Abstract
Donor lymphocyte infusion (DLI) is an option for relapsed hematologic malignancies or incomplete chimerism of non-malignant diseases following allogeneic hematopoietic cell transplantation (HCT). We analyzed the incidence of acute graft versus host disease (aGVHD) in patients treated with DLI. From 1995-2013, 171 DLIs were given to 120 patients. The cumulative incidence of post-DLI grade II-IV aGVHD was 31.6% (CI 25-42%, n=40; 12 grade II); grade III-IV 23.3% (CI 16-32%, n=28). GVHD after DLI (n=46) involved the skin in 70% (n=32), lower gastrointestinal (GI) 65% (n=30), upper GI 43% (n=20), and liver 35% (n=16). Patients receiving chemotherapy accompanying the DLI (chemo-DLI)(n=37) had more frequent aGVHD and particularly lower GI GVHD. Risk factors for grade II-IV aGVHD included: age > 40, chemo-DLI, malignant disease, and time from HCT to DLI < 200 days. aGVHD response to treatment at 8 weeks was complete in 40% and complete/partial (CR/PR) in 52%.
We observed frequent, yet therapy-responsive aGVHD following DLI. Gastrointestinal GVHD in particular is a significant risk when giving chemotherapy prior to DLI. Improvements in DLI efficacy and GVHD management are still needed.
Keywords: Donor lymphocyte infusion, acute graft vs. host disease
Introduction
For over 20 years, donor lymphocyte infusion (DLI) has been a therapeutic option for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation (HCT). It is most effective in treatment of relapsed chronic phase chronic myelogenous leukemia (CML), with complete response (CR) rates >70%. (1) DLI has been applied to other hematological malignancies with results falling short of the responses observed for CML. (2, 3) DLI has also been given successfully in non-malignant disorders post-HCT for incomplete T-cell chimerism to prevent graft failure. (4)
Acute graft vs. host disease (aGVHD) causes frequent morbidity and mortality after HCT, with an estimated incidence of 40-50% and subsequent compromised survival. (5) The role of T-lymphocytes in perpetuating a graft-versus-leukemia (GVL) effect was suggested when T-cell depleted grafts were reported to yield lower risks of GVHD, yet higher rates of graft failure and relapse. This confirmed the dual role of T-cells in maintaining engraftment and directly contributing to anti-tumor effects. (6) DLI is usually administered without immunosuppression to potentiate a maximal GVL effect. The reported incidence of aGVHD is 40-60% in patients treated with DLI after HCT. (1, 7)
We reviewed 171 donor lymphocyte infusions in 120 patients at the University of Minnesota (1995 - 2013) to determine the incidence and manifestations of aGVHD.
Materials and Methods
Study Design
We reviewed the outcome of all patients receiving DLI from February 1995 to October 2013 using the University of Minnesota Blood and Marrow Transplant Database supplemented by detailed review of all available clinical and laboratory records. We identified 120 patients receiving 171 DLIs. Based upon active clinical trials, 37 patients (31%) received immunodepleting chemotherapy prior to DLI including fludarabine 25 mg/m2 IV × 5 doses on days −6 to −2 and cyclophosphamide 60 mg/kg IV for 1 dose on day −5. (7) All patients receiving DLI were tapered off immunosuppression at least 2 weeks prior to DLI. All patients were followed for a minimum of 1-year post-DLI (median 2 years, range 1 to 14).
Patients
Patient characteristics (Table 1) include 25 patients with CML, 27 with acute myeloid leukemia (AML), 12 with myelodysplastic syndrome (MDS), 10 with lymphoma, 4 with acute lymphoid leukemia (ALL), 3 with myeloproliferative disease, 5 with multiple myeloma, 4 with plasma cell leukemia, 3 with Juvenile CML, 2 with chronic lymphocytic leukemia (CLL), 1 with prolymphocytic leukemia, and 1 with renal cell carcinoma. Also, 24 patients with non-malignant disorders included adrenoleukodystrophy, thalassemia, mucupolysaccharidosis I, immunodysregulation polyendocrinopathy enteropathy X-linked syndrome, aplastic anemia, sickle cell anemia, Fanconi anemia, I-cell Mucolipidosis, hemophagocytic lymphohistiocytosis, and dystrophic epidermolysis bullosa. Indication for DLI in non-malignant disease was incomplete chimerism in the majority of cases. One patient with CML was non-evaluable for aGVHD and excluded from analysis of aGVHD. Six patients who died <1 month after receiving DLI were excluded from analysis of disease response. A total of 113 patients were included in analyses of disease response to DLI and aGVHD.
