Abstract
With over 1 million people living with HIV, the US faces national challenges in HIV care delivery due to an inadequate HIV specialist workforce and the increasing role of non-communicable chronic diseases in driving morbidity and mortality in HIV-infected patients. Alternative HIV care delivery models, which include substantial roles for advanced practitioners and/or coordination between specialty and primary care settings in managing HIV-infected patients, may address these needs. We aimed to systematically review the evidence on patient-level HIV-specific and primary care health outcomes for HIV-infected adults receiving outpatient care across HIV care delivery models. We identified randomized trials and observational studies from bibliographic and other databases through March 2016. Eligible studies met pre-specified eligibility criteria including on care delivery models and patient-level health outcomes. We considered all available evidence, including non-experimental studies, and evaluated studies for risk of bias. We identified 3605 studies, of which 13 met eligibility criteria. Of the 13 eligible studies, the majority evaluated specialty-based care (9 studies). Across all studies and care delivery models, eligible studies primarily reported mortality and antiretroviral use, with specialty-based care associated with mortality reductions at the clinician and practice levels and with increased antiretroviral initiation or use at the clinician level but not the practice level. Limited and heterogeneous outcomes were reported for other patient-level HIV-specific outcomes (e.g., viral suppression) as well as for primary care health outcomes across all care delivery models. No studies addressed chronic care outcomes related to aging. Limited evidence was available across geographic settings and key populations. As redesign of care delivery in the US continues to evolve, better understanding of patient-level HIV-related and primary care health outcomes, especially across different staffing models and among different patient populations and geographic locations, is urgently needed to improve HIV disease management.
Keywords: HIV/AIDS, health care delivery, specialty care, primary care, primary care redesign, nursing, telemedicine, systematic review
BACKGROUND
Combination antiretroviral therapy (ART) has dramatically improved life expectancy for persons with HIV (Antiretroviral Therapy Cohort Collaboration, 2008). However, among the 1.2 million people living with HIV in the United States (US), nearly two-thirds are unengaged in HIV care and fewer than one-third are virologically suppressed (Bradley et al., 2014). Care engagement initiatives (Obama, 2013), treatment advances (Antiretroviral Therapy Cohort Collaboration, 2008), and stable new HIV infections annually (Centers for Disease Control and Prevention (CDC), 2012) increase demand for HIV care. Moreover, as ART evolves and patients age (CDC, 2014), chronic disease increases care complexity (Deeks, Lewin, & Havlir, 2013). On the supply side, professional organizations report declining HIV specialist clinicians, difficulties in specialist recruitment and retention, and increasing HIV patient caseloads (Carmichael et al., 2009). These trends occur amidst national primary care clinician shortages.
In response, a spectrum of HIV care delivery models has been suggested (CDC & Health Resources and Services Administration (HRSA), 2011; HRSA, 2010; Institute of Medicine (IOM), 2011). These recommendations are timely, given the re-design of care delivery toward multidisciplinary team-based care across primary and specialty settings (Affordable Care Act (ACA), 2010). In this context, we systematically reviewed and synthesized evidence on patient-level health outcomes associated with different service delivery models for adult outpatient HIV care in the US.
METHODS
The protocol for conducting this systematic review was developed and registered with PROSPERO (Chang & Slutsky, 2012) (PROSPERO number CRD42013005096 at http://www.crd.york.ac.uk/prospero/). The review was conducted according to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines (Table S1) (Moher, Liberati, Tetzlaff, Altman, & Group, 2009). Detailed information on our methodologic approach is in the supplementary content.
Care delivery model definitions
We considered four delivery models (Box 1) for HIV treatment and care in the US, based on current and historical practice patterns in both US and international settings (Auerbach et al., 2013; Institute of Medicine (IOM), 2011; World Health Organization (WHO), 2014). We defined specialty-based care as HIV care delivered by physicians who primarily care for HIV-infected patients, making no assumptions regarding degree (e.g., Medicinae Doctor (MD), Doctor of Osteopathy (DO)) or specialty (infectious diseases, internal medicine, family medicine). We assumed they managed most of their patients’ HIV, chronic disease, wellness, and acute care needs. We defined advanced practitioner-based care as nurse practitioners and physician assistants who primarily care for HIV-infected patients. Similar to specialty-based care, advanced practitioners provide comprehensive HIV- and non-HIV care, with support from specialists as needed, thus expanding the workforce. Team-based care is the comprehensive, patient-centered management of HIV-infected patients by a multidisciplinary team, including HIV specialists, primary care clinicians, advanced practitioners, case managers, social workers, and others. Teams are co-located, as in Ryan White Part C-funded practices (Saag, 2009). We defined shared care as care co-management by HIV specialists, primary care clinicians, and others, similar to team-based care. However, we assumed different team members were located in different settings, with systems in place for communication and care coordination. While we considered each care delivery model distinctly, we acknowledge the fluid nature of care delivery models and their overlap in practice, as well as the challenge of assigning a single care delivery model definition to studies examining physician or practice experience. For the latter, we assumed studies examining greater physician experience or expertise reflected specialty-based care, unless it was reported explicitly that the sample population (i.e., the physicians) worked in a team-based or shared care setting.
