Abstract
Clinicians managing sports-related concussions are left to their clinical judgment in making diagnoses and return-to-play decisions. This study was designed to evaluate the utility of a novel measure of functional brain networking for concussion management. 24 athletes with acutely diagnosed concussion and 21 control participants were evaluated in a research laboratory. At each of the 4 post-injury time points, participants completed the Axon assessment of neurocognitive function, a self-report symptom inventory, and the auditory oddball and go/no-go tasks while electroencephalogram (EEG) readings were recorded. Brain Network Activation (BNA) scores were calculated from EEG data related to the auditory oddball and go/no-go tasks. BNA scores were unable to differentiate between the concussed and control groups or by self-report symptom severity. These findings conflict with previous work implementing electrophysiological assessments in concussed athletes, suggesting that BNA requires additional investigation and refinement before clinical implementation.
Keywords: Concussion, Brain network activation (BNA), Electrophysiology
Introduction
Over the previous decade, sports-related concussions have become a significant concern among the public and sports medicine professionals. Injury incidence estimates from 2006 suggest that 1.6–3.8 million sports- and recreation-related injuries were occurring annually [32]. Since that time, every state in the United States and the District of Columbia has enacted concussion legislation, resulting in a 75–92 % increase in medical system utilization [21]. Despite increased attention and research on the injury, health care providers continue to struggle with injury identification [41], diagnosis, and post-injury management.
Concussion is described as a functional disturbance of the cerebral tissue [22] following the direct or indirect transmission of force to the head [43]. To aid in the assessment and diagnosis of injury, a number of sports medicine organizations recommend the clinician employ a multifaceted approach to both injury diagnosis and management that includes assessments of self-reported symptoms, motor control (e. g., balance), and neurocognitive functioning [4, 23, 25, 28, 43]. To date however, no single or combined set of measures has the requisite sensitivity to be implemented diagnostically, making the concussion diagnosis a clinical one based on patient history and the physical examination.
The use of symptom, motor control, and neurocognitive assessments are implemented collectively to reduce false negative findings, but their accuracy is largely predicated on the collection of, and comparison to, a valid baseline assessment [17, 48, 54]. Although the value of baseline measures is debated [48], when these data are available, collegiate athletes have been shown to typically return to pre-injury levels on symptoms (day 7), balance (day 3 to 5), and cognitive functioning (day 5 to 7) [39]. Indeed, it is widely accepted that 90 % of concussed young adults will return to pre-injury levels of clinical functioning within 10 days of injury [40], with adolescents taking slightly longer [19, 57, 61].
Restoration of pre-injury levels of functioning, however, may not represent complete metabolic recovery of the cerebral tissue. Indeed, athletes are known to suppress symptom reports as they may be unaware they are related to concussion, or they have a strong desire to return to play prior to recovery [18, 34, 41, 44, 55]. In addition, the media has reported on athletes intentionally performing poorly on baseline assessments [33] to mask postconcussion deficits, and the reliability of both neurocognitive and postural control assessments may be less than optimal for clinical purposes [5, 9, 49, 53]. Most recently, investigations have demonstrated changes in brain functioning in the absence of clinical decline following head impact exposure [1–3, 36, 59], leading some to speculate that true metabolic recovery may extend past the point of clinical recovery on standard concussion assessment tools [38]. To further complicate injury management, athletes may present with concussion-like symptoms that are due to a condition other than concussion [29].
With growing public concern over sports-related concussions and increasing recognition of the limitations of standard concussion assessment tools, the need for sensitive, objective measures has increased. The ElMindA Brain Network Activation (BNA) algorithm is a novel assessment of functional brain connectivity that has been described in detail by Reches et al. (2013) [50]. In short, BNA is a combined analysis derived from electroencephalogram (EEG) readings of the brain activity spectrum across the scalp and over time. Unlike traditional analyses based on EEG or event-related potential (ERP), measurement sites (i. e., electrodes) are collectively analyzed in response to a cognitive demand and are thought to represent the networking capacity of the brain or how distinct regions of the brain interact in response to an event or to complete a specific task. The platform has the potential to inform the medical provider with objective measures of brain functioning during concussion recovery. Previous investigations implementing this technology have reported its ability to differentiate between those with and without attention deficit hyperactivity disorder [56], document brain networking changes that coincided with improved working memory performance with donepezil administration [51], decreased networking with scopolamine administration that coincided with increased response times and suppressed response accuracy [52], differentiate between those with and without post-traumatic migraine following concussion [31], and a case study found BNA declines following concussion relative to pre-injury measures [30]. These findings suggest that BNA technology may have the ability to monitor brain networking changes associated with concussion and be beneficial to clinical concussion management. Therefore, the purpose of this investigation was to compare BNA scores in conjunction with standard symptom reports and neurocognitive assessments in concussed and control athletes.
Methods
A total of 45 athletes (n = 24 concussed) were enrolled in this investigation (20 males, 16.7 ± 2.5 years, 167.9 ± 19.7 cm, 68.9 ± 18.5 kg). Concussed athletes were recruited from a concussion clinic and all concussions were confirmed by a physician prior to enrollment. Control participants matched for gender, sport, age, height, and weight were recruited from local high schools and universities. Following the completion of a demographics form on the first visit, all participants completed the following assessments at 4 time points: EEG/event-related potential (ERP) recordings during auditory oddball and go/no-go tasks, the Axon assessment of neurocognitive function, and a self-report symptom inventory (described below).
Following a clinical care model, concussed participants were evaluated at 4 time points: symptomatic, self-report asymptomatic, return to play (RTP), and one month post-asymptomatic. The symptomatic time point was completed within 10 days of injury if the athlete continued to report symptoms. The asymptomatic time point was defined as within 4 days of the athlete reporting complete concussion symptom resolution. The RTP time point was within 4 days of medical clearance to return to sport, and the one-month time point was at one month post-asymptomatic. Control participants completed the test sessions within one week of their matched injured athlete’s test sessions. Informed consent and/or assent was obtained based on the University of Michigan Institutional Review Board protocol prior to any data collection and ethical standards were maintained throughout the study [26].
