Summary
The prevailing paradigm is that tuberculosis infection is initiated when patrolling alveolar macrophages and dendritic cells within the terminal alveolus ingest inhaled M. tuberculosis (Mtb). However, definitive data for this model are lacking. Among the epithelial cells of the upper airway a specialized epithelial cell known as a microfold cell (M-cell) overlies various components of mucosa associated lymphatic tissue. Here we show using multiple mouse models that Mtb invades via M-cells to initiate infection. Intranasal Mtb infection in mice lacking M-cells either genetically or by antibody depletion resulted in reduced invasion and dissemination to draining lymph nodes. M-cell depleted mice infected via aerosol also had delayed dissemination to lymph nodes and reduced mortality. Translocation of Mtb across two M-cell transwell models was rapid and transcellular. Thus, M-cell translocation is a vital entry mechanism that contributes to the pathogenesis of Mtb.
Graphical abstract
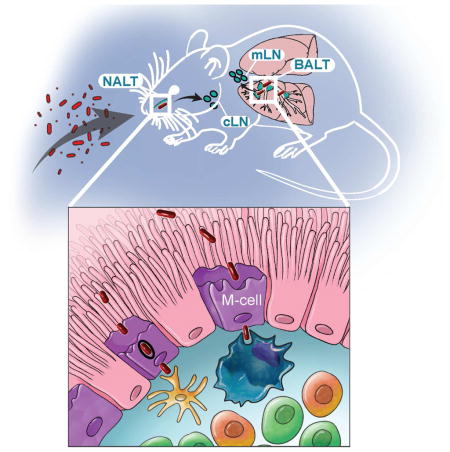
Introduction
Mtb (Mtb) causes 8 million cases of tuberculosis and 1.3 million deaths worldwide annually. Although person-to-person Mtb transmission requires an obligate airborne route, once inhaled the precise mechanism used by Mtb to penetrate the mucosal barrier remains unknown. Importantly, about 10% of all cases of active tuberculosis involve isolated infection of cervical lymph nodes (LN) suggesting that pulmonary infection per se may not be required for infection. This form of tuberculosis, also called scrofula commonly manifests in children (Fontanilla et al., 2011).
When airborne particles are inhaled, medium and large particles are trapped in the nasopharyngeal and tracheobronchial region, while smaller particles can reach the distal lung (Roy and Milton, 2004). Mtb particles range in size from 0.65 (small) to >7.0 μm (medium-large) (Fennelly et al., 2004). Thus, some Mtb-containing particles are likely trapped in the proximal airway while other particles distribute distally. Although the current paradigm is that tuberculosis is initiated by primary infection of alveolar macrophages (AMs), alternative routes of entry such as via epithelial cells or via mucosa-associated lymphatic tissue (MALT) have been proposed (Behr and Waters, 2014; Bermudez and Goodman, 1996).
Oropharyngeal and airway MALT is prevalent in childhood including nasal-associated lymphatic tissue (NALT), the tonsils and adenoids of Waldeyer’s ring and bronchus-associated lymphatic tissue (BALT), but tends to regress in adulthood (Debertin et al., 2006). Whether BALT is present in adults is controversial (Randall, 2010); however, BALT-like structures, also known as induced BALT (iBALT) (Foo and Phipps, 2010), can be induced by bacterial and viral infections (Halle et al., 2009). Overlying MALT is a rare, specialized cell called a microfold or M-cell whose primary function is to deliver mucosal particles to submucosal antigen presenting cells (Mabbott et al., 2013). M-cells are found primarily in the gastrointestinal tract overlying Peyer’s patches. M-cells can also be found in the upper airway overlying NALT, adenoids, and BALT. Though significantly less is known about airway than gastrointestinal M-cells (Kanaya and Ohno, 2014), a recent study demonstrated shared expression of vital differentiation molecules in mouse NALT M-cells (Mutoh et al., 2015).
Airway M-cells can mediate infection by bacteria such as Streptococcus pyogenes (Park et al., 2003) and Bacillus anthracis (Plaut et al., 2012). Infection of cattle with Mycobacterium bovis can be achieved by direct inoculation of bovine tonsils (Palmer et al., 2007), and drinking unpasteurized milk contaminated with M. bovis can cause human tuberculosis that frequently manifests as cervical lymphadenitis (Cosivi et al., 1998). At the cellular level, Mtb (Kumagai, 1922), Mycobacterium bovis var Bacillus Calmette-Guerin (BCG) (Fujimura, 1986) and M. avium (Secott et al., 2004) can translocate across Peyer’s patches and it was reported over 15 years ago that in experimentally infected mice Mtb is occasionally found inside cells with the morphologic appearance of M-cells (Teitelbaum et al., 1999). However, a functional analysis of M-cell mediated translocation of Mtb has not been performed. Here we show that airway M-cells can directly mediate primary infection by Mtb and facilitate dissemination beyond the mucosa.
