Abstract
Rate of nicotine metabolism has been identified as an important factor influencing nicotine intake and can be estimated using the nicotine metabolite ratio (NMR), a validated biomarker of CYP2A6 enzyme activity. Individuals who metabolize nicotine faster (higher NMR) may alter their smoking behavior to titrate their nicotine intake in order to maintain similar levels of nicotine in the body compared to slower nicotine metabolizers. There are known racial differences in the rate of nicotine metabolism with African Americans on average having a slower rate of nicotine metabolism compared to Whites. The goal of this study was to determine if there are racial differences in the relationship between rate of nicotine metabolism and measures of nicotine intake assessed using multiple biomarkers of nicotine and tobacco smoke exposure. Using secondary analyses of the screening data collected in a recently completed clinical trial, treatment-seeking African American and White daily smokers (10 or more cigarettes per day) were grouped into NMR quartiles so that the races could be compared at the same NMR, even though the distribution of NMR within race differed. The results indicated that rate of nicotine metabolism is a more important factor influencing nicotine intake in White smokers. Specifically, Whites were more likely to titrate their nicotine intake based on the rate at which they metabolize nicotine. However, this relationship was not found in African Americans. Overall there was a greater step down, linear type relationship between NMR groups and cotinine or cotinine/cigarette in African Americans, which is consistent with the idea that differences in blood cotinine levels between the African American NMR groups were primarily due to differences in CYP2A6 enzyme activity without titration of nicotine intake among faster nicotine metabolizers.
Keywords: nicotine metabolism, nicotine intake, nicotine titration, biomarkers, racial differences, African American smokers
1. INTRODUCTION
Smokers can manipulate both the number of cigarettes per day (CPD) they consume, and how they smoke a cigarette, to titrate their nicotine intake to obtain desired rewards and prevent withdrawal symptoms.1–2 Furthermore, differences in rates of nicotine metabolism have been found to affect nicotine intake. Approximately 70–80% of nicotine is metabolized into cotinine by the liver enzyme CYP2A6.3 Cotinine is also metabolized via CYP2A6 to trans-3′ hydroxycotinine (3HC). The ratio of metabolite to parent (3HC/cotinine), termed the nicotine metabolite ratio (NMR) is a validated biomarker for CYP2A6 activity.4 A higher NMR indicates greater CYP2A6 enzyme activity (i.e., faster rate of nicotine metabolism). Previous research indicates that individuals with faster versus slower rates of nicotine metabolism smoke more cigarettes per day.5–7 Additionally, individuals who metabolize nicotine faster may alter their nicotine intake by smoking cigarettes more intensively compared to slow metabolizers, who can take in less nicotine to maintain the same nicotine levels in the body.8–9 This would suggest that smokers in general are titrating their nicotine intake to maintain desired levels in the brain.10
On average, African American smokers have lower NMRs (slower rate of nicotine metabolism) and higher cotinine levels than White smokers.11–13 While previous research in Whites indicates that faster nicotine metabolizers smoke more cigarettes per day, it remains unclear if this relationship occurs in African Americans as well. 5–7 African Americans smoke fewer cigarettes per day but tend to smoke those cigarettes more intensely than Whites and as a consequence take in more nicotine per cigarette.11, 14 In addition, African American light smokers with slower nicotine metabolism were found to have higher plasma nicotine levels compared to faster metabolizers.15 This suggests that African Americans may not titrate nicotine intake as closely based on rate of nicotine metabolism compared to what has been reported in White smokers. However, this study15 was only in African Americans, without a direct comparison between African Americans and Whites in terms of the relationship between NMR and nicotine intake from cigarette smoking.
Differences in motivations for cigarette smoking may contribute to racial differences in tobacco use characteristics. Two distinct types of cigarette smokers have been described. One includes smokers who seek intermittent high blood levels of nicotine (termed “peak-seekers”), presumably motivated primarily by the positive reinforcing effects of nicotine. A second type includes those who seek to maintain steady levels of nicotine throughout the day (termed “trough-maintainers”), presumably motivated primarily to avoid nicotine withdrawal symptoms and/or to obtain other desirable effects of persistently desensitizing nicotinic receptors.16–17 We hypothesize that African Americans are more likely to be “peak-seekers,” smoking primarily to achieve a high nicotine boost (for positive reinforcement), while Whites, who smoke more frequently are more likely to be “trough-maintainers,” smoking primarily to maintain consistent nicotine levels throughout the day. If one is smoking to maintain a steady level of nicotine in the body, this level is influenced substantially by the rate of nicotine metabolism. Thus, Whites would be more likely to titrate their nicotine intake to maintain a certain optimal level of nicotine. If one is smoking to achieve a particular peak level of nicotine after smoking a cigarette, this level is minimally influenced by the rate of nicotine metabolism. Thus, African American smoking would be less influenced by the rate of nicotine metabolism. Understanding racial differences in the relationship between nicotine metabolism and smoking behavior has important implications for understanding racial disparities in efficacy of smoking cessation interventions and prevention strategies.
The primary aim of our analysis was to examine racial differences in the relationship between rate of nicotine metabolism and measures of nicotine intake. As “trough-maintainers,” we hypothesized that, within Whites, individuals who are faster metabolizers of nicotine would show greater nicotine intake to achieve nicotine levels similar to Whites who are slow metabolizers. This would indicate a strong titration effect. On the other hand, we hypothesized that in African American smokers, titration would be weaker, reflecting similar nicotine intake between African Americans with different rates of nicotine metabolism.
