Abstract
Protective postural responses to external perturbations are hypokinetic in people with Parkinson’s disease (PD) and improving these responses may reduce falls. However, the ability of people with PD to improve postural responses with practice is poorly understood. Our objective was to determine whether people with PD can improve protective postural responses similarly to healthy adults through repeated perturbations, and whether improvements are retained or generalize to untrained perturbations. Twelve healthy adults and 15 people with PD underwent 25 forward and 25 backward translations of the support surface, eliciting backward and forward protective steps, respectively. We assessed whether: 1) performance improved over one day of practice, 2) changes were retained 24 hours later, and 3) improvements generalized to untrained (lateral) postural responses. People with PD and healthy adults improved postural response characteristics including center of mass displacement after perturbations (p<0.001), margin of stability at first foot-fall (p=0.001), step latency (p=0.044), and number of steps (p=0.001). However, unlike controls, improvements in people with PD occurred primarily in the first block of trials. Improvements were more pronounced during backward protective stepping than forward, and with the exception of step latency, were retained 24 hours later. Improvements in forward-backward stepping did not generalize to lateral protective stepping. People with PD can improve protective stepping over the course of one day of perturbation practice. Improvements were generally similar to healthy adults, and were retained in both groups. Perturbation practice may represent a promising approach to improving protective postural responses in people with PD, however additional research is needed to understand how to enhance generalization.
Keywords: Parkinson’s disease, motor learning, posture, stepping
INTRODUCTION
Falls are a common and disabling consequence of Parkinson’s disease (PD). Automatic postural responses to loss of equilibrium are critical for fall prevention [27], particularly in people with PD [3]. Such movements can include upper limb grasp, a feet-in-place postural adjustment, and/or protective steps. Previous research has shown that protective steps in people with PD are shorter compared to healthy older adults (HO) [7, 18, 20, 24]. The preparation phase in compensatory stepping is also altered in people with PD, as severe PD patients often exhibit one or multiple lateral weight shifts prior to compensatory stepping, delaying the onset of step initiation [20, 24].
How PD affects motor learning (defined as the relatively permanent change in performance through practice [43]) is controversial, however most investigations suggest that learning, while present, is less pronounced in people with PD with respect to healthy controls. For example, upper extremity learning studies suggest that people with PD do not improve motor performance with practice as well as healthy controls [14, 31, 44], possibly due to dysfunction of the basal ganglia, an area associated with motor learning [51, 53]. Considerably less research has been conducted on lower extremity motor learning in people with PD, and results have been somewhat mixed. Roemmich et al. recently showed that locomotor adaptation does occur in PD, although to a lesser degree than healthy controls [39]. However, Hayes & colleagues demonstrated that PD had little effect on postural sequence learning [13].
Given the importance of protective postural responses in fall prevention, and the poor protective stepping performance in people with PD, it is critical to understand whether this population can improve automatic postural responses through practice. However, few studies have addressed this question. Two recent investigations characterized changes in postural responses over the course of <10 perturbations, showing people with PD improve protective stepping [49], although in some cases less than healthy elderly subjects [30, 45]. However, improvements in these first trials have been suggested to be related to habituation to perturbations [1]. One clinical study investigated effects of practice of postural stepping responses in people with PD. Jobges and colleagues exposed people with PD to 2 weeks of training in which they experienced brisk, manual pushes or pulls at the trunk in multiple directions [22]. People with PD improved stepping responses after perturbation training, and retained improvements over 2 months. Although this study suggests people with PD can improve protective postural responses, it provides little insight how people with PD learn compared to older people without PD, or whether practice can be generalized. Specifically, the slope of improvements through practice in healthy and parkinsonian individuals is unknown. In addition, a recent trial by Schlenstedt and colleagues showed that balance training that included perturbations did not have a superior effect on the Fullerton Advanced Balance Scale, a measure that includes reactive postural control, than freely coordinated resistance training [42]. Overall, it remains unknown whether people with PD improve stepping responses similarly to age matched controls, or whether improvements generalize to non-trained protective postural responses.
The purpose of this study is to determine whether PD affects improvements in protective stepping responses over the course of practice. Understanding short-term learning (both improvement and retention of performance), is a first step toward identifying whether postural rehabilitation may improve protective stepping responses in people with PD. We hypothesize that people with PD will improve postural responses through practice and that these improvements will be retained, however improvements may not be as pronounced as in age-matched control subjects [22, 31].
METHODS
Participants
Fifteen individuals with PD and 12 HO participated in the study. Participants were recruited through the OHSU Movement Disorders Clinic and surrounding geographic area. Inclusion criteria for people with PD were currently taking levodopa and a confirmed diagnosis of PD from a movement disorders specialist. Exclusion criteria for all participants were: 1) inability to stand without aid for at least 1hr, 2) neurological diagnoses other than PD, and 3) orthopedic injuries interfering with balance. Individuals with PD were mild to moderately affected and tested in the ON state of medication (UPDRS-III 25.4±13.8, mean±SD). HO participants were similar with respect to age, Montreal Cognitive Assessment, and MiniBESTest to the PD participants (Table 1). Data from HO subjects have been compared to younger adults previously [9]. All participants provided informed consent, and the research protocol was approved by the OHSU institutional review board.
Table 1.
Subject Characteristics. Data represented as mean (SD).
| HO | PD | p-values | |
|---|---|---|---|
| N (#female) | 12 (6) | 15 (3) | 0.13* |
| Age | 68.04 (6.61) | 66.34 (6.02) | 0.49α |
| MoCA | 27.17 (1.64) | 27.2 (3.0) | 0.97α |
| MiniBEST | 25.25 (2.18) | 23.2 (3.8) | 0.11α |
| Years with Disease | -- | 6.38 (4.75) | -- |
| UPDRS-III | -- | 25.4 (13.8) | -- |
| Hoehn & Yahr Stage | -- | 2.00 (0.38) | -- |
| Levodopa Dosage | -- | 598.67 (228.41) | -- |
Fisher’s Exact Test;
Independent Samples T-test
Abbreviations: HO- Healthy older adults; PD- Parkinson’s disease; UPDRS-III- Unified Parkinson’s Disease Rating Scale- Part III (motor) score; MiniBEST- Mini Balance Evaluation System Test; MoCA- Montreal Cognitive Assessment; COM- Center of Mass; PD- Parkinson’s disease; HO- Healthy older adult;
Experimental protocol
This protocol has been described previously [9]. Briefly, participants completed two visits on consecutive days. Visit 1 included: 1) familiarization perturbations (n=14), which included 4 perturbations in the forward and leftward direction, and 3 perturbations in both the backward and rightward directions, 2) a baseline test for stepping in response to medio-lateral perturbations: 5 rightward and 5 leftward support surface perturbations (random order), and 3) the motor practice of forward-backward perturbations: 25 forward and 25 backward perturbations (random order). The same perturbation sequence was administered to all participants. Participants were given breaks after every 10 perturbations, or more often if requested.
