SUMMARY
Despite a positive prognosis for seizure remission, children with Benign Epilepsy with Centrotemporal Spikes (BECTS) have been reported to exhibit subtle neuropsychological difficulties. We examined the relationship between patterns of centrotemporal spikes (typical EEG finding in BECTS) and neuropsychological and motor outcomes in children with new-onset BECTS. Thirty-four patients with new-onset BECTS (not taking antiepileptic medication) and 48 typically-developing children participated in the study. In BECTS patients, centrotemporal spikes (CTS) were evaluated in the first hour awake and first two hours of sleep in a 24-hour EEG recording and left or right-sided origin was noted. General intellectual function, language, visuospatial skill, processing speed and fine motor skill were assessed in all participants. We found no significant difference between BECTS patients and controls on measures of general intellectual function, visuospatial or language testing. There were significant differences in Processing Speed Index and non-dominant hand fine motor scores between groups. Significant negative relationships were observed between rates of left-sided CTS and right hand fine motor scores. This suggests that psychomotor and fine motor speed are affected in BECTS, but the extent of affected domains may be more limited than previously suggested, especially in untreated patients early in the course of their epilepsy.
Keywords: Benign Epilepsy with Centrotemporal Spikes, Electroencephalography, Neuropsychology
INTRODUCTION
Benign Epilepsy with Centrotemporal Spikes (BECTS), one of the most common pediatric epilepsy syndromes, is assumed to have a relatively benign course with onset between ages 4 and 10 years and likely cessation of seizures by adulthood. The infrequent, brief seizures typical of BECTS manifest with hemifacial motor and somatosensory symptoms, but may generalize. Seizures tend to occur in drowsiness or sleep, and the typical EEG in BECTS shows frequent high-voltage centro-temporal spikes (CTS) that are much more frequent during drowsiness and sleep.1 In contrast to the assumed benign course, a growing literature has documented cognitive and/or behavioral problems in children with BECTS (see 2 for a recent review).
Specifically, general intellectual function (Full Scale IQ) is in the average range in most studies of BECTS patients,3 as is true of the larger population of individuals with epilepsy, but specific cognitive domains may be more affected. Most consistently reported is poorer performance on tests of language and verbal memory compared to healthy controls,3; 4 as well as attention5 and processing speed.6 These problems may underlie difficulties in academic achievement.3 However, most existing studies include heterogeneous groups of BECTS participants; including some patients on anti-epileptic medications, and some years after their first recognized seizure that may or may not continue to have CTS3–5; 7; 8. Cognitive problems in BECTS have been suggested to emerge6, or remain stable9 with longer duration of epilepsy, so examining more homogenous groups of patients is important for understanding the natural course of BECTS.
Also, these studies have not clearly explained how frequent CTS in BECTS may contribute to particular profiles of cognitive problems. Associations have been observed between left-sided CTS and phonological language skills, and between right-sided CTS and poorer visuospatial skills and other aspects of language skill 8; 10; 11, but other studies did not find such relationships.3; 4 Other studies have focused on the role of frequent CTS during sleep in BECTS and their impact on cognition; more frequent CTS during sleep have been associated with poorer reading scores and Verbal IQ12, learning disabilities3 and poorer visual attention scores5. In these studies, CTS frequency is often described categorically; e.g. a sleep CTS rate over 40/minute is “frequent”, below is “infrequent” (though some studies have used more than two categories12). This approach allows comparison between groups of patients, but does not address whether CTS rates affect cognition in a continuous way. In fact, Ebus and colleagues7, examining CTS as a continuous variable, found that more frequent CTS during wakefulness, rather than sleep, correlated with decreased processing speed.
Another gap in this literature is that fine motor speed and dexterity is rarely tested in BECTS. Lundberg and colleagues13 documented oromotor problems in BECTS compared to controls, and Overvliet and colleagues14 note that over 20% of BECTS patients had a parent-reported history of motor development problems.