Results
Genetic depletion of M-cells reduces mycobacterial dissemination to cervical lymph nodes
To determine if murine NALT inoculation is sufficient to initiate infection with Mtb, we established an intranasal infection model and found that doses of 106 CFU or greater could lead to consistent dissemination to the draining cervical lymph nodes (cLN) 30 days after infection (Supplemental Fig. 1, related to Fig. 1). Using a small volume (10 μl) isolated the initial inoculum to the NALT (Supplemental Fig. 1). The earliest time after infection we could detect Mtb in the cLN was 7 days. Between day 0 (inoculum) and day 7, Mtb were rarely recovered from the lungs, and at day 7 dissemination occurs prior to the onset of cell-mediated immunity. Thus, Mtb can disseminate from the NALT to initiate infection.
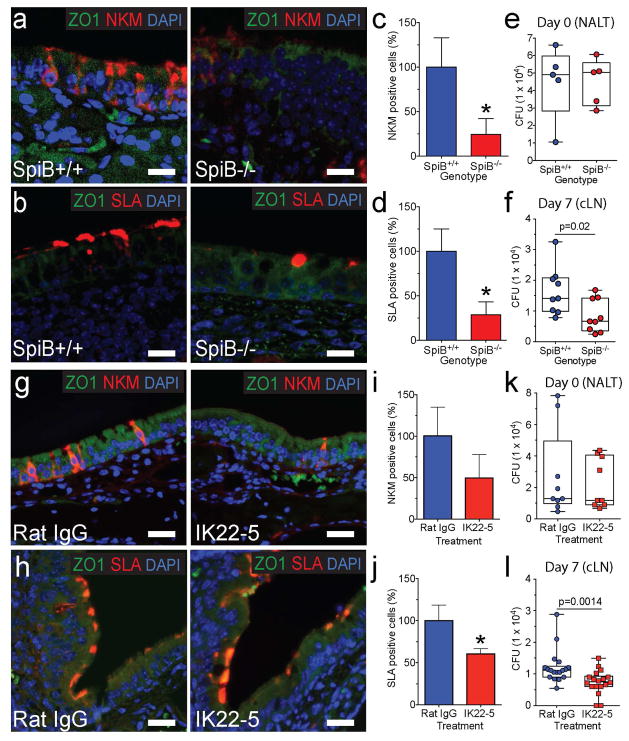
Fig. 1. Genetic and antibody-mediated loss of M-cells reduces Mtb translocation to cervical lymph nodes.
a,b, NALT from Spi-B+/+ (WT) and Spi-B−/− mice was stained with anti-ZO1 (cell marker) and NKM 16-2-4 (a, top) or anti-SLA (b, bottom). Scale bars are 20 μm. c, d, M-cells were quantified in the NALT of Spi-B+/+ and Spi-B−/− mice. The number of M-cells in WT mice was averaged and used to normalize percent positive cells for individual mice (n=3 mice/group). *p<0.05 compared to WT by Student’s t-test. e, f WT or Spi-B−/− mice were intranasally infected with Mtb and CFU determined in the NALT at day 0 (e) (n=5/group) and in LN at day 7 (f) (n=9/group). g, h NALT from control and IK22-5 treated BALB/c mice was stained with anti-ZO1 and NKM 16-2-4 (g, top) or anti-SLA (h, bottom). Scale bars are 20 μm. i, j M-cells were quantified in the NALT of wild-type and IK22-5 treated mice as above. *p<0.05 compared to WT by Student’s t-test. k, l Mice were intranasally infected with Mtb and CFU determined in the NALT at day 0 (k) (n=9/treatment) and in LN at day 7 (l) (n=18/treatment). Results are pooled from two independent infection experiments per mouse model. Statistical significance was determined by the Mann-Whitney U test.