First we evaluated racial differences in the relationship between nicotine metabolism and smoke exposure using cigarettes per day (CPD) and expired carbon monoxide (CO). Since CPD is not the most sensitive measure of nicotine exposure we also evaluated racial differences in the relationship between nicotine metabolism and two biomarkers of nicotine exposure: plasma cotinine and plasma [cotinine + 3HC] concentrations. Cotinine is the most widely used biomarker of daily nicotine intake. However cotinine levels in relation to daily nicotine intake are influenced by CYP2A6 enzyme activity. Nicotine’s metabolism to cotinine, and cotinine’s metabolism to 3HC are both mediated largely by CYP2A6. On balance, lower CYP2A6 activity results in slower metabolism of cotinine to 3HC compared to the rate of generation of cotinine from nicotine, such that for any given levels of nicotine intake cotinine levels would be expected to be higher in slower compared to faster metabolizers.18–19 Taking the sum of cotinine and 3HC helps to compensate for individual differences in CYP2A6 activity. Empirical studies show that the sum of cotinine and 3HC in plasma correlates more strongly with daily nicotine intake compared to cotinine alone.20
One would expect that if daily nicotine intake is similar, individuals with faster (versus slower) rate of nicotine metabolism (higher NMR) would have lower levels of cotinine but the same levels of cotinine + 3HC. We hypothesized that this relationship would be found in African Americans (similar nicotine intake between NMR groups) but not in Whites (individuals with higher NMR take in more nicotine).
2. METHODS
2.1 Overview of study design
The parent study was a randomized, placebo controlled clinical trial examining the efficacy of nicotine replacement therapy versus varenicline for smoking cessation in a sample stratified by NMR. Results from the parent trial are published elsewhere.21 The data for this secondary analysis were taken from the screening visit, prior to determination of eligibility for the clinical trial.
2.2 Participants
As described previously,22 participants were recruited to participate in a free smoking cessation trial. The study consisted of participants aged 19–65 who smoked at least 10 cigarettes per day, had a exhaled breath carbon monoxide (CO) level of > 10ppm, and were interested in quitting smoking. Participants were excluded if they had substance abuse or dependence, use of contraindicated medications (e.g., smoking cessation medication), had a history of psychiatric disorder (e.g., bipolar, major depression, or suicide attempt) or were unwilling to reside in the area for the following 12 months. African Americans and Whites who completed the screening assessment for the clinical trial were included in the current analyses, even if not included in the final intent to treat sample. Because the main clinical trial included a sample that was stratified based on NMR, the screening sample was used for the current analyses as it better represents the distribution of NMR found in the population. The study population used in the current analyses consisted of 591 self-identified African Americans and 1102 Whites. Participants indicating mixed ethnicity were excluded from the present analysis (N= 17).
2.3 Measures
The NMR was determined as the ratio of free (unconjugated) 3HC/free cotinine in plasma. This ratio is a validated biomarker of CYP2A6 metabolic activity and nicotine clearance.4 We assessed the level of nicotine exposure in each group with the following variables: Cigarettes per day (CPD), expired carbon monoxide (CO), and biomarkers of nicotine (cotinine, and the molar sum of cotinine+3HC). CPD was assessed by self-report and CO was assessed using a CO breath meter. Biomarkers of nicotine exposure (cotinine, and the molar sum of cotinine+3HC) were also examined using the plasma sample provided by the participant.
CO, cotinine and cotinine + 3HC were examined in two ways: as an absolute level assessed, and as amount per cigarette smoked. These two analyses provide different information: (1) absolute value of the biomarkers were used to determine if individuals were adjusting their daily nicotine intake to maintain a similar optimal desired level of nicotine between NMR quartiles; and (2) biomarkers corrected for CPD were used as an estimate of intensity of smoking individual cigarettes, that is, nicotine/tobacco exposure per cigarette smoked.
2.4 Data analysis
Cut-offs for NMR quartiles were generated from the combined baseline screening sample of African American and White smokers. This was done so that races could be compared at the same NMR quartile, even though the distribution of NMR within race differed and, thus, the number of people in each quartile differed by race.
Biomarkers and tobacco use variables were initially analyzed using ANOVAs with race, sex, and NMR quartile as predictors. Although there were significant main effects for sex, there were no significant interactions between sex and NMR quartile or three-way interactions between NMR quartile, race, and sex. Therefore, subsequent analyses included sex in the ANOVA model but are presented collapsed by sex. Significant interactions between race and NMR quartile for each dependent variable were examined by determining if there were differences: (1) Between NMR quartile within each race, and (2) Between races for each NMR group. For within race comparisons, ANOVAs were used to determine if there was a significant main effect of NMR quartile within race, followed by Bonferroni post hoc comparisons between NMR quartiles within race. For the between race comparisons, t-tests were used to compare races at each NMR quartile. Effects were considered significant at an alpha level of 0.05 or less. Statistical analyses were performed using SPSS 22 (IBM Corporation, Armonk, NY, USA).
