Summary
Objective
Tuberous sclerosis complex (TSC) is a genetic disorder, characterized by tumor formation in multiple organs and severe neurological manifestations, including epilepsy, intellectual disability, and autism. Abnormalities of both neurons and astrocytes have been implicated in contributing to the neurological phenotype of TSC, but the role of microglia in TSC has not been investigated. The objectives of this study were to characterize microglial activation in a mouse model of TSC, involving conditional inactivation of the Tsc1 gene predominantly in glial cells (Tsc1GFAPCKO mice), and to test the hypothesis that microglial activation contributes to epileptogenesis in this mouse model.
Methods
Microglial and astrocyte activation was examined in Tsc1GFAPCKO mice by Iba1 and GFAP immunohistochemistry. Cytokine and chemokine expression was evaluated with QPCR. Seizures were monitored by video-EEG. The effect of minocycline in inhibiting microglial and astrocyte activation, cytokine expression, and seizures was tested.
Results
Microglial cell number and size were increased in cortex and hippocampus of 3–4 wk old Tsc1GFAPCKO mice, which correlated with the onset of seizures. Minocycline treatment prevented the increase in number and cell size of microglia in 4 week old Tsc1GFAPCKO mice. However, minocycline treatment had no effect on astrocyte proliferation and cytokine/chemokine expression and the progression of seizures in Tsc1GFAPCKO mice.
Significance
Microglia cell number and size are abnormal in Tsc1GFAPCKO mice, and minocycline treatment inhibits this microglia activation, but does not suppress seizures. Microglia may play a role in the neurological manifestations of TSC, but additional studies are needed in other models and human studies to determine whether microglia are critical for epileptogenesis in TSC.
Keywords: epilepsy, seizure, microglia, astrocyte, inflammation
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder, resulting from a germline mutation in one of two genes, TSC1 or TSC2.1; 2 TSC is primarily characterized by tumor or hamartoma (tuber) formation in multiple organs including the brain, skin, eyes, heart, kidneys, and lungs. Although tumors can lead to symptoms in various organs, neurological manifestations, such as intractable epilepsy, intellectual disability, and autism, are especially common and typically the most disabling problems of TSC and in many instances are independent of tumor growth per se. Epilepsy occurs in ~80% of TSC patients, with about two-thirds having medically-intractable seizures.3 Intellectual disability, autism, and other neuropsychiatric symptoms are seen in about 50% of TSC patients. Treatments for all neurological manifestations of TSC are purely symptomatic and often ineffective.
Since the discovery of the TSC genes, there has been considerable progress in defining the molecular pathogenesis of this disorder, in particular the involvement of the mechanistic target of rapamycin (mTOR) signaling pathway.1; 2; 4 However, the cellular and pathophysiological mechanisms causing the neurological symptoms of TSC remain poorly understood. Abnormalities in neuron, astrocytes, and immature neuroglia progenitor cells have been identified in tubers and non-tuber brain tissue from TSC patients and are hypothesized to contribute to the neurological manifestations of TSC.5 In addition, microglia activation has been reported in cortical tuber specimens,6; 7 but little is known about the role of microglia in the pathophysiology of TSC. We have recently found evidence that inflammatory changes may contribute to epileptogenesis in a mouse model of TSC, Tsc1GFAPCKO mice. Antiinflammatory treatments, which reduced cytokine and chemokine production, caused a moderate inhibition of seizure progression in this mouse model.8 While astrocytes may play a central role in mediating inflammatory mechanisms and epileptogenesis in Tsc1GFAPCKO mice, microglia may also be involved in reactive inflammatory changes in the brain. Thus, in this study, we investigated the role of microglia in the neurological phenotype of Tsc1GFAPCKO mice.
Materials and Methods
Animals and drug treatment
Care and use of animals were conducted according to an animal protocol approved by the Washington University Animal Studies Committee. Tsc1flox/flox-GFAP-Cre knock-out (Tsc1GFAPCKO) mice with conditional inactivation of the Tsc1 gene predominantly in glia were generated as described previously.9 Tsc1flox/+-GFAP-Cre and Tsc1flox/flox littermates have previously been found to have no abnormal phenotype and were used as control animals in these experiments. One to four-week-old Tsc1GFAPCKO mice and control mice were used for a developmental time course study of Iba1 immunohistochemistry analysis.
Minocycline (Sigma, St. Louis, MO), was initially dissolved in DMSO, stored at 20 °C, and diluted with saline, and administrated by intraperitoneal injection. Mice were randomized into treatment (minocycline) or control (vehicle) groups, and results analyzed in a blinded fashion. In some experiments, three-week-old Tsc1GFAPCKO mice were treated with minocycline (50 mg/kg/day) or vehicle (saline) for one week for western blot, quantitative PCR, and Iba1 immunohistochemistry analysis (based on expected molecular effects of minocycline on microglia); for four weeks for GFAP immunohistochemistry analysis (based on time course of astrocyte proliferation in Tsc1GFAPCKO mice10); and for up to 12 weeks for video-EEG monitoring (until death). In other experiments, three-week-old Tsc1GFAPCKO mice were treated with vehicle (saline) or minocycline at different dosages (1, 10 and 50 mg/kg/day) for one week for Iba1 immunohistochemistry analysis.