Box 1. Definitions of HIV care delivery models.
| Care delivery model | Definition |
|---|---|
| Specialty-based care | HIV care delivered by physicians (e.g., MD, DO), regardless of specialty, who care primarily for HIV-infected patients and manage the majority of patients’ HIV, chronic disease, wellness, and acute care needs. |
| Advanced practitioner based care | Nurse practitioners and physician assistants who care primarily for HIV-infected patients and who provide comprehensive HIV- and non- HIV care to HIV-infected patients. |
| Team-based care | Comprehensive, patient-centered management of HIV-infected patients by a multidisciplinary team that can include HIV specialists, primary care clinicians, advanced practitioners, case managers, behavioral health providers, social workers, and others and that are generally co-located. |
| Shared care | Co-management of HIV care by HIV specialists, primary care clinicians, and others, but with team members located in different settings and systems in place for communication and coordination of care. |
RESULTS
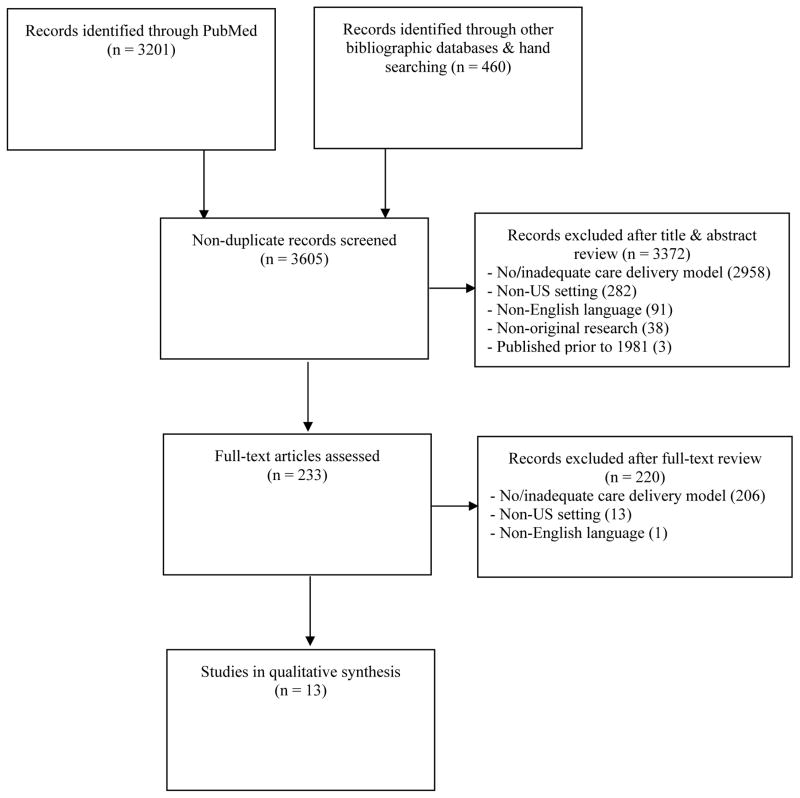
Our literature search resulted in 3,661 records screened, of which 3,641 were identified from databases, 20 from manual searches, and 56 were duplicates. After initial screening, 233 studies met inclusion criteria, thus excluding 3,372 studies; in a second round of review, we eliminated an additional 220 from further consideration. The most common exclusion criterion was lack of care delivery model studied or described (3,164). Thirteen studies were fully evaluated (Figure 1) (Aiken et al., 1993; Chu, Umanski, Blank, Grossberg, & Selwyn, 2010; Ding et al., 2008; Gardner et al., 2002; Irvine et al., 2015; Keitz, Box, Homan, Bartlett, & Oddone, 2001; Kitahata et al., 1996; Kitahata et al., 2003; Laine et al., 1998; Landon et al., 2003; Landon et al., 2005; Lê, Winter, Boyd, Ackerson, & Hurley, 1998; Young et al., 2014).
Figure 1.
Flow diagram of the study selection process
Care delivery models
Of 13 included studies, 9 evaluated specialty-based care (Table 1). Of these, 7 examined only specialty-based care at the clinician (Keitz et al., 2001; Kitahata et al., 1996; Kitahata et al., 2003; Landon et al., 2003; Landon et al., 2005) and/or practice levels (Gardner et al., 2002; Keitz et al., 2001; Laine et al., 1998; Landon et al., 2005), while 2 focused on specialty-based care and either advanced practitioner care (Ding et al., 2008) or shared care (Chu et al., 2010). One study each evaluated only advanced practitioner care (Aiken et al., 1993), team-based care (Irvine et al., 2015), and shared care (Young et al., 2014), while another assessed both team-based and shared care (Lê et al., 1998).
Table 1.
Characteristics of studies on US HIV care delivery models
| Study* | Care delivery model(s) (level assessed) | Treatment era† | Target population | Setting | Design | Intervention | Control |
|---|---|---|---|---|---|---|---|
| Aiken 1993 | Advanced practitioner (clinician) | Monotherapy | HIV patients having ≥1 practice visit in prior year | Outpatient HIV practice at university hospital | Cross-sectional survey | Care provided by nurse practitioner | Care provided by medical doctor (MD) |
| Kitahata 1996 | Specialty (clinician) | Monotherapy, combination therapy | HIV patients with AIDS as defined by CDC’s 1987 case surveillance definition | Nonprofit health care system | Retrospective cohort study | Primary care physicians trained in internal medicine, family medicine, or general practice with moderate or most HIV care experience | Primary care physicians trained in internal medicine, family medicine, or general practice with least HIV care experience |
| Laine 1998 | Specialty (practice) | Monotherapy, combination therapy | Female AIDS patients receiving hospital outpatient care or care from independent diagnostic and treatment center providing services under a physician | New York State practices delivering longitudinal medical care | Retrospective cohort study | Patients seen in high experience practices (i.e., >100 cumulative Medicaid patients with AIDS) and moderate experience practices (i.e., 20–99 cumulative Medicaid patients with AIDS) | Patients in low experience practices (i.e., <20 cumulative Medicaid patients with advanced HIV disease) |
| Keitz 2001 | Specialty (practice, clinician) | Combination therapy, potent combination therapy | HIV patients who are uninsured, self-pay, or receive public insurance (Medicaid or Medicare) | University hospital | Randomized controlled trial | HIV patient care in a general medicine practice with internal medicine residents and non-infectious disease attending physicians, with physician education component | HIV patient care in an infectious diseases practice staffed by residents and infectious diseases fellows and attending physicians |
| Gardner 2002 | Specialty (practice) | Potent combination therapy | HIV-infected patients without AIDS | Medical settings (Bronx & Maryland) | Cross-sectional analysis of HIV Epidemiology Research Study cohort | HIV care received from a practice specializing in HIV care | HIV care received from a practice not specializing in HIV care |
