Abstract
Hypothesis. This study examines moderators and mediators of a yoga intervention targeting quality-of-life (QOL) outcomes in women with breast cancer receiving radiotherapy.Methods. Women undergoing 6 weeks of radiotherapy were randomized to a yoga (YG; n = 53) or stretching (ST; n = 56) intervention or a waitlist control group (WL; n = 54). Depressive symptoms and sleep disturbances were measured at baseline. Mediator (posttraumatic stress symptoms, benefit finding, and cortisol slope) and outcome (36-item Short Form [SF]-36 mental and physical component scales [MCS and PCS]) variables were assessed at baseline, end-of-treatment, and 1-, 3-, and 6-months posttreatment. Results. Baseline depressive symptoms (P = .03) and sleep disturbances (P < .01) moderated the Group × Time effect on MCS, but not PCS. Women with high baseline depressive symptoms in YG reported marginally higher 3-month MCS than their counterparts in WL (P = .11). Women with high baseline sleep disturbances in YG reported higher 3-months MCS than their counterparts in WL (P < .01) and higher 6-month MCS than their counterparts in ST (P = .01). YG led to greater benefit finding than ST and WL across the follow-up (P = .01). Three-month benefit finding partially mediated the effect of YG on 6-month PCS. Posttraumatic stress symptoms and cortisol slope did not mediate treatment effect on QOL. Conclusion. Yoga may provide the greatest mental-health–related QOL benefits for those experiencing pre-radiotherapy sleep disturbance and depressive symptoms. Yoga may improve physical-health–related QOL by increasing ability to find benefit in the cancer experience.
Keywords: breast cancer, QOL, yoga, moderation, mediation
Introduction
Moderators of Mind-Body Interventions
Despite the large number of behavioral intervention studies for individuals diagnosed with cancer, the overall efficacy of these treatments in addressing patient symptom burden has been heavily debated.1-5 Though an estimated 20%-40% of cancer patients experience depression, the typical cancer patient enrolled in psychosocial trials tends to be not depressed.4,6 Thus, the frequently used “all-comers” approach to patient recruitment may result in negligible treatment gains for quality of life (QOL) indicators such as depression. In fact, a recent meta-analysis of 61 trials demonstrated that psychological distress moderated the efficacy of psychosocial treatments regarding mood management for cancer patients.7 Furthermore, a meta-analysis of trials targeting depressed cancer patients indicated that psychotherapeutic and pharmacological interventions are effective in reducing depressive symptoms with sustained effects.8
Although these meta-analyses have been instrumental in identifying the importance of examining moderators of treatment efficacy, they have rarely included trials of complementary medicine interventions such as yoga and meditation. Such exclusion is surprising in light of the rapid proliferation of Eastern-influenced behavioral interventions in oncology research and practice.9-17 Yoga in particular has gained popularity in the cancer setting, and several systematic reviews and meta-analyses have examined the QOL benefits associated with cancer patients’ and survivors’ yoga practice.14-17 For instance, a meta-analysis of 13 randomized controlled trials (RCTs) of yoga in cancer patients and survivors revealed large effects for psychological health; medium effects for fatigue, general QOL, and psychosocial well-being; and small effects for sleep disturbances and physical function.17 It is important to note that the reviewed trials did not select for elevated symptom burden (ie, used an “all-comer” approach). Thus, yoga may lead to even greater grains in at-risk participants. However, with the exception of some limited evidence,18 it is largely unknown if patients with elevated distress derive greater QOL benefit from a yoga intervention compared with their less-distressed counterparts.
The benefits of yoga are multifaceted, targeting not only psychological but also physical and spiritual dimensions of QOL.17 Thus, participant characteristics beyond psychological distress may moderate the efficacy of a yoga intervention. Sleep disturbances, potentially caused by the cancer process itself or cancer treatments, are commonly experienced among women with breast cancer19-21 and are associated with impaired QOL in cancer patients even when controlling for depression and fatigue.20,22,23 Because yoga is an effective treatment to improve sleep in cancer patients and survivors,9,24,25 it may be particularly efficacious for patients reporting high levels of sleep disturbances. In fact, a multicenter RCT involving 410 survivors with moderate to high sleep disturbances demonstrated that an 8-session yoga intervention improved self-reported and actigraphy-assessed sleep relative to standard care.9 However, to the best of our knowledge, sleep disturbances have not yet been examined as a yoga intervention moderator.