Development of mouse NALT M-cells was recently associated with expression of the transcription factor Spi-B and receptor activator of nuclear factor kappa-B ligand (RANKL) signaling (Mutoh et al., 2015). Because mice deficient in the transcription factor Spi-B lack Peyer’s patch M-cells (Kanaya et al., 2012) we examined the NALT of Spi-B−/− mice (Su et al., 1997) to determine if they also lack NALT M-cells. Although several cell surface markers have been used to identify Peyer’s patch M-cells, those for NALT M-cells are less well characterized. Therefore, we tested several established M-cell markers and found that the antibodies NKM 16-2-4 (Nochi et al., 2007), anti-Sialyl Lewis A (SLA) (Giannasca et al., 1999) identified two populations of M-cells on diffuse or organized NALT respectively (Supplemental Fig 1). By immunohistochemistry, NALT NKM 16-2-4 staining was similar to a previous report (Nochi et al., 2007) and anti-SLA staining identified cell surface staining of dome-shaped cells characteristic of M-cells (Supplemental Fig 1). By immunofluorescence microscopy (Fig. 1a,b), Spi-B−/− mice had a significant reduction in M-cells (Fig. 1c,d), with a 75% reduction in cells detected by NKM 16-2-4 overlying the diffuse NALT and an 80% reduction in SLA-positive cells overlying the organized NALT. Because Spi-B deficient mice have pleiotropic immune defects (Garrett-Sinha et al., 1999), we limited our Mtb NALT infection to 7 days post infection. Although the initial inocula in the NALT were the same for Spi-B−/− mice and wild-type littermates (Fig 1e), by 7 days Spi-B−/− mice had significantly fewer bacteria in cLN (Fig 1f). No bacteria were isolated from the lungs of either wild-type or Spi-B−/− mice. Thus, we conclude that genetic inhibition of M-cell differentiation decreased Mtb translocation and dissemination to draining cervical lymph nodes.
Antibody mediated depletion of M-cells in wild-type mice reduces mycobacterial dissemination to cervical lymph nodes
To determine if depletion of M-cells in wild-type mice affects Mtb infection via NALT, we took advantage of the rat anti-mouse RANKL monoclonal antibody IK22-5, which can deplete M-cells in the gastrointestinal tract (Knoop et al., 2009). We injected Balb/c mice intraperitoneally with the IK22-5 mAb or an isotype-matched nonspecific rat IgG2a mAb control (control Ig) every 2 days for 8 days (Knoop et al., 2009) and collected NALT 24 h after the last treatment or infected the mice with Mtb. IK22-5 treatment resulted in the expected loss of Peyer’s patch M-cells (data not shown). NALT M-cells were reduced by immunofluorescence to ~50% of wild-type using the NKM16-2-4 and anti-SLA antibodies (Fig. 1g–j) which was not as robust as the ~90% reduction in gastrointestinal M-cells reported previously (Knoop et al., 2009). A similar ~60% reduction of NALT M-cells was observed by immunofluorescence microscopy using an antibody against another M-cell marker, glycoprotein 2 (GP2) (Mutoh et al., 2015) (Supplemental Fig 1). Following intranasal infection of Mtb, equal numbers of bacteria were enumerated from the NALT of M-cell depleted mice at time zero (Fig. 1k), but at 7 days these mice demonstrated an ~50% reduction in CFU in the cLN (Fig. 1l). Thus, depletion of NALT M-cells by IK22-5 treatment reduced Mtb dissemination to cLN.
Dendritic cells have been proposed to transport Mtb to draining lymph nodes (Wolf et al., 2008) and thus an alternative explanation for the reduced cLN dissemination might be that IK22-5 treatment depleted NALT DCs. However, using flow cytometry we found that IK22-5 treatment did not affect the absolute number of CD11c+ DCs in the NALT (Supplemental Fig. 2, related to Fig. 1). As an additional control for potential M-cell independent affects of IK22-5 treatment, we infected IK22-5 treated mice intravenously with 107 CFU Mtb via retro-orbital injection. The dose was chosen to approximate the intranasal dose, and the route was chosen for anatomic proximity to the NALT. Both initial seeding of lungs and NALT and CFU 7 days after infection in lung, liver, spleen and lymph node were equal between groups (Supplemental Fig. 2).