| Kitahata 2003 | Specialty (clinician) | Monotherapy, combination therapy, potent combination therapy | HIV-infected individuals with AIDS as defined by CDC’s 1987 case surveillance definition | Nonprofit health care system | Retrospective cohort study | Primary care physicians trained in internal medicine, family medicine, or general practice with moderate or most experience in HIV care | Primary care physicians trained in internal medicine, family medicine, or general practice with least experience in HIV care |
| Landon 2003 | Specialty (clinician) | Combination therapy, potent combination therapy | Clinicians serving a national random sample of HIV- infected patients | Not reported | Observational cohort from HIV Cost and Services Utilization Study | Infectious disease HIV specialist, primary care HIV specialist | Non-HIV-specialist primary care clinician |
| Landon 2005 | Specialty (practice, clinician) | Potent combination therapy | HIV-infected patients ≥18 years at Ryan White CARE Act-funded practices with >100 HIV patients | Ryan White CARE Act– funded practices | Cross-sectional analysis | Care from infectious disease specialists and HIV specialist primary care clinicians at an HIV specialty practice | Care from non-HIV- specialist primary care clinician at a non-HIV-specialist primary care practice |
| Irvine 2015 | Team (practice, clinician) | Multi-drug resistant virus | Ryan White clients at risk for or with a history of suboptimal outcomes and alive one year after program enrollment | Ryan White Care Coordination Program- funded agencies | Pre-post retrospective cohort | Comprehensive care coordination program‡ | No comprehensive care coordination program |
| Young 2014 | Shared (clinician) | Multi-drug resistant virus | HIV-infected individual >18 yrs and an offender in correctional facility | Correctional facility | Observational cohort with historical controls | HIV subspecialty care from infectious disease physician, infectious disease pharmacist, and case manager, and correctional nurse via telemedicine | On-site HIV care from a correctional physician without HIV subspecialty training |
| Ding 2008 | Specialty, advanced practitioner (practice, clinician) | Combination therapy, potent combination therapy | HIV-infected individual >18 yrs and with >1 visit to a nonmilitary, nonprison medical clinician | HIV specialty sites with >20,000 outpatient visits per year | Cross-sectional analysis of HIV Cost and Services Utilization Study | HIV care from an identified clinician (physician, nurse practitioner, or physician’s assistant) | No identified HIV care clinician |
| Chu 2010 | Specialty, shared (practice, clinician) | Potent combination therapy, multi-drug resistant virus | HIV patients presenting for care in hospital-based specialty center or individuals in community-based site later testing HIV positive | University hospital and community affiliates | Retrospective cohort study | Collaborative care at community-based sites, where patients have appointments with an infectious disease specialist as well as routine primary care visits, with clinician consulting specialists | Hospital-based HIV specialty clinic, staffed by infectious disease physicians, with patient followed by a single clinician |
| Lê 1998 | Team, shared (clinician) | Combination therapy | HIV-positive adults ≥18 years receiving care from Kaiser Permanente medical centers in Northern California | Kaiser Permanente medical centers in Northern California (managed care) | Retrospective cohort study | HIV care from primary care physicians and HIV interdisciplinary team (coordinator, infectious disease physician, nurse practitioner, pharmacist, social worker, home health manager, nutritionist) | Usual care through a primary care physician, with clinical guidelines, continuing education opportunities, and specialist consults made available when requested by a primary care physician |
Studies are grouped by care delivery model and, within each group, ordered by publication year.
The antiretroviral era was classified into 4 treatment eras: monotherapy (1987 – 1991), combination therapy (1992 – 1996), potent combination therapy (1997 – 2005), and therapy for multi-drug resistant virus (2006 – present). We report any antiretroviral eras that overlap with the study start and end dates. Further details on how we defined these eras is described in the main text.
The program included: case finding after a missed appointment, case management, multidisciplinary team communication and conference-based decision making, patient navigation (including accompaniment to primary care visits), antiretroviral adherence support (including directly observed therapy), and formal health promotion education.
Study characteristics
Study description and qualitative characteristics are in Table 1. Data came from each treatment era considered, although primarily the combination therapy (7 of 13 studies) (Ding et al., 2008; Keitz et al., 2001; Kitahata et al., 1996; Kitahata et al., 2003; Laine et al., 1998; Landon et al., 2003; Lê et al., 1998) and potent combination therapy (also 7 of 13 studies) (Chu et al., 2010; Ding et al., 2008; Gardner et al., 2002; Keitz et al., 2001; Kitahata et al., 2003; Landon et al., 2003; Landon et al., 2005) eras. All except one study (Keitz et al., 2001) were non-experimental. Only 4 of 13 studies were published in the last decade (Chu et al., 2010; Ding et al., 2008; Irvine et al., 2015; Young et al., 2014).