Table 1.
Clinical Characteristics of Patients who received DLI
| Total | CML | AML+ | MDS | Lymphoma | Other Malignancies* | Non Malignancies | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| ALL | |||||||||
| Factors | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Total N | 120 | 25 | 31 | 12 | 10 | 18 | 24 | ||
| Age (years) | <20 | 32(27%) | 0 | 3(10%) | 2(17%) | 1(10%) | 3(17%) | 23(96%) | <0.01 |
| 20-40 | 25(21%) | 13(52%) | 7(23%) | 1(8%) | 2(20%) | 1(6%) | 1(4%) | ||
| ≥40 | 63(53%) | 12(48%) | 21(68%) | 9(75%) | 7(70%) | 14(78%) | 0 | ||
| Sex | Male | 69(58%) | 11(44%) | 19(61%) | 5(42%) | 7(70%) | 13(72%) | 14(58%) | 0.36 |
| Female | 51(43%) | 14(56%) | 12(39%) | 7(58%) | 3(30%) | 5(28%) | 10(42%) | ||
| Year of DLI | 1990-1999 | 28(23%) | 18(72%) | 5(16%) | 3(25%) | 0 | 1(6%) | 1(4%) | <0.01 |
| 2000-2009 | 62(52%) | 5(20%) | 19(61%) | 5(42%) | 7(70%) | 12(67%) | 14(58%) | ||
| 2010-2013 | 30(25%) | 2(8%) | 7(23%) | 4(33%) | 3(30%) | 5(28%) | 9(38%) | ||
| Pre-DLI treatment | Chemotherapy | 37(31%) | 3(12%) | 19(61%) | 4(33%) | 7(70%) | 4(22%) | 0 | <0.01 |
| None | 83(69%) | 22(88%) | 12(39%) | 8(67%) | 3(30%) | 14(78%) | 24(100%) | ||
| Donor Type | HLA matched sibling | 83(69%) | 16(64%) | 25(81%) | 8(67%) | 7(70%) | 13(72%) | 14(58%) | 0.6 |
| Unrelated | 37(31%) | 9(36%) | 6(19%) | 4(33%) | 3(30%) | 5(28%) | 10(42%) | ||
| Dose of DLI (CD3/kg) | <1×107 | 27(23%) | 6(24%) | 6(19%) | 2(17%) | 0 | 0 | 13(54%) | <0.01 |
| 1×107-1×108 | 65(54%) | 14(56%) | 14(45%) | 7(58%) | 6(60%) | 13(72%) | 11(46%) | ||
| ≥1×108 | 28(23%) | 5(20%) | 11(36%) | 3(25%) | 4(40%) | 5(28%) | 0 | ||
| Time from HCT to DLI | < 200 days | 45(38%) | 3(12%) | 13(42%) | 6(50%) | 3(30%) | 7(39%) | 13(54%) | 0.05 |
| ≥ 200 days | 75(63%) | 22(88%) | 18(58%) | 6(50%) | 7(70%) | 11(61%) | 11(46%) | ||
Other malignancies include myeloproliferative disease (n= 3), multiple myeloma (n=5), plasma cell leukemia (n= 4), Juvenile CML (n= 3), chronic lymphocytic leukemia (n= 2), prolymphocytic leukemia (n= 1), and renal cell carcinoma (n= 1).
AGVHD assessment
Acute GVHD was assessed clinically using the University of Minnesota grading system. (8) Grading was performed weekly by clinical assessment and supplemented with retrospective chart analysis, including laboratory records, with biopsy confirmation in the majority of cases.
Statistical Analysis
Data on transplantation patient characteristics, post-transplantation complications and outcomes were prospectively collected by the University of Minnesota Biostatistical Support Group using standardized collection procedures. Patient and transplant characteristics were summarized using descriptive statistics. Statistical comparisons between disease groups used the Chi-square test. All patients were followed longitudinally until death or last follow up. The cumulative incidence of aGVHD (grades II-IV and III-IV) was calculated using death prior to GVHD as a competing risk. The proportional hazards model of Fine and Gray (9) was used to assess the independent factors of aGVHD (grade II-IV). The factors considered are listed in Table 3. Kaplan Meier curves were used to estimate overall survival calculated from time of first DLI until death or last follow-up evaluation. All statistical analyses were performed with Statistical Analysis System statistical software version 9.3 (SAS Institute, Inc., Cary, NC).