3. RESULTS
3.1 Demographic characteristics
Baseline demographic and smoking history information is provided in Table 1. The average cigarettes per day (CPD) was 16 for African Americans and 20 Whites. Although African Americans were more likely to smoke within 5 minutes of waking (47% of African Americans vs. 32% of Whites), nicotine dependence scores were similar between the races (Fagerström Test of Cigarette Dependence = 5.25).
Table 1.
Baseline smoking characteristics and biomarkers in African Americans and Whites by NMR quartile.
| Whites | African Americans | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Variable (N) | Q1 (194) |
Q2 (277) |
Q3 (306) |
Q4 (325) |
Total (1102) |
Q1 (230) |
Q2 (148) |
Q3 (115) |
Q4 (98) |
Total (598) |
t-statistic† (p-value) |
| Age | 44.6 ± 12.2 | 44.3 ± 11.6 | 46.1 ± 10.7 | 46.7 ± 11.2 | 45.6 ± 11.4 | 45.6 ± 10.5 | 44.8 ± 11.1 | 46.9 ± 10.1 | 49.7 ± 8.8 | 46.3 ± 10.4 | 1.33 (1.81) |
|
| |||||||||||
| % Female | 34.5% | 35.7% | 48.7% | 48.6% | 42.9% | 43.9% | 49.3% | 56.5% | 55.1% | 49.6% | 7.32 (0.007) |
|
| |||||||||||
| ≥High school education | 94.8% | 96.4% | 94.4% | 93.2% | 94.6% | 86.1% | 87.2% | 90.4% | 93.9% | 88.5% | 22.05† (<0.001) |
|
| |||||||||||
| Income > US $50, 000 | 50.0% | 52.0% | 52.5% | 45.5% | 49.9% | 17.4% | 18.8 % | 13.9 % | 14.4 % | 16.6% | 179.02† (<0.001) |
|
| |||||||||||
| CPD | 19.0 ± 6.0 | 19.7 ± 7.3 | 20.6 ± 7.3 | 20.2 ± 7.4 | 20.0 ± 7.1 | 15.9 ± 6.0 | 16.0 ± 6.1 | 16.4 ± 5.9 | 16.4 ± 6.6 | 16.1 ± 6.1 | −11.78 (<0.001) |
|
| |||||||||||
| Expired CO (ppm) | 24.0 ± 10.0 | 23.4 ± 9.7 | 24.9 ± 10.2 | 24.1 ± 10.5 | 24.1 ± 10.1 | 21.7 ± 9.9 | 22.2 ± 10.6 | 22.2 ± 11.3 | 22.3 ± 10.0 | 22.0 ± 10.4 | −3.95 (<0.001) |
|
| |||||||||||
| Menthol smoker* | 18.8% | 25.2% | 23.5% | 24.0% | 22.9% | 87.6% | 86.0% | 79.7% | 75.5% | 84.4% | 429.80† (<0.001) |
|
| |||||||||||
| FTCD score | 5.2 ± 2.1 | 5.3 ± 1.9 | 5.5 ± 1.9 | 5.3 ± 2.0 | 5.3 ± 2.0 | 5.3 ± 1.9 | 5.3 ± 1.6 | 5.2 ± 2.1 | 4.8 ± 2.0 | 5.2 ± 1.9 | −1.21 (0.227) |
|
| |||||||||||
| TTFC | |||||||||||
| Within 5 min | 32.0% | 30.7% | 32.7% | 32.3% | 31.9 % | 42.6% | 51.4% | 53.9% | 41.8% | 46.9 % | 38.21 (<0.001) |
| 6–30 min | 45.4% | 52.0% | 50.0% | 49.8% | 49.6 % | 41.7% | 37.8% | 30.4% | 36.7% | 37.7 % | |
| 31–60 min | 16.0% | 12.3% | 13.1% | 10.2% | 12.5 % | 10.9% | 7.4% | 8.7% | 11.2% | 9.6 % | |
| After 60 min | 6.7% | 5.1% | 4.2% | 7.7% | 5.9 % | 4.8% | 3.4% | 7.0% | 10.2% | 5.8 % | |
|
| |||||||||||
| NMR | .17 ± .05 | .30 ± .03 | .41 ± .04 | .66 ± .18 | 0.41 ± 0.20 | .16 ± .05 | .29 ± .03 | .41 ± .04 | .69 ± .22 | 0.33 ± 0.21 | −8.19 (<0.001) |
|
| |||||||||||
| Blood cotinine (ng/mL) | 239.8 ± 101.6 | 252.4 ± 113.0 | 246.5 ± 105.3 | 214.1 ± 99.1 | 237.2 ± 105.9 | 300.0 ± 148.1 | 269.4 ± 121.3 | 253.4 ± 111.0 | 206.2 ± 103.5 | 267.7 ± 131.9 | 5.16 (<0.001) |
|
| |||||||||||
| Blood 3HC (ng/mL) | 42.0 ± 21.1 | 75.9 ± 35.5 | 101.4 ± 45.1 | 138.2 ± 70.9 | 95.4 ± 59.9 | 49.2 ± 30.7 | 78.1 ± 34.4 | 102.3 ± 43.3 | 134.9 ± 69.1 | 81.0 ± 52.7 | −5.13 (<0.001) |
|
| |||||||||||
| Blood cotinine + 3HC (nmols/mL) | 1.6 ± 0.7 | 1.8 ± 0.8 | 1.9 ± 0.8 | 1.9 ± 0.9 | 1.8 ± 0.8 | 2.0 ± 1.0 | 1.9 ± 0.9 | 2.0 ± 0.9 | 1.9 ± 0.9 | 1.9 ± 0.9 | 2.27 (0.02) |
|
| |||||||||||
| Blood cotinine/ CPD | 13.2 ± 5.5 | 13.7 ± 6.6 | 12.7 ± 5.6 | 11.5 ± 6.2 | 12.7 ± 6.1 | 20.5 ±11.1 | 19.1 ± 12.2 | 17.0 ± 8.9 | 13.7± 7.6 | 18.3 ± 10.7 | 13.77 (<0.001) |
|
| |||||||||||
| Blood [Cotinine + 3HC]/ CPD | .09 ± .04 | .10 ± .05 | .10 ± .04 | .10 ± .06 | 0.10 ± 0.05 | .13 ± .07 | .14 ± .09 | .13 ± .07 | .12 ± .07 | 0.13 ± 0.07 | 11.40 (<0.001) |
Note. T-tests were calculated comparing total Caucasian vs African American smokers.