Quantitative RT-PCR
Real-time quantitative RT-PCR was used to assay inflammatory cytokine and chemokine markers, CCL2, IL-1 β and CXCL10, as described previously.8 Total RNA was prepared from brains of four-week-old Tsc1GFAPCKO or control mice. Following DNase I treatment (Invitrogen, Grand Island, NY), cDNA was synthesized using iScript Reverse Transcription Kit (BIO-RAD, Hercules, CA). The following conditions were used for reverse transcription: 25 °C for 10 min, 48 °C for 30 min, and 95 °C for 5 min. Each 25 μl PCR contained 2 μl cDNA, 12.5 μl of 2 × SYBR Green PCR Master Mix (Applied Biosystems, Foster city, CA), and 12.5 pmol of each primer. Quantitative PCR was performed in 96-well optical reaction plates on the AB1 7000 Real-Time PCR System (Applied Biosystems) under the following conditions: 50 °C for 2 min, 95 °C for 10 min, and then 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Emitted fluorescence for each reaction was measured at the annealing/extension phase. All oligonucleotide primers used for quantitative PCR were designed using Primer Express v2.0 (Applied Biosystems). Calculated copies were normalized against copies of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Forward and reverse primer sets are listed as following: GAPDH forward: 5'-GGC AAA TTC AAC GGC ACA GT-3', reverse: 5'-AGA TGG TGA TGG GCT TCC C-3'; CCL2 forward: 5'-TGG CTC AGC CAG ATG CAG T-3', reverse: 5'-TTG GGA TCA TCT TGC TGG TG-3'; IL-1β forward: 5'-ACC TGC TGG TGT GTG ACG TT-3', reverse: 5'-TCG TTG CTT GGT TCT CCT TG-3'; CXCL10 forward: 5'-TGG CTC AGC CAG ATG CAG T-3', reverse: 5'-TTG GGA TCA TCT TGC TGG TG-3'.
Western blotting
Western blot analysis was used to measure protein levels of CXCL10 in the brains of Tsc1GFAPCKO and control mice, using standard methods as described previously.10 Briefly, brains were dissected and homogenized separately. Equal amounts of total protein extract were separated by gel electrophoresis and transferred to nitrocellulose membranes. β-actin was used as a loading control. After incubating with primary antibodies to CXCL10 (1:1,000; Abcam, Cambridge, MA), or β-actin (1:2,000; Cell Signaling Technology), the membranes were reacted with a peroxidase-conjugated secondary antibody (1:1,000, Cell Signaling Technology). Signals were detected by enzyme chemiluminescence (GE Healthcare Life Science, Little Chalfont Buckinghamshire, UK) and quantitatively analyzed with ImageJ software (NIH, Bethesda, MD).
Immunohistochemistry
Histological analysis was performed in a blinded fashion to assess glial proliferation and neuronal organization by standard methods, as previously described.10 In brief, brains were perfusion-fixed with 4% paraformaldehyde and cut into 45 μm sections with a cryotome. Sections were labeled with GFAP antibody (anti-GFAP, rabbit; 1:1,000; Cell Signaling Technology, Beverly, MA), and then Alexa-488 conjugated goat anti-rabbit IgG (1:1,000; Jackson Immuno, West Grove, PA). In other experiments, immunohistochemistry was performed for Iba1 to assess microglial cells, by labeling with primary antibody (anti-Iba1, rabbit, 1:200; Wako), followed by labeling with secondary antibody Alexa-488 conjugated goat anti-rabbit IgG (1:500; Life Technologies, Grand Island, NY). In addition, sections were counterstained with TO-PRO-3 Iodide (1:1,000; Life Technologies) for the nonspecific nuclear staining of all cells.
For blinded analysis, images were acquired with a Zeiss LSM PASCAL confocal microscope (Zeiss Thornwood, NY). In images from coronal sections at approximately 2 mm posterior to bregma and approximately 1 mm from midline, regions of interest were marked in neocortex by a 200 μm-wide box spanning from the neocortical surface to the bottom of layer VI and in hippocampus by areas up to 0.04 mm2 within the striatum radiatum of CA1 and dentate gyrus. GFAP-immunoreactive cells were quantified in the regions of interest from two sections per mouse from a total of five mice per group. Iba1 positive cells and TO-PRO-3 Iodide stained cell numbers were counted in the sections of each groups, and Iba1 positive cell numbers were normalized to TO-PRO-3 Iodide stained cell number, and were presented as Iba1 positive cells/100 TO-PRO-3 positive cells11. The size of Iba1 positive cells in each group was measured by outlining the cell body and calculating area using ImageJ software (NIH, Bethesda, MD).
Video-electroencephalography monitoring
Vehicle- and minocycline-treated Tsc1GFAPCKO mice underwent video-EEG monitoring starting at 3 weeks of age (48 hrs at 3 weeks, and then continuous 24/7 starting at 4 weeks), using established methods for implanting epidural electrodes and performing continuous video-EEG recordings, as described previously.10; 12 Briefly, mice were anesthetized with isoflurane and placed in a stereotaxic frame. Epidural screw electrodes were surgically implanted and secured using dental cement for long term EEG recordings. Four electrodes were placed on the skull: one right and one left central electrodes (1 mm lateral to midline, 2 mm posterior to bregma), one frontal electrode (0.5 mm anterior and 0.5 mm to the right or left of bregma) and one occipital electrode (0.5 mm posterior and 0.5 mm to the right or left lambda). The typical recording montage involved two EEG channels with the right and left central “active” electrodes being compared to either the frontal or occipital “reference” electrode. Video and EEG data were acquired simultaneously with an AD Instruments PowerLab video-EEG system. Continuous 24/7 video-EEG data were obtained every week from each mouse, for the life of the animal, and were analyzed for seizures by a blinded reviewer. Electrographic seizures were identified by their characteristic pattern of discrete periods of rhythmic spike discharges that evolved in frequency and amplitude lasting at least 10 s, typically ending with repetitive burst discharges and voltage suppression. On video analysis, the behavioral correlate to these seizures typically involved head bobbing, rearing with forelimb clonus, and occasional generalized convulsive activity. Seizure frequency (number of seizures per week period, based on analysis of the entire EEG record) was calculated from each week epoch.
