translational approaches to develop targeted therapies. Hepatology. doi: 10.1002/hep.28530. Published Online First: 3 Mar 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondili LA, Taliani G, Cerga G, et al. Correlation of alcohol consumption with liver histological features in non-cirrhotic patients. Eur J Gastroenterol Hepatol. 2005;17:155–9. doi: 10.1097/00042737-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Badaloo A, Reid M, Soares D, et al. Relation between liver fat content and the rate of VLDL apolipoprotein B-100 synthesis in children with protein-energy malnutrition. Am J Clin Nutr. 2005;81:1126–32. doi: 10.1093/ajcn/81.5.1126. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Iqbal J, Saha PK, et al. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007;46:147–57. doi: 10.1002/hep.21632. [DOI] [PubMed] [Google Scholar]
- 7.Xu MJ, Cai Y, Wang H, et al. Fat-Specific Protein 27/CIDEC Promotes Development of Alcoholic Steatohepatitis in Mice and Humans. Gastroenterology. 2015;149:1030–41. e6. doi: 10.1053/j.gastro.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang L, Zhao M, Xu Z, et al. Molecular cloning and characterization of CIDE-3, a novel member of the cell-death-inducing DNA-fragmentation-factor (DFF45)-like effector family. Biochem J. 2003;370:195–203. doi: 10.1042/BJ20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsusue K, Kusakabe T, Noguchi T, et al. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7:302–11. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong J, Sun Z, Li P. CIDE proteins and metabolic disorders. Curr Opin Lipidol. 2009;20:121–6. doi: 10.1097/MOL.0b013e328328d0bb. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Zhou L, Li P. CIDE proteins and lipid metabolism. Arterioscler Thromb Vasc Biol. 2012;32:1094–8. doi: 10.1161/ATVBAHA.111.241489. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Park JG, So JS, et al. Transcriptional activation of Fsp27 by the liver-enriched transcription factor CREBH promotes lipid droplet growth and hepatic steatosis. Hepatology. 2015;61:857–69. doi: 10.1002/hep.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller P, Petrie JT, De Rose P, et al. Fat-specific protein 27 regulates storage of triacylglycerol. J Biol Chem. 2008;283:14355–65. doi: 10.1074/jbc.M708323200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg AS, Coleman RA, Kraemer FB, et al. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–10. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno M, Shen WJ, Patel S, et al. Fat-specific protein 27 modulates nuclear factor of activated T cells 5 and the cellular response to stress. J Lipid Res. 2013;54:734–43. doi: 10.1194/jlr.M033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puri V, Konda S, Ranjit S, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–18. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 17.Grahn TH, Kaur R, Yin J, et al. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J Biol Chem. 2014;289:12029–39. doi: 10.1074/jbc.M113.539890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh M, Kaur R, Lee MJ, et al. Fat-specific protein 27 inhibits lipolysis by facilitating the inhibitory effect of transcription factor Egr1 on transcription of adipose triglyceride lipase. J Biol Chem. 2014;289:14481–7. doi: 10.1074/jbc.C114.563080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robenek H, Robenek MJ, Troyer D. PAT family proteins pervade lipid droplet cores. J Lipid Res. 2005;46:1331–8. doi: 10.1194/jlr.M400323-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Danesch U, Hoeck W, Ringold GM. Cloning and transcriptional regulation of a novel adipocyte-specific gene, FSP27. CAAT-enhancer-binding protein (C/EBP) and C/EBP-like proteins interact with sequences required for differentiation-dependent expression. J Biol Chem. 1992;267:7185–93. [PubMed] [Google Scholar]
- 21.Williams PM, Chang DJ, Danesch U, et al. CCAAT/enhancer binding protein expression is rapidly extinguished in TA1 adipocyte cells treated with tumor necrosis factor. Mol Endocrinol. 1992;6:1135–41. doi: 10.1210/mend.6.7.1508226. [DOI] [PubMed] [Google Scholar]
- 22.Yu S, Matsusue K, Kashireddy P, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 23.Puri V, Ranjit S, Konda S, et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci USA. 2008;105:7833–8. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–78. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 25.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 26.Vilà-Brau A, De Sousa-Coelho AL, Gonçalves JF, et al. Fsp27/CIDEC is a CREB target gene induced during early fasting in liver and regulated by FA oxidation rate. J Lipid Res. 2013;54:592–601. doi: 10.1194/jlr.M028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aibara D, Matsusue K, Matsuo K, et al. Expression of hepatic fat-specific protein 27 depends on the specific etiology of fatty liver. Biol Pharm Bull. 2013;36:1766–72. doi: 10.1248/bpb.b13-00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertola A, Mathews S, Ki SH, et al. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–37. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naveau S, Dobrin AS, Balian A, et al. Body fat distribution and risk factors for fibrosis in patients with alcoholic liver disease. Alcohol Clin Exp Res. 2013;37:332–8. doi: 10.1111/j.1530-0277.2012.01927.x. [DOI] [PubMed] [Google Scholar]
- 30.Hart CL, Morrison DS, Batty GD, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Lai KK, Verlinsky A, et al. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol. 2011;55:673–82. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang B, Xu MJ, Zhou Z, et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: an important role for CXCL1. Hepatology. 2015;62:1070–85. doi: 10.1002/hep.27921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiedemann MS, Wueest S, Item F, et al. Adipose tissue inflammation contributes to short-term high-fat diet-induced hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2013;305:E388–95. doi: 10.1152/ajpendo.00179.2013. [DOI] [PubMed] [Google Scholar]
- 34.Langhi C, Baldán Á. CIDEC/FSP27 is regulated by peroxisome proliferator-activated receptor alpha and plays a critical role in fasting- and diet-induced hepatosteatosis. Hepatology. 2015;61:1227–38. doi: 10.1002/hep.27607. [DOI] [PMC free article] [PubMed] [Google Scholar]