= χ2 used for categorical variables. Means are presented ± standard deviation.
Menthol smoking was not assessed in all participants and prevalence of use is based on 474 African Americans and 704 Whites. Q = Nicotine metabolite ratio (NMR) quartile based on the full sample; CPD= Cigarettes per day; CO= Carbon Monoxide in parts per million; FTCD = Fagerström Test for Cigarette Dependence; TTFC = Time to first cigarette after waking; NMR= Nicotine Metabolite Ratio; 3HC= trans 3′ hydroxyl cotinine.
It should be noted that menthol cigarette data were not included in the baseline data collected at the screening visit. Thus, the menthol data presented in Table 1 are based on 1178 participants (African Americans, N= 474; Whites, N= 704), who were participants selected for low and high NMR and enrolled in the parent clinical trial study. Our data are consistent with previous literature indicating that African Americans are more likely to be menthol cigarette smokers than Whites. There is also an interesting trend among African Americans indicating that a greater percentage of slow metabolizers (NMR Q1) are menthol smokers (86%) compared to faster metabolizers (NMR Q4; 75%); χ2 (1, N=340) = 5.04, p = 0.03). This relationship was not significant among White smokers.
3.2 NMR quartiles
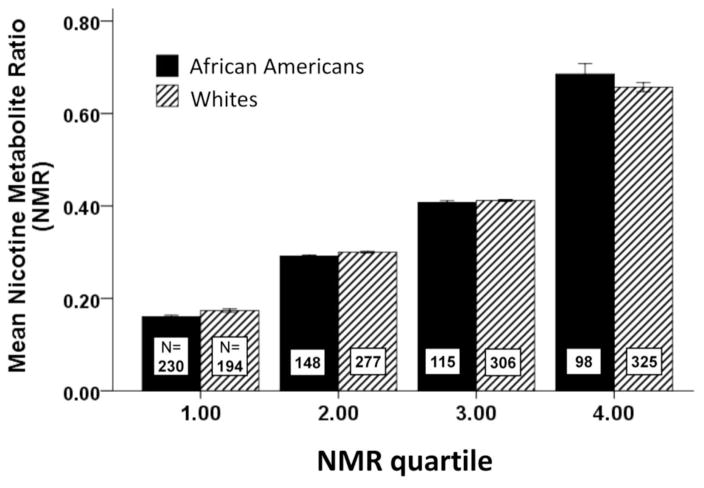
The mean NMR values for African Americans and Whites for each NMR quartile are presented in Figure 1. The cut-offs for each quartile were: Q1 (NMR 0.240), Q2 (NMR≤0.241–0.350, Q3 (NMR=0.35–0.485), Q4 (NMR≥0.486) using the NMR quartiles based on the full sample. Consistent with a previous study22, the distribution of NMR values within race was different, which is also shown in Figure 1. Overall, there was a greater distribution of Whites in the higher NMR quartile vs. the lower NMR quartiles, with the opposite for African Americans. However, African Americans and Whites have similar mean NMR values within each NMR quartile. Thus, using NMR quartiles derived by the combined race sample corrects for the difference in distribution of NMR within race and was used in the subsequent analyses. Demographics, tobacco use characteristics, and biomarkers of exposure by NMR quartile within African Americans and Whites are presented in Table 1.
Figure 1.