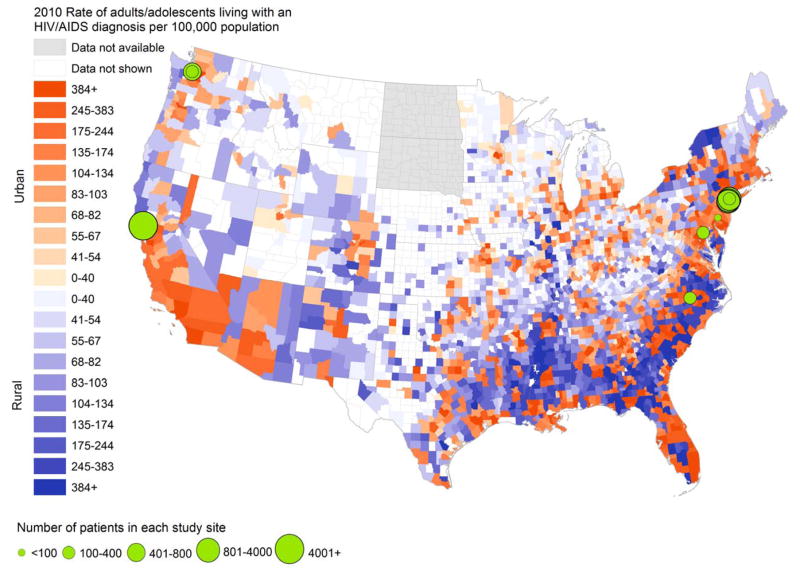
We evaluated study site location in the context of HIV burden and urbanicity. Five studies included practices located in the Northeast region (Pennsylvania, New York) (Aiken et al., 1993; Chu et al., 2010; Gardner et al., 2002; Irvine et al., 2015; Laine et al., 1998), three in the West (California, Washington) (Kitahata et al., 1996; Kitahata et al., 2003; Lê et al., 1998), one in the Midwest (Illinois) (Young et al., 2014) and two in the South (Maryland, North Carolina) (Gardner et al., 2002; Keitz et al., 2001) (Figure 2). Three were nationally representative (Ding et al., 2008; Landon et al., 2003; Landon et al., 2005), although only regional-level outcomes were reported. Of nine studies explicitly reporting specific study site location, six were in urban metropolitan areas and three in metropolitan areas serving a wider catchment area. One study, which included incarcerated individuals, reported on subjects from correctional facilities across the state of Illinois, although specific study sites or catchment areas were not reported (Young et al., 2014).
Figure 2. Geographic variation in study sites evaluating HIV care delivery models and county-level rates of HIV diagnosis.
This map shows county-level cases of HIV per 100,000 population, with counties shaded in darker colors indicating higher county-level rates of HIV. Counties shaded in orange are classified as urban, while those shaded in purple are classified as rural. The urban-rural classification occurs according to the 2013 Urban-Rural Continuum Codes from the United States Department of Agriculture from the Area Health Resources File, 2010 release. For this visualization, we included metropolitan counties (defined by population size of their metro area, and considered “urban”) and non-metropolitan counties (defined by degree of urbanicity and adjacency to metropolitan areas, and considered “rural”). Superimposed on this map are the sites of 9 studies meeting selection criteria. The sites for the remaining studies were either nationally representative or involved numerous sites statewide for which individual site sample sizes were not reported and therefore are not shown. Sources: AIDSVu (www.aidsvu.org), Emory University, Rollins School of Public Health on March 2, 2015, for county-level rates of HIV. Area Health Resources File (AHRF), 2010–2011, Rockville, MD: US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce.
Patient characteristics
Sample size ranged from 87 (Aiken et al., 1993) to 5,247 patients (Lê et al., 1998) (median 871, interquartile range 2475 subjects). While one study included only females (Gardner et al., 2002), the majority in the remaining studies were male (range 57% (Chu et al., 2010) – 100% (Kitahata et al., 1996; Kitahata et al., 2003)). African Americans represented 30%–78% of the study sample in 6 studies (Aiken et al., 1993; Chu et al., 2010; Ding et al., 2008; Irvine et al., 2015; Keitz et al., 2001; Landon et al., 2003); 3 studies reported <15% of the study sample as African American (Kitahata et al., 1996; Kitahata et al., 2003; Lê et al., 1998). While Hispanics and Latinos represented 44% of patients in one study and 38% in another (Chu et al., 2010; Irvine et al., 2015), this group did not exceed 15% in five other studies (Ding et al., 2008; Kitahata et al., 1996; Kitahata et al., 2003; Landon et al., 2003; Lê et al., 1998) and was not represented in the remaining studies (Aiken et al., 1993; Gardner et al., 2002; Keitz et al., 2001; Laine et al., 1998; Landon et al., 2005). Two studies did not report race and ethnicity (Laine et al., 1998; Young et al., 2014). Key populations included men who have sex with men (7 of 13 studies) (Chu et al., 2010; Ding et al., 2008; Keitz et al., 2001; Kitahata et al., 1996; Kitahata et al., 2003; Landon et al., 2003; Lê et al., 1998), injection drug users (5 of 13 studies) (Chu et al., 2010; Ding et al., 2008; Gardner et al., 2002; Keitz et al., 2001; Lê et al., 1998), and prison inmates (1 of 13 studies) (Young et al., 2014).
Patient outcomes
We found limited evidence on patient health outcomes associated with different HIV care delivery models (Table 2). Evidence from three studies indicated greater clinician and practice experience with HIV care was associated with reduced mortality (Kitahata et al., 1996; Kitahata et al., 2003; Laine et al., 1998). Four studies reported on retention in care, showing increased retention among patients receiving care from more experienced HIV clinicians (Keitz et al., 2001; Kitahata et al., 2003; Landon et al., 2005) or when enrolled in a comprehensive care coordination program (Irvine et al., 2015); there was no statistically significant effect at the practice level. Eight studies reported findings related to initiation or use of antiretroviral therapy (Chu et al., 2010; Ding et al., 2008; Gardner et al., 2002; Keitz et al., 2001; Kitahata et al., 1996; Kitahata et al., 2003; Laine et al., 1998; Landon et al., 2003; Landon et al., 2005), with evidence of increased antiretroviral use with more experienced or specialist HIV clinicians (Chu et al., 2010; Gardner et al., 2002; Kitahata et al., 1996; Landon et al., 2003; Landon et al., 2005) but no statistically significant findings for antiretroviral use among more experienced practices (Ding et al., 2008). Two studies indicated no statistically significant differences in referral for or use of mental health services (Aiken et al., 1993; Lê et al., 1998), although patients who identified a regular HIV care clinician were less likely to receive care at facilities with a mental health professional or substance abuse counselors (Ding et al., 2008). No chronic disease management outcomes—including for cardiovascular disease, hypertension, and/or diabetes screening and/or treatment—were reported. Hepatitis C screening did not differ by clinician type (HIV specialist vs. primary care) (Landon et al., 2005); however, tuberculosis screening occurred more frequently in primary care practices versus infectious disease practices (Keitz et al., 2001) as did influenza vaccination (Landon et al., 2005). Only three studies (Ding et al., 2008; Keitz et al., 2001; Lê et al., 1998) and five studies (Aiken et al., 1993; Chu et al., 2010; Keitz et al., 2001; Kitahata et al., 2003; Lê et al., 1998) reported any patient-centeredness or resource utilization outcomes (Figure S1).