Mediators of Mind-Body Interventions
In addition to lacking a clear understanding of for whom mind-body interventions are most helpful, no cohesive theoretical framework has been proposed to explain how and why yoga interventions produce change.26,27 A recent systematic review reports that few potential mechanisms of yoga have been explored to date, and no mechanisms of yoga have been tested in cancer populations.28,29 The exception is one study on mindfulness-based stress reduction for cancer patients, which includes some yoga. This study found that increasing mindfulness partially mediated the intervention’s beneficial effects on stress and posttraumatic avoidance.30 However, in light of the growing RCTs examining yoga in cancer and the limited exploration of mediators of this intervention, further examination of yoga mediators guided by a conceptual model is needed.
We propose a stress response model as a way to understand how yoga produces change in cancer patients. A diagnosis of cancer and its treatment are typically experienced as stressful, or even traumatic, events, and yoga interventions are generally conceptualized within the framework of a stress reduction program. Thus, practicing yoga may improve health outcomes in cancer patients via modulating the stress response.28 Put another way, yoga may affect posttraumatic stress symptoms, such as cognitive interference and avoidance, while also increasing posttraumatic growth or benefit finding. Importantly, both constructs of trauma response (ie, posttraumatic stress symptoms and benefit finding) have been consistently and prospectively associated with psychological and physical QOL outcomes in various cancer samples.31-39
With this model in mind, we previously examined the effects of yoga versus usual care on intrusive thoughts/avoidance behaviors and benefit finding in a small pilot trial in women with breast cancer undergoing radiotherapy.10 Differences between groups in benefit finding did not emerge until the last 3-month assessment time point, precluding the examination of benefit finding as an intervention mediator. Surprisingly, the yoga group reported increased intrusive thoughts 1 month after the end of radiotherapy compared with the women in the usual care group, with subsequent reduction at the 3-month time point, and nonsignificant reductions in avoidance behaviors. Interestingly, intrusive thoughts at 1 month were positively associated with benefit finding at 3 months. There is some evidence to suggest that heightened levels of intrusive thoughts experienced during the aftermath of a traumatic event may help individuals more effectively adjust to the stressor and ultimately to find benefit in the traumatic experience.40 Thus, the increase in intrusive thoughts associated with yoga may have led to better, more mindful processing of the cancer experience, ultimately fostering finding meaning in the cancer experience.
Moreover, within a trauma response model, cortisol rhythmicity may represent one biological pathway by which mind-body interventions improve health and well-being. Both types of trauma response (posttraumatic stress symptoms and benefit finding) are associated with hypothalamic-pituitary-adrenal axis function in cancer patients, which in turn is associated with behavioral symptoms (eg, fatigue, sleep disturbance, and depression), making changes to cortisol rhythmicity a potential mechanism of yoga.41-44 Although previous studies have shown group main effects of a yoga intervention on diurnal cortisol and speculated a mediating effect, no empirical evidence exists to date.28
The Present Study
The goal of the current study was to examine moderators and mediators of a previously reported 3-arm yoga RCT for women diagnosed with breast cancer undergoing radiotherapy.45 We focused only on mediators and moderators of the primary outcome variable: health-related QOL. First, we hypothesized that, compared with active and waitlist control groups, the yoga program would be especially beneficial at improving posttreatment QOL for women with elevated pretreatment depressive symptoms and sleep disturbance. Second, we hypothesized that the beneficial QOL effects of the intervention would be mediated by improved trauma responses (ie, short-term increases in intrusive thoughts and reduction in avoidance behaviors and increased benefit finding) as well as better stress hormone regulation (ie, a steeper cortisol slope).
Method
Participants
Women were recruited prior to radiotherapy treatment (XRT), with inclusion criteria being the following: ≥18 years old; ability to read, write, and speak English; diagnosed with stage 0 to III breast cancer; and scheduled to undergo daily adjuvant XRT for 6 weeks at MD Anderson Cancer Center. Patients with lymphedema, metastatic bone disease, deep-vein thrombosis, documented diagnosis of a formal thought disorder, and extreme mobility problems or those who had practiced yoga in the year before diagnosis were excluded. The protocol was approved by the institutional review board.