Statistics
All statistical analysis was performed using GraphPad Prism (GraphPad Software). Quantitative differences between groups were analyzed by Student's t test or one-way ANOVA with Tukey's multiple comparisons post hoc tests when comparing one factor over more than two groups (or two-way ANOVA over time). Chi-Square test was used for survival analysis. Quantitative data are expressed as mean ± SEM. Statistical significance was defined as p < 0.05.
Results
Microglial activation occurs in Tsc1GFAPCKO mice
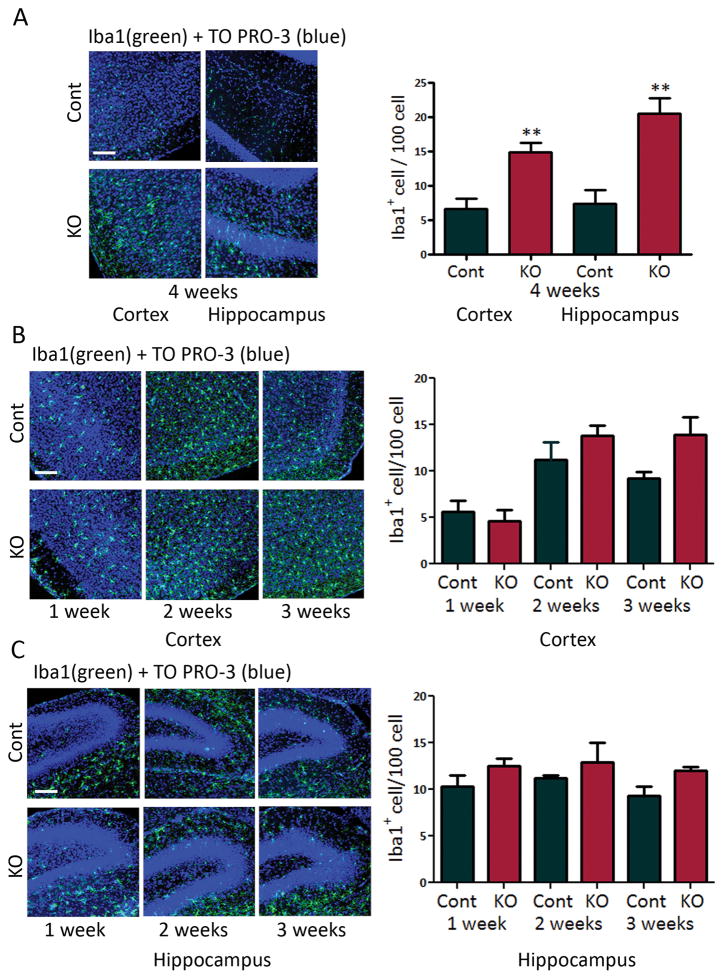
Microglial activation was assessed by Iba1 immunohistochemistry. Four week-old and seven week-old Tsc1GFAPCKO mice had significantly increased Iba1-positive cells in hippocampus and neocortex compared with control mice (Fig. 1A, Fig. 3E,F). A developmental time course revealed that increased microglial numbers was first detectable around 3–4 weeks of age (Fig. 1B,C), which correlates with the earliest age of onset of seizures in Tsc1GFAPCKO mice.12; 13 Microglia in four week-old Tsc1GFAPCKO mice also exhibited increased cell size (Fig. 2), indicating the presence of activated, as opposed to resting, microglia.
Figure 1.
Tsc1GFAPCKO mice exhibit increased microglial cell number. (A) Microglial activation was assessed by Iba1 (green) immunohistochemistry. Four week-old Tsc1GFAPCKO mice had significantly increased Iba1-positive cells in neocortex and hippocampus, compared with control mice. ** p<0.01, versus control mice by student’s t-test (n = 6–8 mice/group). (B,C) Developmental time course suggests that the increase in Iba1-positive cells in cortex and hippocampus of Tsc1GFAPCKO mice starts around 3–4 weeks of age, although the difference with control is not quite statistically significant at 3 weeks (p=0.07, versus control mice by two-way ANOVA. Cont = control mice, KO = Tsc1GFAPCKO mice. Scale bar = 100 μm.