M-cells translocate mycobacteria in vitro
To test the ability of M-cells to translocate Mtb in vitro, we used an established model of M-cell differentiation that relies on coculturing human Caco-2 epithelial cells with RajiB cells on two sides of a porous transwell (Supplemental Fig 3, related to Fig 2) (Kerneis et al., 1997). Whereas Caco-2 cells cultured alone form a homogenous impermeable polarized monolayer (hereafter called “control”), coculture with RajiB cells induces some Caco-2 cells to differentiate into M-cells (hereafter called RajiB). We validated the model and found that monolayers were impermeable to fluorescently labeled dextrans as small as 2 kD, that Raji-B treated cells translocated significantly more inert beads than control cells and that RajiB cocultured cells increased the number of cells lacking microvilli and demonstrated a flatter and smoother surface consistent with M-cells (Supplemental Fig 3).
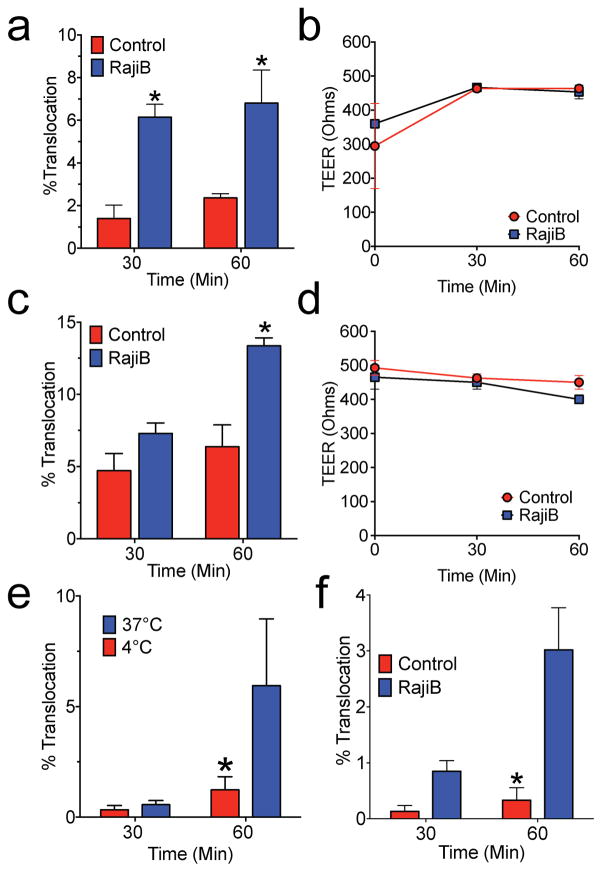
Fig. 2. Mycobacteria translocate across M-cells in vitro.
a,b M. marinum or Mtb (c,d) were added to the apical compartment of Caco-2 transwells with or without coculture with RajiB cells. Percent translocation was determined by dividing CFU recovered from the basolateral compartment by the apical compartment inoculum (a,c) and TEER (b,d) was measured directly. e, Mtb was added to RajiB treated transwells maintained at either 4 or 37°C and CFU enumerated as above. f, Human bronchial epithelial (HBE) cells were treated as above and Mtb translocation determined. Experiments are representative of at least 3 independent experiments. * p<0.05 by Student’s t-test compared to control.
We next measured the translocation of mycobacteria across control and RajiB cocultured cells. When M-cell formation was enhanced by the presence of Raji-B cells, significantly more Mycobacterium marinum (Fig 2a,b) and Mtb (Fig 2c,d) translocated across the transwell. Neither organism damaged the monolayer, as TEER was stable during translocation (Fig 2b,d). Mtb translocation did not occur by passive diffusion through intercellular tight junctions or through small leaks in the monolayer, as translocation of Mtb was significantly diminished at 4°C (Fig 2e). To confirm the role of airway M-cells in Mtb translocation, we established a second M-cell model of translocation using the human bronchial epithelial cell line 16HBE14o- (hereafter called HBE cells) (Cozens et al., 1994). We grew HBE cells on 3.0 μm transwells with and without RajiB cell coculture, and found that they behaved similarly as Caco-2 cells with respect to adherence, TEER development over time, bead translocation, Gram positive and Gram negative bacterial translocation and surface morphologic changes in response to RajiB coculture (Supplemental Fig 4, related to Fig 2). Importantly, as for Caco-2 cells, coculture with RajiB cells significantly increased Mtb translocation (Fig 2f). Finally, we studied Mtb translocation across control and RajiB cocultured Caco-2 cells by electron microscopy. Using scanning electron microscopy, we observed only rare Mtb associated with the surface microvilli on control Caco-2 transwells (Fig 3a). In contrast, many more bacteria were observed on the surface of RajiB-cocultures, predominantly on the flattened, non-villous M-cells (Fig. 3a). Occasionally we also observed groups of bacteria overlying 5–10 μm defects in the cell surface, suggesting a possible mode of entry via phagocytosis or pore formation (Fig 3a, right panel). Using transmission electron microscopy, we found Mtb entering and exiting cells, apparently surrounded by a membrane (Fig 3b). Thus, we conclude that Mtb translocated across M-cells in vitro.