Mean NMR level for African Americans and Whites within each NMR quartile. NMR quartiles were based on the full sample of African Americans and Whites. The distribution of participants within each NMR quartile is represented by the N’s shown within each bar. Distribution of African Americans and Whites across the NMR quartiles based on the combined sample
3.3 Cigarettes per day (CPD)
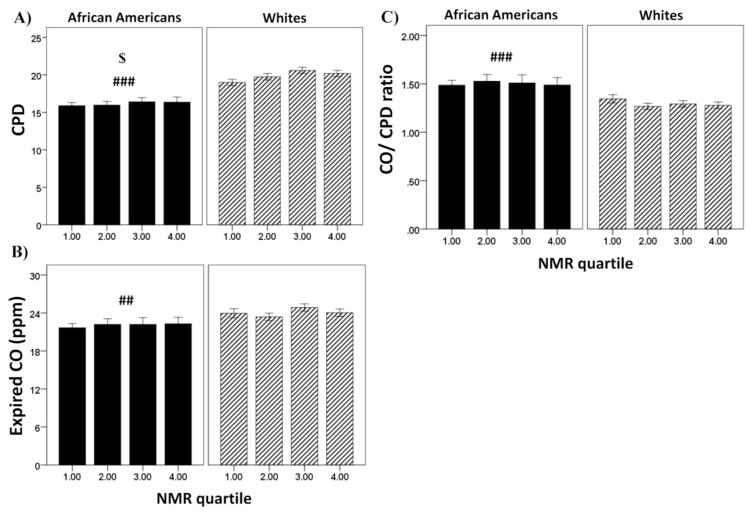
For CPD (Figure 2A), there was a significant main effect of both NMR quartile (F(3, 1677)=2.70, p<0.05, partial η2= .005) and race (F(1, 1677)=93.04, p<0.001, partial η2= .053); African Americans smoked fewer CPD than Whites (16.1 ± 0.3 vs. 19.9 ± 0.2) and, overall, higher NMR was associated with smoking more CPD (1st =17.4 ±0.3, 2nd = 17.9 ±0.3; 3rd = 18.5 ±0.4; 4th = 18.3 ±0.4). However, there was not a significant interaction between NMR quartile and race for CPD.
Figure 2.
Relationship between NMR quartile and tobacco use characteristics in African Americans and Whites. Shown are the means ± SEM for (A) CPD, (B) expired CO, and (C) expired CO per cigarette. Data are combined for men and women as sex did not significantly influence the results. No significant interactions by NMR quartile. Because there were no interactions, main effects of race (###)= p<0.001, (##)= p<0. 01) and NMR quartile ($)= p<0.05) are presented in the figure.
3.4 Carbon Monoxide (CO)
There was a significant main effect of race for expired CO (F(1, 1677)=10.36, p=0.001, partial η2= .006; Fig. 2B) but no main effect or interaction with NMR quartile. Overall, African Americans had lower levels of expired CO compared to Whites (22.1±0.4 vs. 24.1±0.3).
Overall, similar results were found when looking at levels of each biomarker per cigarette smoked. There was a significant main effect of race for expired CO/cigarette (F(1, 1677)=30.98, p<0.001; Figure 2C), but no main effect or interaction with NMR quartile. Overall, African Americans were taking in more CO/cigarette (1.50 ±0.03 vs. 1.30 ±0.02).
3.5 Cotinine
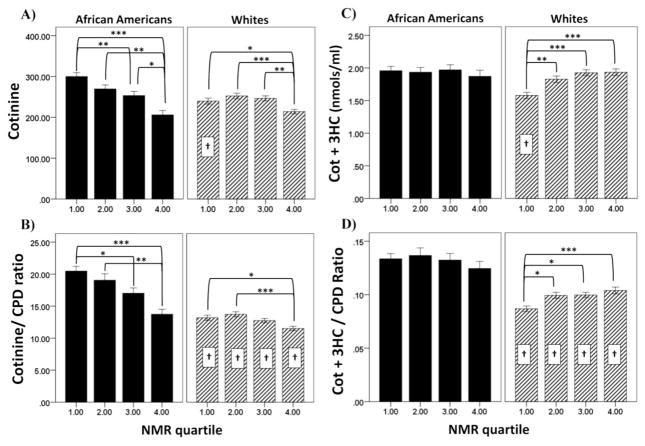
There were differences by race in the relationship between NMR quartile and the biomarkers of exposure. For cotinine (Figure 3A), there was a significant interaction between race and NMR quartile (F(3, 1677)=6.40, p<0.001, partial η2= .011). Within African Americans there was a step-down relationship between NMR quartile and cotinine; lower NMR quartiles had higher cotinine levels. Within Whites, only the 4th NMR quartile had significantly lower cotinine levels compared to the other NMR quartiles. The only significant differences between African Americans and Whites for cotinine level was found in the 1st NMR quartile; African Americans in the 1st NMR quartile had higher cotinine levels (p<0.001) compared to Whites in the first NMR quartile.
Figure 3.
Relationship between NMR quartile and biomarkers of nicotine exposure in African Americans and Whites. Shown are the means ± SEM for (A) cotinine, (B) [COT+3HC], (C) cotinine per cigarette, and (D) [COT+3HC] per cigarette. Biomarkers are measured in blood. Data are combined for men and women as sex did not significantly influence the results. Significance of within race comparisons between NMR quartiles are indicated by: (*)= p<0.05, (**)= p<0.01, and(***)= p<0.001. Significance for the comparison between African Americans and Whites for the individual NMR quartile are indicated by: (†)= p<0.01.
There was a significant interaction between race and NMR quartile for cotinine/cigarette (F(3, 1677)=6.00, p<0.001; Figure 3B.) Overall, African Americans had a larger decrease in cotinine/ cigarette by NMR quartile compared to Whites. Within African Americans, there was a step down relationship between NMR quartile and cotinine/cigarette. Within Whites, lower cotinine per cigarette was only found in the 4th NMR quartile compared to the 1st and 2nd quartiles. African Americans had higher cotinine/cigarette at each NMR quartile compared to Whites.