Table 3.
Risk factors for Grade II-IV aGVHD
| Risk Factor | N | Cumulative Incidence | 95% CI | P-value | |
|---|---|---|---|---|---|
| Age (years) | <20 | 32 | 22% | 8- 36% | <0.01 |
| 20-40 | 25 | 16% | 2- 30% | ||
| ≥40 | 63 | 46% | 33- 59% | ||
| Sex | Male | 69 | 36% | 25- 48% | 0.45 |
| Female | 51 | 29% | 17- 42% | ||
| Malignant Disease | Yes | 96 | 39% | 28- 49% | 0.01 |
| No | 24 | 13% | 0- 25% | ||
| Previous aGVHD | Yes | 61 | 39% | 27- 52% | 0.32 |
| No | 59 | 27% | 16- 39% | ||
| Previous chronic GVHD | Yes | 44 | 43% | 28- 58% | 0.11 |
| No | 76 | 28% | 17- 38% | ||
| Chemo-DLI | Yes | 37 | 59% | 42- 77% | <0.01 |
| No | 83 | 22% | 13- 31% | ||
| Time from HCT to DLI (days) | <200 | 45 | 44% | 29- 60% | 0.01 |
| ≥200 | 75 | 27% | 17- 37% | ||
| Donor Type | Sibling | 83 | 30% | 20- 40% | 0.29 |
| URD | 37 | 41% | 24- 57% | ||
| DLI Dose (CD3 /kg) | <1×107 | 27 | 19% | 4- 33% | 0.23 |
| 1×107-1×108 | 65 | 35% | 23- 47% | ||
| ≥1×108 | 28 | 43% | 24- 62% | ||
AGVHD Therapy and Clinical Endpoints
Initial aGVHD treatment included prednisone at 60 mg/m2/d (or methylprednisolone IV equivalent) for 14 days followed by taper over 8 weeks for responding patients. Response to treatment of aGVHD was classified at 4 and 8 weeks as either: no response, partial response (PR), or complete response (CR). No response was defined as death prior to the time of evaluation, no improvement, or worsening symptoms. CR was defined as complete resolution of aGVHD signs and symptoms in all organs without need for secondary therapy. PR was defined as improvement in GVHD stage in all initially involved organs without development of new organ involvement.
Complete remissions (CR) of the DLI-treated disease definitions were disease-specific. For CML patients, CR was defined as hematological, cytogenetic, or molecular remission. Persistent disease was defined as residual Ph+ cells or evidence of peripheral blood or bone marrow CML. For acute leukemias, CR required <5% marrow blasts, hematologic recovery, and absence of any previous cytogenetic abnormality. For myeloma, CR required disappearance of M-protein by electrophoresis and absence of monoclonal marrow plasmacytosis. For lymphoma, CR was defined by clinical resolution by physical exam, imaging, and bone marrow examination.
Cause of death was assessed by reviewing death registries and the medical chart and assigned as secondary to disease recurrence, aGVHD, or other. Any infectious cause of death that occurred while on immunosuppression for treatment of aGVHD was classified as a GVHD-related death.
Donor Lymphocyte Infusions
DLI were collected from the donor by steady state leukapheresis. In all cases, the original allogeneic transplant donor was used for DLI. Infused doses ranged from 4.7 × 105 to 2.29 × 108 CD3+ cells/kg. Median DLI dose was 5 × 107 CD3+ cells/kg. Multiple DLI infusions were given × 2 (n=33 patients), × 3 (n=21), × 4 (n=6), × 5 (n=3), and × 6 (n=1). Patients with either malignant or non-malignant diseases received multiple DLIs. For patients receiving multiple DLIs, the largest single cell dose was used for analysis of outcomes.
Results
The patients’ clinical characteristics are shown in Table 1 and included 120 patients; 79% (n=96) treated for relapsed hematological malignancy and 21% (n=24) for non-malignant conditions. The largest disease subgroups were CML (n=25) and AML (n=27). The median age at time of DLI was 36 years (range, 9 months-66 years). The group included more males (57%) than females. Thirty seven (31%) patients received lymphodepleting chemotherapy immediately prior to DLI, half in the AML subgroup (n=19). 83 donors (69%) were HLA-matched siblings (MSD) and 37 (31%) unrelated donors (URD). Most DLIs were performed > 200 days after HCT (median 255, range 29-8536 days); only 26 were given beyond 3 years post HCT.