BNA analysis
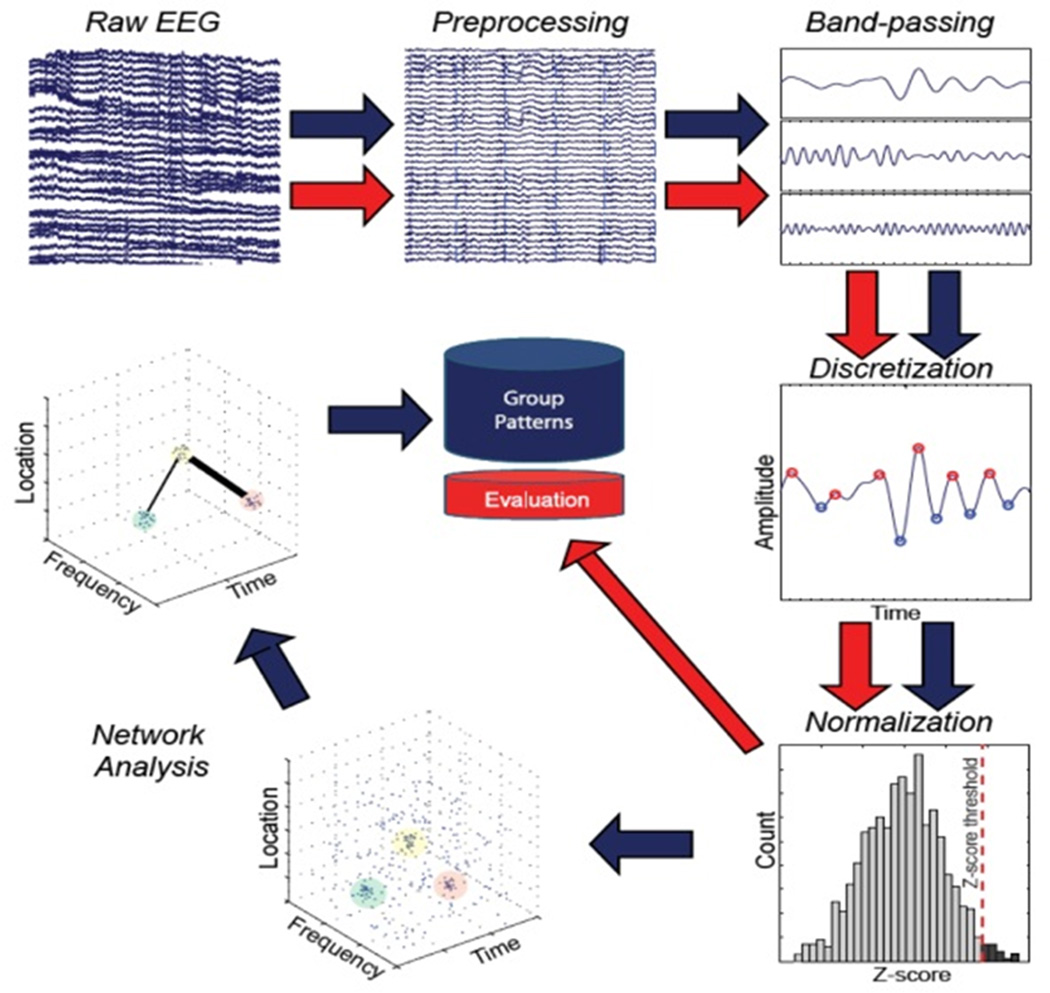
The Brain Network Activation (BNA) algorithm is a proprietary analysis applied to standard ERP data (ElMindA, Ltd., Herzliya, Israel). A sub-component of EEG, ERP, represents the patterns of neuroelectric activation that occur in preparation for, or in response to, an event (typically visual or auditory). In this investigation, data were collected using a 256-channel Ag-AgCl wet lead cap and 300-amp amplifier (Electrical Geodesics, Inc, Eugene, OR). Data were recorded at 256 Hz, bandpass-filtered at 0.1–100 Hz, and stored for BNA analysis at a later date. Eye motion and blinks were removed as previously described [50]. The BNA score was completed in 2 separate stages (Fig. 1): the normative group pattern analysis stage (blue arrows) and the individual subject evaluation stage (red arrows). Separate from this investigation, normative group EEG data were collected and underwent a 5-step analysis: (1) data preprocessing, (2) bandpass filtering, (3) discretization, (4) normalization, and (5) network analysis to form a set of patterns that characterize the group. In this investigation, individual subject evaluation of the raw EEG underwent 5 steps of evaluation: stages 1–4 as performed in the group analysis stage and stage 5 based on a set of patterns collected during the group analysis stage. The individual patterns were then compared to group patterns derived. This process has been described in detail elsewhere [31]. BNA scores range from 0 to 100, and represent the percent similarity between the individual subject’s BNA pattern and the normative group pattern.
Figure 1.
The process by which standard EEG signals are processed to determine brain networks associated with a given task.
Oddball task
During the auditory 3-stimulus oddball task, participants respond as quickly and accurately as possible with a right hand button press only to a randomly occurring, infrequent target stimulus tone while ignoring all other auditory stimuli. Target stimuli were presented as a 1 000-Hz tone that occurred with a 10 % probability. Frequent stimuli were a 2 000-Hz tone occurring with 80 % probability. In addition to the target and frequent stimuli, novel stimuli (e. g., white noise, phone ring, knock on door, etc.) were presented with a 10 % probability. Stimuli were presented using a headset over a total duration of 16 min. A total of 5 BNA scores representing distinct activation networks were calculated: 2 associated with the frequent stimulus (OB-F1, OB-F2), 2 with the target stimulus (OB-T1, OB-T2), and one with the novel stimulus (OB-N).
Go/No-go task
Participants were presented serially with blocks of 200 tones through a headset every 1 000–2 000 ms. Subjects depressed a button whenever a target (i. e., go) tone (2 000 Hz) was presented and inhibited a response to non-target (i. e., no-go) tones (1 000 Hz). Targets and non-targets were presented with probabilities of 80 % and 20 %, respectively. The go/no-go task took 18 min and yielded 2 BNA scores representing unique activation networks associated with the go (GNG-G) and no-go (GNG-N) stimuli.
Axon
The Axon Sports Computerized Cognitive Assessment Tool (CCAT) is a common clinical concussion assessment tool that measures the speed and accuracy of different cognitive processes: processing speed, attention, learning, and working memory. Each 15-min test features 4 tasks based on responses to playing cards presented on the computer monitor. The test has been implemented in a number of previous investigations on sports concussion [12, 13, 58] and has demonstrated high sensitivity (71 %) to cognitive declines associated with concussion [37].