Here we focused on the relationship between CTS frequency and lateralization and neuropsychological scores in children with new-onset BECTS. We hypothesized that BECTS patients would perform more poorly than controls in three primary domains: language, visuospatial processing, and fine motor skill. Secondly, we hypothesized that there would be a negative relationship between frequency of CTS and performance in these domains in BECTS patients; specifically, that left-sided CTS might affect language skill, and right-sided CTS visuospatial processing; and further, that fine motor skill may be affected by CTS in the contralateral hemisphere.
METHODS
All study procedures were approved by Cincinnati Children’s Hospital Medical Center Institutional Review Board.
Participants
Patients with clinical presentation and an EEG pattern consistent with BECTS were recruited from Neurology Clinics at Cincinnati Children’s Hospital Medical Center. Typically-developing children were recruited via community advertising, and were not family members of BECTS patients. Informed consent was obtained from a parent/guardian for all participants, including written assent from participants age 11 and up.
Electroencephalographic (EEG) Evaluation
Healthy control participants had a brief EEG recording (10–15 minutes awake) was examined for the presence of epileptiform discharges. One control participant was found to have CTS (but no history of seizures) and was excluded from further participation.
In BECTS patients, CTS were evaluated based on a 24-hour ambulatory EEG recording with 23 electrodes in standard 10–20 positioning (no polygraphic channels such as EMG were included). Participants wore the EEG apparatus home overnight and returned the following day. The first hour of wakefulness after the EEG setup (see Table 2 for setup times) and the first two hours of overnight sleep following the first sleep spindle were examined by a pediatric epileptologist. A twenty-minute period of N2 sleep and a thirty-minute period of N3 sleep during the two-hour sleep period were also marked for comparison. Stage N2 sleep was defined by the first sleep spindle and did not include any slow wave sleep. Stage N3 sleep was defined by slow wave sleep comprised of high amplitude delta frequency activity. CTS were counted by visual analysis and noted as originating from left or right centrotemporal regions.
Table 2.
Gender, Age, Seizure history, time of EEG and CTS/min during 1 hour awake and 2 hours sleep for BECTS patients.
| Participant ID | Gender | Age (years) | Number of Seizures | Months since first seizure | Time of day awake EEG begins | CTS/min during 1 hour awake | CTS/min during 2 hours asleep | Scores −1.5SD or below Control mean | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Bilateral | Right | Left | Bilateral | |||||||
| 1 | Male | 10 | 2 | 12 | 10:52 | 0.88 | 0.11 | 0.00 | 16.13 | 15.62 | 0.31 | VMI, PSI, pegboard D/ND |
| 2 | Male | 9 | 5 | 6 | 10:06 | 2.93 | 0.63 | 0.65 | 24.40 | 14.89 | 3.24 | Pegboard ND |
| 3 | Male | 5 | 1 | 1 | 10:06 | 0.00 | 10.18 | 0.00 | 0.00 | 26.04 | 0.00 | CELF, pegboard D |