Incidence and Clinical Manifestations of AGVHD
In 120 patients, 46 developed aGVHD at a median of 34 days (range, 5-121 days) after DLI. The cumulative incidence of grade II-IV aGVHD was 31.6% (95% confidence interval (CI) 25-42%) (n=40) and grade III-IV aGVHD 23.3% (95% CI 16-32%) (n=28). GVHD organ involvement is shown in Table 2. The maximum GVHD grade was grade I in 13% (n=6), grade II in 26% (n=12), grade III in 28% (n=13) and grade IV in 33% (n=15).
Table 2.
AGVHD post-DLI: Maximal Organ Stage
| Organ Stage | Skin | Liver | Upper GI | Lower GI |
|---|---|---|---|---|
| 0 | 14 (30%) | 30 (65%) | 26 (46%) | 16 (35%) |
| 1 | 3 (7%) | 3 (7%) | 20 (44%) | 6 (13%) |
| 2 | 8 (17%) | 1 (2%) | - | 2 (4%) |
| 3 | 18 (39%) | 4 (9%) | - | 9 (20%) |
| 4 | 3 (7%) | 8 (17%) | - | 13 (28%) |
Percentages represent organ involvement in patients with aGVHD after DLI (n= 46).
Of those receiving DLI, skin aGVHD developed in 70% (n=32), lower GI in 65% (n=30), upper GI in 44% (n=20), and liver in 35% (n=16). Chemo-DLI recipients (n=37) had higher rates of aGVHD II-IV than DLI without chemotherapy (59% vs. 22%, p < 0.01). Patients receiving chemo-DLI had similar rates of liver and upper GI aGVHD, but more frequent lower GI aGVHD (51% vs. 13%).
Chronic GVHD after DLI was observed in 24% (29/120). 46 patients survived free of chronic GVHD for > 1 year. 18 patients had both acute and chronic GVHD, while 11 had chronic GVHD only.
Risk factors for aGVHD
Risk factors for aGVHD are shown in Table 3. Age > 40, chemotherapy prior to DLI, malignant disease, and shorter time from BMT to DLI (< 200 days) were significantly associated with higher risks of grade II-IV aGVHD. We did not observe a statistically significant increase in aGVHD risk with higher DLI cell doses, either in the entire group or in a subgroup analysis of the chemo-DLI vs. non-chemo-DLI cohorts. A prior history of aGVHD trended towards increased risk of aGVHD after DLI, but did not reach statistical significance. Within the subgroup with malignant diseases, age did not significantly influence the risk for aGVHD.
Response to treatment of aGVHD
Of 44 evaluable patients treated for aGVHD after DLI, 6 died within 4 weeks and thus were not evaluable for response; 3 of these had GVHD as a cause of death. Of 40 patients with grade II-IV aGVHD, 28% (n=11) achieved a CR and 55% (n=22) had a CR/PR at 4 weeks. The overall response rate at 4 weeks is similar to that described for aGVHD after HCT (64% CR/PR) where most patients had a CR (49%) in contrast with the lower CR rate we observed post DLI. (10)
At 8 weeks, CR was observed in 40% (n=16) and CR/PR in 52% (n=21). At 8 weeks, 10% (n=4) had no response and 35% (n=14) of patients had died. Treatment responses for grade II-IV and III-IV aGVHD were similar. At 8 weeks, liver GVHD responded poorly (only 25% CR/PR, n=16) while patients with skin or GI involvement had 45-59% CR/PR. Age, malignancy, time from HCT to DLI, time from DLI to aGVHD, donor type, chemo-DLI, and the infused DLI dose did not influence the efficacy of aGVHD treatment.
Disease Response to DLI
CR after DLI was achieved in 54% (48/89) of all patients with malignancies. In CML (n=25), CR was 80% (20/25) with most treatment failure in patients with accelerated phase disease. In non-CML malignancies (n=65), rates of CR ranged from 25% to 55% for disease subgroups with overall similar CR at 45% and 46% for chemo-DLI and non-chemo DLI, respectively. However, higher CR rates with chemo-DLI were reported in lymphoma (28% CR with chemo-DLI vs. 0% without, n= 7).