Procedures
Details of the study procedures have been reported elsewhere.45 Briefly, after receiving written informed consent, self-report and saliva samples (for cortisol data) were collected from participants at baseline before randomization, during the last week of XRT, and 1, 3, and 6 months later.
Participants were randomly assigned to 1 of 3 groups: (1) yoga (YG); (2) stretching control (ST); or waitlist control (WL) using a form of adaptive randomization,46 according to age, stage of disease, time since diagnosis, type of surgery, and chemotherapy (neoadjuvant or adjuvant). Participants in the WL group received usual care, completed all assessments on the same timeline as the active groups, and were offered yoga classes at the end of their study participation. All participants were asked to refrain from participating in any other yoga classes while on study. Participants in the YG and ST groups attended up to three 60-minute classes per week during their 6 weeks of XRT. Each participant received an audio CD and a written manual of the program to encourage at-home practice.
The integrated yoga program, described previously,10 included the following: (1) preparatory warm-up synchronized with breathing; (2) selected postures, or asanas (forward-, backward-, and side-bending asanas in sitting and standing positions, cobra posture, crocodile, and half-shoulder-stand with support); (3) deep relaxation (supine posture); (4) alternate-nostril breathing or pranayama; and (5) meditation. The program was taught by Vivekananda Yoga Anusandhana Samsthana–trained teachers with specific oncology training.
The stretching program included exercises recommended specifically for women undergoing or recovering from breast cancer treatment.47,48 The exercises approximated the gross movements of the yoga exercises and were taught by cancer center physiotherapists.
Measures
Primary Intervention Outcome: Health-Related QOL
Overall QOL was assessed by the Medical Outcomes Study 36-item Short Form survey (SF-36) and was the primary outcome of the clinical trial published previously.45 The SF-36 assesses physical functioning, physical impediments to role functioning, bodily pain, general health perceptions, vitality, social functioning, emotional impediments to role functioning, and mental health and includes an overall physical and mental component scale (PCS and MCS).49,50 To reduce the number of analyses, only the component scales are included in outcome analyses. Higher scores reflect better QOL.
Proposed Moderators
Depressive symptoms were assessed using the Centers for Epidemiological Studies–Depression measures (CES-D),51 a well-validated measure focusing on affective components of depression. Lower scores reflect fewer depressive symptoms. In this study sample, the internal reliability was high (Cronbach’s α = .89).
Sleep disturbances were assessed using the Pittsburgh Sleep Quality Index (PSQI),52 a questionnaire that assesses sleep disturbances over a 1-month period. We report on the total score, with lower scores reflecting fewer sleep disturbances. Acceptable internal reliability was found in this study sample (Cronbach’s α = .70).
Proposed Mediators: Posttraumatic Responses
Posttraumatic stress symptoms were measured by the Impact of Event Scale (IES), a scale that assesses the 2 most common categories of responses to traumatic events: intrusion (intrusively experienced ideas, images, feelings, or bad dreams) and avoidance behaviors (conscious efforts to avoid certain ideas, feelings, or situations).53 We report on the intrusive thoughts and avoidance behaviors subscales and the total score. Lower scores reflect fewer symptoms. Adequate internal reliability was found for the total scale (Cronbach’s α = .85) as well as intrusive (Cronbach’s α = .85) and avoidance (Cronbach’s α = .79) subscales.
Participants’ ability to find benefit was measured by the Benefit Finding Scale (BFS),54,55 a scale assessing acceptance of life’s imperfections, change in priorities, and development of a sense of purpose in life as a result of having been diagnosed with cancer. Higher scores reflect greater benefit finding. In this study sample, the internal reliability was high (Cronbach’s α = .94).
Cortisol Rhythmicity
Cortisol was obtained via 5 saliva samples (waking, 45 minutes later, approximately 8 and 12 hours after waking, and at bedtime) for 3 consecutive days at each assessment. Participants chewed on a cotton swab (Salivette) and placed it in a plastic tube (Sarstedt), which was then frozen at −80°C for later time-resolved immunoassay with fluorescence detection performed at the University of Dresden. Values <0.0001 and >70 nmol/L were classified as missing. If patients missed a collection point, they were told to leave the tube empty. Reliability for each collection time point within a day across the 3 days and the 5 assessment time points ranged from 0.20 to 0.87, with only 6 out of 25 α values (5 samples a day at 5 time points) dropping below 0.50. Reliability was directly related to the sample size, which dropped off by the 6-month follow-up. A steeper, more-negative cortisol slope indicates better cortisol regulation.