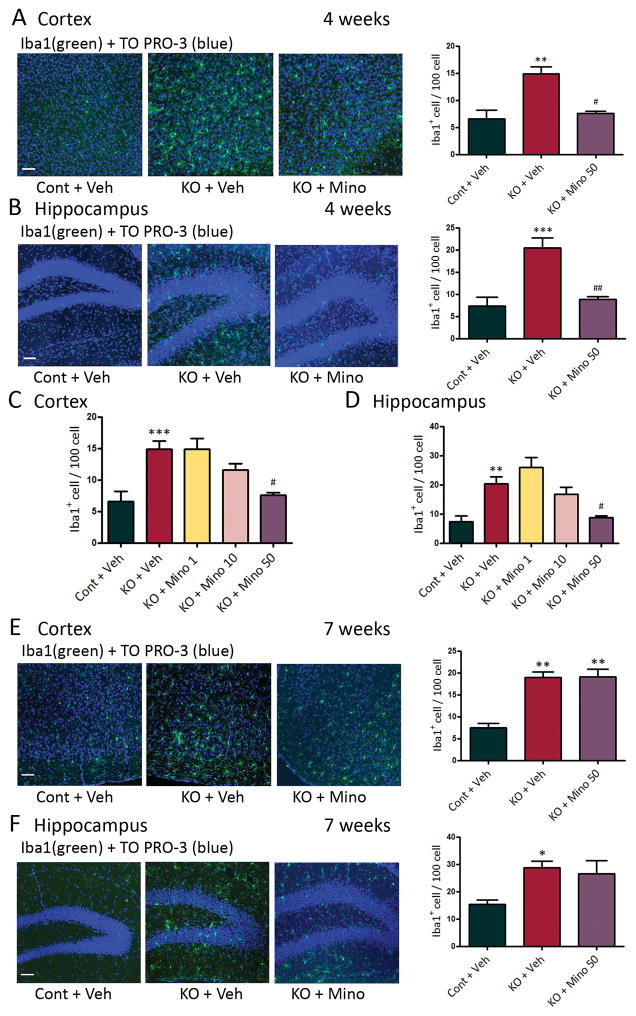
Figure 3.
Minocycline inhibits increased microglial number in Tsc1GFAPCKO mice during early but not late epileptogenesis. (A,B) Microglial cell number was assessed by Iba1 (green) immunohistochemistry, and the effect of minocycline was tested. Four week-old Tsc1GFAPCKO mice had significantly increased numbers of Iba1-positive cells in hippocampus and neocortex compared with control mice. Minocycline treatment (50 mg/kg/day, i.p. for one week starting at 3 weeks of age) resulted in control number of Iba1-positive cells in Tsc1GFAPCKO mice. (C,D) Minocycline caused a dose-dependent inhibition of the increased microglial cell number in Tsc1GFAPCKO mice. (E,F) Seven week-old Tsc1GFAPCKO mice had significantly increased numbers of Iba1-positive cells in hippocampus and neocortex compared with control mice. However, minocycline treatment (50 mg/kg/day, i.p. for four weeks starting at 3 weeks of age) was no longer effective in reducing the elevated Iba1-positive cells in seven-week old Tsc1GFAPCKO mice. *p<0.05, ** p<0.01, *** p<0.001, versus vehicle-treated control mice; # p<0.05, ## p<0.01, versus vehicle-treated Tsc1GFAPCKO mice by one-way ANOVA (n = 4–8 mice/group). Cont = control mice, KO = Tsc1GFAPCKO mice, Veh = vehicle, Mino = Minocycline. Scale bar = 100 μm.
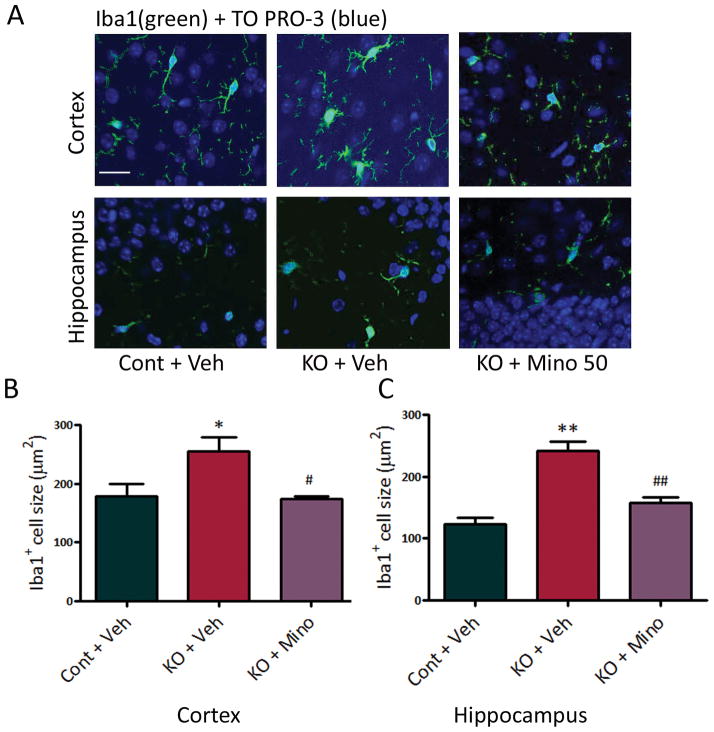
Figure 2.
Tsc1GFAPCKO mice exhibit increased microglial cell size, which is reversed by minocycline. (A) Microglial cell size was assessed by Iba1 (green) immunohistochemistry. (B,C) Four week-old Tsc1GFAPCKO mice had significantly increased size of Iba1-positive cells in hippocampus and neocortex compared with control mice. Minocycline treatment (50 mg/kg/day, i.p. for one week starting at 3 weeks of age) resulted in control size of Iba1-positive cells in Tsc1GFAPCKO mice. * p<0.05, ** p<0.01, versus vehicle-treated control mice; # p<0.05, ## p<0.01, versus vehicle-treated Tsc1GFAPCKO mice by one-way ANOVA (n = 4–5 mice/group). Cont = control mice, KO = Tsc1GFAPCKO mice, Veh = vehicle, Mino = Minocycline. Scale bar = 50 μm.