Twenty-four hours after visit 1, participants returned to assess retention of improvement in forward-backward stepping, and generalization to medio-lateral stepping. Participants repeated familiarization and medio-lateral perturbations exactly as in day 1, followed by 10 forward-backward perturbations (5 per direction, random order). These 10 forward-backward perturbations were exactly the same size and sequence as the first 10 forward-backward perturbations on day 1. Medio-lateral protective stepping was chosen as the generalization task because it is an important movement for fall-prevention. In addition, despite the relatively distinct nature of this movement from anterior-posterior stepping, generalization across tasks may be possible. For example, finger tapping reaction time to random sequences of visually presented stimuli have been shown to improve over training [5]. Given this “nonspecific” improvement in reaction time in upper extremity learning, it is not unreasonable to expect some generalization across protective postural responses.
Participants were instrumented with reflective markers on boney landmarks and EMG electrodes on bilateral tibialis anterior and medial gastrocnemius to assess muscle onset latencies. Participants stood with arms crossed, eyes open-staring straight ahead, and feet together. Foot position was marked and held constant through all trials. Participants were instructed “not to anticipate upcoming perturbations, and to react naturally to the perturbation when trying to keep balance.” Open-ended instructions were chosen to avoid altering natural protective postural responses.
Perturbations consisted of translations of the support surface. Forward translations of the support surface resulted in backward displacement of the center of mass and backward stepping responses. Thus, we will refer to “backward” perturbations as those resulting in backward stepping responses, and “forward” perturbations as those resulting in forward stepping responses. Familiarization perturbations ranged from 9cm, 14.6cm/s to 15cm, 56cm/s. All lateral perturbations were 15cm, 21cm/s. All forward-backward practice perturbations were 15cm and 56cm/s, with an average acceleration of 2.25m/s2.
Data analysis
Our primary variable of interest was the total displacement of the center of mass (COM) after perturbations, as this measure has been used to characterize the global postural control performance in response to perturbations [9, 33, 50]. COM displacement was calculated by identifying the summed position of the COM of all body segments. COM for each segment was calculated using segment kinematics and anthropometric data[6, 48]. Segment kinematics were captured via markers placed on boney landmarks. Markers were tracked via a Motion Analysis system (Motion Analysis Corporation, Santa Rosa, California) sampling at 120 Hz. All marker data were low-pass filtered at 5Hz via a 4th order butterworth filter. COM displacement was then calculated as the maximum anterior-posterior displacement of the vertical projection of the COM to the ground with respect to its position at perturbation onset. This value included the total COM displacement after completion of all necessary protective steps.
Given the importance of the first protective step after external perturbations, secondary variables characterized first step function. The margin of stability (MOS) is the distance between the extrapolated center of mass (XCOM) and the base of support (either heel or toe-markers for backward and forward perturbations, respectively) at first foot fall [15] (Figure 1). XCOM incorporates the position and velocity of the center of mass, and is defined as:
Figure 1.
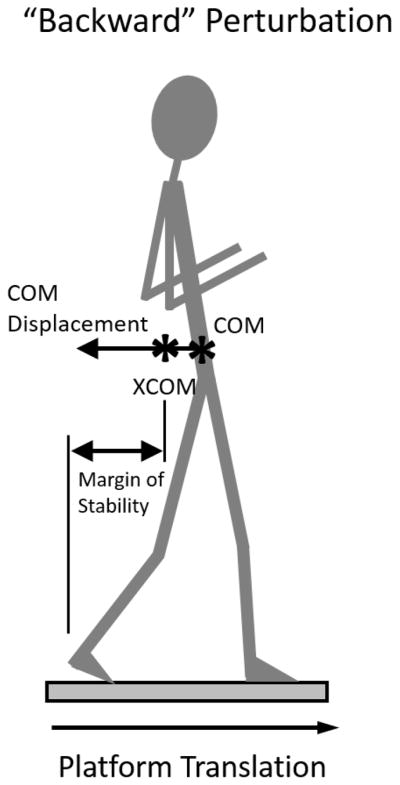
Schematic of primary outcome variables including center of mass (COM) and margin of stability, which is calculated using the extrapolated center of mass (XCOM). See text for further details.
In which x is the position (vertical projection to the ground), and Vx is the velocity of the COM. Wo represents eigenfrequency of the inverted pendulum and is defined as:
Where g is gravity (9.81m/s2) and L represents the effective pendulum length (trochanteric height times 1.24[52]).
Other step characteristics analyzed were: the first step length (distance between the stance and swing feet at foot contact), step onset (the time at which the first foot left the ground, as measured via force-plates), and number of steps (the number of steps until COM reached its maximum displacement). Step characteristics were calculated via marker and force-plate data.
Finally, the onset of muscle activity after perturbations were calculated via EMG data. These data were band-pass filtered from 75–470 Hz and full-wave rectified. A linear envelope was created via 100Hz low pass filtering. Muscle onset latency was calculated as the first instance in which activity moved above 2 standard deviations of the baseline (standing EMG), and remained above this threshold for at least 25ms. All outcome variables were calculated via interactive functions programmed in MATLAB (Natick, MA).
Data were averaged in blocks of 5 perturbations of the same direction. Therefore, over the 50 practice perturbations (25 forward & 25 backward, randomly ordered), we consolidated data into 5 blocks of 5 forward perturbations, and 5 blocks of 5 backward perturbations.
Statistical analysis
Data were tested for skewness using the Shapiro-Wilk test. If data exhibited small or modest skewness (p>0.01), parametric assessments were used. To assess practice effects, group effects, and practice by group interaction effects, we used a mixed design ANOVA with fixed effects on group (between subjects factor; 2 levels) and block (within subject factor; 5 levels). For retention, we assessed whether performance on day 2 was significantly different than performance on day 1, block 1 via a mixed model ANOVA with repeated measures on time (Block1, day 1 to Block 2, day 2). Retention was only assessed if significant improvement was observed over the course of day 1.