The development of aGVHD influenced the success in achieving CR in non-CML malignancies. Patients with aGVHD after DLI had higher CR rates. Only 9% (6/71) of patients had CR after DLI without aGVHD. In acute leukemias (AML + ALL), CR rate was 73% (8/11) with aGVHD and 33% (6/18) without. In lymphoma, CR rate was 25% (1/5) with aGVHD and 17% (1/6) without. In other malignancies, CR rate was 89% (8/9) with aGVHD and 29% (5/17) without. CR rate for malignant disease overall was 73% (22/30) with aGVHD and 44% (26/ 59) without. Of 7 patients with non-CML malignancies who had no response to GVHD treatment at 4 weeks, 4 of 7 had a response of their malignant disease.
Amongst patients with non-malignant diseases, 79% (19/24) survive >1 year post-DLI with follow up between 1 and 14 years. Of 5 deaths, 4 patients had developed aGVHD. Causes of death included 3 from aGVHD, 1 from infection, and 1 from secondary B-cell lymphoma. Only 1 of 19 of surviving patients developed aGVHD. DLI was successful in attaining complete engraftment as only 3 out of 24 patients failed to engraft.
Overall survival (OS) at 2 years for patients with CML was similar (83% vs 79%) with or without aGVHD (Figure 2A). However, for non-CML malignancies, OS at 2 years was significantly better without aGVHD at 41% vs. 22% (Figure 2B).
Figure 2.
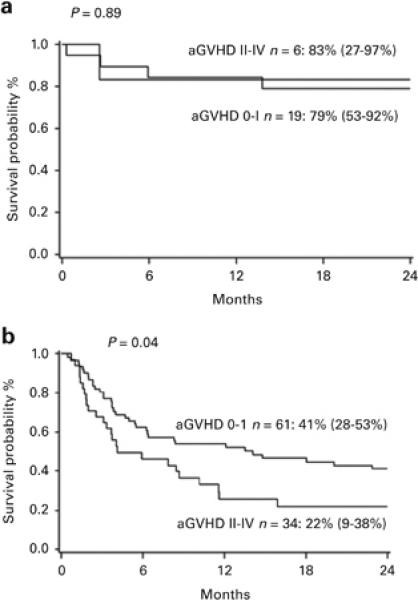
A. 2 year overall survival of CML patients with or without aGVHD grade II-IV.
Figure 2B. 2 year overall survival of patients with non-CML malignancies with or without aGVHD grade II-IV. Survival was significantly increased in patients without aGVHD (41% vs 22%).
Of patients who died without malignant relapse (n=25), the median time from DLI to death was 4 months. Only 25% survived at 1 year and 12% at 2 years. Of patients who achieved CR after DLI (n=41), survival at 2 years was 51%; 88% for those with CML and 25% for non-CML malignancies.
Discussion
In this large consecutive series of patients receiving DLI, the cumulative incidence of grade II-IV and III-IV aGVHD was 32% and 23%, respectively; similar though somewhat lower than earlier reports describing 41-46% aGVHD after DLI (1, 4) and 37%-63% after allogeneic HCT. (10, 11) The chemo-DLI subgroup had more frequent, advanced grade aGVHD, particularly with lower GI involvement. The toxic and pro-inflammatory effects of chemotherapy on the GI mucosa may have contributed to this higher incidence of GI GVHD. We attempted amelioration in part by infusion of lower cell doses of DLI after the preceding chemotherapy; our earlier report noted 25% vs. 66% aGVHD with the lower cell dose chemo-DLI (12) while achieving high rates of disease response using chemo-DLI. However, our current analysis does not support a direct relationship between DLI cell dose and development of aGVHD.
In comparison to post-HCT GVHD, aGVHD after DLI appears to have more frequent organ involvement, particularly upper and lower GI and liver at time of initiation of therapy. (5, 6, 10) The frequency and severity of liver, upper GI, and lower GI GVHD were higher after DLI compared to a large series (n = 1723) of patients with aGVHD after HCT described by MacMillan et al. (13) In this cohort, incidence of any liver GVHD lower at 8% and upper GI GVHD lower at 27%. The post HCT incidence of lower GI GVHD was similar at 65%, but Gr III-IV GI GVHD comprised 26% of total GI GVHD vs. 73% in our study of post-DLI GVHD. We did not observe the hepatitic variant of liver aGVHD after DLI. (14) Higher rates of aGVHD are likely a result of withholding immunosuppressive medications at time of DLI administration in effort to induce maximum graft versus tumor effect.