| 4 | Female | 9 | 1 | 2 | 13:00 | 0.58 | 0.58 | 0.00 | 21.53 | 15.34 | 0.28 | None |
| 5 | Male | 11 | 2 | 9 | 10:55 | 1.45 | 0.00 | 0.08 | 22.43 | 0.02 | 0.01 | CTOPP phono |
| 6 | Female | 8 | 3 | 6 | 11:10 | 0.25 | 0.00 | 0.00 | 26.87 | 0.00 | 0.01 | None |
| 7 | Female | 8 | 1 | 0 | 10:59 | 0.07 | 0.00 | 0.00 | 9.36 | 0.00 | 0.00 | VMI |
| 8 | Female | 9 | 2 | 13 | 11:07 | 0.32 | 0.00 | 0.15 | 23.57 | 0.01 | 7.98 | None |
| 9 | Male | 5 | 1 | 2 | 15:25 | 0.00 | 0.47 | 0.00 | 0.00 | 20.60 | 0.00 | None |
| 10 | Female | 5 | 1 | 0 | 15:05 | 0.03 | 0.00 | 7.05 | 5.87 | 0.00 | 0.00 | None |
| 11 | Female | 9 | 3 | 1 | 9:58 | 0.02 | 0.00 | 0.00 | 0.18 | 0.03 | 0.00 | None |
| 12 | Male | 10 | 2 | 1 | 14:18 | 0.00 | 0.00 | 0.00 | 1.70 | 0.01 | 0.00 | VMI, PSI |
| 13 | Female | 8 | 1 | 5 | 12:54 | 1.58 | 0.88 | 0.00 | 24.98 | 18.53 | 0.05 | PSI |
| 14 | Female | 5 | 2 | 1 | 11:28 | 13.22 | 8.43 | 0.08 | 39.62 | 36.38 | 1.57 | VMI, PSI |
| 15 | Female | 7 | 2 | 8 | 10:53 | 0.02 | 10.13 | 0.00 | 0.02 | 39.83 | 0.00 | None |
| 16 | Male | 6 | 3 | 0 | 9:13 | 5.08 | 1.18 | 0.00 | 18.40 | 5.93 | 0.04 | None |
| 17 | Female | 11 | 2 | 12 | 14:16 | 0.03 | 2.83 | 0.02 | 0.08 | 17.70 | 0.01 | None |
| 18 | Male | 10 | 2 | 9 | 12:21 | 7.38 | 0.02 | 0.02 | 25.56 | 0.00 | 0.03 | None |
| 19 | Male | 6 | 6 | 0 | 14:41 | 19.45 | 0.00 | 0.00 | 40.30 | 0.00 | 0.00 | None |
| 20 | Male | 6 | 5 | 0 | 14:50 | 0.00 | 17.18 | 0.00 | 0.02 | 38.87 | 0.00 | PSI, pegboard D |
| 21 | Male | 8 | 1 | 1 | 14:36 | 0.50 | 2.33 | 0.00 | 11.47 | 11.08 | 0.57 | None |
| 22 | Female | 12 | 2 | 0 | 11:57 | 0.00 | 0.00 | 0.00 | 0.00 | 15.23 | 0.00 | VMI, FSIQ, CELF |
| 23 | Male | 10 | 2 | 34* | 11:48 | 0.00 | 0.00 | 0.00 | 2.72 | 0.00 | 0.00 | FSIQ, PSI, CELF,pegboard ND |
| 24 | Female | 9 | 1 | 1 | 8:43 | 0.87 | 0.20 | 0.00 | 15.21 | 12.28 | 0.72 | None |
| 25 | Male | 5 | 3 | 1 | 14:43 | 0.00 | 16.53 | 0.00 | 0.00 | 27.97 | 0.00 | FSIQ,pegboard D/ND |
| 26 | Male | 10 | 3 | 11 | 9:18 | 0.00 | 0.98 | 0.00 | 11.15 | 30.42 | 0.44 | None |
| 27 | Male | 8 | 3 | 1 | 10:07 | 6.52 | 2.93 | 0.00 | 11.99 | 3.35 | 0.07 | None |
| 28 | Female | 5 | 1 | 1 | 16:48 | 9.71 | 0.02 | 0.00 | 24.38 | 0.00 | 0.00 | FSIQ, CELF |
| 29 | Female | 5 | 1 | 6 | 13:42 | 4.68 | 0.92 | 0.00 | 32.86 | 0.01 | 0.01 | None |
| 30 | Male | 9 | 1 | 0 | 13:41 | 0.08 | 0.02 | 0.00 | 0.03 | 0.00 | 0.01 | VMI, PSI |
| 31 | Male | 9 | 1 | 2 | 9:28 | 4.13 | 0.00 | 0.00 | 39.79 | 0.00 | 0.00 | PSI |
| 32 | Female | 10 | 2 | 2 | 14:45 | 6.80 | 0.00 | 0.00 | 38.63 | 0.00 | 0.00 | PSI |
| 33 | Female | 5 | 2 | 5 | 16:11 | 2.00 | 5.00 | 0.00 | 29.18 | 32.98 | 0.18 | FSIQ, PSI,pegboard D/ND |
| 34 | Female | 7 | 2 | 6 | 12:42 | 3.62 | 2.22 | 0.00 | 21.89 | 0.00 | 0.00 | None |
Patient 23 had a first recognized seizure 34 months before study enrollment, but clinical EEG was found to be normal at that time. A second recognized seizure took place less than one month before study enrollment, and clinical EEG at that time confirmed the typical pattern for BECTS. CELF = Clinical Evaluation of Language Fundamentals. VMI = Visuomotor Integration. D=dominant hand, ND=nondominant hand. PSI = Processing Speed Index.