3.6 Cotinine + 3HC
There was a significant interaction between race and NMR quartile for [cotinine + 3HC] (F(3, 1677)=4.72, p<0.01; Figure 3C), a marker of total nicotine exposure. In African Americans there was no change in [cotinine + 3HC] across NMR quartiles. In Whites, on the other hand, those in the 1st NMR quartile had significantly lower levels of [cotinine + 3HC] than NMR quartiles 2–4. African Americans in the 1st NMR quartile had higher levels [cotinine + 3HC] compared to Whites in the 1st NMR quartile.
There was also a significant interaction between race and NMR quartile for [cotinine + 3HC]/cigarette smoked (F(3, 1677)=2.97, p<0.05; Figure 3D). African Americans at each NMR quartile had higher levels of [cotinine + 3HC]/cigarette compared to Whites in the same NMR quartile.
4. DISCUSSION
Using a large sample of White and African American daily smokers (10 or more CPD), our analyses determined that there are racial differences in the relationship between rate of nicotine metabolism (NMR) and biomarkers of nicotine exposure. Rate of nicotine metabolism appears to be a more important factor influencing nicotine intake in Whites smokers. Specifically, Whites appear to be more likely to titrate their nicotine intake [cotinine + 3HC] based on the rate at which they metabolize nicotine compared to African Americans. Furthermore, it has been posited that Whites who take in more nicotine are doing so to consistently maintain a specific level of systemic nicotine (aka “trough maintainers”), while African Americans tend to be “peak seekers” and smoke for the positive reward of nicotine from each cigarette and not to maintain a consistent level of nicotine in the blood stream.16 Although we did not see any effect on CPD or CO, our biomarker analysis supports this hypothesis. Whites may be titrating nicotine levels for a desired “maintenance” effect, while African Americans who are more likely to be “peak seekers” are less likely to maintain consistent levels of nicotine and thus nicotine intake would be less influenced by rate of nicotine metabolism.
Overall there was a greater step down, linear type relationship between NMR groups and cotinine or cotinine/cigarette in African Americans vs. Whites, as seen in Figure 3. Based on previous work by Zhu et al.18, we expected that cotinine/cigarette would decrease as rate of nicotine metabolism increases (higher NMR). The African American NMR groups did not differ in the sum of cotinine + 3HC or in [cotinine + 3HC]/cigarette. This is consistent with the idea that differences in blood cotinine levels between the African American NMR groups were primarily due to differences in CYP2A6 enzyme activity. This relationship was not found in Whites. Whites who are faster nicotine metabolizers appear to titrate their nicotine intake to compensate for rate of nicotine metabolism to maintain desired levels of nicotine. Since we do not see significant differences in CPD or CO by NMR quartile in Whites, this effect is likely due to differences in intensity of smoking, as CPD and CO are not sensitive measures of nicotine exposure.23 NMR was unrelated to nicotine intake in African Americans, suggesting no titration.
Participants’ nicotine dependence and use of menthol cigarettes also provided interesting insights that warrant further exploration. First, African Americans were more likely to smoke their first cigarette within the first 5 minutes of waking, which is suggestive of greater physical dependence.24 One might expect that smoking sooner after waking (TTFC) would be expected to be associated with trough maintaining, however, African Americans did not show a titration effect across NMR quartiles. There was no significant difference between races for FTCD. The higher prevalence of smoking mentholated cigarettes among African Americans compared to Whites is consistent with previous reports.25 Although there were no significant differences in the distribution of menthol cigarette smoking by NMR quartile, there was greater menthol cigarette smoking among African Americans in the 1st NMR quartile (slower nicotine metabolizers) compared to those in the 4th NMR quartile (faster nicotine metabolizers).
Smoking mentholated cigarettes may lead to differences in nicotine intake in several ways. Menthol has been found to have anesthetizing effects that can attenuate tobacco smoke irritants,26 which could allow for deeper inhalation of tobacco smoke (more nicotine per puff). Menthol has also been found to inhibit CYP2A6 activity and decrease rate of nicotine metabolism.27 Smoking mentholated cigarettes may contribute to the overall difference in rate of nicotine metabolism between African Americans and Whites. It is important to note that use of mentholated cigarettes was only assessed in the individuals entering the treatment arm of the study. This limited our ability to use menthol status in the analyses using the full screening sample. The relationship between NMR, menthol status, and nicotine intake warrants further study. This study also supports other research showing that cotinine is a biased biomarker of nicotine exposure when there is variability in CYP2A6 activity. Results from our study suggest that plasma [cotinine+3HC] may be a better biomarker of nicotine exposure as it is less influenced by individual differences in CYP2A6 enzyme activity. However, a potential limitation in the use of the sum of plasma [cotinine + 3HC] to examine racial differences is that there are racial differences in the extent of glucuronidation of cotinine. In addition to the primary C-oxidation pathway of nicotine metabolism, nicotine and cotinine also undergo glucuronidation via UGT (uridine diphosphate-glucuronosyltransferase), which converts them into nicotine and cotinine glucuronides, respectively.28 African Americans, on average, glucuronidate cotinine more slowly than Whites due to genetic differences in UGT2B10 activity.19, 29 In addition, UGT2B17 glucuronidates 3HC, and racial differences in the activity of this enzyme have also been reported.30 However, variation in 3HC glucuronidation was not found to be associated with NMR or nicotine intake31 and we have no reason to believe that there is any association between genetically determined slow glucuronidation and low CYP2A6 activity. Thus, we believe it is valid to use [cotinine + 3HC] as a measure to examine the relationship between NMR and nicotine intake within race.