Symptom inventory
The SCAT2 symptom inventory [42] was used to track the presence and severity of concussion-related symptoms at each assessment time point. The symptom inventory asks the athlete about 22 different symptoms that are commonly associated with concussion (e. g., nausea, dizziness, headache, etc.), and grades them on a scale of 0–6 (none to severe) with the values summed for a severity score.
Data analysis
The primary analyses for this investigation implemented group (concussed and control) by time (symptomatic, asymptomatic, RTP, and one month) analyses of variance (ANOVA) with repeated measures for each BNA score, Axon output variable, and the SCAT2 severity scores. Oddball and go/no-go data were unavailable for analysis. Because BNA is an emerging technology for concussion clinical care that may detect ongoing changes in functional brain networking in the absence of overt symptoms, a secondary analysis was completed to evaluate the relationship between SCAT2 symptom severity reports and BNA performance using Pearson correlations. Additionally, the concussed and control athletes were categorized based on symptom severity scores suggested by Lovell et al. [35] [0 (Low-normal), 1–5 (Broadly Normal), 6–12 (Borderline), 13–26 (Very High), > 26 (Extremely High)] and the BNA scores were revaluated using group-by-time ANOVAs. Violations to sphericity were evaluated using Mauchly’s test and corrected with the Greenhouse-Geisser technique as needed. Significance was noted when p < 0.05 and posthoc analyses were corrected using the Bonferroni method. All analyses were performed using SPSS v22.
Results
24 athletes with acutely diagnosed concussion (11 males, 16.3 ± 2.2 years, 165.5 ± 25.1 cm, 71.2 ± 18.5 kg, 1.2 ± 1.1 previously diagnosed concussions (range 0–4), n = 3 with migraine history, n = 1 with anxiety and depression) and 21 control participants (9 males, 17.1 ± 2.9 years, 170.9 ± 9.7 cm, 66.0 ± 18.2 kg, 0.8 ± 1.1 previously diagnosed concussions (range 0–5), n = 1 with migraine history, n = 0 with anxiety and depression) were enrolled. Concussed athletes completed the symptomatic time point assessment within 6.2 ± 2.4 days of injury, followed by the asymptomatic (20.0 ± 46.3 days later; 26.2 ± 43.8 days postinjury), RTP (23.0 ± 31.6 days later; 49.2 + 60.9 days post-injury), and one month assessments (30.3 ± 8.6 days following the RTP visit; 79.5 ± 60.1 days post-injury). Control athletes followed a similar schedule with the asymptomatic visit completed 21.4 ± 46.3 days following the initial visit, followed by the RTP (27.7 ± 33.4 days later) and one month (26.7 ± 11.3 days later) assessments. There were no significant differences between participant group demographics (i. e., age, height, weight, previous concussions, migraine, anxiety/depression) or assessment times (ps > 0.05).
BNA findings
In primary analyses, no significant group or time effects were present for the OB-N, OB-F1, OB-F2, OB-T2, GNG-G, and GNG-N BNA markers (ps > 0.05). A significant time effect (F(3,126) = 5.13, p = 0.002) was present for the OB-T1 marker, with post-hoc analyses indicating significantly higher scores at the symptomatic time point (58.2 ± 34.6) compared to the asymptomatic (45.6 ± 37.2, p = 0.03), RTP (42.0 ± 36.1, p = 0.03), and one month (39.9 ± 39.3, p = 0.01). No significant group effect was present for the OB-T1 marker (p > 0.05). Mean BNA scores by group and assessment point are presented in Fig. 2.
Figure 2.
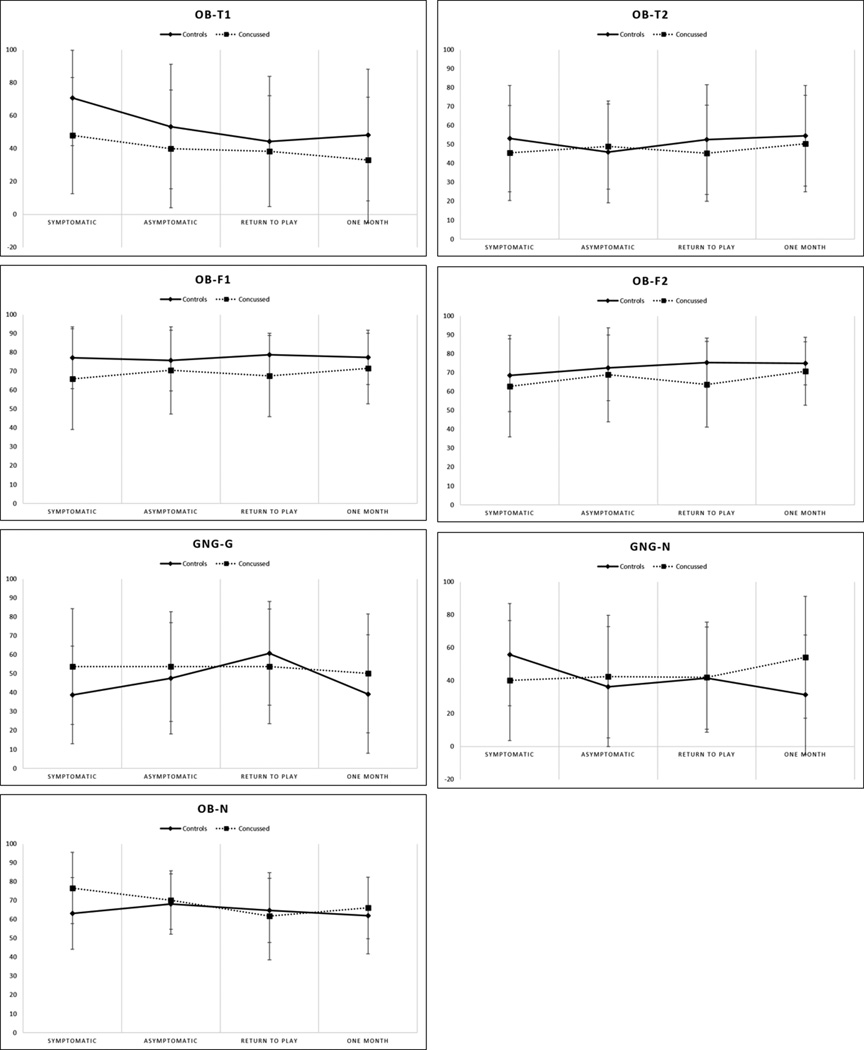
Mean and standard deviation BNA scores presented by assessment point for each network. Dashed lines represent concussed athletes and the solid lines represent controls. No significant differences were noted (p’s>0.05).