Covariates
Patients also completed basic demographic information (eg, age, marital status, education). Medical information was obtained from electronic medical records.
Data Analyses
Hypothesis 1: To evaluate whether the intervention was more effective in regard to health-related QOL (SF-36 MCS and PCS) for participants with high depressive symptoms (Hypothesis 1A) or greater sleep disturbance (Hypothesis 1B) at baseline, we used an ANCOVA framework to first examine the Group × Baseline moderator effect using PROC MIXED in SAS v9.2. This was followed by a Group × Time × Baseline moderator ANCOVA to see if the moderator effect varied by time. We controlled for the respective baseline outcome as well as randomization factors (age, stage of disease, time since diagnosis, type of surgery, and chemotherapy type). We also controlled for baseline SF-36 general health scores in all analyses as a result of imbalances across groups. We treated time as a categorical variable and the intercept as a random effect. We specified an unstructured covariance structure. For significant 3-way interactions, we decomposed the interaction according to high and low (mean ± ½SD) baseline depressive symptoms or sleep disturbance and compared the least-squared means (LSM) for each group at each time point using a general linear model analysis, controlling for baseline levels of the outcome variable and illustrating the interaction by plotting the LSM.
Hypothesis 2: We were interested in determining mediators of significant group effects on health-related QOL. The original trial45 demonstrated group differences in SF-36 PCS at 1 and 3 months, and a subscale of the SF-36 PCS (physical functioning) at those time points and 6 months. We chose to examine mediators of SF-36 PCS at 1, 3, and 6 months, rather than the physical functioning subscale because the SF-36 PCS is a more comprehensive index of physical health–related QOL.
To determine the mediator variables, we regressed each proposed mediator (ie, IES, BFS, and cortisol slope) on group, time, and the Group × Time interaction using the model and covariates described above. Where there was a group or Group × Time effect on the proposed mediator variable, we chose the time point associated with the largest group or Group × Time effect as the mediator. If a proposed mediator variable did not significantly differ by group, it was not further examined. Only primary outcome variables assessed after our chosen mediators were included as dependent variables in the mediation models to enable a predictive relationship between the mediator and the dependent variable to be established.56
To test for mediation, we calculated indirect effects using Hayes and Preacher’s bias-corrected bootstrap procedure via the MEDIATE macro for SPSS, which is designed to estimate indirect effects of multicategorical independent variables.57 Indicator coding of the grouping variable was used, with WL functioning as the reference group. D1 codes the effect of YG compared with WL controlling for ST, and D2 codes the effect of ST compared with WL controlling for YG. Indirect effects were determined significant when the mean of the indirect effect across all 5000 bootstrap samples was associated with a bias-corrected confidence interval that did not include 0.57
Results
Attrition and Adherence
Out of 294 eligible women approached, 191 consented to participate; 13 dropped out before, and 15 after, randomization, for a baseline sample size of 163 (YG = 53; ST = 56; WL = 54). Measurements were obtained for 80% of the sample at 6 months (n = 131; YG = 43, ST = 42, WL =46; see original clinical trial45 for CONSORT). Out of a maximum possible 18 classes, 87% of YG and 85% of ST participants attended ≥12 classes (YG = 13.8; ST = 14.7). Only 3 patients in each group attended fewer than half the classes. There were no significant differences in demographic, medical, or baseline self-report scores between those who attended ≥75% of classes compared with those who did not. For the YG group, self-reported practice outside of class was high (≥twice per week) at 1 month posttreatment and then declined at 3 and 6 months (71%, 55%, and 45%, respectively). For the ST group, self-reported practice outside of class (≥twice per week) was lower at 1 month and then increased somewhat at 3 and 6 months (53%, 69%, and 60%, respectively). WL participants were offered the YG program after data collection was complete, but no data were collected from the WL during or after yoga.
There were also no group, demographic, or baseline self-report differences between those who completed the 6-month follow-up assessment and those who did not (Ps > .14), with the exception that older adults were more likely to complete the 6-month assessment.