If skewness was observed, non-parametric statistical assessments determined group, direction, and group by direction interactions. Practice effects (collapsed across group) were assessed via Wilcoxon Signed-Rank tests. Group effects (collapsed across time) were assessed via Mann-Whitney U tests. To assess interaction effects between practice and group (i.e. whether HO learned differently than people with PD), we first calculated the change in performance through practice by taking the difference between day1-block1 and day1-block5 for each group separately (PD and HO). This difference score was then compared across groups via a Mann-Whitney U test. Therefore, in instances where variables were skewed and non-parametric tests were required, only blocks 1 and 5 were analyzed.
If a significant practice by group interaction was observed, two post-hoc across-group assessments were run. First, we assessed whether improvements across blocks could be observed for each group separately. Second, post-hoc contrasts were performed comparing practice by group interactions for each block to determine where interactions were most pronounced.
Generalization was assessed by determining whether COM displacement after lateral perturbations was different before and after forward-backward perturbation practice.
Finally, in an attempt to understand the basis for variability of improvement across participants, Spearman’s rho correlation statistics related improvement in COM displacement, the primary variable of interest with participant cognitive, balance and PD severity characteristics (MoCA, MiniBESTest, UPDRS) as well as with baseline protective stepping performance (COM displacement). Given the a-priori determination of a single primary variable of interest (COM displacement), multiple comparison corrections were not utilized.
RESULTS
No participants exhibited freezing of gait at any time during the visit. One individual with PD attempted to withhold a step after perturbations, resulting in a very long step latency. Step latency data from this participant were removed from the analysis. Other data from this participant (i.e. COM displacement) remained in the analysis. All other data from all participants were included.
Backward stepping
Both PD and control subjects improved postural motor performance throughout practice. Stepping performance and statistics for all backward perturbation outcome variables are shown in Table 2. Retention of improvements are shown in Table 3.
Table 2.
Changes in stepping performance, represented as mean (SD), in people with PD and healthy adults (HO) across blocks 1–5 (day 1).
| Grp | Start-train (block 1) | End-train (block 5) | Group Effect | Practice Effect | Grp X Practice Interaction | Post-Hoc within group (p-values) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| BACKWARD PERTURBATIONS | |||||||
| Primary variable | |||||||
|
|
|||||||
| COM displacement (m) | HO | 0.27(0.05) | 0.23(0.03) | F1,25=0.27 | F3,63=10.8 | F3,65=2.27 | -- |
| PD | 0.31(0.11) | 0.25(0.09) | p=0.610 | p<0.001 | p=0.097 | -- | |
|
|
|||||||
| Secondary Variables | |||||||
|
|
|||||||
| Margin of Stability (m) | HO | 0.11(0.8) | 0.15(0.03) | F1,25=4.3 | F2,44=8.25 | F2,44=1.83 | -- |
| PD | 0.07(0.04) | 0.10(0.05) | p=0.049 | p=0.001 | p=0.167 | -- | |
|
|
|||||||
| Step Latency (ms) | HO | 293(31) | 281(21) | F1,24=0.9 | F2,52=3.23 | F2,52=0.93 | -- |
| PD | 300(28) | 293(37) | p=0.352 | p=0.044 | p=0.828 | -- | |
|
|
|||||||
| Step Length (m) | HO | 0.24(0.11) | 0.27(0.08) | F1,25=4.7 | F2,45=2.62 | F2,45=1.15 | -- |
| PD | 0.17(0.05) | 0.19(0.07) | p=0.039 | p=0.089 | p=0.321 | -- | |
|
|
|||||||
| *Number of Steps | HO | 2.03(0.63) | 1.15(0.28) | Z=−1.47 | Z=−3.26 | Z=−2.21 | HO: p=0.003 |
| PD | 2.17(0.84) | 1.90(0.89) | p=0.152 | p=0.001 | p=0.028 | PD: p=0.145 | |
|
|
|||||||
| EMG onset (ms) | HO | 110(10) | 108(9) | F1,25=0.05 | F4,100=1.71 | F4,100=1.88 | -- |
| PD | 111(6) | 109(7) | p=0.831 | p=0.153 | p=0.121 | -- | |
|
|
|||||||
| FORWARD PERTURBATIONS | |||||||
| Primary Variable | |||||||
|
|
|||||||
| COM displacement (m) | HO | 0.28(0.11) | 0.23(0.05) | F1,25=1.94 | F3,64=5.82 | F3,64=2.48 | -- |
| PD | 0.23(0.05) | 0.22(0.06) | p=0.176 | p<0.001 | p=0.078 | -- | |
|
|
|||||||
| Secondary Variables | |||||||
|
|
|||||||
| Margin of Stability (m) | HO | 0.12(0.06) | 0.12(0.05) | F1,25=6.26 | F2,54=0.70 | F2,54=0.43 | -- |
| PD | 0.08(0.06) | 0.08(0.05) | p=0.019 | p=0.511 | p=0.670 | -- | |
|
|
|||||||
| Step Latency (ms) | HO | 291(42) | 287(28) | F1,24=0.46 | F2,52=0.61 | F2,52=0.39 | -- |
| PD | 297(36) | 293(34) | p=0.503 | p=0.559 | p=0.700 | -- | |
|
|
|||||||
| Step Length (m) | HO | 0.32(0.11) | 0.31(0.09) | F1,25=8.94 | F2,56=0.42 | F2,56=1.06 | -- |
| PD | 0.23(0.08) | 0.24(0.07) | p=0.006 | p=0.618 | p=0.359 | -- | |
|
|
|||||||
| *Number of Steps | HO | 1.53(0.52) | 1.08(0.29) | Z=−0.03 | Z=−2.62 | Z=−0.95 | -- |
| PD | 1.52(0.62) | 1.27(0.51) | p=0.980 | p=0.009 | p=0.344 | -- | |
|
|
|||||||
| EMG onset (ms) | HO | 120(22) | 113(14) | F1,25=0.23 | F4,100=1.90 | F4,100=0.33 | -- |
| PD | 116(6) | 113(8) | p=0.591 | p=0.116 | p=0.793 | -- | |
Non-parametric statistical assessments used
EMG- Electromyograhy; COM- Center of mass; HO- Healthy older adult; PD- Parkinson’s disease
Table 3.