Minocycline reduces microglial activation, but not astrocyte proliferation and cytokine/chemokine production, in Tsc1GFAPCKO mice
Minocycline is a pharmacological inhibitor of microglia activation.14 Minocycline treatment (50 mg/kg/day, i.p., starting at 3 weeks of age) prevented the increase in both microglial cell size (Fig. 2) and cell number (Fig. 3A,B) in 4 week old Tsc1GFAPCKO mice, indicating that minocycline inhibited microglial activation in these mice. Minocycline showed a dose-dependent inhibition of microglia cell number in Tsc1GFAPCKO mice, with 50 mg/kg/day i.p. showing maximal effect in restoring control levels of Iba1-positive cells in Tsc1GFAPCKO mice (Fig. 3C,D). However, the effect of minocycline on the elevated microglia in Tsc1GFAPCKO mice was diminished by 7 weeks of age (Fig. 3E,F). Higher doses of minocycline (100 mg/kg) caused premature death, likely associated with liver toxicity (data not shown).
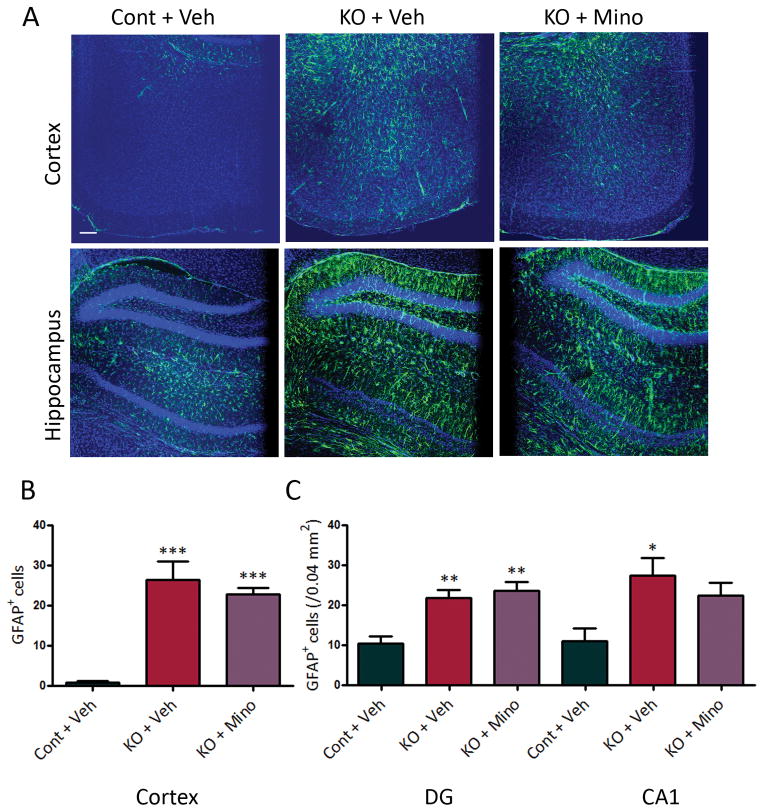
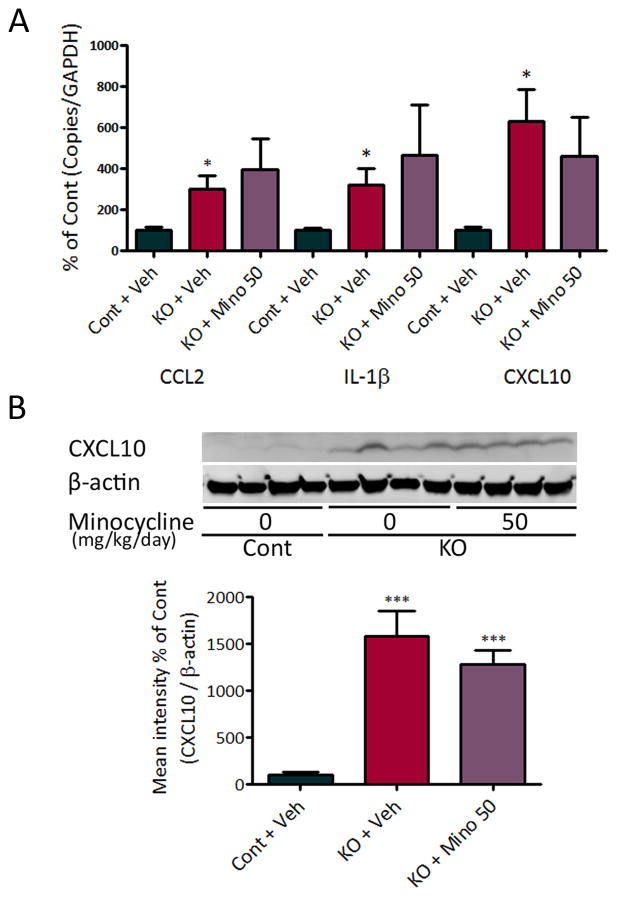
Tsc1GFAPCKO mice also exhibit progressive astrocyte proliferation as seen by a dramatic increase in GFAP-positive cells.9 Despite inhibiting microglial activation, minocycline had no effect on astrocyte proliferation in Tsc1GFAPCKO mice as assayed by GFAP immunohistochemistry (Fig. 4), indicating that minocycline is selective for microglia. In addition, consistent with previous studies,9; 10 Tsc1GFAPCKO mice had widely dispersed pyramidal cell layers compared with control mice, but minocycline treatment had no effect (CA pyramidal layer width: 68.1 ± 3.2 μm in control mice; 113.6 ± 3.2 μm in vehicle-treated Tsc1GFAPCKO mice; 108.1 ± 3.9 μm in minocycline-treated Tsc1GFAPCKO mice; p<0.001 by ANOVA). Inflammatory markers, such as elevated cytokine and chemokine expression, are seen in Tsc1GFAPCKO mice, which may be primarily localized to astrocytes,8 but are often also expressed by microglia. Minocycline had no effect on chemokine and cytokine expression in Tsc1GFAPCKO mice as assessed by quantitative PCR or western blot (Fig. 5).