Baseline Sample Characteristics
The 3 groups were similar on all medical and demographic variables (Table 1). There were no statistically significant differences among the groups on any of the self-reported variables at baseline, apart from the SF-36 general health subscale. Women in YG reported lower baseline general health compared with those in ST (P = .01). Depending on the time point, 21% to 34% of participants did not provide saliva samples. There were no differences between patients providing samples and those who did not based on group assignment, medical, demographic, or outcome measures. We present the baseline and follow-up means of self-reported variables in Table 2.
Table 1.
Baseline Characteristics of Study Participants, by Group.
| Yoga |
Stretch |
Waitlist |
||||
|---|---|---|---|---|---|---|
| n = 53 (33%) | n = 56 (34%) | n = 54 (33%) | ||||
| Disease stage, n (%) | ||||||
| 0 | 5 | 10 | 6 | 11 | 7 | 13 |
| I | 16 | 30 | 18 | 32 | 17 | 31 |
| II | 15 | 28 | 14 | 25 | 15 | 28 |
| III | 17 | 32 | 18 | 32 | 15 | 28 |
| ER/PR status (n = 156), n (%) | ||||||
| ER+/PR+ | 32 | 62 | 33 | 62 | 31 | 61 |
| ER+/PR− | 7 | 13 | 7 | 13 | 6 | 12 |
| ER−/PR+ | 1 | 2 | 0 | 0 | 3 | 6 |
| ER−/PR− | 12 | 23 | 13 | 25 | 11 | 21 |
| Surgery, n (%) | ||||||
| Mastectomy (without reconstruction) | 12 | 23 | 17 | 31 | 12 | 22 |
| Mastectomy (with reconstruction) | 6 | 11 | 3 | 5 | 5 | 9 |
| Breast conserving | 35 | 66 | 36 | 64 | 37 | 69 |
| Chemotherapy, n (%) | ||||||
| Yes | 36 | 68 | 34 | 61 | 34 | 63 |
| Hormone treatment (n = 156), n (%) | ||||||
| Yes | 33 | 62 | 38 | 70 | 34 | 67 |
| Marital status (n = 151), n (%) | ||||||
| Married and living together | 31 | 67 | 37 | 71 | 34 | 64 |
| Not cohabiting | 15 | 33 | 15 | 29 | 19 | 36 |
| Ethnicity (n = 150), n (%) | ||||||
| Black/African American | 9 | 19 | 9 | 17 | 7 | 13 |
| White/Caucasian | 32 | 68 | 28 | 55 | 37 | 71 |
| Latino/Hispanic/Mexican | 4 | 9 | 8 | 16 | 5 | 10 |
| Asian/Oriental/Pacific Islander | 2 | 4 | 4 | 8 | 1 | 2 |
| Other | 0 | 0 | 2 | 4 | 2 | 4 |
| Employment status (n = 140), n (%) | ||||||
| Employed part-/full-time | 15 | 33 | 21 | 43 | 18 | 39 |
| Employed, taken time off | 11 | 25 | 10 | 20 | 5 | 11 |
| Not employed | 19 | 42 | 18 | 37 | 23 | 50 |
| Education (n = 152), n (%) | ||||||
| Some college or lower | 27 | 57 | 26 | 50 | 32 | 60 |
| College and higher education | 20 | 43 | 26 | 50 | 21 | 40 |
| Income (n = 149), n (%) | ||||||
| >$75 000 | 31 | 67 | 26 | 51 | 26 | 50 |
| <$75 000 | 15 | 33 | 25 | 49 | 26 | 50 |
| Age (mean, SE) | 52.38 | 1.35 | 51.14 | 1.32 | 52.11 | 1.34 |
Abbreviations: ER = Estrogen receptor; PR = Progesterone receptor
Table 2.