Axon findings
Evaluation of the Axon output scores did not reveal significant group or time differences for processing speed (ps > 0.05). Analysis of the attention output score revealed a significant group effect (F(1,43) = 4.88, p = 0.03). Additional attention analysis indicated concussed athlete performed significantly worse when compared to controls at the symptomatic time point (100.1 ± 5.9 vs. 104.5 ± 4.6, p = 0.01), but there were no differences at the asymptomatic (p = 0.15), RTP (p = 0.12), or one-month (p = 0.29) time points. Significant time effects were noted for learning (F(3,129) = 7.40, p < 0.0001) with performance at the symptomatic time point (101.1 ± 10.2) being significantly lower than RTP (106.8 ± 10.1, p < 0.0001) and at one month (109.0 ± 10.8, p < 0.0001). There was no significant group effect (p = 0.35) for learning. Following a Greenhouse-Geisser correction for a sphericity violation (W(5) = 0.13, p < 0.0001), a significant time effect (F(1.38, 59.45) = 3.69, p = 0.05) was noted for the working memory- speed variable. Performance at the symptomatic time point (99.1 ± 6.8) was significantly lower than RTP (102.7 ± 6.2, p < 0.0001) and the one month (103.4 ± 6.1, p < 0.0001). There was no significant difference between the symptomatic and asymptomatic (p = 1.00) time points or a significant group effect (p = 0.22). Significant group effects (F(1,43) = 5.34, p = 0.026) were noted for the working memory – accuracy variable, with control athletes performing better overall than concussed (105.7 ± 8.17 vs. 101.8 ± 10.75, p = 0.03). Additional analyses of the working memory – accuracy scores indicated control athletes performed significantly better than concussed at the symptomatic time point (105.8 ± 7.19 vs. 100.3 ± 9.42, p = .0.35), but there were no significant differences at the asymptomatic (p = 0.10), RTP (p = 0.61), or one-month (p = 0.51) time points. There was no significant time effect for the working memory – accuracy variable (p = 0.11). Mean Axon scores by group and assessment point are presented in Table 1.
Table 1.
Mean (standard deviation) for each Axon score and SCAT2 Symptom Severity Scores for Concussed and Control athletes at each post-injury time point.
| Symptomatic | Asymptomatic | Return to Play | One Month | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Concussed | Control | Concussed | Control | Concussed | Control | Concussed | |
| Processing Speed | 97.4 (11.5) | 97.4 (7.8) | 101.0 (4.3) | 97.2 (17.7) | 100.9 (4.6) | 99.8 (5.2) | 100.4 (4.7) | 100.1 (4.8) |
| Attention | 104.5 (4.6) | 100.1 (5.9)* | 104.5 (5.4) | 99.2 (15.6) | 104.4 (4.6) | 102.3 (4.1) | 104.5 (5.6) | 102.9 (4.3) |
| Learning | 103.2 (8.9) | 99.3 (11.1) | 106.2 (8.1) | 102.0 (20.7) | 108.0 (9.6) | 105.8 (10.6) | 109.4 (9.9) | 108.6 (11.7) |
| Working Memory - Speed | 100.4 (6.4) | 98.0 (7.1) | 102.6 (6.7) | 97.3 (18.2) | 103.7 (6.1) | 101.8 (6.2) | 103.4 (6.8) | 103.3 (5.6) |
| Working Memory - Accuracy | 105.8 (7.2) | 100.3 (9.4)* | 105.1 (8.2) | 97.8 (18.2) | 104.9 (8.6) | 103.7 (7.5) | 107.2 (8.7) | 105.6 (7.8) |
| SCAT2 Symptom Severity | 1.2 (1.5) | 31.4 (18.4)* | 0.5 (0.9) | 3.4 (5.6)* | 0.7 (1.5) | 2.6 (4.7) | 1.0 (2.9) | 0.9 (2.0) |
indicates significantly worse concussed group performance for that time point (p<0.05)
Symptom findings
Analysis of the SCAT2 total symptom severity score revealed significant group (F(1,43) = 36.83, p < 0.0001) and time effects (F(3,129) = 59.15, ps < 0.00). As expected, concussed athletes reported significantly greater symptom severity relative to controls at the symptomatic (31.4 ± 18.4 vs. 1.2 ± 1.5, p < 0.0001) and asymptomatic (3.4 ± 5.6 vs. 0.5 ± 0.9, p = 0.24) time points. Within the concussed athlete group, significantly higher symptom severity was reported at the symptomatic time point (31.4 ± 18.4) compared to the asymptomatic (3.4 ± 5.6, p = 0.004) and RTP (2.6 ± 4.7, p = 0.024) time points. The concussed group also had significantly greater symptoms at the asymptomatic time point (3.4 ± 5.6) compared to the RTP time point (2.6 ± 4.7, p < 0.0001). There were no differences between the RTP or one-month time points. Mean SCAT2 symptom scores by group and assessment point are presented in Table 1.
BNA symptom findings
In the secondary analyses, there were significant negative associations between total symptom severity and OB-F1 (r = − 0.49, p = 0.001) and OB-F2 (r = − 0.40, p = 0.006) BNA scores at the symptomatic time point. In addition, there was a positive relationship between the Axon Working Memory Speed and OB-F1 (r = 0.34, p = 0.02). No other significant correlations were noted between symptom severity or Axon scores and BNA scores at the asymptomatic, RTP, or one-month time points (ps > 0.05).