Neuropsychological Testing
Eleven children with BECTS completed neuropsychological testing the day prior to the overnight EEG recording, and 21 participated the following day. Two participants split testing over the two days. Primary outcomes were the core subtests of the Clinical Evaluation of Language Fundamentals15 to assess language skill, the Developmental Test of Visuomotor Integration16, and the Grooved Pegboard Test (Lafayette Instrument Company) to assess fine motor skill. The Grooved Pegboard Test is scored separately for each hand; we analyzed it both in terms of dominant handedness and left versus right hand, since three participants were left-handed.
Secondary outcomes included the Wechsler Abbreviated Scale of Intelligence17 to measure general intelligence (5-year-olds were administered the Wechsler Preschool and Primary Scale of Intelligence, Third Edition, WPPSI-III)18. Phonological language skills were assessed using and the Phonological Awareness and Rapid Naming subtests of the Comprehensive Test of Phonological Processing (CTOPP)19, and speed of processing was assessed using Symbol Search and Coding subtests from the Wechsler Intelligence Scale for Children, Fourth Edition20 or from the WPPSI-III for 5-year-olds. Not all participants completed all testing; see Table 3.
Table 3.
Neuropsychological Scores, including means, standard deviation (SD), n, and number of participants −1.5 SD or below the Control mean.
| Primary Outcomes | ||||||
|---|---|---|---|---|---|---|
| BECTS | Controls | Raw p | FDR-corrected p | |||
| CELF | Core | mean | 96.03 | 101.79 | 0.082 | 0.109 |
| SD | 14.34 | 14.52 | ||||
| n | 33 | 48 | ||||
| −1.5 SD or below | 4 | 3 | ||||
| Beery VMI | mean | 94.82 | 96.29 | 0.570 | 0.570 | |
| SD | 12.06 | 10.95 | ||||
| n | 34 | 48 | ||||
| −1.5 SD or below | 6 | 4 | ||||
| Grooved Pegboard | Dominant Hand z-score* | mean | −0.94 | −0.09 | 0.100 | 0.084 |
| median | −0.43 | 0 | ||||
| SD | 2.04 | 1.02 | ||||
| n | 30 | 45 | ||||
| −1.5 SD or below | 5 | 5 | ||||
| Non-Dominant Hand z-score* | mean | −1.15 | −0.19 | 0.008 | 0.032 | |
| median | −0.55 | 0.20 | ||||
| SD | 2.08 | 1.35 | ||||
| n | 30 | 45 | ||||
| −1.5 SD or below | 5 | 2 | ||||
| Secondary Outcomes | ||||||
| BECTS | Controls | p | ||||
| WASI/WPPSI | Full scale IQ | mean | 106.64 | 109.44 | 0.357 | |
| SD | 13.30 | 12.84 | ||||
| n | 31 | 48 | ||||
| −1.5 SD or below | 5 | 4 | ||||
| WISC/WPPSI Processing Speed Index | mean | 95.77 | 103.17 | 0.005 | ||
| SD | 12.56 | 9.53 | ||||
| n | 31 | 47 | ||||
| −1.5 SD or below | 10 | 3 | ||||
| CTOPP | Phono Awareness | mean | 99.28 | 100.59 | 0.653 | |
| SD | 11.27 | 13.40 | ||||
| n | 32 | 46 | ||||
| −1.5 SD or below | 1 | 4 | ||||
| RapidNaming | mean | 100.58 | 97.72 | 0.271 | ||
| SD | 10.32 | 11.61 | ||||
| n | 31 | 46 | ||||
| −1.5 SD or below | 0 | 2 | ||||
Wilcoxon Rank-sum Test
Statistical Analysis
Initially, distributions of neuropsychological outcomes were examined to assess normality and potential outliers using Shapiro-Wilk test and graphical displays. When the tests showed no gross deviations from the assumption of normality, an independent two-sample t-test was used to compare differences in group means. For variables that were not normally distributed, group comparisons were made using a non-parametric Wilcoxon rank-sum test. Similarly, correlation between CTS rates and neuropsychological outcomes were examined using Pearson correlation. Group differences and correlation results for primary outcomes were corrected for multiple comparisons using a false discovery rate approach. For secondary outcomes, exploratory analyses were conducted to examine differences in group means and correlation with CTS rates.