Table 2.
HIV-specific and selected primary care outcomes across U.S. HIV care delivery models
| Study* | Mortality | HIV outcomes
|
Primary care outcomes | ||
|---|---|---|---|---|---|
| Retention | ART | HIV RNA suppression | |||
| Aiken 1993 | -- | -- | -- | -- | Difference in use of mental health services not statistically significant; more reported symptoms for patients of nurse practitioners vs. MDs (β=0.235, p=0.04) |
| Kitahata 1996 | Higher mortality among patients of least experience vs. most experience physicians (adjusted RR=0.69, 95% CI 0.59–0.80) | -- | Percentage of patients with AIDS on ART 59%, 49%, and 42% (most, moderate, and least experienced HIV clinicians) (p<0.001) | -- | -- |
| Laine 1998 | Decreased mortality for patients receiving care from high (>100 HIV patients) vs. low experience practices (<20 HIV patients) (53% decrease in relative hazard of death, 95% CI 0.35–0.82) | -- | No statistically significant difference for receiving ART by practice experience, 49.5% of patients overall receiving ART at baseline | -- | ‡ |
| Keitz 2001 | -- | No statistically significant difference in loss to follow-up among patients receiving care in general medicine clinic vs. infectious disease clinic | No statistically significant difference in number receiving ART by clinic type (80% in general medicine clinic vs. 76% in infectious disease clinic) | -- | More patients had TB screening in general medicine clinic (89%) than in infectious disease clinic (68%) (p=0.001) No statistically significant difference in pneumococcal vaccination across treatment groups |
| Gardner 2002 | -- | -- | Lower rates of guideline-concordant ART for patients with care from non-specialist HIV clinicians vs. patients receiving care from specialist HIV clinicians (p<0.001) | -- | Predictors of HIV specialist care are having depression (adjusted OR = 2.8, 95% CI 1.3–6.2) and current injection drug use (adjusted OR=0.4, 95% CI 0.1–0.95) |
| Kitahata 2003 | Higher risk of death among patients of least experienced physicians and who received infrequent primary primary care visits versus patients of most- experienced physicians (adjusted HR = 15.34 (95% CI 1.67–140.79)) | Patients of least and moderately experienced HIV physicians more likely to receive no primary or specialty care visits (adjusted OR=2.46, p<0.001) | -- | -- | Patients receiving care from more experienced physicians more likely to have a monthly primary care visit vs. patients of less experienced (adjusted OR = 0.50, 95% CI 0.32–0.77) and moderate experience physicians (adjusted OR=0.54, 95% CI 0.39–0.74) |
| Landon 2003 | -- | -- | Patients of non-HIV- specialist clinicians less likely to receive PI - based ART than those of infectious diseases specialists (adjusted OR=0.32, 95% CI 0.17–0.61) (findings attenuate) | -- | -- |
| Landon 2005 | -- | Percentage of patients with outpatient visits to infectious diseases physicians and HIV specialist primary care clinicians in last 3 quarters similar (both >65%) compared to 57% among non-HIV- specialist primary care clinicians (adjusted, p<0.01) | Probability of ART use for patients of infectious diseases specialists (0.83), HIV specialist primary care physicians (0.82) vs. non-HIV-specialist primary care clinicians (0.73) (p<0.05) | Probability of HIV RNA <400 copies/mL 0.41 (infectious diseases specialists), 0.39 (HIV specialist primary care clinicians), and 0.31 (non-HIV-specialist primary care clinicians) (p<0.01) | Probability of hepatitis C screening 0.86 (infectious diseases specialists), 0.81 (HIV specialist primary care clinicians), and 0.81 (non-HIV- specialist primary care clinicians) (results not statistically significant) Probability of influenza vaccination 0.54 (infectious diseases specialists), 0.49 (HIV- specialist primary care clinicians), and 0.41 (non-HIV- specialist primary care clinicians) (p<0.01) Probability of PPD testing and Pap smears not statistically different across clinician type |
| Irvine 2015 | -- | Percentage of previously diagnosed program clients engaged in care increased from 73.7% to 91.3% (relative risk=1.24, 95% CI 1.21 – 1.27)† | -- | Percentage of previously diagnosed program clients with HIV RNA <200 copies/mL increased from 32.3% to 50.9% (relative risk=1.58, 95% CI 1.50 – 1.66)† | -- |
| Young 2014 | -- | -- | -- | Higher proportion with complete HIV RNA suppression for patients with HIV care via telemedicine (91.1%) vs on-site (59.3%) (OR=7.0, 95% CI 5.1 – 9.8) | -- |
| Ding 2008 | -- | -- | Patients self-reporting no primary HIV care clinician less likely to receive ART vs. patients with care from a physician (adjusted, p=0.04) or an NP or PA (adjusted, p=0.012) | -- | Patients identifying a primary HIV care clinician less likely to receive care at sites with a mental health professional or substance abuse counselor available (p<0.01) |
| Chu 2010 | -- | -- | 178 (42%) patients with community-based HIV care and 237 (55%) patients receiving hospital-based HIV care initiated combination ART (statistical significance not reported) | Likelihood of HIV RNA <400 copies/mL for patients receiving community vs. hospital-based HIV care not statistically significant (adjusted OR=1.24, 95% CI 0.69–2.33) | -- |
| Lê 1998 | -- | -- | -- | Rate ratio of psychologist visits for patients receiving team- based care compared to usual care not statistically significant (rate ratio = 0.80, 95% CI 0.56–1.10) | |
Abbreviations: ART = antiretroviral therapy; PI = protease inhibitor; CI = confidence interval; HR = hazard ratio; OR = odds ratio.