Importantly, we noted that treatment of aGVHD after DLI yielded similar overall response rates to aGVHD following HCT. We observed CR/PR rates of 55% at 4 weeks and 53% at 8 weeks. This is similar to response rates after HCT, but was more frequently a PR rather than CR. Liver GVHD was associated with the lowest response rates to treatment.
We observed a correlation between disease remission and aGVHD in post-DLI patients, except in those with CML who had frequent responses, even without GVHD. In an early multi-center analysis, Collins et al. reported that 93% of complete responders had aGVHD, while only 13% of those without acute or chronic GVHD achieved CR. (4) Kolb et al. reported durable remissions in chronic phase CML and a correlation of response with aGVHD (91% responses with GVHD versus 45% without). (1)
The relationship of aGVHD to disease response was most notable in the cohort with acute leukemias and other malignancies including plasma cell disorders. CML patients responded with or without aGVHD, possibly reflecting the bcr-abl fusion protein acting as a leukemia-specific antigenic target. However, of 71 non-CML patients without aGVHD, only 6 had a disease response. In contrast to some series, the development of aGVHD and an associated remission did not translate into increased 2-year survival, either in CML or non-CML malignancies. (15)
Whether some GVHD is permissive for a maximal graft versus tumor effect has been debated. Some groups have administered interferon-alpha to promote GVHD in hopes of improving disease response. (16, 17) DLI has been given along with chemotherapy and concurrent immunosuppression to reduce GVHD rates. Using this approach, Yan et al. reported higher CR rates compared to chemotherapy alone, suggesting that despite the post-DLI immunosuppression, the GVL effect was still clinically evident. Modified DLI techniques have been explored to maximize GVL while minimizing GVHD including ex vivo T-cell activation with dose escalation (18) and transduction of suicide genes to lymphocytes to selectively control GVHD. (19) T-cell receptor transfer of donor lymphocytes may also enhance the specificity of antigen recognition and thus augment GVL while reducing GVHD. (20) The clinical value and reproducibility of these techniques in vivo remain uncertain.
Our analysis did show that about half of patients with aGVHD had persisting GVHD at 4 weeks despite treatment, yet had CR of their disease. Pre-DLI chemotherapy might contribute to tumor cell eradication, but also to depletion of immunosuppressive regulatory elements such as regulatory T cells or myeloid derived suppressor cells. This suggests the presence of complex immunologic interactions that remain incompletely understood and not well regulated by current clinical interventions. These interactions need further study to improve clinical safety and anti-tumor responses.
Chronic GVHD after DLI has been previously shown to be positively associated with improved survival in relapsed acute leukemia (21) and with no impact on mortality in a large multicenter study of CML. (22) As both acute and chronic GVHD are a significant risk following DLI, optimal treatment to improve both survival and quality of life are still an active area of investigation.
We identified some subgroups at particularly high risk for aGVHD following DLI, including older patients, those receiving chemo-DLI, and DLI given < 200 days after HCT. Whether these groups require different management remains a question for future studies. Prior acute or chronic GVHD did not significantly influence the risk for developing aGVHD, so DLI should be not be withheld due to history of GVHD if it has resolved. Other comorbidities besides chemotherapy exposure may also play a role in the development of aGVHD, although it is not clear how such comorbidities may contribute to the outcomes of DLI. (23)
The development of tyrosine kinase inhibitors (TKI) for molecular-targeted treatment of CML has markedly reduced the need for HCT for CML. In the 2010 EBMT survey, 62% of allogeneic HCTs were performed for AML, ALL, and MDS/MPS; only 3% for CML. (24) In addition, TKI or other targeted therapy may supplant the need for DLI for CML patients who relapse after HCT. Such effective targeted therapies are not available for patients with non-CML hematologic malignancies. As a result, DLI remains an important component of contemporary post-HCT relapse therapy. New methods of enhancing responses while allowing time for the development of GVL, possibly including the use of DLI in combination with molecularly targeted therapies, may further improve outcomes. Alternatively, direct enhancement of donor-derived anti-tumor activity using immunostimulatory cytokines (e.g. IL-2 or IL-15) or through inhibition of immune checkpoints (with anti-CTLA4 or anti-PD1/PDL1) might be of value, though potentially augmenting risks of GVHD. Although we observed that aGVHD after DLI was associated with disease response, it ultimately compromised survival. Therefore, future efforts should also focus on minimizing GVHD, particularly visceral GVHD, after DLI.
Figure 1.