RESULTS
Demographics
Thirty–four (ages 5–12, 17 females) patients with BECTS and 48 healthy controls (ages 5–13, 23 females) participated. BECTS patients did not differ significantly from the control group in age, estimated household income (parent report) or mother’s education level. All participants were native speakers of English with no history of neuropsychological or learning disorders (based on parent report). Two BECTS patients and one control were left-handed; all other participants were right-handed. See Table 1 for summary information. Patients had a history of 1–9 seizures at time of participation, and none were taking anti-epileptic medications; median time from first recognized seizure was two months (Table 2).
Table 1.
Demographic information for BECTS patients and healthy controls.
| n | Gender | Age (years) | Handedness | Median income range | Maternal Education (Associate Degree or above) | ||
|---|---|---|---|---|---|---|---|
| BECTS | 34 | 17 F | mean | 7.94 | 32R, 2L | $50,001 to $75,000 | 21 (62%) |
| SD | 2.15 | ||||||
| Healthy Controls | 48 | 23 F | Mean | 8.08 | 47R, 1L | $50,001 to $75,000 | 35 (73%) |
| SD | 2.21 | ||||||
Group differences in performance
Primary Outcomes
Mean CELF Language scores in children with BECTS were approximately six points lower than the control group, but there was no statistically significant difference observed between the groups. There was also no significant group difference in scores on Beery VMI (p>0.1). Fine motor scores on the Grooved Pegboard Test differed for the non-dominant hand (corrected p=0.032).
Secondary Outcomes
Processing Speed Index scores differed significantly between groups (p<0.005). There was no statistically significant difference observed between children with BECTS and controls in full-scale IQ or verbal or performance subscale scores, nor on either CTOPP subtest See Table 3 for a summary of scores for both groups. The measures that differed between the groups were ones where participants explicitly instructed to perform the task as quickly as possible while still being accurate. Therefore, we explored the possibility that a composite measure based on all the speeded measures (Grooved Pegboard (dominant and non-Dominant hand z-score), WISC processing speed standard score, and CTOPP rapid naming standard score) might best represent the pattern of poorer performance in BECTS. Among the measures, Processing Speed Index was correlated with Grooved Pegboard score for the dominant hand (r=0.51, p<0.0001); the other measures did not show a significant relationship. We used a multivariate factor analysis to explore the group difference in a composite score reflecting the combined effect of the speeded measures. This resulted in a mean score of 0.18 (SD 0.65) in Controls and −0.29 (SD 1.17) BECTS patients. There was a trend toward poorer scores in BECTS patients, p=0.060.
EEG Results
Eight patients showed a preponderance of left-sided spikes (at least 60% left), eighteen patients showed a preponderance of right-sided spikes (at least 60% right), and eight patients showed a bilateral independent distribution with no predominant lateralization (Table 2). Total CTS rates during one hour of wakefulness ranged from 0–21.73/min, during two hours sleep 0.04–77.57/min, were highest during N2 sleep ranging 0=108.30/min, and during N3 sleep ranged from 0–79.07/min. CTS rates and lateralization during both N2 and N3 sleep were highly correlated with the rates during the entire two hours (N2 right and two hours right r=0.81, N2 left and two hours left r=0.89, N3 right and two hours right r=0.93, N3 left and two hours left r=0.94, all p<.0001.)