Raw Means and SDs of Measures at Baseline and Follow-up.a
| Yoga |
Stretch |
Waitlist |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Primary outcome variables | SF-36 PCS | ||||||
| Baseline | 41.9 | 9.6 | 43.4 | 8.6 | 44.5 | 9.4 | |
| Post-XRT | 42.3 | 9.2 | 44.5 | 8.1 | 44.1 | 8.4 | |
| 1 Month | 47.0* | 8.1 | 46.9 | 9.1 | 45.4** | 7.6 | |
| 3 Months | 48.2* | 7.3 | 47.6 | 8.8 | 45.8** | 7.8 | |
| 6 Months | 46.9b | 9.3 | 47.5 | 8.6 | 46.6 | 7.5 | |
| SF-36 MCS | |||||||
| Baseline | 42.1 | 12.4 | 45.6 | 10.3 | 42.2 | 12.9 | |
| Post-XRT | 47.2 | 13.5 | 49.7 | 8.9 | 46.8 | 11.5 | |
| 1 Month | 46.2 | 13.1 | 47.1 | 11.2 | 49.0 | 10.1 | |
| 3 Months | 46.5 | 12.6 | 49.8 | 10.2 | 46.9 | 12.2 | |
| 6 Months | 46.8 | 12.7 | 50.8 | 9.5 | 48.8 | 9.9 | |
| Moderator variables | CES-D | ||||||
| Baseline | 15.5 | 10.5 | 11.9 | 5.9 | 14.9 | 10.2 | |
| Post-XRT | 12.2 | 9.7 | 10.3 | 7.5 | 12.4 | 9.6 | |
| 1 Month | 13.1 | 10.7 | 11.6 | 9.6 | 12.3 | 8.3 | |
| 3 Months | 13.9 | 10.8 | 9.6 | 8.8 | 12.9 | 10.5 | |
| 6 Months | 13.9 | 11.8 | 10.4 | 9.3 | 11.5 | 9.0 | |
| PSQI | |||||||
| Baseline | 8.3 | 3.9 | 8.5 | 4.0 | 8.2 | 3.7 | |
| Post-XRT | 6.7 | 3.1 | 8.3 | 4.0 | 7.3 | 3.7 | |
| 1 Month | 7 | 3.8 | 7.5 | 4.2 | 5.9 | 3.6 | |
| 3 Months | 6.5 | 3.1 | 7.2 | 3.3 | 6.5 | 3.8 | |
| 6 Months | 7 | 3.5 | 7.2 | 3.9 | 6.4 | 4.1 | |
| Mediator variables | IES total | ||||||
| Baseline | 22 | 15.2 | 20.1 | 13.3 | 20.4 | 13.3 | |
| Post-XRT | 17.3 | 13.9 | 17.8 | 15 | 15.8 | 12.1 | |
| 1 Month | 17.1 | 13.5 | 16.9 | 13 | 14.4 | 12.1 | |
| 3 Months | 18.2 | 13 | 17.1 | 15.7 | 15.7 | 13.7 | |
| 6 Months | 18.7 | 16.6 | 16.4 | 12.8 | 11.9 | 10.6 | |
| IES intrusive thoughts | |||||||
| Baseline | 10.4 | 8.5 | 8.9 | 7.3 | 8.7 | 7.6 | |
| Post-XRT | 6.5 | 6.3 | 7.5 | 7.2 | 6.8 | 6.4 | |
| 1 Month | 5.8 | 6.5 | 6.9 | 6.4 | 5.8 | 6.5 | |
| 3 Months | 6.9 | 6.3 | 7.4 | 8.7 | 5.8 | 5.9 | |
| 6 Months | 7.2 | 8.0 | 6.8 | 6.3 | 4.6 | 4.9 | |
| IES avoidance | |||||||
| Baseline | 11.6 | 8.7 | 11.2 | 7.9 | 11.8 | 8.8 | |
| Post-XRT | 11.1 | 8.9 | 10.4 | 9 | 9 | 7.6 | |
| 1 Month | 11.3 | 8.2 | 10 | 7.9 | 8.6 | 7.8 | |
| 3 Months | 11.3 | 8.4 | 9.7 | 8.7 | 9.9 | 9.5 | |
| 6 Months | 11.6* | 10.4 | 9.7 | 8.4 | 7.3** | 7.0 | |
| BFS | |||||||
| Baseline | 42.5 | 13.4 | 44.1 | 16 | 44.3 | 13.8 | |
| Post-XRT | 46.3 | 14.1 | 45.9 | 16.7 | 42.9 | 15.1 | |
| 1 Month | 44.4* | 16.1 | 43.8 | 17.2 | 41.6** | 13.7 | |
| 3 Months | 43.9* | 17.7 | 42.7** | 18.1 | 40.8** | 16.3 | |
| 6 Months | 41.8* | 16.9 | 42.1** | 17.4 | 38** | 16.4 | |
| Cortisol slope | |||||||
| Baseline | −0.104 | 0.04 | −0.118 | 0.04 | −0.113 | 0.04 | |
| Post-XRT | −0.104* | 0.04 | −0.084** | 0.05 | −0.084** | 0.05 | |
| 1 Month | −0.098* | 0.04 | −0.090 | 0.05 | −0.065** | 0.05 | |
| 3 Months | −0.086 | 0.06 | −0.091 | 0.04 | −0.078 | 0.05 | |
| 6 Months | −0.090 | 0.06 | −0.095 | 0.04 | −0.099 | 0.04 | |
Abbreviations: SF-36, 36-item Short Form; PCS, physical component scale; MCS, mental component scale; XRT, radiotherapy treatment; CES-D, Centers for Epidemiological Studies–Depression; PSQI, Pittsburgh Sleep Quality Index; IES, Impact of Event Scale; BFS, Benefit Finding Scale.