Studies are grouped by care delivery model and, within each group, ordered by publication year.
Engagement in care was defined as ≥2 CD4 or viral load tests administered at least 90 days apart, with at least 1 test in each half of the 12-month enrollment period. The definition of viral load suppression was met if HIV RNA ≤200 copies/mL occurred at the most recent viral load test in the second half of the 12-month evaluation period.
Risk of bias
Across all eligible studies, selection bias was the primary identified bias, appearing in three of four study designs represented. Studies with a cross-sectional (Aiken et al., 1993; Ding et al., 2008; Gardner et al., 2002; Landon et al., 2005), retrospective cohort (Chu et al., 2010; Kitahata et al., 1996; Kitahata et al., 2003; Laine et al., 1998; Lê et al., 1998), and randomized controlled trial (Keitz et al., 2001) study design generally received medium quality ratings; two studies (Landon et al., 2003; Young et al., 2014) with an observational cohort study design received high quality ratings (Table S7). We identified no bias across studies (e.g., publication bias) but found that the quality of the available data was limited by overall study design.
DISCUSSION
We systematically reviewed and qualitatively synthesized evidence on patient health outcomes associated with different service delivery models for outpatient US HIV care. The evidence primarily addressed specialty-based care, supporting that better clinical outcomes are associated with increased clinician experience; limited data were available for other care models. Mainly mortality and clinical outcomes along the HIV care continuum were reported, but not chronic disease outcomes. Evidence was inconsistently available for key populations. Most studies were published over a decade ago and reflected study sites, and therefore patient populations, in Northeast and West metropolitan areas. Data quality was limited by overall study design.
Comparable questions on HIV service delivery have been posed for other complex, chronic diseases experiencing workforce shortages, fragmented care delivery, and escalating costs. For example, a larger, more equal role for advanced practitioners and team-based care across the cancer care continuum (IOM, 2013), and comprehensive, coordinated management including nurse-directed care for diabetes mellitus type 2 (Kahn & Anderson, 2009), have been emphasized. As in HIV, limited US evidence exists.
HIV workforce challenges highlight a need for care delivery reform. Declines in the number of infectious diseases training programs and positions between 1994 and 2002 suggest difficulties in retaining infectious disease physicians (Knobler, Burroughs, Mahmoud, Lemon, & (eds), 2006). This trend persists: in the July 2015 National Residency Matching Program, only half of infectious diseases programs filled their slots (Chandrasekar, 2015). Similarly, projected shortfalls have been estimated in the supply of primary care physicians by 2020 (DHHS, November 2013). Logistical, legal, and policy challenges may limit ability of non-physician clinicians, particularly nurse practitioners, to manage HIV care. While the nurse practitioner workforce is projected to increase (DHHS, November 2013), scope of practice varies. Only 20 US states authorize full practice authority (American Association of Nurse Practitioners, 2015).
Little is known about non-HIV-specialist primary care clinicians’ willingness to manage HIV. However, persons at risk for HIV, and therefore potential candidates for pre-exposure prophylaxis (PrEP), typically receive care from primary care clinicians, who may feel discomfort with prescribing PrEP and that it may not fall within their clinical purview (Hoffman et al., 2015; Krakower, Ware, Mitty, Maloney, & Mayer, 2014). The literature also indicates educational (i.e., lack of knowledge or misperceptions) (Sison et al., 2013) and financial barriers (i.e., inadequate reimbursement) (Korthuis et al., 2011; White et al., 2015) to primary care physicians offering HIV testing and counseling. Many primary care physicians remain unaware of CDC’s HIV testing recommendations (Arya et al., 2014), and increased educational and outreach opportunities not only for HIV testing but also for HIV management may be required. Similarly, HIV specialists report feeling uncomfortable providing primary care to their HIV patients (Cheng, Engelage, Grogan, Currier, & Hoffman, 2014; Fultz et al., 2005). For example, infectious disease and primary care clinicians practicing in infectious disease clinics are less comfortable providing care for HIV-related comorbidities—hyperlipidemia, diabetes, hypertension, and depression—than general medicine physicians practicing in primary care settings (Fultz et al., 2005). Mutual discomfort in providing care outside of their respective areas of expertise suggests the need for new models of HIV care that promote collaborative arrangements between HIV specialists and primary care physicians (Chu & Selwyn, 2011; Fultz et al., 2005).
Finally, while federal legislation promotes team-based medicine, it is unclear how HIV care will be integrated into these reforms. HIV care received at Ryan White Part C-funded practices reflects such patient-centered, coordinated care (Saag, 2009), but the future of Ryan White funding remains unknown (Martin, Meehan, & Schackman, 2013).
This study has several limitations. Although we included comprehensive search terms in our bibliographic search and supplemented with hand searching, few studies met selection criteria, and it is possible we missed some eligible studies. We also found inconsistent reporting of outcomes. Both restricted our ability to quantitatively synthesize the literature. While new care delivery models may be in use programmatically, they are absent from the literature and could benefit from rigorous implementation research. We included non-experimental studies, which may have bias compared to randomized controlled trials. There is possible bias due to a non-English language exclusion criterion. However, we do not believe this criterion materially affected our search or review findings, since the review was limited to a US context. Therefore, we would have expected that the vast majority of studies were published in English. We also excluded articles from non-US settings to limit cultural and health system factors potentially affecting the applicability of results to the US context. Finally, this review did not address intrapersonal outcomes, such as psychological resilience and social support, that are associated with improved well-being and reduced HIV-related risk behaviors among some key populations (Fang et al., 2015), as well as stress, which is associated with poorer clinical outcomes (e.g., higher viral load) (Weinstein & Li, 2016). As HIV care delivery models evolve, the role of advanced practitioners and social support in promoting resilience may warrant consideration (De Santis, Florom-Smith, Vermeesch, Barroso, & DeLeon, 2013).