Figure 4.
Minocycline has no effect on astrocyte proliferation in Tsc1GFAPCKO mice. Vehicle-treated Tsc1GFAPCKO mice (KO + Veh) displayed a diffuse increase in GFAP-positive cells (green) in neocortex and hippocampus, compared with vehicle-treated control mice (Cont + Veh). Minocycline treatment (50 mg/kg/day, i.p. for four weeks starting at 3 weeks of age) had no effect in preventing this increase in GFAP-positive cells in Tsc1GFAPCKO mice (KO + Mino). *p<0.05, **p<0.01, *** p<0.001 versus vehicle-treated control mice by one-way ANOVA (n = 5 mice per group). Cont = control mice, KO = Tsc1GFAPCKO mice, Veh = vehicle, Mino = Minocycline, DG = dentate gyrus, CA1 = CA1 pyramidal cell layer of hippocampus.
Figure 5.
Minocycline has no effect on the elevated inflammatory markers in Tsc1GFAPCKO mice. (A) mRNA expression of cytokines and chemokines was evaluated by quantitative RT-PCR in the brains of Tsc1GFAPCKO and control mice. The mRNA levels of CCL2, IL-1beta, and CXCL10 were increased in four-week old Tsc1GFAPCKO mice compared with control mice. Minocycline treatment (50 mg/kg/day, i.p. for one week starting at 3 weeks of age) had no effect on the elevated cytokine and chemokine levels of Tsc1GFAPCKO mice (n = 9–11 mice/group). (B) Protein expression of CXCL10 was assessed by western blotting in the brains of Tsc1GFAPCKO mice, and the effect of minocycline was tested. Vehicle-treated Tsc1GFAPCKO mice have significantly increased CXCL10 levels compared with control mice. Minocycline treatment (50 mg/kg/day, i.p. for one week starting at 3 weeks of age) had no significant effect on CXCL10 levels in Tsc1GFAPCKO mice (7 mice/group). The ratio of CXCL10/beta-actin was normalized to the vehicle-treated control group. * p<0.05, *** p<0.001, versus vehicle-treated control mice by one-way ANOVA. Cont = control mice, KO = Tsc1GFAPCKO mice, Veh = vehicle, Mino = Minocycline.
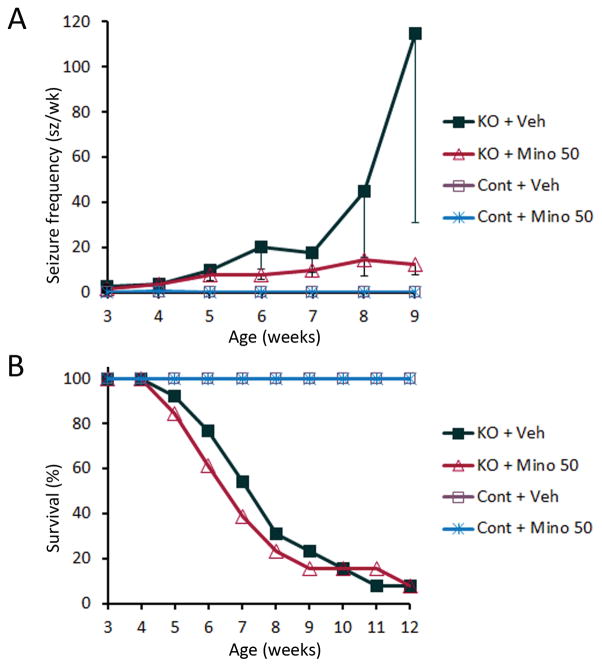
Minocycline has no effect on progression of seizures or survival in Tsc1GFAPCKO mice
Tsc1GFAPCKO mice exhibit progressive epilepsy, with onset of seizures around 3–4 weeks of age followed by a progressive increase in seizures, leading to premature death typically by 3 months of age.10; 12 Despite inhibiting microglial activation at early stages, minocycline (50 mg/kg) had no effect in preventing epilepsy, as all Tsc1GFAPCKO mice in both the minocycline-treated and vehicle-treated groups developed seizures (13 of 13 mice in both groups). Although there was a trend towards decreased seizure frequency in the minocycline group at 8–9 weeks of age, 50 mg/kg minocycline had no significant effect on seizure frequency or survival of Tsc1GFAPCKO mice (Fig. 6), suggesting that inhibition of early microglial activation does not prevent progressive epileptogenesis in Tsc1GFAPCKO mice. By comparison, vehicle or minocycline-treated (50 mg/kg) control mice had normal survival and no seizures, except very rare post-op seizures.