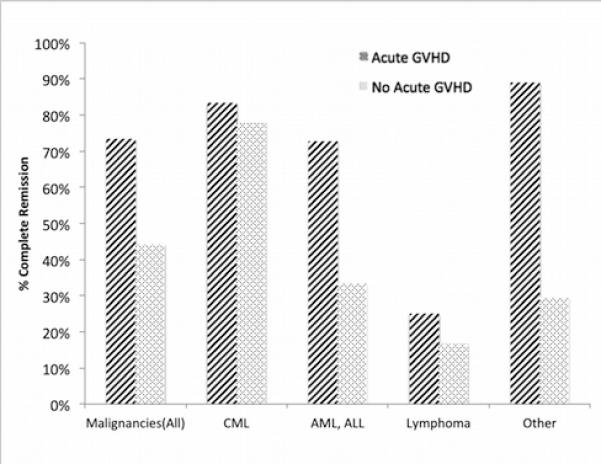
Complete remission rates of malignancies in patients with or without aGVHD after DLI.
Table 4.
Treatment of aGVHD: response and risk factors for response
| Response at 4 wks | Response at 8 wks | ||||||
|---|---|---|---|---|---|---|---|
| Acute GVHD (no.) | CR | CR+PR | CR | CR+PR | p-value | ||
| Age (years) | <20 | 9 | 3 (33%) | 3 (33%) | 3 (33%) | 4 (44%) | 0.95 |
| 20-40 | 6 | 2 (33%) | 3 (50%) | 3 (50%) | 3 (50%) | ||
| >40 | 31 | 10 (32%) | 21 (68%) | 15 (48%) | 19 (61%) | ||
| Malignancy | Yes | 40 | 12 (30%) | 23 (58%) | 17 (43%) | 21 (53%) | 0.42 |
| No | 6 | 3 (50%) | 4 (67%) | 4 (67%) | 5 (83%) | ||
| AGVHD Grade | II-IV | 40 | 11 (28%) | 22 (55%) | 16 (40%) | 21 (53%) | - |
| III-IV | 18 | 5 (28%) | 12 (67%) | 8 (44%) | 12 (67%) | - | |
| Target Organs | Skin | 32 | 11 (24%) | 22 (48%) | 16 (35%) | 19 (59%) | - |
| Liver | 16 | 0 | 4 (9%) | 1 (2%) | 4 (25%) | - | |
| Upper GI | 20 | 7 (15%) | 11 (24%) | 9 (20%) | 11 (46%) | - | |
| Lower GI | 30 | 8 (27%) | 14 (47%) | 10 (33%) | 14 (47%) | - | |
| Time from HCT to DLI (days) | <200 | 21 | 5 (24%) | 11 (52%) | 8 (38%) | 11 (52%) | 0.44 |
| ≥200 | 25 | 10 (40%) | 16 (64%) | 13 (52%) | 15 (60%) | ||
| Time from DLI to aGVHD (days) | 1-60 | 37 | 10 | 22 | 16 | 21 | 0.74 |
| 61-121 | 9 | 5 | 5 | 5 | 5 | ||
| Chemotherapy with DLI | No | 23 | 7 (30%) | 11 (48%) | 9 (39%) | 11 (48%) | 0.18 |
| Yes | 23 | 8 (35%) | 16 (70%) | 11 (48%) | 14 (61%) | ||
| Donor type | Sibling | 30 | 13 (43%) | 21 (70%) | 16 (53%) | 20 (67%) | 0.22 |
| URD | 16 | 2 (13%) | 6 (38%) | 5 (31%) | 6 (38%) | ||
| DLI dose (CD3/kg) | <1×107 | 6 | 2 (33%) | 2 (33%) | 2 (33%) | 3 (50%) | 0.19 |
| 1×107-1×108 | 28 | 9 (32%) | 17 (61%) | 12 (43%) | 16 (57%) | ||
| ≥1×108 | 12 | 4 (33%) | 8 (67%) | 7 (58%) | 7 (58%) | ||
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 2.Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working party. J Clin Oncol. 2007;25(31):4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 3.Collins RH, Goldstein S, Girait S, Levine J, Porter D, Drobyski W, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000;26(5):511–516. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 4.Frugnoli I, Cappelli B, Chiesa R, Biral E, Noé A, Evangelio C, et al. Escalating doses of donor lymphocytes for incipient graft rejection following SCT for thalassemia. Bone Marrow Transplant. 2010;45(6):1047–51. doi: 10.1038/bmt.2009.298. [DOI] [PubMed] [Google Scholar]
- 5.Jagasia M, Arora M, Mary ED, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apperley JF, Mauro FR, Goldman JM, Gregory W, Arthur CK, Hows J, et al. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: importance of a graft-versus-leukaemia effect. Br J Haematol. 1988;69(2):239–245. doi: 10.1111/j.1365-2141.1988.tb07628.x. [DOI] [PubMed] [Google Scholar]
- 7.Collins RH, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15(2):433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 8.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 9.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 10.Macmillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versushost disease. Biol Blood Marrow Transplant. 2002;8(7):387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 11.Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76(8):1464–1472. [PubMed] [Google Scholar]