As HIV treatment management advances, HIV patients and the HIV workforce age, and national care delivery reforms are further implemented, adequate evidence to inform the future of HIV care delivery is imperative. We found that the limited and largely outdated data on patient health outcomes associated with HIV care delivery models are inadequate to inform future care delivery. A coordinated, detailed, and peer-reviewed effort to better understand the HIV workforce and that addresses future workforce training and policies is crucial. Improved understanding of how different clinician roles and relationships affect patient outcomes and differences across target populations and geographic settings is critical to further improving health along the HIV care continuum.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institutes of Health (CTSA award number KL2TR000057 from the National Center for Advancing Translational Sciences and NIAID R01 AI42006) and Health Resources and Services Administration (H97HA27534). The funding sources played no role in the study, including study design; collection, analysis, and/or interpretation of data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Preliminary results for this manuscript were presented in part at the AcademyHealth Annual Research Meeting [abstract 4089], June 14 – 16, 2015, Minneapolis, USA, and the 8th International Conference on HIV Pathogenesis, Treatment & Prevention [abstract MOPED693], July 19 – 22, 2015, Vancouver, Canada.
References
- Aiken LH, Lake ET, Semaan S, Lehman HP, O’Hare PA, Cole CS, … Frank I. Nurse practitioner managed care for persons with HIV infection. Image--the journal of nursing scholarship. 1993;25(3):172–177. doi: 10.1111/j.1547-5069.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- American Association of Nurse Practitioners. Nurse Practitioner State Practice Environment. 2015. [Google Scholar]
- Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya M, Zheng MY, Amspoker AB, Kallen MA, Street RL, Viswanath K, Giordano TP. In the routine HIV testing era, primary care physicians in community health centers remain unaware of HIV testing recommendations. J Internat Assoc Providers AIDS Care. 2014;13(4):296–299. doi: 10.1177/2325957413517140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach DI, Chen PG, Friedberg MW, Reid R, Lau C, Buerhaus PI, Mehrotra A. Nurse-managed health centers and patient-centered medical homes could mitigate expected primary care physician shortage. Health Aff (Millwood) 2013;32(11):1933–1941. doi: 10.1377/hlthaff.2013.0596. [DOI] [PubMed] [Google Scholar]
- Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, … Valleroy LA. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morbidity and mortality weekly report. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- Carmichael JK, Deckard DT, Feinberg J, Gallant JE, Hoffman-Terry ML, Lee SD, … Squires KE. Averting a crisis in HIV care: a joint statement of the American Academy of HIV Medicine (AAHIVM) and the HIV Medicine Association (HIVMA) on the HIV medical workforce 2009 [Google Scholar]
- CDC. HIV Surveillance Report, 2012. 2014:24. [Google Scholar]
- CDC & Health Resources and Services Administration (HRSA) Record of the Proceedings. 2011. CDC/HRSA Advisory Committee on HIV and STD Prevention and Treatment May 10 2011. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Estimated HIV Incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012;17(4) [Google Scholar]
- Chandrasekar PH. Bad news to worse news: 2015 infectious diseases fellowship match results. Clin Infect Dis. 2015;60(9):1438. doi: 10.1093/cid/civ037. [DOI] [PubMed] [Google Scholar]
- Chang SM, Slutsky J. Debunking myths of protocol registration. Syst Rev. 2012;1:4. doi: 10.1186/2046-4053-1-4. 2046-4053-1-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng QJ, Engelage EM, Grogan TR, Currier JS, Hoffman RM. Who Provides Primary Care? An Assessment of HIV Patient and Provider Practices and Preferences. J AIDS Clin Res. 2014;5(11) doi: 10.4172/2155-6113.1000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Selwyn PA. An epidemic in evolution: the need for new models of HIV care in the chronic disease era. J Urban Health. 2011;88(3):556–566. doi: 10.1007/s11524-011-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Umanski G, Blank A, Grossberg R, Selwyn PA. HIV-infected patients and treatment outcomes: an equivalence study of community-located, primary care-based HIV treatment vs. hospital-based specialty care in the Bronx, New York. AIDS care. 2010;22(12):1522–1529. doi: 10.1080/09540121.2010.484456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis JP, Florom-Smith A, Vermeesch A, Barroso S, DeLeon DA. Motivation, management, and mastery: a theory of resilience in the context of HIV infection. J Am Psychiatr Nurses Assoc. 2013;19(1):36–46. doi: 10.1177/1078390312474096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS. Projecting the Supply and Demand for Primary Care Practitioners Through 2020. Nov, 2013. [Google Scholar]
- Ding L, Landon BE, Wilson IB, Hirschhorn LR, Marsden PV, Cleary PD. The quality of care received by HIV patients without a primary provider. AIDS care. 2008;20(1):35–42. doi: 10.1080/09540120701439295. [DOI] [PubMed] [Google Scholar]
- Fang X, Vincent W, Calabrese SK, Heckman TG, Sikkema KJ, Humphries DL, Hansen NB. Resilience, stress, and life quality in older adults living with HIV/AIDS. Aging Ment Health. 2015;19(11):1015–1021. doi: 10.1080/13607863.2014.1003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz SL, Goulet JL, Weissman S, Rimland D, Leaf D, Gibert C, … Justice AC. Differences between infectious diseases-certified physicians and general medicine-certified physicians in the level of comfort with providing primary care to patients. Clin Infect Dis. 2005;41(5):738–743. doi: 10.1086/432621. [DOI] [PubMed] [Google Scholar]