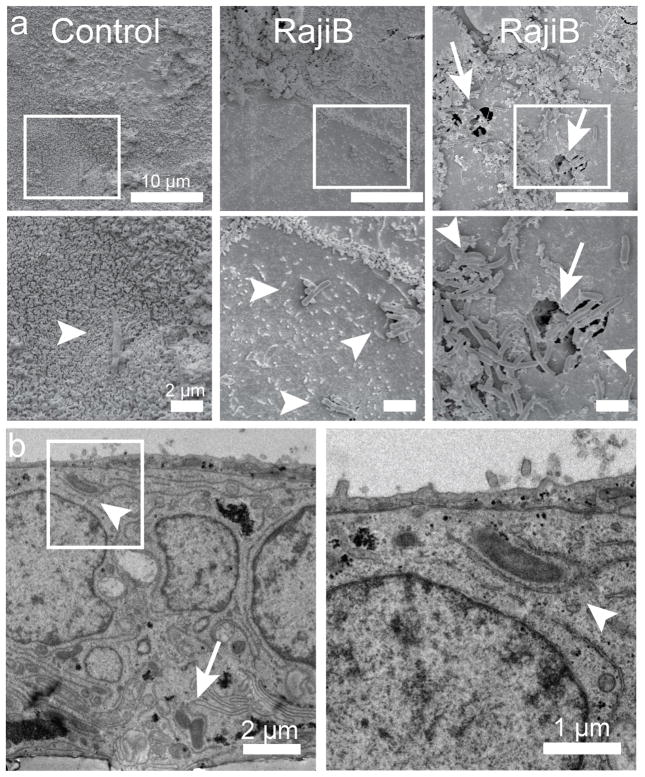
Fig. 3. Electron microscopy of Mtb transcytosis by M-cells in tissue culture.
Control or RajiB-co-cultured transwells were infected with Mtb for 30 minutes, fixed and imaged by scanning (a) and transmission (b) electron microscopy. Boxes (a, top) are magnified in lower panels (a, bottom). a, Rare Mtb (arrowheads) were observed overlying cells with microvilli in control wells. In RajiB cocultures, bacteria were visualized predominantly on M-cells. Occasionally, large clumps of bacteria were observed entering large cell surface pores (arrow). Scale bars are 10 μm (a, top), 2 μm (a, bottom and panel b, left) and 1 μm (panel b, right). In (b), only RajiB co-cultured cells are shown. Entering (arrowhead) and exiting (arrow) bacteria can be observed inside an M-cell. Results are representative images from two independent experiments.
Antibody mediated depletion of M-cells prevents Mtb dissemination and delays mortality during aerosol infection
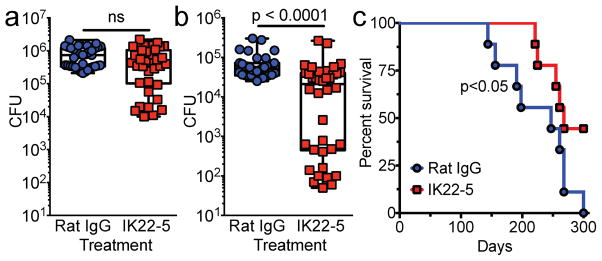
M-cells can be found not only in the NALT, where they are concentrated and easily identified, but also in the BALT. To determine if airway M-cells can mediate Mtb dissemination via BALT and whether they are important for mouse survival, we conducted a low-dose aerosol infection (~200 CFU of Mtb) of IK22-5 treated mice and determined CFU in the lungs and mediastinal lymph nodes at day zero and 18 days after infection. The 18-day time point was chosen as it represents an early time point for detection of Mtb in draining mediastinal lymph nodes (Wolf et al., 2008). Despite having an equal number of bacteria in the lungs at day zero (not shown), at day 18 there was a trend towards reduced Mtb CFUs in the lungs and liver, though this was not statistically significant (Fig 4a and not shown). In contrast, we found an approximately one-log reduction of Mtb dissemination to mediastinal LN (mLN) (Fig 4b). We also observed a bimodal distribution in the mLN of IK22-5 treated mice, with about half of the mice showing a 2–3 log10 reduction in CFUs, suggesting the possibility of a bottleneck effect. An alternative explanation for the reduced LN dissemination is that IK22-5 treatment prevented the development or recruitment of Ly6C+/CD11b+ inflammatory monocytes (IMs) to the lung, as IMs have been reported to transport Mtb from the lungs to draining lymph nodes (Samstein et al., 2013). In uninfected mice, less than 0.1% of the cells in the NALT were Ly6C+/CD11b+ (data not shown). Pulmonary IM recruitment during aerosol Mtb infection was unaffected by IK22-5 treatment (Supplementary Fig 2, related to Fig 4). Thus, IK22-5-mediated depletion of M-cells prevented Mtb dissemination beyond the oropharynx and airways to draining lymph nodes.