Secondary ANOVA analyses compared BNA performance when the participants were divided by symptom severity reports at the first assessment point [Low-normal (n = 9), Broadly Normal (n = 13), Borderline (n = 3), Very High (n = 4), Extremely High (n = 14)]. Mean BNA scores by symptom group and assessment time point are presented in Fig. 3. Analysis of the OB-N marker revealed a significant time main effect (F(3, 114) = 3.77, p = 0.01), but no group main effect (p = 0.25). Post-hoc analysis of the time effect indicated a significantly higher OB-N BNA score at the symptomatic time point (70.1 ± 20.2) compared to the return-to-play (62.2 ± 20.4, p = 0.02) and one-month (63.4 ± 17.5, p = 0.01) time points. A significant group × time interaction effect was found for the GNG-N measure (F(12,144) = 1.97, p = 0.033), however the main effects for group (p = 0.56) and time (p = 0.27) were both non-significant, precluding additional analyses. No significant group or time effects were found for OB-F1, OB-F2, OB-T1, OB-T2, or GNG-G (Fig. 3).
Figure 3.
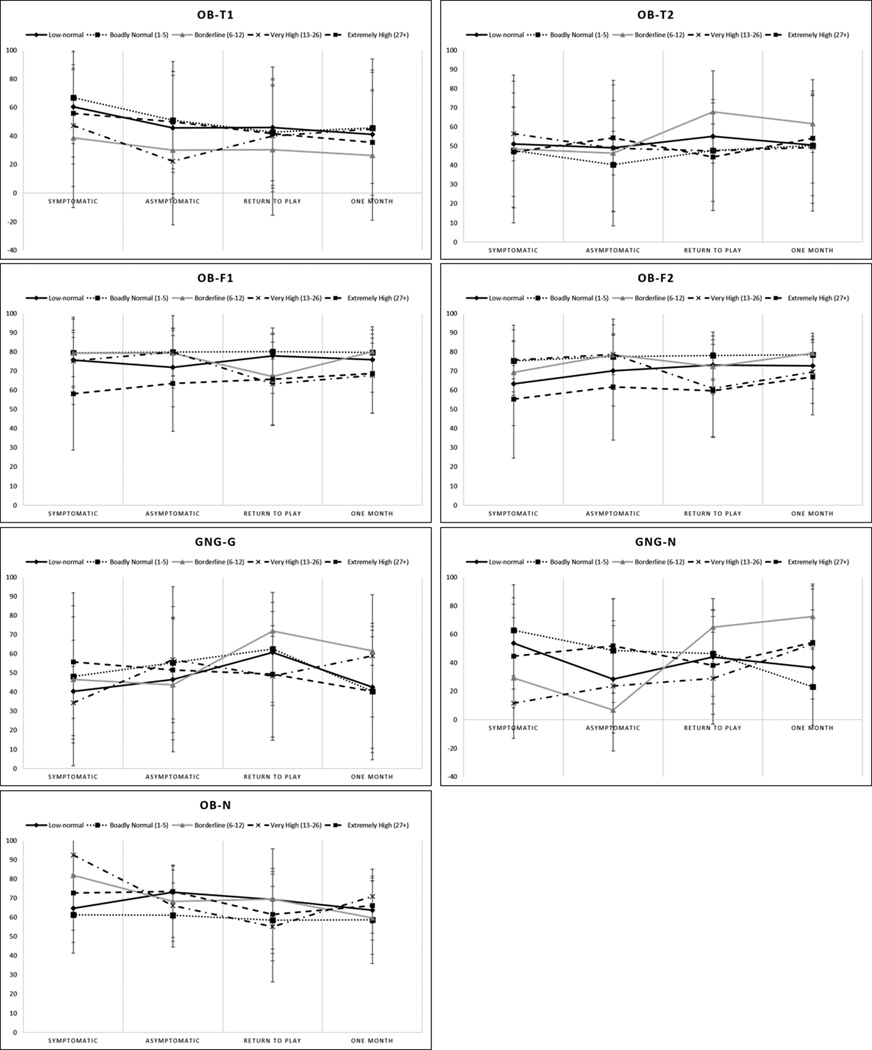
Mean and standard deviation BNA scores presented by assessment point and symptom severity group for each network. Symptom severity was determined by self-report on the SCAT2 symptom list with a report of 0 (Low-normal), 1-5 (Broadly Normal), 6-12 (Borderline), 13-26 (Very High), 4 (Extremely High).
Discussion
This investigation sought to compare BNA scores between concussed and control athletes as a step toward their potential use as a clinical management tool in athletes with sports-related concussion. The BNA scores evaluated in this investigation were unable to differentiate between concussed and control athlete groups (Fig. 2) or those with varying symptom severity reports (Fig. 3). OB-N scores were shown to differ over time, but with the highest scores reported at the symptomatic time point and declining performance moving forward, clinical utility is not implied. The OB-F1 and OB-F2 networks were negatively related to symptom reports, suggesting a link between symptoms and the cognitive networking associated with the oddball task. Interpreting the relationship between symptom reports and oddball performance, however, is not entirely clear. That is, the oddball test has traditionally been implemented as a means to elicit attention and response inhibition [7], which do not explicitly overlay with symptom reports, but the relationship does suggest a common underlying etiology that presents in both measures. Previous investigations implementing advanced neuroimaging and electrophysiological techniques have demonstrated functional brain changes associated with symptom presentation. Most germane to this investigation, Kontos et al. [31] reported on BNA performance among 37 concussed athletes (15 with post-traumatic migraine (PTM) and 22 without) and 20 control subjects up to one month post-injury. Using a go/no-go task, those with PTM were found to have significantly lower BNA scores relative to both the concussed non-PTM and control groups on the go and no-go components 3 weeks post-injury, as well as the no-go component at week 4. The weeks 3 and 4 assessments also demonstrated impaired performance by the PTM group on the ImPACT visual memory, visual processing speed, reaction-time components, and symptom reports relative to the control group. Traditional ERP measures have also been implemented to evaluate concussion-related symptoms. Indeed, Dupuis et al. [16] found that concussed individuals who remained symptomatic 1.7 months post-injury had a smaller ERP amplitude relative to a non-symptomatic concussed group at 9.8 months post-injury and a non-concussed control group. Similarly, ERP latency has been shown to increase in those with concussion reporting symptoms of ‘memory problems’ and ‘taking longer to think’ [20].