Retention of improvements, represented as mean (SD), in backward and forward protective stepping performance in people with PD and healthy older adults (HO). Retention was only analyzed if a significant improvement was observed across day 1 practice.
| Grp | Start Train (block 1) | Retention | Group Effect | Time Effect (Retention) | Group x Retention Interaction | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| BACKWARD PERTURBATIONS | |||||||
| Primary Variable | |||||||
|
|
|||||||
| *COM displacement (m) | HO | 0.28(0.05) | 0.25(0.03) | Z=0.922 | Z=−2.86 | Z=−0.586 | |
| PD | 0.31(0.11) | 0.26(0.11) | p=0.943 | p=0.004 | p=0.581 | ||
|
|
|||||||
| Secondary Variables | |||||||
|
|
|||||||
| *Margin of Stability (m) | HO | 0.11(0.08) | 0.12(0.04) | Z=−0.878 | Z=−3.12 | Z=−1.03 | |
| PD | 0.07(0.04) | 0.11(0.04) | p=0.399 | p=0.002 | p=0.323 | ||
|
|
|||||||
| Step Latency (ms) | HO | 293(9) | 293(11) | F1,23=0.238 | F1,23=0.015 | F1,23=0.068 | |
| PD | 300(7) | 297(10) | p=0.63 | p=0.903 | p=0.796 | ||
|
|
|||||||
| Number of Steps | HO | 2.03(0.63) | 1.60(0.48) | F1,25=0.46 | F1,25=28.9 | F1,25=0.22 | |
| PD | 2.17(0.85) | 1.81(0.70) | p=0.504 | p<0.001 | p=0.64 | ||
|
|
|||||||
| FORWARD PERTURBATIONS | |||||||
| Primary Variable | |||||||
|
|
|||||||
| COM displacement (m) | HO | 0.28(0.11) | 0.23(0.05) | F1,25=2.0 | F1,25=6.832 | F1,25=2.591 | |
| PD | 0.23(0.05) | 0.21(0.06) | p=0.167 | p=0.015 | p=0.12 | ||
|
|
|||||||
| Secondary Variables | |||||||
|
|
|||||||
| *Number of Steps | HO | 1.53(0.52) | 1.00(0.37) | Z=−0.20 | Z=−3.27 | Z=−1.71 | |
| PD | 1.52(0.62) | 1.30(0.41) | p=0.843 | p=0.001 | p=0.093 | ||
Non-parametric statistical assessments used
EMG- Electromyograhy; COM- Center of mass; HO- Healthy older adult; PD- Parkinson’s disease
Group by practice interactions
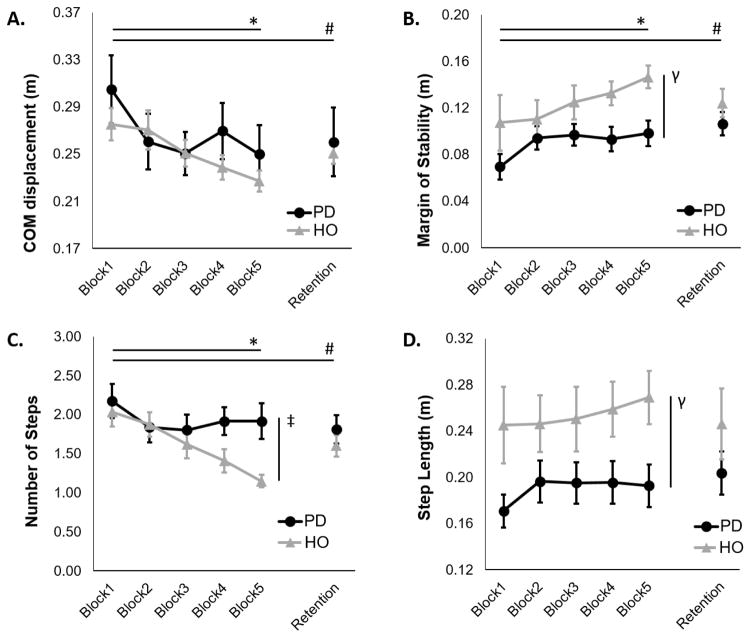
COM displacement showed a nonsignificant trend (p=0.097, Table 2) toward an interaction effect due to more pronounced reduction in COM displacement with practice in HO compared subjects with PD (Table 2). A significant group by practice interaction for number of steps after perturbations was found, due to more pronounced reduction in number of steps in HO compared to PD. Post-hoc analyses confirmed this interaction effect, as HO exhibited significant improvement in number of steps taken from the beginning to the end of practice, whereas people with PD did not (Table 2, Figure 2). For both COM displacement and number of steps, these interactions were driven by PD exhibiting improvements primarily during the first block of perturbations, while HO improved throughout the practice session. Indeed, post-hoc contrasts demonstrated that the most pronounced group by practice interaction was noted between blocks 2 and 5 for both COM displacement (p=0.092) and number of steps (p=0.001). The significant interaction between blocks 2 and 5 was also noted for margin of stability (p=0.009; Figures 2, 3). In other words, while both groups seemed to improve performance from blocks 1–2, HO continued to improve performance across blocks 2–5 while PD did not.
Figure 2.
Protective stepping performance after backward perturbations: A) Center of mass (COM) displacement, B) Margin of stability, C) Number of steps, and D) Step Length in people with PD and healthy older adults (HO). Error bars represent standard error. * Significant practice effect across day 1; γ Significant group effect; ‡ Significant group by practice interaction; # Significant retention over 24 hours.