- Gardner LI, Holmberg SD, Moore J, Arnsten JH, Mayer KH, Rompalo A, … Smith DK. Use of highly active antiretroviral therapy in HIV-infected women: impact of HIV specialist care. J Acquir Immune Defic Syndr. 2002;29(1):69–75. doi: 10.1097/00126334-200201010-00010. [DOI] [PubMed] [Google Scholar]
- Hoffman S, Guidry JA, Collier KL, Mantell JE, Boccher-Lattimore D, Kaighobadi F, Sandfort TG. A Clinical Home for Preexposure Prophylaxis: Diverse Health Care Providers’ Perspectives on the “Purview Paradox”. J Int Assoc Provid AIDS Care. 2015 doi: 10.1177/2325957415600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRSA. Workforce Capacity in HIV. HRSA Care Action 2010 [Google Scholar]
- HRSA. Area Health Resources File. 2010–2011. [Google Scholar]
- Institute of Medicine (IOM) HIV Screening and Access to Care: Health Care System Capacity for Increased HIV Testing and Provision of Care. 2011. [PubMed] [Google Scholar]
- IOM. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. 2013. [PubMed] [Google Scholar]
- Irvine MK, Chamberlin SA, Robbins RS, Myers JE, Braunstein SL, Mitts BJ, … Nash D. Improvements in HIV care engagement and viral load suppression following enrollment in a comprehensive HIV care coordination program. Clin Infect Dis. 2015;60(2):298–310. doi: 10.1093/cid/ciu783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R, Anderson JE. Improving diabetes care: the model for health care reform. Diabetes Care. 2009;32(6):1115–1118. doi: 10.2337/dc09-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitz SA, Box TL, Homan RK, Bartlett JA, Oddone EZ. Primary care for patients infected with human immunodeficiency virus: a randomized controlled trial. J Gen Intern Med. 2001;16(9):573–582. doi: 10.1046/j.1525-1497.2001.016009573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahata MM, Koepsell TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH. Physicians’ experience with the acquired immunodeficiency syndrome as a factor in patients’ survival. New Engl J Med. 1996;334(11):701–706. doi: 10.1056/NEJM199603143341106. [DOI] [PubMed] [Google Scholar]
- Kitahata MM, Van Rompaey SE, Dillingham PW, Koepsell TD, Deyo RA, Dodge W, Wagner EH. Primary care delivery is associated with greater physician experience and improved survival among persons with AIDS. J Gen Intern Med. 2003;18(2):95–103. doi: 10.1046/j.1525-1497.2003.11049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler SL, Burroughs T, Mahmoud A, Lemon SM, editors. Ensuring an Infectious Disease Workforce: Education and Training Needs for the 21st Century - Workshop Summary. 2006. [PubMed] [Google Scholar]
- Korthuis PT, Berkenblit GV, Sullivan LE, Cofrancesco J, Jr, Cook RL, Bass M, … Sosman JM. General internists’ beliefs, behaviors, and perceived barriers to routine HIV screening in primary care. AIDS Educ Prev. 2011;23(3 Suppl):70–83. doi: 10.1521/aeap.2011.23.3_supp.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakower D, Ware N, Mitty JA, Maloney K, Mayer KH. HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav. 2014;18(9):1712–1721. doi: 10.1007/s10461-014-0839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine C, Markson LE, McKee LJ, Hauck WW, Fanning TR, Turner BJ. The relationship of clinic experience with advanced HIV and survival of women with AIDS. AIDS. 1998;12(4):417–424. doi: 10.1097/00002030-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Landon BE, Wilson IB, Cohn SE, Fichtenbaum CJ, Wong MD, Wenger NS, … Cleary PD. Physician specialization and antiretroviral therapy for HIV. J Gen Intern Med. 2003;18(4):233–241. doi: 10.1046/j.1525-1497.2003.20705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon BE, Wilson IB, McInnes K, Landrum MB, Hirschhorn LR, Marsden PV, Cleary PD. Physician specialization and the quality of care for human immunodeficiency virus infection. Arch Intern Med. 2005;165(10):1133–1139. doi: 10.1001/archinte.165.10.1133. 165/10/1133 [pii] [DOI] [PubMed] [Google Scholar]
- Lê CT, Winter TD, Boyd KJ, Ackerson L, Hurley LB. Experience with a managed care approach to HIV infection: effectiveness of an interdisciplinary team. Am J Manage Care. 1998;4(5):647–657. [PubMed] [Google Scholar]
- Martin EG, Meehan T, Schackman BR. AIDS Drug Assistance Programs: managers confront uncertainty and need to adapt as the Affordable Care Act kicks in. Health Aff. 2013;32(6):1063–1071. doi: 10.1377/hlthaff.2012.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obama B. Executive Order -- HIV Care Continuum Initiative. 2013. [Google Scholar]
- Saag MS. Ryan White: an unintentional home builder. The AIDS Reader. 2009;19(5):166–168. [PubMed] [Google Scholar]
- Sison N, Yolken A, Poceta J, Mena L, Chan PA, Barnes A, … Nunn A. Healthcare provider attitudes, practices, and recommendations for enhancing routine HIV testing and linkage to care in the Mississippi Delta region. AIDS Pat Care STDS. 2013;27(9):511–517. doi: 10.1089/apc.2013.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein TL, Li X. The relationship between stress and clinical outcomes for persons living with HIV/AIDS: a systematic review of the global literature. AIDS Care. 2016;28(2):160–169. doi: 10.1080/09540121.2015.1090532. [DOI] [PubMed] [Google Scholar]
- White BL, Walsh J, Rayasam S, Pathman DE, Adimora AA, Golin CE. What Makes Me Screen for HIV? Perceived Barriers and Facilitators to Conducting Recommended Routine HIV Testing among Primary Care Physicians in the Southeastern United States. J Internat Assoc Providers AIDS Care. 2015;14(2):127–135. doi: 10.1177/2325957414524025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) A Universal Truth: No Health Without a Workforce. 2014. [Google Scholar]
- Young JD, Patel M, Badowski M, Mackesy-Amiti ME, Vaughn P, Shicker L, … Ouellet LJ. Improved virologic suppression with HIV subspecialty care in a large prison system using telemedicine: an observational study with historical controls. Clin Infect Dis. 2014;59(1):123–126. doi: 10.1093/cid/ciu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.