Fig. 4. M-cell depletion in mice prevents mediastinal lymph node dissemination and prolongs survival during aerosol infection.
a–c, Balb/c mice (n=35–40/group in total) were aerosol infected with ~200 CFU Mtb and CFU in lung (a) or mediastinal LN (b) measured 18 days later. Results are pooled from 4 independent experiments conducted with 8–10 mice per treatment. c, Mice infected as above were monitored for survival (n=10/group). For CFU, statistical significance was determined by the Mann-Whitney U test. For survival, Kaplan-Meier analysis was performed.
In conjunction with the CFU studies, we infected IK22-5 treated mice via aerosol and monitored mice for survival. Surprisingly, survival from Mtb infection was significantly improved (Fig 4c), suggesting that translocation via M-cells and dissemination beyond the lungs has a profound impact on Mtb pathogenesis.
Discussion
Our findings reveal that M-cells are a portal of entry for Mtb. Using two different mouse models of M-cell loss, namely Spi-B deficiency and IK22-5 mediated depletion as well as two different infection models we show that a reduction in M-cells is associated with reduced lymph node dissemination, and in the low-dose aerosol model is also associated with improved survival. M-cell mediated entry may not only be relevant for Mtb, but also for other respiratory pathogens that can disseminate from the respiratory tree to cause systemic disease such as S. aureus and B. anthracis, the causative agent of anthrax.
It was demonstrated previously that cells with the morphology of respiratory M-cells harbor Mtb during experimental infection (Teitelbaum et al., 1999). In that study, Mtb translocation to paratracheal lymph nodes was rapid (i.e. occurring within 24 hours) and dependent on the presence of macrophages. Macrophage deficient Csfmop/Csfmop mice were more susceptible to Mtb disease despite reduced lymph node involvement but equal lung CFUs, as they succumbed to experimental aerosol infection with 103 CFU significantly earlier than wild-type control mice (Teitelbaum et al., 1999). In contrast to infected Csfmop/Csfmop mice, M-cell depleted mice had significantly delayed mortality as compared to wild-type mice, suggesting that reducing dissemination via M-cells in the context of a maintained cell-mediated immune response is protective during murine tuberculosis.
Despite reducing the number of airway M-cells, some Mtb was still able to translocate to draining lymph nodes. One potential explanation is that in our hands, loss of Spi-B or treatment with IK22-5 reduced but did not eliminate M-cells, and thus residual M-cells in Spi-B−/− mice or IK22-5 treated mice could be mediating Mtb entry. In that case, it is possible that complete M-cell loss, either via genetic or antibody-mediated depletion, could more significantly protect mice from disease. Alternatively, there may be fewer residual M-cells in Spi-B−/− mice or IK22-5 treated mice than we quantified using NKM 16-2-4, anti-SLA and anti-GP2 antibodies, suggesting an additional M-cell independent mucosal entry route such as via epithelial cells (Bermudez and Goodman, 1996).
Although the so-called ‘pulmonary model’ of tuberculosis is attractive (Orme, 2014), there is evidence to suggest that initial tuberculosis infection manifests as a lymphatic disease (Behr and Waters, 2014). Interestingly, it was recently shown that lymphatic endothelial cells could be directly infected by Mtb (Lerner et al., 2016), though how extracellular bacteria reach the endothelium is not known. Thus, applying our data to the lymphatic model, we propose that medium and large bacteria-containing droplets can initially attach to the nasopharyngeal and bronchial epithelium, contact and translocate across adjacent M-cells, and then travel to draining lymph nodes, possibly via dendritic cells or inflammatory monocytes. These findings have implications not only for understanding the general pathogenesis of pulmonary tuberculosis, but also for tuberculosis cases like scrofula that occur in the absence of obvious pulmonary involvement. Tuberculosis remains a major global pandemic, and there is an urgent need to understand how Mtb spreads from person to person. A therapeutic approach aimed at preventing early Mtb contact with M-cells could represent a novel vaccination strategy in humans to reduce Mtb infection, dissemination and mortality.