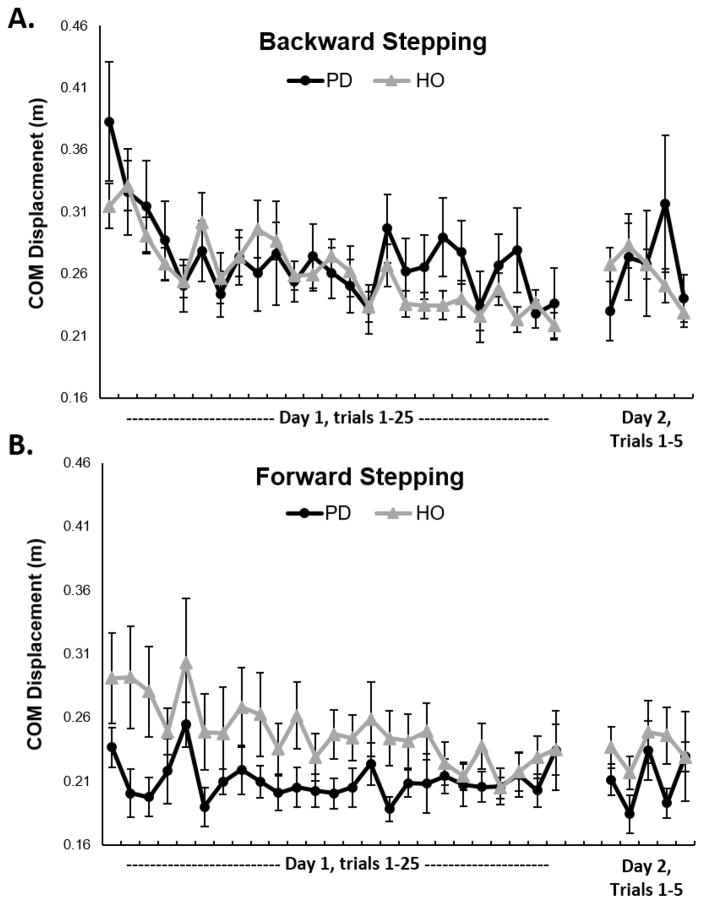
Figure 3.
Mean center of mass (COM) displacement performance across participants for each backward (A) and forward (B) perturbation in people with PD and healthy older adults (HO). Error bars represent standard error.
Group effects
MOS and step length were smaller in people with PD compared to HO, representing worse stepping performance.
Practice effects
COM displacement, MOS, step latency, and number of steps were improved over the course of practice (Table 2). Improvements were retained for all variables 24 hours later, except step latency (Table 3).
Forward stepping
Stepping performance and statistics for all outcome variables after forward perturbations are shown in Table 2, and retention of improvements are shown in Table 3.
Group by practice interactions
No significant interaction effects were noted, however there was a nonsignificant trend (p=0.078) in COM displacement toward an interaction effect due to more pronounced reduction in HO compared to people with PD (Table 2).
Group effects
MOS and step length were smaller in people with PD with respect to HO, representing worse stepping performance throughout practice (Table 2).
Practice effects
COM displacement and number of steps became smaller (improved) over the course of practice (Table 2). Improvements for both variables were retained 24 hours later (Table 3).
Correlation analyses
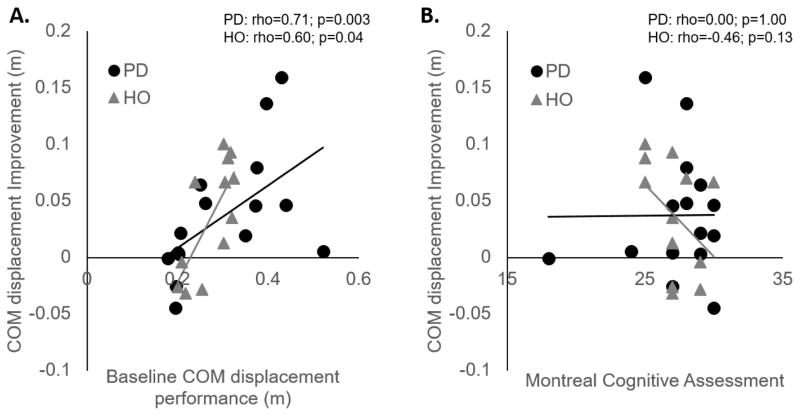
Balance characteristics (MiniBEST), general cognitive ability (MoCA), and PD sign severity (UPDRS) were not correlated to the degree of improvement in protective postural responses. However, unsurprisingly, baseline performance after perturbations (COM displacement) was significantly related to the improvement in performance, such that worse baseline performance predicted more improvement over practice (Table 4; Figure 4).
Table 4.
Spearman’s rho correlation statistics between improvement in COM displacement over the course of practice and baseline performance measures in people with PD and healthy older adults (HO).
| Spearman’s Rho | ||
|---|---|---|
| PD (n=15) | HO (n=12) | |
| UPDRS-III | −0.15 | -- |
| MiniBESTest | −0.13 | −0.12 |
| MoCA | 0.00 | −0.46 |
| Baseline COM displacement | 0.71** | 0.60* |
p<0.05;
p<0.005
UPDRS-III- Unified Parkinson’s Disease Rating Scale Part III (motor) score; MiniBEST- Mini Balance Evaluation System Test; MoCA- Montreal Cognitive Assessment; COM- Center of Mass;
Figure 4.
Correlations between the amount of improvement in center of mass (COM) displacement over practice and (A) baseline COM performance, and (B) global cognitive performance (Montreal Cognitive Assessment) in people with PD and healthy adults (HO).
Generalization
No group effects were observed during lateral perturbations (Z=−1.76; p=0.08). COM displacement after untrained medial-lateral perturbations were not different before or after practice (Z=−1.84; p=0.07). Group by time interaction was also non-significant (Z=−0.98; p=0.35; Online Resource 1).
DISCUSSION
Our data show that people with PD are able to improve postural response performance over the course of a single practice session of repeated perturbations. Although overall improvements for people with PD were similar to HO, improvements in the PD group were primarily over the first two blocks of trials whereas HO gradually improved over 5 blocks of trials. Like HO, improvements in postural stepping responses made by people with PD were retained 24 hours later, were larger in the backward, than forward direction, and did not generalize from forward-backward stepping to untrained, lateral stepping.
In agreement with previous results, we observed people with PD to exhibit smaller, less efficient protective steps than HO [7, 24]. The cause of this poorer performance is not fully understood, but is likely the result of bradykinesia, rigidity and central neural control of posture [17, 19]. Protective postural control, including stepping, is thought to involve brainstem (e.g. pontomedulary reticular formation), subcortical (e.g. basal ganglia and cerebellum) and cortical structures, such as the supplementary motor area (for reviews, see [4, 19, 32]). Several recent investigations have observed alterations in the structure and function of these regions in people with PD [11, 21, 37], contributing to the altered postural performance during perturbations in this population.