Concussion-related symptoms were also evaluated by Chen et al. [10], who reported on a group of 18 concussed (mean 5 months post-injury, divided by low and moderate symptom score) and 10 control athletes who completed the CogSport assessment and functional MRI (fMRI) during verbal and non-verbal working memory tasks. The low symptom group performed equivalently to the controls on the CogSport battery, whereas the moderate symptom group demonstrated impaired performance relative to the other groups. The moderate symptom group also performed worse than the low symptom group on the verbal and non-verbal working memory task. fMRI findings indicated decreased activation among the low and moderate symptom groups during the verbal and non-verbal working memory task. In another investigation by Chen et al. [11], 15 concussed athletes (mean 4.8 months post-injury) with on-going concussion-related symptoms and 8 control participants were evaluated on a working memory task while fMRI was completed. The 2 groups performed equally on the working memory task, but the symptomatic concussed athletes displayed lower BOLD activity in the mid-dorsolateral prefrontal cortex, the area associated with working memory information monitoring. These results suggest the potential for cerebral-level physiological changes that underlie post-concussion symptoms to be indexed by advanced neuroimaging and electrophysiological techniques. It is unclear then why similar results were not seen here, suggesting that the clinical application of BNA hinges on additional investigation and refinement. In the interim, because athletes displaying evidence of metabolic and electrophysiological alterations also reported concussion-related symptoms, the more prudent clinical measure is the continued implementation of a symptom checklist and other commonly implemented clinical assessments.
Should the BNA be refined and able to differentiate between those with and without a concussion shortly following injury, particularly when an athlete denies any concussion-related symptoms, its use as a return-to-play decision-making tool will need to be carefully interpreted. As BNA is a measure of cerebral electrophysiology, it is unclear if scores should ever be expected to entirely return to pre-injury levels. A number of investigations have shown persistent deficits (i. e., decreased amplitude and/or increased latency) to various ERP components years after injury. For example, alterations in the P3b component have been documented following concussion at 5 to 15 weeks [16, 24], 20 to 59 months [20], and 31 to 56 months [14]. Similar findings related to persistent changes in the P3a component have been reported in previously concussed participants 22 to 60 months [60], 3.4 years [8], and 26 years [15] post-injury. To a lesser extent, suppressed Ne has been shown at 2.9 years [47], suppressed P1 amplitude at 6.7 years [45], and an altered N2 component at 7.1 years post-injury [46]. While these findings were generated in response to the target stimuli during a visual task, compared to the auditory task implemented here, it is possible that ongoing deficits may need to be accounted for by clinicians when interpreting post-concussion results.
As with all investigations, this study is not without its limitations. Perhaps most notable is the protracted recovery period by our concussion cohort. Indeed, typical concussion recovery is estimated at less than 14 days [39], yet our sample did not report being asymptomatic until day 26. How this may influence BNA performance is not known, but the longer-than-expected recovery period may be interpreted as a more severe injury reported in other clinic based studies [27]. In addition, while individualized BNA scores are compared to the normative dataset, there is research benefit in collecting pre-morbid information for direct comparison in the post-injury state.
Although higher symptom severity scores were associated with worsening OB-F1 and OB-F2 networks, this investigation failed to show the capability of the BNA networks to differentiate between concussed and non-concussed athletes or between those with varying levels of self-report symptoms. While the BNA technology has strong underpinnings in electrophysiological science, its use in the clinical management of concussed athletes is not prudent. Indeed, the multi-faceted concussion assessment battery has been shown to have a high level of sensitivity to concussion in a prior investigation of 94 concussed collegiate football athletes where the individual sensitivities of a symptom scale (89 %), balance assessment (34 %), and Standardized Assessment of Concussion (SAC) (80 %) were found to increase to 94 % when considered in combination at the time of injury [37]. Another investigation of 75 collegiate athletes reported similar individual and combined sensitivities within 24 h of injury [symptoms scale (68 %), computerized postural control (62 %), pen-and-paper or computerized neurocognitive assessments (43 to 78 %), combined sensitivity (89–96 % )] [6]. At this time, these standard clinical assessments are more cost-effective than advanced measures, but lose sensitivity as the athlete recovers [37]. It is conceivable therefore, that as the technology improves, more advanced measures may add information to the injury management process and better inform the return-to-play decision when signal detection of standard clinical tests diminishes. Indeed, additional BNA networks have been identified and are commercially available since the inception of this investigation that may provide additional information to the injury management process. Thus to better clarify this issue, investigations collecting pre-injury levels of BNA performance and functioning on clinical and structural measures (e. g., DTI) and protracted post-injury assessments should be conducted.
Acknowledgments
The authors would like to thank Max Zeiger, Samantha Zetlin, Susan Brimbcombe, Richelle Williams MS, ATC and Jeffery Kutcher MD for their assistance with this investigation. The study was funded by ElMindA, Ltd.
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
References