Relationships with frequency and lateralization of CTS
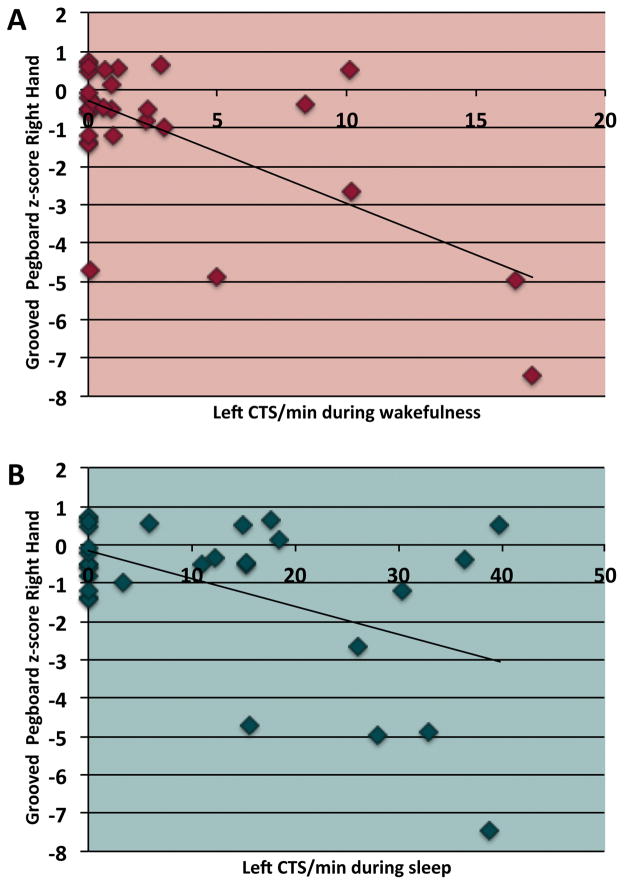
In children with BECTS, we also examined the relationship between rates of left- and right- sided CTS during sleep and wakefulness with primary neuropsychological outcomes. We hypothesized that more frequent CTS would be associated with poorer scores, and that relationships would be dependent on CTS lateralization. Significant negative relationships were observed between the rate of left CTS during wakefulness and sleep and Grooved Pegboard scores for the right hand (wakefulness r= −0.65, p=0.0001; sleep, r=−0.50, p=0.02, corrected). No other significant relationships were observed with the primary or secondary outcomes.
DISCUSSION
We assessed neuropsychological and motor status in 34 children with new-onset BECTS compared to age-matched healthy controls. BECTS patients scored lower than controls on processing speed, and had slower performance on the Grooved Pegboard Test for the non-dominant hand. A composite score based on all the speeded measures (Processing Speed Index, Grooved Pegboard, and CTOPP rapid naming) did not show a significant group difference. In terms of the relationship with CTS patterns, more frequent left CTS were associated with poorer fine motor performance with the right hand. However, there was no relationship between left or right CTS frequency and language or visuospatial performance.
Neuroimaging studies in BECTS have shown functional and structural abnormalities that in some cases are specific to peri-rolandic regions and in others extend beyond these regions. Ciumas et al., using diffusion tensor imaging, showed reduced white matter integrity in peri-rolandic regions ipsilateral to the patients’ centrotemporal spikes 6. During both resting state and task-based functional MRI, connectivity between sensorimotor (Rolandic) regions and language networks has also been found to be altered in BECTS compared to healthy controls 21,22. Another resting-state study found increased regional synchronization in sensorimotor regions in new-onset BECTS patients compared to controls, but found a similar effect in specific frontal, temporal and occipital regions23. Similarly, other studies have found that BECTS patients differ from controls in perisylvian regions in frontal or temporal cortex in both structure 24 and in function as engaged during language tasks 25–27. A longitudinal study of brain structure in BECTS 28 found that over two years of epilepsy, children with BECTS showed only small regions of cortical thinning in isthmus cingulate and frontal cortex, versus control participants, who showed a more typical developmental pattern of widespread cortical thinning in both hemispheres.