Materials and Methods
Strains and media
Mtb Erdman and M. marinum were grown in Middlebrook 7H9 medium or on Middlebrook 7H11 plates supplemented with 10% oleic acid-albumin-dextrose-catalase. Tween-80 was added to liquid medium to a final concentration of 0.05%. Streptococcus pneumonia and Pseudomonas aeruginosa were clinical isolates obtained from the microbiology laboratory and grown in Brain-Heart-Infusion broth or LB respectively.
Mice
Spi-B−/− mice (Su et al., 1997) on a C57BL/6J background were obtained from the Sinha lab (State University of New York at Buffalo, NY) and housed under specific pathogen-free conditions. Mice were bred as heterozygotes and age and sex-matched littermates were used for experiments. BALB/c mice (6–8 week females) were obtained from Jackson Labs. Animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas (UT) Southwestern.
Statistical analysis
Statistical analysis was performed using GraphPad Prism. For in vitro studies, one-tailed Analysis of Variance (ANOVA) tests were used for experiments with multiple comparisons using Dunnett’s test. For experiments with single comparisons, two-tailed unpaired Student’s t-test was used. For in vivo CFU measurements, the non-parametric Mann-Whitney U test was used. Kaplan-Meier analysis was used to analyze survival studies.
Supplementary Material
Acknowledgments
We thank R. Sumpter for help with microscopy and the UTSW Microscopy core for assistance with Electron Microscopy. We thank L. Desvignes and J. Ernst (NYU) for sharing their flow cytometry protocol for pulmonary cells. This work was supported by NIH grants R01 AI099439 and R21 AI111023 (M.U.S.), U19 AI109725 (B.L and M.U.S.), and T32AI005284 (V.M.Z., C.E.S). M.U.S. is a Disease Oriented Clinical Scholar at UT Southwestern Medical Center.
Footnotes
Author Contributions
V.R.N., L.H.F., C.E.S., H.K. and V.M.Z. performed mouse intranasal and aerosol infections with Mtb. V.R.N. and D.K.M. performed immunofluorescence microscopy. V.R.N. developed the in vitro M-cell model and performed all of the mycobacterial translocation experiments. W.Y. performed bead assays. H.Y. provided reagents and was involved in study design. B.L. was involved in study design. V.R.N. and M.U.S. developed the hypothesis, designed and analyzed the study and wrote the paper. All authors discussed and commented on the manuscript.
Author information
The authors declare no competing financial interests.
Supplementary Information includes 4 figures, supplementary experimental procedures, and references.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Behr MA, Waters WR. Is tuberculosis a lymphatic disease with a pulmonary portal? The Lancet infectious diseases. 2014;14:250–255. doi: 10.1016/S1473-3099(13)70253-6. [DOI] [PubMed] [Google Scholar]
- Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, Cousins D, Robinson RA, Huchzermeyer HF, de Kantor I, Meslin FX. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerging infectious diseases. 1998;4:59–70. doi: 10.3201/eid0401.980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. American journal of respiratory cell and molecular biology. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- Debertin AS, Tschernig T, Schurmann A, Bajanowski T, Brinkmann B, Pabst R. Coincidence of different structures of mucosa-associated lymphoid tissue (MALT) in the respiratory tract of children: no indications for enhanced mucosal immunostimulation in sudden infant death syndrome (SIDS) Clin Exp Immunol. 2006;146:54–59. doi: 10.1111/j.1365-2249.2006.03190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennelly KP, Martyny JW, Fulton KE, Orme IM, Cave DM, Heifets LB. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am J Respir Crit Care Med. 2004;169:604–609. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- Fontanilla JM, Barnes A, von Reyn CF. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:555–562. doi: 10.1093/cid/cir454. [DOI] [PubMed] [Google Scholar]
- Foo SY, Phipps S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal immunology. 2010;3:537–544. doi: 10.1038/mi.2010.52. [DOI] [PubMed] [Google Scholar]
- Fujimura Y. Functional morphology of microfold cells (M cells) in Peyer’s patches--phagocytosis and transport of BCG by M cells into rabbit Peyer’s patches. Gastroenterologia Japonica. 1986;21:325–335. [PubMed] [Google Scholar]