Figure 6.
Minocycline has no effect on the development of seizures and survival in Tsc1GFAPCKO mice. (A) Seizures start to develop in vehicle-treated Tsc1GFAPCKO mice (KO + Veh) around 3 weeks and become progressively more frequent with age. Minocycline treatment (50 mg/kg/d i.p. starting at 3 weeks of age (KO + Mino) had no significant effect on seizure frequency in Tsc1GFAPCKO mice compared with vehicle-treated Tsc1GFAPCKO mice (n = 13 mice per group). Vehicle- and minocycline-treated control mice essentially had no seizures, except very rare postoperative seizures in the first week after surgery. (B) Survival analysis showed that vehicle-treated Tsc1GFAPCKO mice die prematurely with 50% mortality around 7 weeks of age and 100% mortality by 13 weeks. Minocycline treatment (50 mg/kg/day, i.p. starting at 3 weeks of age) had no significant effect on the survival of Tsc1GFAPCKO mice compared to vehicle treated Tsc1GFAPCKO mice (n = 13 mice per group). Vehicle- and minocycline-treated control mice had normal survival. KO = Tsc1GFAPCKO mice, Veh = vehicle, Mino = minocycline.
Discussion
The present study demonstrates for the first time evidence of microglial activation in a mouse model of TSC. Tsc1GFAPCKO mice, which involve conditional inactivation of the Tsc1 gene primarily in astrocytes, exhibit increased number and size of Iba1 positive cells, which are reversed by minocycline, consistent with microglial activation. While minocycline could inhibit microglial activation during early epileptogenesis, it had no significant effect on the development or progression of epilepsy in Tsc1GFAPCKO mice, suggesting that microglia are not necessary for epileptogenesis in Tsc1GFAPCKO mice. Further studies are required to demonstrate the pathophysiological role of microglia in other animal models and in human TSC.
Microglia serve as the resident macrophages of the brain that mediate innate immunity and inflammatory reactions in the brain, such as in response to central nervous infection and other brain injuries.15; 16 Furthermore, microglia are also involved in more basic aspects of brain development and function, including synaptogenesis and synaptic pruning.17 However, in some disease states, microglia may create pathological conditions that promote neurological dysfunction, including seizures. Microglial activation commonly occurs in patients and animal models of temporal lobe epilepsy.18; 19 Pharmacological inhibition of microglia with minocycline decreases epileptogenesis in models of temporal lobe epilepsy.20; 21 In TSC patients, abnormalities in microglia have been reported in cortical tuber specimens,6; 7 but there is minimal information on the potential role and mechanisms of microglia in epileptogenesis in TSC. The present study indicates that microglia are abnormally activated in Tsc1GFAPCKO mice, as indicated by increases in both cell size and number. However, while minocycline was effective in inhibiting microglial activation during early epileptogenesis, it had no significant effect on progression of epilepsy, suggesting that microglial activation is not necessary for epileptogenesis in Tsc1GFAPCKO mice.
Although we originally hypothesized that microglia might contribute, at least secondarily, to epileptogenesis in Tsc1GFAPCKO mice, it is not that surprising that inhibition of microglial activation by minocycline had no apparent effect on epilepsy in these mice. Tsc1GFAPCKO mice involve targeted inactivation of the Tsc1 gene predominantly in astrocytes, with some additional involvement of neurons due to GFAP expression in embryonic neuroprogenitor cells.22 However, as GFAP is not expressed in microglia,23 GFAP-mediated Tsc1 inactivation should not directly target microglial cells. Therefore, the microglial activation observed in Tsc1GFAPCKO mice almost certainly represents a secondary process due to primary astrocyte or neuronal abnormalities or possibly due to seizures themselves. The earliest increase in microglia observed at 3 weeks coincides with the development of astrocyte proliferation and onset of epilepsy, so it is difficult to distinguish cause and effect based on the temporal relationship of these events alone. However, a number of abnormalities have been identified in astrocytes in Tsc1GFAPCKO mice that likely contribute to epileptogenesis, including inflammatory cytokine and chemokine expression.8; 24–27 Minocycline had no effect on cytokine and chemokine expression, suggesting that microglia may not contribute to this inflammatory response in Tsc1GFAPCKO mice. On the other hand, it’s possible that minocycline could inhibit microglia activation, at least as assayed by change in morphology or number, without affecting cytokine or chemokine secretion. Furthermore, the effect of minocycline on microglial activation diminished by 7 weeks of age when the mice were having frequent seizures, suggesting that seizures themselves might exacerbate microglial activation at later times and make them more resistant to minocycline. In contrast, mTOR inhibition with rapamycin can reverse or prevent astrocytic abnormalities and correspondingly prevent epilepsy,10 again suggesting that astrocytic mechanisms may be more central to epileptogenesis than microglia in Tsc1GFAPCKO mice.