In contrast to previous studies, we did not find that patients with BECTS had poorer language scores than controls. However, studies showing differences in language skill3; 4 had more limited numbers of participants, particularly of healthy controls, limiting generalization of the results. Further, as mentioned above, these studies included BECTS patients with a wide range of duration of epilepsy, and included patients on medication. Language problems were less apparent in our sample of untreated, new-onset BECTS patients, and our larger control group may better represent the range of language skill in the typical population. For example, a previous study by Overvliet et al.29 found a mean CELF composite score in healthy controls of 106, while our control participants had a mean score of 101. Other recent studies examining processing speed in BECTS patients and healthy controls5; 6 found mean Processing Speed Index scores in healthy controls of 111 or higher, while we found a mean Processing Speed Index of 103 in our control group. A brief awake EEG recording was used to verify that epileptiform discharges were not present in controls; however, since sleep was not recorded, there is the possibility that some controls had epileptiform activity during sleep. Rather than significant differences in language function, we found that processing speed was most affected in BECTS patients, consistent with previous results,6; 7 though scores were still in the normal range on average. Ten of 31 BECTS patients (32%) who completed the WISC processing speed subtests had scores one standard deviation or greater below the control mean, while only 3 of 47 control participants (6%) had scores in this range.
Interestingly, we found that children with BECTS performed more poorly than controls on the Grooved Pegboard Test for the non-dominant hand. Five of 30 BECTS patients (17%) who completed this test had scores over one standard deviation below the control mean as compared to 2 of 45 control participants (4%). While all but four of these participants had some CTS recorded in the hemisphere contralateral to their non-dominant hand; the frequency of these CTS did not correlate directly with non-dominant hand pegboard scores. In contrast, more frequent left-sided CTS during sleep were associated with poorer right hand (dominant for most patients) pegboard scores. This suggests that the non-dominant hand motor skill is affected by the presence of CTS in general; we speculate that the non-dominant (and therefore less-skilled for most participants) side may be more susceptible to CTS-related disruption. However, the dominant hand is only affected as CTS become more frequent in the left hemisphere. Overall, left-hemisphere CTS were less frequent than right, across our patient group (see Table 2), so this may partially explain the lack of group difference in the dominant hand. This pattern has not previously been described in BECTS and may warrant further investigation.
Overall, our results show that children with BECTS have subtle difficulties in cognitive and fine motor skills, but the extent of affected domains may be more limited than previously suggested, especially in untreated patients early in the course of their epilepsy. Longitudinal neuropsychological and EEG investigation of these patients will allow for a more complete understanding of their neuropsychological outcomes, the relationship with CTS patterns, and risk factors that might indicate a need for intervention.
Figure 1.
Scatterplots of Grooved Pegboard z-scores for the right hand and (A) left CTS rates during one hour of wakefulness and (B) two hours of sleep in BECTS patients.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke, R01-NS065840 (Vannest, PI).
Footnotes
Disclosures
None of the authors has any conflict of interest to disclose (No commercial interests relevant to this research activity).
References
- 1.ILAE. Commission on Classification and Terminology of the International League Against Epilepsy: Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 2.Vannest J, Tenney JR, Gelineau-Morel R, et al. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2015 doi: 10.1016/j.yebeh.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Piccinelli P, Borgatti R, Aldini A, et al. Academic performance in children with rolandic epilepsy. Developmental Medicine & Child Neurology. 2008;50:353–356. doi: 10.1111/j.1469-8749.2007.02040.x. [DOI] [PubMed] [Google Scholar]
- 4.Jurkeviciene G, Endziniene M, Laukiene I, et al. Association of language dysfunction and age of onset of benign epilepsy with centrotemporal spikes in children. Eur J Paediatr Neurol. 2012;16:653–661. doi: 10.1016/j.ejpn.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Kim EH, Yum MS, Kim HW, et al. Attention-deficit/hyperactivity disorder and attention impairment in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2014;37:54–58. doi: 10.1016/j.yebeh.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Ciumas C, Saignavongs M, Ilski F, et al. White matter development in children with benign childhood epilepsy with centro-temporal spikes. Brain. 2014;137:1095–1106. doi: 10.1093/brain/awu039. [DOI] [PubMed] [Google Scholar]