- 12.Warlick ED, DeFor T, Blazar BR, Burns L, Verneris MR, Ustun C, et al. Successful remission rates and survival after lymphodepleting chemotherapy and donor lymphocyte infusion for relapsed hematologic malignancies postallogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):480–486. doi: 10.1016/j.bbmt.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761–767. doi: 10.1016/j.bbmt.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akpek G, Boitnott JK, Lee LA, Hallick JP, Torbenson M, Jacobsohn DA, et al. Hepatitic variant of graft-versus-host disease after donor lymphocyte infusion. Blood. 2002;100(12):3903–3907. doi: 10.1182/blood-2002-03-0857. [DOI] [PubMed] [Google Scholar]
- 15.Yan CH, Wang JZ, Liu DH, Zu LP, Chen H, Liu KY, et al. Chemotherapy followed by modified donor lymphocyte infusion as a treatment for relapsed acute leukemia after haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion: superior outcomes compared with chemotherapy alone and an analysis of prognostic factors. Eur J Haematol. 2013;91(4):304–314. doi: 10.1111/ejh.12168. [DOI] [PubMed] [Google Scholar]
- 16.Posthuma EF, Marijt EW, Barge RM, van Soest RA, Baas IO, Starrenburg CW, et al. Alpha-Interferon with very-low-dose donor lymphocyte infusion for hematologic or cytogenetic relapse of chronic myeloid leukemia induces rapid and durable complete remissions and is associated with acceptable graftversus- host disease. Biol Blood Marrow Transplant. 2004;10(3):204–212. doi: 10.1016/j.bbmt.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Eefting M, von dem Borne PA, de Wreede LC, Halkes CJ, Kersting, Marijt EW, et al. Intentional donor lymphocyte induced limited acute graft versus host disease is essential for long-term survival of relapsed acute myeloid leukemia after allogeneic stem cell transplantation. Haematologica. 2014;99(4):751–758. doi: 10.3324/haematol.2013.089565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter DL, Levine BL, Bunin N, Stadtmauer EA, Luger SM, Goldstein S, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107(4):1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 19.Traversari C, Marktel S, Magnani Z, Mangia P, Russo V, Ciceri F, et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109(11):4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov R, Hol S, Aarts T, Hagenbeek A, Slager EH, Ebeling S. UTY-specific TCRtransfer generates potential graft-versus-leukaemia effector T cells. Brit J Haematol. 2005;129(3):392–402. doi: 10.1111/j.1365-2141.2005.05461.x. [DOI] [PubMed] [Google Scholar]
- 21.Curley C, Hill GR, McLean A, Kennedy GA. Immunotherapy following relapse of acute leukaemia after T-cell-replete allogeneic peripheral blood progenitor cell transplantation: importance of new onset of chronic graft-versus-host disease. Int J Lab Hematol. 2014;36(2):197–204. doi: 10.1111/ijlh.12153. [DOI] [PubMed] [Google Scholar]
- 22.Chalandon Y, Passweg JR, Schmid C, Olavarria E, Dazzi F, Simula MP, et al. Outcome of patients developing GVHD after DLI given to treat CML relapse: a study by the Chronic Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2010;45(3):558–564. doi: 10.1038/bmt.2009.177. [DOI] [PubMed] [Google Scholar]
- 23.Sorror ML, Martin PJ, Storb RF, Bhatia S, Maziarz RT, Pulsipher MA, et al. Pretransplant cormorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood. 2014;124(2):287–295. doi: 10.1182/blood-2014-01-550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P, et al. The EBMT activity survey: 1990–2010. Bone Marrow Transplant. 2012;47(7):906–923. doi: 10.1038/bmt.2012.66. [DOI] [PubMed] [Google Scholar]