In addition to impaired stepping ability, people with PD exhibit poorer motor learning, particularly implicit learning [14, 31], due in part to dysfunction of the basal ganglia [44, 51]. However, previous investigations have focused primarily on upper extremity motor learning. Thus, there is a relative lack of literature focusing on postural motor learning in PD, which may be implicit when automatically triggered externally [46]. In particular, the ability of people with PD to improve protective stepping responses, which are critical for fall avoidance, is poorly understood. Our study is consistent with previous results, showing that despite the poorer performance, people with PD were able to improve stepping, and retain these improvements over 24 hours [22]. We extend previous findings, showing that although improvements (and retention of improvements) were largely similar with people with PD and age-matched HO, people with PD show an altered time course of learning.
Unlike HO who showed robust learning throughout the training period, people with PD improved stepping primarily over the first block of perturbation practice, with relatively little improvement through the rest of the practice session (Figures 2 & 3, backward perturbations). Interestingly, this result is partially consistent with a recent investigation into adaptation and adaptive learning in people with PD. Roemmich & colleagues investigated the ability of people with PD to adapt step characteristics during split-belt walking. When people with PD were exposed to split-belt walking, the initial adaptation period of step length asymmetry was similar between people with PD and HO. However, while HO continued to improve step asymmetry with additional practice, people with PD did not [39]. In addition, Beeler and colleagues recently demonstrated that dopamine depleted mice show preserved learning of rotarod gait with early practice, with complete absence of learning thereafter [2]. Nutt et al. [34] also demonstrated that during repeated reciprocal finger tapping, people with PD and healthy controls improve tapping speed with 3 consecutive trials, With continued practice over the course of 26 hours, controls, but not people with PD, continued to improve performance. Finally, the ability of people with PD to use prior experience to improve postural responses early in practice, but plateau later, has also been reported for learning to scale up feet-in-place postural responses for predicted amplitudes of surface translations [16]. Together, these studies suggest that for some tasks, people with PD may exhibit early, but not continued improvement in performance with training.
The underlying reason why subjects with PD show robust early improvements but slower later improvements with practice are unknown. We propose two non-exclusive hypotheses to explain this finding. First, it is possible that the early performance improvement in people with PD is primarily due to habituation of postural responses to startling perturbations [1, 12]. We feel this explanation is unlikely, as participants were exposed to 14 perturbation trials prior to training to reduce the effects of habituation to initial ‘startling’ perturbations. However, as reported previously, startle effects can, to some degree, reemerge with new perturbation types [35]. Therefore, it remains possible that the improvement in performance over the first trials may have been related to habituation to startling support surface perturbations. Alternatively, early, but not late performance improvements may be related to a disproportionate dysfunction of the posterior sensorimotor striatum. Recent investigations in mouse models show that the dorsomedial striatum, homologous to the caudate in primates, is preferentially related to early motor learning, while the dorsolateral striatum, homologous to the putamen, is related to more gradual improvements and automatic behavior [54]. People with PD generally exhibit a posterior (i.e. putamen) to anterior (i.e. caudate) striatal degeneration such that the putamen is effected sooner, and the caudate later in the disease course [29]. Given the mild to moderate disease severity of participants in the current study, it is possible that the relatively in-tact ventral striatum (i.e. caudate) may have contributed to early performance improvements. However, dysfunction of posterior striatum (i.e. putamen) resulted in less continued, gradual improvements. Increased activity of the cerebellum, which plays a critical role in early learning and adaptation, may have further contributed to the short term improvements from blocks 1 to 2 in people with PD [23]. Indeed, PD has been suggested to be associated with compensatory increases in cerebellar activity [10]. Clearly, further research is necessary to more thoroughly understand the underlying mechanisms of postural improvement in people with PD. However, it is important to note that in the current study, both groups maintained improvements over 24 hours suggesting that, regardless of the cause or course of improvement, it was retained for at least one day.
Improvements from the trained perturbation task (i.e. forward and backward perturbations), did not generalize to a non-trained, lateral, perturbation task, for either the subjects with PD or HO. This result is not entirely surprising, given the distinct nature of lateral vs. forward/backward stepping and our earlier study showing the same in young adults. Lateral perturbations can elicit different stepping strategies (e.g. cross over step, side step, etc. [26]), which may incorporate more cortical control than forward backward stepping. Similarly, adaptation of the vestibulo-ocular reflex also does not generalize across different directions of stimuli [36]. Additional variability of practice, e.g. incorporating lateral perturbations or varying the velocity and amplitude of perturbations may improve the degree of generalization, however these approaches remain to be tested.
Interestingly, people with PD showed less movement in the ML direction after forward or backward perturbations than HO (Table 3). This was not due to differences in the size of perturbations, nor was it due to differences in strategy (data not shown). This reduction may have been related to increased stiffness (axial rigidity) in the ML direction in those with PD. ML displacements in response to forward or backward postural perturbations have been shown to be associated with fall risk in the elderly so stiffness may be protective, especially for people with PD who have difficulty widening their base of support to step sideways [28].
During forward stepping, control subjects started with larger COM displacement, and this decreased to the level of people with PD over the course of practice. It is notable that despite the larger COM movement early in practice, HO took the same number of steps as PD, and these steps were larger than those of people with PD. Therefore, the larger COM in HO during forward falling is likely related to an increased limit of stability and step length in HO as opposed to poor COM control.
Broad measures of symptom severity including balance, cognition, and motor signs were not related to the improvement in performance over practice, suggesting these measures are unable to predict learning in this population. Unsurprisingly, baseline protective postural response performance was related to improvement over time, such that poorer baseline performance predicted more improvement. Although this analysis is limited by small sample size, it contrasts previous reports suggesting cognitive ability may be related to learning [8, 47]. However, recent work shows a relatively weak relationship between global cognitive performance and motor learning [40, 41]. Additional work will be necessary to understand the relationship between cognitive ability and postural learning.
Several limitations must be acknowledged. First, participants were not given explicit instructions on how or whether to alter stepping throughout the course of practice. We chose not to give specific/explicit instructions to avoid focusing conscious attention toward the stepping process, and also in an attempt to increase the degree of implicit learning. However, this approach may have increased the variability in stepping responses across trials. Further, the instructions “try to keep balance” may have been interpreted as “try not to take a step”, possibly altering results. Second, people with PD completed all data collection ON levodopa. Previous studies have indicated that levodopa may have positive [38], or perhaps negative [25] effects on learning depending on the type learning evaluated (i.e. explicit, implicit, upper or lower limb, etc.). Finally, the practice and retention periods were short (1-day of practice and 24 hour retention). Follow-up studies should examine improvement and retention over longer periods.