- Garrett-Sinha LA, Su GH, Rao S, Kabak S, Hao Z, Clark MR, Simon MC. PU.1 and Spi-B are required for normal B cell receptor-mediated signal transduction. Immunity. 1999;10:399–408. doi: 10.1016/s1074-7613(00)80040-0. [DOI] [PubMed] [Google Scholar]
- Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR. Human intestinal M cells display the sialyl Lewis A antigen. Infect Immun. 1999;67:946–953. doi: 10.1128/iai.67.2.946-953.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hammerling G, Garbi N, Sutter G, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya T, Hase K, Takahashi D, Fukuda S, Hoshino K, Sasaki I, Hemmi H, Knoop KA, Kumar N, Sato M, et al. The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nature immunology. 2012;13:729–736. doi: 10.1038/ni.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya T, Ohno H. The Mechanisms of M-cell Differentiation. Bioscience of microbiota, food and health. 2014;33:91–97. doi: 10.12938/bmfh.33.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, Akiba H, Yagita H, Kiyono H, Williams IR. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–5747. doi: 10.4049/jimmunol.0901563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K. Uber den Resorptionsvergang der corpuscularen Bestandteile im Darm. Kekkaku-Zassi (Japan) 1922;4:429–431. [Google Scholar]
- Lerner TR, de Souza Carvalho-Wodarz C, Repnik U, Russell MR, Borel S, Diedrich CR, Rohde M, Wainwright H, Collinson LM, Wilkinson RJ, et al. Lymphatic endothelial cells are a replicative niche for Mycobacterium tuberculosis. The Journal of clinical investigation. 2016;126:1093–1108. doi: 10.1172/JCI83379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal immunology. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh M, Kimura S, Takahashi-Iwanaga H, Hisamoto M, Iwanaga T, Iida J. RANKL regulates differentiation of microfold cells in mouse nasopharynx-associated lymphoid tissue (NALT) Cell Tissue Res. 2015 doi: 10.1007/s00441-015-2309-2. [DOI] [PubMed] [Google Scholar]
- Nochi T, Yuki Y, Matsumura A, Mejima M, Terahara K, Kim DY, Fukuyama S, Iwatsuki-Horimoto K, Kawaoka Y, Kohda T, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med. 2007;204:2789–2796. doi: 10.1084/jem.20070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme IM. A new unifying theory of the pathogenesis of tuberculosis. Tuberculosis (Edinb) 2014;94:8–14. doi: 10.1016/j.tube.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MV, Waters WR, Thacker TC. Lesion development and immunohistochemical changes in granulomas from cattle experimentally infected with Mycobacterium bovis. Veterinary pathology. 2007;44:863–874. doi: 10.1354/vp.44-6-863. [DOI] [PubMed] [Google Scholar]
- Park HS, Francis KP, Yu J, Cleary PP. Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A streptococcus. J Immunol. 2003;171:2532–2537. doi: 10.4049/jimmunol.171.5.2532. [DOI] [PubMed] [Google Scholar]
- Plaut RD, Kelly VK, Lee GM, Stibitz S, Merkel TJ. Dissemination bottleneck in a murine model of inhalational anthrax. Infect Immun. 2012;80:3189–3193. doi: 10.1128/IAI.00515-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Advances in immunology. 2010;107:187–241. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CJ, Milton DK. Airborne transmission of communicable infection--the elusive pathway. N Engl J Med. 2004;350:1710–1712. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- Samstein M, Schreiber HA, Leiner IM, Susac B, Glickman MS, Pamer EG. Essential yet limited role for CCR2(+) inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. eLife. 2013;2:e01086. doi: 10.7554/eLife.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secott TE, Lin TL, Wu CC. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect Immun. 2004;72:3724–3732. doi: 10.1128/IAI.72.7.3724-3732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su GH, Chen HM, Muthusamy N, Garrett-Sinha LA, Baunoch D, Tenen DG, Simon MC. Defective B cell receptor-mediated responses in mice lacking the Ets protein, Spi-B. EMBO J. 1997;16:7118–7129. doi: 10.1093/emboj/16.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum R, Schubert W, Gunther L, Kress Y, Macaluso F, Pollard JW, McMurray DN, Bloom BR. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity. 1999;10:641–650. doi: 10.1016/s1074-7613(00)80063-1. [DOI] [PubMed] [Google Scholar]
- Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.