- 1.Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging. 2011;30:171–180. doi: 10.1016/j.mri.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazarian JJ, Zhu T, Zhong J, Janigro D, Rozen E, Roberts A, Javien H, Merchant-Borna K, Abar B, Blackman EG. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS One. 2014;9:e94734. doi: 10.1371/journal.pone.0094734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breedlove EL, Robinson M, Talavage TM, Morigaki KE, Yoruk U, O'Keefe K, King J, Leverenz LJ, Gilger JW, Nauman EA. Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J Biomech. 2012;45:1265–1272. doi: 10.1016/j.jbiomech.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Broglio SP, Cantu RC, Gioia GA, Guskiewicz KM, Kutcher JS, Palm M, Valovich-Mcleod T. National Athletic Trainers’ Association position statement: management of sport concussion. J Athl Train. 2014;49:245–265. doi: 10.4085/1062-6050-49.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broglio SP, Ferrara MS, Macciocchi SN, Baumgartner TA, Elliott R. Testretest reliability of computerized concussion assessment programs. J Athl Train. 2007;42:509–514. [PMC free article] [PubMed] [Google Scholar]
- 6.Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050–1057. doi: 10.1227/01.NEU.0000255479.90999.C0. [DOI] [PubMed] [Google Scholar]
- 7.Broglio SP, Moore RD, Hillman CH. A history of sport-related concussion on event-related brain potential correlates of cognition. Int J Psychophysiol. 2011;82:16–23. doi: 10.1016/j.ijpsycho.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Broglio SP, Pontifex MB, O’Connor P, Hillman CH. The persistent effects of concussion on neuroelectic indices of attention. J Neurotrauma. 2009;26:1463–1470. doi: 10.1089/neu.2008.0766. [DOI] [PubMed] [Google Scholar]
- 9.Broglio SP, Zhu W, Sopiarz K, Park Y. Generalizability theory analysis of balance error scoring system reliability in healthy young adults. J Athl Train. 2009;44:497–502. doi: 10.4085/1062-6050-44.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JK, Johnston KM, Collie A, McCrory P, Ptito A. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurol Neurosurg Psychiatry. 2007;78:1231–1238. doi: 10.1136/jnnp.2006.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Collie A, Makdissi M, Maruff P, Bennell KL, McCrory P. Cognition in the days following concussion: comparison of symptomatic versus asymptomatic athletes. J Neurol Neurosurg Psychiatry. 2006;77:241–245. doi: 10.1136/jnnp.2005.073155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collie A, Maruff P, Makdissi M, McCrory P, McStephen M, Darby D. Cog- Sport: Reliability and correlation with conventional cognitive tests used in postconcussion medical evaluations. Clin J Sport Med. 2003;13:28–32. doi: 10.1097/00042752-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 14.De Beaumont L, Brisson B, Lassonde M, Jolicoeur P. Long-term electrophysiological changes in athletes with a history of multiple concussions. Brain Inj. 2007;21:631–644. doi: 10.1080/02699050701426931. [DOI] [PubMed] [Google Scholar]
- 15.De Beaumont L, Theoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, Ellemberg D, Lassonde M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- 16.Dupuis F, Johnston KM, Lavoie M, Lepore F, Lassonde M. Concussion in athletes produce brain dysfunction as revealed by event-related potentials. Neuroreport. 2000;11:4087–4092. doi: 10.1097/00001756-200012180-00035. [DOI] [PubMed] [Google Scholar]
- 17.Echemendia RJ, Bruce JM, Bailey CM, Sanders JF, Arnett P, Vargas G. The utility of post-concussion neuropsychological data in identifying cognitive change following sports-related MTBI in the absence of baseline data. Clin Neuropsychol. 2012;26:1077–1091. doi: 10.1080/13854046.2012.721006. [DOI] [PubMed] [Google Scholar]
- 18.Fedor A, Gunstad J. Limited knowledge of concussion symptoms in college athletes. Appl Neuropsychol Adult. 2015;22:108–113. doi: 10.1080/23279095.2013.860604. [DOI] [PubMed] [Google Scholar]
- 19.Field M, Collins MW, Lovell MR, Maroon JC. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142:546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- 20.Gaetz M, Weinberg H. Electrophysiological indices of persistent postconcussion symptoms. Brain Inj. 2000;14:815–832. doi: 10.1080/026990500421921. [DOI] [PubMed] [Google Scholar]
- 21.Gibson TB, Herring SA, Kutcher JS, Broglio SP. Analyzing the effect of state legislation on health care utilization for children with concussion. JAMA Pediatr. 2015;169:163–168. doi: 10.1001/jamapediatrics.2014.2320. [DOI] [PubMed] [Google Scholar]
- 22.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl 4):S24–S33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, Gronseth GS, Guskiewicz KM, Mandel S, Manley G, McKeag DB, Thurman DJ, Zafonte R. Summary of evidence-based guideline update: Evaluation and management of concussion in sports: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250–2257. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gosselin N, Theriault M, Leclerc S, Montplaisir J, Lassonde M. Neurophysiological anomalies in symptomatic and asymptomatic concussed athletes. Neurosurgery. 2006;58:1151–1161. doi: 10.1227/01.NEU.0000215953.44097.FA. [DOI] [PubMed] [Google Scholar]
- 25.Harmon KG, Drezner J, Gammons M, Guskiewicz KM, Halstead M, Herring SA, Kutcher JS, Pana A, Putukian M, Roberts W. American Medical Society for Sports Medicine. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;23:1–18. doi: 10.1097/JSM.0b013e31827f5f93. [DOI] [PubMed] [Google Scholar]
- 26.Harriss DJ, Atkinson G. Ethical Standards in Sport and Exercise Science Research: 2016 Update. Int J Sports Med. 2015;36:1121–1124. doi: 10.1055/s-0035-1565186. [DOI] [PubMed] [Google Scholar]
- 27.Henry LC, Elbin RJ, Collins MW, Marchetti G, Kontos AP. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78:232–241. doi: 10.1227/NEU.0000000000001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herring SA, Cantu RC, Guskiewicz KM, Putukian M, Kibler WB, Bergfeld JA, Boyajian-O’Neill LA, Franks RR, Indelicato PA. American College of Sports Medicine. Concussion (mild traumatic brain injury) and the team physician: a consensus statement – 2011 update. Med Sci Sports Exerc. 2011;43:2412–2422. doi: 10.1249/MSS.0b013e3182342e64. [DOI] [PubMed] [Google Scholar]
- 29.Iverson GL, Lange RT. Examination of “postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol. 2003;10:137–144. doi: 10.1207/S15324826AN1003_02. [DOI] [PubMed] [Google Scholar]
- 30.Kiefer AW, Barber Foss K, Reches A, Gadd B, Gordon M, Rushford K, Laufer I, Weiss M, Myer GD. Brain network activation as a novel biomarker for the return-to-play pathway following sport-related brain injury. Front Neurol. 2015;6:243. doi: 10.3389/fneur.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kontos AP, Reches A, Elbin RJ, Dickman D, Laufer I, Geva AB, Shacham G, DeWolf R, Collins MW. Preliminary evidence of reduced brain network activation in patients with post-traumatic migraine following concussion. Brain Imaging Behav. doi: 10.1007/s11682-015-9412-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Leahy S. USA Today. Peyton Manning admits to tanking NFL’s baseline concussion test. 4/27/2011 ed. [Google Scholar]