CONCLUSIONS
People with Parkinson’s disease were able to improve protective postural responses through one day of perturbation practice, although most of their improvements where in the first phase of practice. These improvements were largely similar to HO, and were retained 24 hours later. However, improvements did not generalize to a non-practiced perturbation (lateral stepping). These results suggest that people with PD, like people without PD, would benefit from rehabilitation that incorporates postural perturbations. Given the current results, along with the mixed results of recent trials, additional studies to investigate the efficacy of perturbation practice to improve balance and reduce falls in people with PD is warranted.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by grants from the United States Department of Veteran’s Affairs Rehabilitation Research and Development Service (Career Development Award-1: #I01BX007080; PI: DP) & VA Merit Award (E1075-R; PI: FH), the National Institutes of Health (R01 AG006457 29 PI: FH), and the Medical Research Foundation of Oregon (Early Investigator Award; PI: DP). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
CONFLICT OF INTEREST
Dr. Horak and OHSU have an equity/interest in APDM, a company that may have a commercial interest in the results of the study. This potential conflict of interest has been reviewed and managed by the Research & Development Committee at the Portland VA Medical Center and OHSU.
Literature Cited
- 1.Allum JH, Tang KS, Carpenter MG, Oude Nijhuis LB, Bloem BR. Review of first trial responses in balance control: influence of vestibular loss and Parkinson’s disease. Hum Mov Sci. 2011;30:279–295. doi: 10.1016/j.humov.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Beeler JA, Cao ZF, Kheirbek MA, Ding Y, Koranda J, Murakami M, Kang UJ, Zhuang X. Dopamine-dependent motor learning: insight into levodopa’s long-duration response. Ann Neurol. 2010;67:639–647. doi: 10.1002/ana.21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol. 2001;248:950–958. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- 4.Bolton DA. The role of the cerebral cortex in postural responses to externally induced perturbations. Neurosci Biobehav Rev. 2015;57:142–155. doi: 10.1016/j.neubiorev.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Bonzano L, Tacchino A, Roccatagliata L, Mancardi GL, Abbruzzese G, Bove M. Structural integrity of callosal midbody influences intermanual transfer in a motor reaction-time task. Hum Brain Mapp. 2011;32:218–228. doi: 10.1002/hbm.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandler R, Clauser C, McConville J, Reynolds H, Young J. Investigation of the Inertial Properties of the Human Body. National Technical Information Service; Springfield, VA: 1975. [Google Scholar]
- 7.de Kam D, Nonnekes J, Oude Nijhuis LB, Geurts AC, Bloem BR, Weerdesteyn V. Dopaminergic medication does not improve stepping responses following backward and forward balance perturbations in patients with Parkinson’s disease. J Neurol. 2014;261:2330–2337. doi: 10.1007/s00415-014-7496-3. [DOI] [PubMed] [Google Scholar]
- 8.Deroost N, Kerckhofs E, Coene M, Wijnants G, Soetens E. Learning sequence movements in a homogenous sample of patients with Parkinson’s disease. Neuropsychologia. 2006;44:1653–1662. doi: 10.1016/j.neuropsychologia.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Dijkstra BW, Horak FB, Kamsma YPT, Peterson DS. Older adults can improve compensatory stepping with repeated postural perturbations. Frontiers in aging neuroscience. 2015;7 doi: 10.3389/fnagi.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Festini SB, Bernard JA, Kwak Y, Peltier S, Bohnen NI, Muller ML, Dayalu P, Seidler RD. Altered cerebellar connectivity in Parkinson’s patients ON and OFF L-DOPA medication. Front Hum Neurosci. 2015;9:214. doi: 10.3389/fnhum.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, Shibasaki H. Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain. 1999;122(Pt 7):1271–1282. doi: 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- 12.Hansen PD, Woollacott MH, Debu B. Postural responses to changing task conditions. Exp Brain Res. 1988;73:627–636. doi: 10.1007/BF00406622. [DOI] [PubMed] [Google Scholar]
- 13.Hayes HA, Hunsake N, Schaefer SY, Shultz B, Schenkenberg T, Boyd L, White AT, Foreman KB, Dyer P, Maletsky R, Dibble LE. Does Dopamine Replacement Medication Affect Postural Sequence Learning in Parkinson’s Disease? Motor control. 2015;19:325–340. doi: 10.1123/mc.2014-0039. [DOI] [PubMed] [Google Scholar]
- 14.Hayes HA, Hunsaker N, Dibble LE. Implicit Motor Sequence Learning in Individuals with Parkinson Disease: A Meta-Analysis. Journal of Parkinson’s disease. 2015;5:549–560. doi: 10.3233/JPD-140441. [DOI] [PubMed] [Google Scholar]
- 15.Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38:1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75:2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 17.Horak FB, Henry SM, Shumway-Cook A. Postural perturbations: new insights for treatment of balance disorders. Phys Ther. 1997;77:517–533. doi: 10.1093/ptj/77.5.517. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs JV, Horak FB. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience. 2006;141:999–1009. doi: 10.1016/j.neuroscience.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, Aziz TZ. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord. 2009;24:319–328. doi: 10.1002/mds.22189. [DOI] [PubMed] [Google Scholar]
- 22.Jobges M, Heuschkel G, Pretzel C, Illhardt C, Renner C, Hummelsheim H. Repetitive training of compensatory steps: a therapeutic approach for postural instability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1682–1687. doi: 10.1136/jnnp.2003.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King BR, Fogel SM, Albouy G, Doyon J. Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front Hum Neurosci. 2013;7:142. doi: 10.3389/fnhum.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King LA, St George RJ, Carlson-Kuhta P, Nutt JG, Horak FB. Preparation for compensatory forward stepping in Parkinson’s disease. Arch Phys Med Rehabil. 2010;91:1332–1338. doi: 10.1016/j.apmr.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak Y, Muller ML, Bohnen NI, Dayalu P, Seidler RD. Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson’s disease. J Neurophysiol. 2010;103:942–949. doi: 10.1152/jn.00197.2009. [DOI] [PubMed] [Google Scholar]