Our research replicates previous findings that nicotine intake per cigarette is significantly higher in African Americans compared to Whites.11, 14 In addition, this work confirms prior observations that African Americans smoke fewer CPD, have lower CO levels, but take in more CO per cigarette compared to Whites. Overall, African Americans had higher cotinine levels per cigarette smoked compared to Whites. This work confirms prior observations on racial distribution in CYP2A6 activity, with African Americans having on average slower rate of nicotine metabolism.
There are several limitations of this work. First, our participants were treatment-seeking daily smokers (10 or more CPD), whereas African Americans in general tend to be light smokers. Our results may not generalize to light and intermittent smokers, and future research should explore this possibility. In addition, the best biomarker of daily nicotine intake, which is independent of metabolic differences, is the sum of all nicotine metabolites in urine (urinary total nicotine equivalents [TNE]).32 However, we did not collect urine from our participants. Future studies should replicate these findings with TNE.
The implications of our study are as follows. If African Americans are less likely to adjust their smoking behavior based on rate of nicotine metabolism, then African Americans with slower rates of nicotine metabolism would have higher levels of nicotine exposure, which could potentially increase risk for dependence. On the other hand, Whites may be more likely to titrate their nicotine intake based on rate of nicotine metabolism. Whites with faster rates of nicotine metabolism may be at greater risk for higher tobacco smoke exposure to increase nicotine intake, which could potentially put them at greater risk for tobacco related diseases, while the health risks of smoking in African American may be unrelated to the rate of nicotine metabolism.
HIGHLIGHTS.
Rate of nicotine metabolism (NMR) is an important factor influencing nicotine intake
We found that NMR influences nicotine intake more in Whites vs. African American smokers
Whites appear to titrate their nicotine intake based on their NMR
NMR was unrelated to nicotine intake in African Americans, suggesting no titration
Acknowledgments
The parent trial was supported by a grant from the National Institute on Drug Abuse, the National Cancer Institute, the National Human Genome Research Institute, and the National Institute on General Medical Sciences (U01 DA20830) to CL and RFT. Funding also came from the Abramson Cancer Center at the University of Pennsylvania (P30 CA16520), a grant from the Commonwealth of Pennsylvania Department of Health to CL, a grant from the Canadian Institutes of Health Research (CIHR TMH109787), an endowed Chair in Addiction from the Department of Psychiatry, CAMH Foundation, the Canada Foundation for Innovation (#20289, #116014), and the Ontario Ministry of Research and Innovation to RFT. The Pennsylvania Department of Health disclaims responsibility for analyses, interpretations, or conclusions. Pfizer provided varenicline and placebo pills at no cost. KCR and NRG were funded by the National Cancer Institute grant CA 113710.
Footnotes
CONFLICTS OF INTEREST
CL received study medication, placebo, and support for medication packaging for the parent trial from Pfizer. CL has also consulted for Gilead, and has been a paid expert witness in litigation against tobacco companies. PC served on the scientific advisory board of Pfizer, did educational talks on smoking cessation sponsored by Pfizer from 2006-2008, and has received grant support from Pfizer. RAS has received medication and placebo free of charge from Pfizer for different projects, and has consulted for Pfizer and GlaxoSmithKline. TPG has had both investigator-initiated, and industry-sponsored grants from Pfizer in the past 12 months, and serves on a data monitoring committee for Novartis. RFT has acted as consultant to pharmaceutical companies, primarily on smoking cessation. NLB has served as a consultant to several pharmaceutical companies that market smoking cessation medications and has been a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benowitz NL. Compensatory smoking of low-yield cigarettes. In: Shopland DR, Burns DM, Benowitz NL, Amacher RH, editors. Smoking and Tobacco Control Monographs. Monograph 13: Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. Bethesda, MD: National Cancer Institute; 2001. [Google Scholar]
- 2.McMorrow MJ, Foxx RM. Nicotine’s role in smoking: An analysis of nicotine regulation. Psychological Bulletin. 1983;93(2):302–327. doi: 10.1037/0033-2909.93.2.302. [DOI] [PubMed] [Google Scholar]
- 3.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994 Nov;56(5):483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 4.Dempsey D, Tutka P, Jacob P, 3rd, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004 Jul;76(1):64–72. doi: 10.1016/j.clpt.2004.02.011S000992360400102X. [DOI] [PubMed] [Google Scholar]
- 5.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics and Genomics. 2004;14(9):615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003 Oct;5(5):621–624. doi: 10.1080/1462220031000158717. 6DCURVYMPL1C6UQ5. [DOI] [PubMed] [Google Scholar]
- 7.Tyndale RF, Sellers EM. Variable CYP2A6-mediated nicotine metabolism alters smoking behavior and risk. Drug Metab Dispos. 2001 Apr;29(4 Pt 2):548–552. [PubMed] [Google Scholar]
- 8.Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007 Apr;9(4):511–518. doi: 10.1080/14622200701239605. [DOI] [PubMed] [Google Scholar]