- 34.Llewellyn T, Burdette GT, Joyner AB, Buckley TA. Concussion reporting rates at the conclusion of an intercollegiate athletic career. Clin J Sport Med. 2014;24:76–79. doi: 10.1097/01.jsm.0000432853.77520.3d. [DOI] [PubMed] [Google Scholar]
- 35.Lovell MR, Iverson GL, Collins MW, Podell K, Johnston KM, Pardini JE, Pardini JE, Norwig J, Maroon JC. Measurement of symptoms following sports-related concussion: reliability and normative data for the postconcussion scale. Appl Neuropsychol. 2006;13:166–174. doi: 10.1207/s15324826an1303_4. [DOI] [PubMed] [Google Scholar]
- 36.McAllister TW, Ford JC, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, Bolander RP, Tosteson TD, Turco JH, Raman R, Jain S. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82:63–69. doi: 10.1212/01.wnl.0000438220.16190.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCrea M, Barr WB, Guskiewicz KM, Randolph C, Marshall SW, Cantu R, Onate JA, Kelly JP. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11:58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 38.McCrea M, Broshek DK, Barth JT. Sports concussion assessment and management: future research directions. Brain Inj. 2015;29:276–282. doi: 10.3109/02699052.2014.965216. [DOI] [PubMed] [Google Scholar]
- 39.McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 40.McCrea M, Guskiewicz KM, Randolph C, Barr WB, Hammeke TA, Marshall SW, Powell MR, Woo AK, Wang Y, Kelly JP. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J Int Neuropsychol Soc. 2013;19:22–33. doi: 10.1017/S1355617712000872. [DOI] [PubMed] [Google Scholar]
- 41.McCrea M, Hammeke T, Olsen G, Leo P, Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14:13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 42.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med. 2009;43:i76–i90. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- 43.McCrory P, Meeuwisse WH, Aubry M, Cantu RC, Dvorak J, Echemendia RJ, Engebretsen L, Johnston K, Kutcher JS, Raftery M, Sills A, Benson BW, Davis GA, Ellenbogen RG, Guskiewicz KM, Herring SA, Iverson GL, Jordan BD, Kissick J, McCrea M, McIntosh AS, Maddocks D, Makdissi M, Purcell L, Putukian M, Schneider K, Tator CH, Turner M. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 44.Meier TB, Brummel BJ, Singh R, Nerio CJ, Polanski DW, Bellgowan PS. The underreporting of self-reported symptoms following sports-related concussion. J Sci Med Sport. 2015;18:507–511. doi: 10.1016/j.jsams.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Moore RD, Broglio SP, Hillman CH. Sport-related concussion and sensory function in young adults. J Athl Train. 2014;49:36–41. doi: 10.4085/1062-6050-49.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore RD, Hillman CH, Broglio SP. The persistent influence of concussive injuries on cognitive control and neuroelectric function. J Athl Train. 2014;49:24–35. doi: 10.4085/1062-6050-49.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pontifex MB, O’Connor PM, Broglio SP, Hillman CH. The association between mild traumatic brain injury history and cognitive control. Neuropsychologia. 2009;47:3210–3216. doi: 10.1016/j.neuropsychologia.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 48.Randolph C. Baseline neuropsychological testing in managing sportrelated concussion: does it modify risk? Curr Sports Med Rep. 2011;10:21–26. doi: 10.1249/JSR.0b013e318207831d. [DOI] [PubMed] [Google Scholar]
- 49.Randolph C, McCrea M, Barr WB. Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train. 2005;40:139–154. [PMC free article] [PubMed] [Google Scholar]
- 50.Reches A, Kerem D, Gal N, Laufer I, Shani-Hershkovitch R, Dickman D, Geva A. A novel ERP pattern analysis method for revealing invariant reference brain network models. Funct Neurol Rehabil Ergon. 2013;3:295–317. [Google Scholar]
- 51.Reches A, Laufer I, Ziv K, Cukierman G, McEvoy K, Ettinger M, Knight RT, Gazzaley A, Geva AB. Network dynamics predict improvement in working memory performance following donepezil administration in healthy young adults. Neuroimage. 2013;88C:228–241. doi: 10.1016/j.neuroimage.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reches A, Levy-Cooperman N, Laufer I, Shani-Hershkovitch R, Ziv K, Kerem D, Gal N, Stern Y, Cukierman G, Romach MK, Sellers EM, Geva AB. Brain Network Activation (BNA) reveals scopolamine-induced impairment of visual working memory. J Mol Neurosci. 2014;54:59–70. doi: 10.1007/s12031-014-0250-6. [DOI] [PubMed] [Google Scholar]
- 53.Resch J, Driscoll A, McCaffrey N, Brown C, Ferrara MS, Macciocchi S, Baumgartner T, Walpert K. ImPact test-retest reliability: reliably unreliable? J Athl Train. 2013;48:506–511. doi: 10.4085/1062-6050-48.3.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt JD, Register-Mihalik JK, Mihalik JP, Kerr ZY, Guskiewicz KM. Identifying impairments after concussion: normative data versus individualized baselines. Med Sci Sports Exerc. 2012;44:1621–1628. doi: 10.1249/MSS.0b013e318258a9fb. [DOI] [PubMed] [Google Scholar]
- 55.Sefton JM, Pirog K, Capitao A, Harackiewicz D, Cordova ML. An examination of factors that influence knowledge and reporting of mild brain injuries in collegiate football. J Athl Train. 2004;39:S52–S53. [Google Scholar]
- 56.Shahaf G, Reches A, Pinchuk N, Fisher T, Ben Bashat G, Kanter A, Tauber I, Kerem D, Laufer I, Aharon-Peretz J, Pratt H, Geva AB. Introducing a novel approach of network oriented analysis of ERPs, demonstrated on adult attention deficit hyperactivity disorder. Clin Neurophysiol. 2012;123:1568–1580. doi: 10.1016/j.clinph.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Sim A, Terrberry-Spohr L, Wilson KR. Prolonged recovery of memory functioning after mild traumatic brain injury in adolescent athletes. J Neurosurg. 2008;108:511–516. doi: 10.3171/JNS/2008/108/3/0511. [DOI] [PubMed] [Google Scholar]
- 58.Straume-Naesheim TM, Andersen TE, Bahr R. Reproducibility of computer based neuropsychological testing among Norwegian elite football players. Br J Sports Med. 2005;39:i64–i69. doi: 10.1136/bjsm.2005.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talavage TM, Nauman EA, Breedlove EL, Yoruk U, Dye AE, Morigaki KE, Feuer H, Leverenz LJ. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J Neurotrauma. 2014;31:327–338. doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theriault M, De Beaumont L, Gosselin N, Filipinni M, Lassonde M. Electrophysiological abnormalities in well functioning multiple concussed athletes. Brain Inj. 2009;23:899–906. doi: 10.1080/02699050903283189. [DOI] [PubMed] [Google Scholar]
- 61.Williams RM, Puetz TW, Giza CC, Broglio SP. Concussion recovery time among high school and collegiate athletes: a systematic review and meta-analysis. Sports Med. 2015;45:893–903. doi: 10.1007/s40279-015-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]