- 26.Maki BE, Edmondstone MA, McIlroy WE. Age-related differences in laterally directed compensatory stepping behavior. J Gerontol A Biol Sci Med Sci. 2000;55:M270–277. doi: 10.1093/gerona/55.5.m270. [DOI] [PubMed] [Google Scholar]
- 27.Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Phys Ther. 1997;77:488–507. doi: 10.1093/ptj/77.5.488. [DOI] [PubMed] [Google Scholar]
- 28.Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture. 2012;36:471–476. doi: 10.1016/j.gaitpost.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson’s disease. Brain. 1996;119(Pt 2):585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- 30.Nanhoe-Mahabier W, Allum JH, Overeem S, Borm GF, Oude Nijhuis LB, Bloem BR. First trial reactions and habituation rates over successive balance perturbations in Parkinson’s disease. Neuroscience. 2012;217:123–129. doi: 10.1016/j.neuroscience.2012.03.064. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwboer A, Rochester L, Muncks L, Swinnen SP. Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord. 2009;15(Suppl 3):S53–58. doi: 10.1016/S1353-8020(09)70781-3. [DOI] [PubMed] [Google Scholar]
- 32.Nonnekes J, Carpenter MG, Inglis JT, Duysens J, Weerdesteyn V. What startles tell us about control of posture and gait. Neurosci Biobehav Rev. 2015;53:131–138. doi: 10.1016/j.neubiorev.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Nonnekes J, Scotti A, Oude Nijhuis LB, Smulders K, Queralt A, Geurts AC, Bloem BR, Weerdesteyn V. Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience. 2013;245:109–120. doi: 10.1016/j.neuroscience.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 34.Nutt JG, Lea ES, Van Houten L, Schuff RA, Sexton GJ. Determinants of tapping speed in normal control subjects and subjects with Parkinson’s disease: differing effects of brief and continued practice. Mov Disord. 2000;15:843–849. doi: 10.1002/1531-8257(200009)15:5<843::aid-mds1013>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Oude Nijhuis LB, Allum JH, Borm GF, Honegger F, Overeem S, Bloem BR. Directional sensitivity of “first trial” reactions in human balance control. J Neurophysiol. 2009;101:2802–2814. doi: 10.1152/jn.90945.2008. [DOI] [PubMed] [Google Scholar]
- 36.Peng GC, Baker JF, Peterson BW. Dynamics of directional plasticity in the human vertical vestibulo-ocular reflex. J Vestib Res. 1994;4:453–460. [PubMed] [Google Scholar]
- 37.Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- 38.Roemmich RT, Hack N, Akbar U, Hass CJ. Effects of dopaminergic therapy on locomotor adaptation and adaptive learning in persons with Parkinson’s disease. Behav Brain Res. 2014;268:31–39. doi: 10.1016/j.bbr.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roemmich RT, Nocera JR, Stegemoller EL, Hassan A, Okun MS, Hass CJ. Locomotor adaptation and locomotor adaptive learning in Parkinson’s disease and normal aging. Clin Neurophysiol. 2014;125:313–319. doi: 10.1016/j.clinph.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaefer SY, Dibble LE, Duff K. Efficacy and Feasibility of Functional Upper Extremity Task-Specific Training for Older Adults With and Without Cognitive Impairment. Neurorehabil Neural Repair. 2015;29:636–644. doi: 10.1177/1545968314558604. [DOI] [PubMed] [Google Scholar]
- 41.Schaefer SY, Duff K. Rapid Responsiveness to Practice Predicts Longer-Term Retention of Upper Extremity Motor Skill in Non-Demented Older Adults. Frontiers in aging neuroscience. 2015;7:214. doi: 10.3389/fnagi.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlenstedt C, Paschen S, Kruse A, Raethjen J, Weisser B, Deuschl G. Resistance versus Balance Training to Improve Postural Control in Parkinson’s Disease: A Randomized Rater Blinded Controlled Study. PLoS One. 2015;10:e0140584. doi: 10.1371/journal.pone.0140584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt RA, Lee TD. Motor Control and learning: A behavioral emphasis. Human Kinetics; Champaign, IL: 1999. [Google Scholar]
- 44.Siegert RJ, Taylor KD, Weatherall M, Abernethy DA. Is implicit sequence learning impaired in Parkinson’s disease? A meta-analysis. Neuropsychology. 2006;20:490–495. doi: 10.1037/0894-4105.20.4.490. [DOI] [PubMed] [Google Scholar]
- 45.Smulders K, Esselink RA, De Swart BJ, Geurts AC, Bloem BR, Weerdesteyn V. Postural inflexibility in PD: does it affect compensatory stepping? Gait Posture. 2014;39:700–706. doi: 10.1016/j.gaitpost.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Van Ooteghem K, Frank JS, Allard F, Horak FB. Aging does not affect generalized postural motor learning in response to variable amplitude oscillations of the support surface. Exp Brain Res. 2010;204:505–514. doi: 10.1007/s00221-010-2316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandenbossche J, Deroost N, Soetens E, Kerckhofs E. Does implicit learning in non-demented Parkinson’s disease depend on the level of cognitive functioning? Brain Cogn. 2009;69:194–199. doi: 10.1016/j.bandc.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan C, Davis B, O’Connor J. Dynamics of Human Gait. Kiboho Publishers; Cape Town, SA: 1992. [Google Scholar]
- 49.Visser JE, Oude Nijhuis LB, Janssen L, Bastiaanse CM, Borm GF, Duysens J, Bloem BR. Dynamic posturography in Parkinson’s disease: diagnostic utility of the “first trial effect”. Neuroscience. 2010;168:387–394. doi: 10.1016/j.neuroscience.2010.03.068. [DOI] [PubMed] [Google Scholar]
- 50.Welch TD, Ting LH. Mechanisms of motor adaptation in reactive balance control. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkinson L, Khan Z, Jahanshahi M. The role of the basal ganglia and its cortical connections in sequence learning: evidence from implicit and explicit sequence learning in Parkinson’s disease. Neuropsychologia. 2009;47:2564–2573. doi: 10.1016/j.neuropsychologia.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Winter D. Biomechanics and Motor Control of Human Movement. John Wiley & Son’s; Hoboken, NJ, USA: 2009. [Google Scholar]
- 53.Yang J, Li P. Brain networks of explicit and implicit learning. PLoS One. 2012;7:e42993. doi: 10.1371/journal.pone.0042993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature neuroscience. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.