- 7.Ebus SC, DMIJ, den Boer JT, et al. Changes in the frequency of benign focal spikes accompany changes in central information processing speed: a prospective 2-year follow-up study. Epilepsy Behav. 2015;43:8–15. doi: 10.1016/j.yebeh.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Riva D, Vago C, Franceschetti S, et al. Intellectual and language findings and their relationship to EEG characteristics in benign childhood epilepsy with centrotemporal spikes. Epilepsy & Behavior. 2007;10:278–285. doi: 10.1016/j.yebeh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Ramos C, Jackson DC, Lin JJ, et al. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2015;56:1615–1622. doi: 10.1111/epi.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedoin N, Herbillon V, Lamoury I, et al. Hemispheric lateralization of cognitive functions in children with centrotemporal spikes. Epilepsy & Behavior. 2006;9:268–274. doi: 10.1016/j.yebeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Wolff M, Weiskopf N, Serra E, et al. Benign partial epilepsy in childhood: selective cognitive deficits are related to the location of focal spikes determined by combined EEG/MEG. Epilepsia. 2005;46:1661–1667. doi: 10.1111/j.1528-1167.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 12.Ebus SC, Overvliet GM, Arends JB, et al. Reading performance in children with rolandic epilepsy correlates with nocturnal epileptiform activity, but not with epileptiform activity while awake. Epilepsy and Behavior. 2011;22:518–522. doi: 10.1016/j.yebeh.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg S, Frylmark A, Eeg-Olofsson O. Children with rolandic epilepsy have abnormalities of oromotor and dichotic listening performance. Dev Med Child Neurol. 2005;47:603–608. [PubMed] [Google Scholar]
- 14.Overvliet GM, Aldenkamp AP, Klinkenberg S, et al. Correlation between language impairment and problems in motor development in children with rolandic epilepsy. Epilepsy and Behavior. 2011;22:527–531. doi: 10.1016/j.yebeh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals® - Fourth Edition (CELF® - 4) Pearson Education, Inc; San Antonio, TX: 2003. [Google Scholar]
- 16.Beery KE, Buktenica NA. Developmental Test of Visual-Motor Integration. Psychological Assessment Resources; Odessa, FL: 1997. [Google Scholar]
- 17.Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- 18.Wechsler D. The Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) The Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- 19.Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processing - Second Edition. Pro-Ed; Austin, TX: 2009. [Google Scholar]
- 20.Wechsler D. Wechsler Intelligence Scale for Children, Fourth Edition. The Psychological Corporation; San Antonio: 2003. [Google Scholar]
- 21.Besseling RM, Jansen JF, Overvliet GM, et al. Reduced functional integration of the sensorimotor and language network in rolandic epilepsy. Neuroimage Clin. 2013;2:239–246. doi: 10.1016/j.nicl.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besseling RM, Overvliet GM, Jansen JF, et al. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res. 2013;107:253–262. doi: 10.1016/j.eplepsyres.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Zeng H, Ramos CG, Nair VA, et al. Regional homogeneity (ReHo) changes in new onset versus chronic benign epilepsy of childhood with centrotemporal spikes (BECTS): A resting state fMRI study. Epilepsy Res. 2015;116:79–85. doi: 10.1016/j.eplepsyres.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overvliet GM, Besseling RM, Jansen JF, et al. Early onset of cortical thinning in children with rolandic epilepsy. Neuroimage Clin. 2013;2:434–439. doi: 10.1016/j.nicl.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillywhite LM, Saling MM, Simon Harvey A, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- 26.Datta AN, Oser N, Bauder F, et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2013;54:487–494. doi: 10.1111/epi.12067. [DOI] [PubMed] [Google Scholar]
- 27.Vannest J, Szaflarski JP, Eaton KP, et al. Functional magnetic resonance imaging reveals changes in language localization in children with benign childhood epilepsy with centrotemporal spikes. J Child Neurol. 2013;28:435–445. doi: 10.1177/0883073812447682. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Ramos C, Jackson DC, Lin JJ, et al. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2015 doi: 10.1111/epi.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overvliet GM, Besseling RM, van der Kruijs SJ, et al. Clinical evaluation of language fundamentals in Rolandic epilepsy, an assessment with CELF-4. Eur J Paediatr Neurol. 2013;17:390–396. doi: 10.1016/j.ejpn.2013.01.001. [DOI] [PubMed] [Google Scholar]