- 9.Strasser AA, Benowitz NL, Pinto AG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011 Feb;20(2):234–238. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 Genetic Variation for Smoking Behaviors and Nicotine Dependence. Clinical Pharmacology & Therapeutics. 2005;77(3):145–158. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Stable EJ, Herrera B, Jacob IP, Benowitz NL. NIcotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 12.Shiffman S, Dunbar MS, Benowitz NL. A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer Epidemiol Biomarkers Prev. 2014 Jul;23(7):1264–1272. doi: 10.1158/1055-9965.EPI-13-1014. 1055-9965.EPI-13-1014 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caraballo RS, Holiday DB, Stellman SD, et al. Comparison of serum cotinine concentration within and across smokers of menthol and nonmenthol cigarette brands among non-Hispanic black and non-Hispanic white U.S. adult smokers, 2001–2006. Cancer Epidemiol Biomarkers Prev. 2011 Jul;20(7):1329–1340. doi: 10.1158/1055-9965.EPI-10-1330. 1055-9965.EPI-10-1330 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011 Sep;13(9):772–783. doi: 10.1093/ntr/ntr072. ntr072 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho MK, Mwenifumbo JC, Al Koudsi N, et al. Association of Nicotine Metabolite Ratio and CYP2A6 Genotype With Smoking Cessation Treatment in African-American Light Smokers. Clin Pharmacol Ther. 2009;85(6):635–643. doi: 10.1038/clpt.2009.19. doi: http://www.nature.com/clpt/journal/v85/n6/suppinfo/clpt200919s1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell MA. Cigarette smoking : a dependence on high-nicotine boli. Drug Metab Rev. 1978;8:29–57. doi: 10.3109/03602537808993776. [DOI] [PubMed] [Google Scholar]
- 17.Shiffman S, Dunbar MS, Li X, et al. Smoking Patterns and Stimulus Control in Intermittent and Daily Smokers. PLoS ONE. 2014;9(3):e89911. doi: 10.1371/journal.pone.0089911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu AZX, Renner CC, Hatsukami DK, et al. The Ability of Plasma Cotinine to Predict Nicotine and Carcinogen Exposure is Altered by Differences in CYP2A6: the Influence of Genetics, Race, and Sex. Cancer Epidemiology Biomarkers & Prevention. 2013 Apr 1;22(4):708–718. doi: 10.1158/1055-9965.epi-12-1234-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., 3rd Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999 Dec;291(3):1196–1203. [PubMed] [Google Scholar]
- 20.Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P., 3rd Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010 Dec;19(12):3013–3019. doi: 10.1158/1055-9965.EPI-10-0706. 1055-9965.EPI-10-0706 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerman C, Schnoll RA, Hawk LW, Jr, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. The Lancet Respiratory Medicine. 3(2):131–138. doi: 10.1016/s2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnoll R, George T, Hawk L, Cinciripini P, Wileyto P, Tyndale R. The relationship between the nicotine metabolite ratio and three self-report measures of nicotine dependence across sex and race. Psychopharmacology. 2014 Jun 01;231(12):2515–2523. doi: 10.1007/s00213-013-3421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benowitz NL, Hukkanen J, Jacob P. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. Handbook of experimental pharmacology. 2009;(192):29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker TB, Piper ME, McCarthy DE, et al. Time to First Cigarette in the Morning as an Index of Ability to Quit Smoking: Implications for Nicotine Dependence. Nicotine & Tobacco Research. 2007 Dec 1;9(Suppl 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones MR, Apelberg BJ, Tellez-Plaza M, Samet JM, Navas-Acien A. Menthol Cigarettes, Race/Ethnicity, and Biomarkers of Tobacco Use in U.S. Adults: The 1999–2010 National Health and Nutrition Examination Survey (NHANES) Cancer Epidemiology Biomarkers & Prevention. 2013 Feb 1;22(2):224–232. doi: 10.1158/1055-9965.epi-12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willis DN, Liu B, Ha MA, Jordt S-E, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. The FASEB Journal. 2011 Dec 1;25(12):4434–4444. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benowitz NL, Herrera B, Jacob P. Mentholated Cigarette Smoking Inhibits Nicotine Metabolism. Journal of Pharmacology and Experimental Therapeutics. 2004 Sep 1;310(3):1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- 28.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005 Mar;57(1):79–115. doi: 10.1124/pr.57.1.3. 57/1/79 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Park SL, Stram DO, et al. Associations Between Genetic Ancestries and Nicotine Metabolism Biomarkers in the Multiethnic Cohort Study. American Journal of Epidemiology. 2015 Dec 1;182(11):945–951. doi: 10.1093/aje/kwv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wassenaar CA, Conti DV, Das S, et al. UGT1A and UGT2B genetic variation alters nicotine and nitrosamine glucuronidation in european and african american smokers. Cancer Epidemiol Biomarkers Prev. 2015 Jan;24(1):94–104. doi: 10.1158/1055-9965.EPI-14-0804. 1055-9965.EPI-14-0804 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu AZ, Zhou Q, Cox LS, Ahluwalia JS, Benowitz NL, Tyndale RF. Variation in trans-3′-hydroxycotinine glucuronidation does not alter the nicotine metabolite ratio or nicotine intake. PLoS ONE. 2013;8(8):e70938. doi: 10.1371/journal.pone.0070938PONE-D-13-17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benowitz NL, Dains KM, Dempsey D, Yu L, Jacob P., 3rd Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev. 2010 May;19(5):1160–1166. doi: 10.1158/1055-9965.EPI-09-1303. 19/5/1160 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]