Future studies utilizing other modulations of microglia and other TSC animal models or human TSC specimens may be required to more thoroughly investigate a potential role of microglia in the pathogenesis of epilepsy and other neurological manifestations in TSC. Human pathological studies have documented both astrocyte and microglia abnormalities in tubers,6; 7; 28 but proving a primary role in the pathogenesis of neurological manifestations of TSC is difficult with human tissue specimens. Microglial abnormalities have not been reported in other TSC animal models involving Tsc gene inactivation specifically in neurons or neuroglial progenitor cells.29–31 The most direct approach to test for a primary role of microglia would be to create a conditional knockout mice targeting Tsc1 or Tsc2 inactivation specifically to microglia cells32; 33, which is currently under development in our lab and shows promising preliminary results. Future studies with microglia-specific TSC knockout mice may establish a more primary contribution of microglia to epileptogenesis or other neurological phenotypes in TSC.
Key Points.
Microglial activation correlates with seizure onset in Tsc1GFAPCKO mice, a mouse model of tuberous sclerosis complex (TSC).
Minocycline inhibits microglial activation, but not astrocyte proliferation or inflammatory chemokine production, in Tsc1GFAPCKO mice.
Minocycline has no effect in slowing the progression of epilepsy in Tsc1GFAPCKO mice.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 NS056872 to MW and S10 RR027552 to Washington University) and the Department of Defense Tuberous Sclerosis Complex Research Program (W81XWH-12-1-0190 to MW), and by the Alafi Neuroimaging Lab at Washington University.
Footnotes
Disclosures
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiMario FJ, Jr, Sahin M, Ebrahimi-Fakhari D. Tuberous Sclerosis Complex. Pediatr Clin North Am. 2015;62:633–648. doi: 10.1016/j.pcl.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Chu-Shore CJ, Major P, Camposano S, et al. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia. 2008;49:8–21. doi: 10.1111/j.1528-1167.2007.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boer K, Jansen F, Nellist M, et al. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 2008;78:7–21. doi: 10.1016/j.eplepsyres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Boer K, Troost D, Jansen F, et al. Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology. 2008;28:577–590. doi: 10.1111/j.1440-1789.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Zou J, Rensing NR, et al. Inflammatory mechanisms contribute to the neurological manifestations of tuberous sclerosis complex. Neurobiol Dis. 2015;80:70–79. doi: 10.1016/j.nbd.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhlmann EJ, Wong M, Baldwin RL, et al. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 10.Zeng LH, Xu L, Gutmann DH, et al. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pong WW, Higer SB, Gianino SM, et al. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73:303–308. doi: 10.1002/ana.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erbayat-Altay E, Zeng LH, Xu L, et al. The natural history and treatment of epilepsy in a murine model of tuberous sclerosis. Epilepsia. 2007;48:1470–1476. doi: 10.1111/j.1528-1167.2007.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng LH, Rensing NR, Zhang B, et al. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet. 2011;20:445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yrjanheikki J, Keinanen R, Pellikka M, et al. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devinsky O, Vezzani A, Najjar S, et al. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36:174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 18.Najjar S, Pearlman D, Miller DC, et al. Refractory epilepsy associated with microglial activation. Neurologist. 2011;17:249–254. doi: 10.1097/NRL.0b013e31822aad04. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia. 2008;49(Suppl 2):33–41. doi: 10.1111/j.1528-1167.2008.01491.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang N, Mi X, Gao B, et al. Minocycline inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neuroscience. 2015;287:144–156. doi: 10.1016/j.neuroscience.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Abraham J, Fox PD, Condello C, et al. Minocycline attenuates microglia activation and blocks the long-term epileptogenic effects of early-life seizures. Neurobiol Dis. 2012;46:425–430. doi: 10.1016/j.nbd.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser MM, Zhu X, Kwon CH, et al. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–7779. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 23.Cerbai F, Lana D, Nosi D, et al. The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PLoS One. 2012;7:e45250. doi: 10.1371/journal.pone.0045250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong M, Ess KC, Uhlmann EJ, et al. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- 25.Jansen LA, Uhlmann EJ, Crino PB, et al. Epileptogenesis and reduced inward rectifier potassium current in tuberous sclerosis complex-1-deficient astrocytes. Epilepsia. 2005;46:1871–1880. doi: 10.1111/j.1528-1167.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Zeng LH, Wong M. Impaired astrocytic gap junction coupling and potassium buffering in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2009;34:291–299. doi: 10.1016/j.nbd.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng LH, Bero AW, Zhang B, et al. Modulation of astrocyte glutamate transporters decreases seizures in a mouse model of Tuberous Sclerosis Complex. Neurobiol Dis. 2010;37:764–771. doi: 10.1016/j.nbd.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sosunov AA, Wu X, Weiner HL, et al. Tuberous sclerosis: a primary pathology of astrocytes? Epilepsia. 2008;49(Suppl 2):53–62. doi: 10.1111/j.1528-1167.2008.01493.x. [DOI] [PubMed] [Google Scholar]
- 29.Meikle L, Talos DM, Onda H, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carson RP, Van Nielen DL, Winzenburger PA, et al. Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiol Dis. 2012;45:369–380. doi: 10.1016/j.nbd.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goto J, Talos DM, Klein P, et al. Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc Natl Acad Sci U S A. 2011;108:E1070–1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wieghofer P, Knobeloch KP, Prinz M. Genetic targeting of microglia. Glia. 2015;63:1–22. doi: 10.1002/glia.22727. [DOI] [PubMed] [Google Scholar]
- 33.Wolf Y, Yona S, Kim KW, et al. Microglia, seen from the CX3CR1 angle. Front Cell Neurosci. 2013;7:26. doi: 10.3389/fncel.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]