Means with asterisk and double asterisk differ at P < .05 based on multilevel modeling analyses.
Though groups did not differ significantly on physical health–related quality of life (SF-36 PCS) at 6 months, the original trial found that women in the yoga group reported significantly higher scores on the physical functioning subscale of the SF-36 PCS at 6 months compared with the waitlist control group.
Hypothesis 1: Baseline Depressive Symptoms and Sleep Disturbance as Moderators of Intervention Outcomes
There were no significant Group × Baseline Depressive Symptom (CES-D) interaction effects on mental health–related QOL (SF-36 MCS). However, there was a significant Group × Time × Baseline CES-D interaction effect on SF-36 MCS (F(6, 324) = 2.40; P < .03), suggesting that the effect varied by time. We decomposed the interaction according to high and low (mean ± ½SD) baseline CES-D scores. There were no statistically significant differences in SF-36 MCS scores between groups for those with low or high baseline depressive symptoms at any assessment point (Figure 1A). However, women with high baseline depressive symptoms in YG had a trend toward higher SF-36 MCS scores at 3 months compared with WL (P = .107), and their 1-, 3-, and 6-month SF-36 MCS scores were no different from those of women scoring low on baseline depressive symptoms. There were no significant Group × Baseline CES-D or Group × Time × Baseline CES-D interaction effects on physical health–related QOL (SF-36 PCS).
Figure 1.
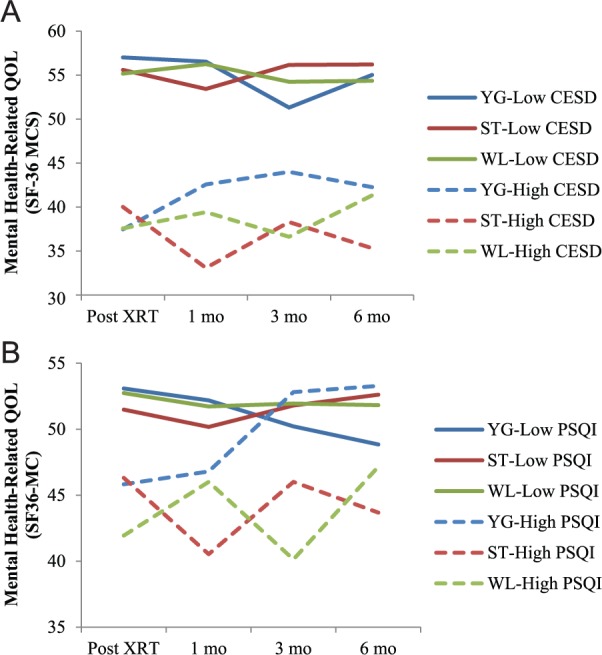
The least-squared means of mental health–related QOL (SF-36 MCS) are from a multilevel modeling analyses controlling for baseline MCS score, baseline SF-36 general health subscale, and randomization factors. Figures illustrate a Group × Time interaction for those with (A) high and low baseline depressive symptoms (mean ± ½SD) on the Center for Epidemiologic Studies (CES-D) and (B) high and low baseline sleep disturbances (mean ± ½SD) on the Pittsburgh Sleep Quality Index (PSQI). Higher SF-36 MCS scores represent greater QOL.
Abbreviations: CES-D, Centers for Epidemiological Studies–Depression; QOL, quality of life; SF-36, 36-item Short Form; MCS, mental component scale; YG, yoga group; ST, stretching control group; WL, or waitlist control group; XRT, radiotherapy treatment; PSQI, Pittsburgh Sleep Quality Index.
There was no significant Group × Baseline sleep disturbance (PSQI) interaction effects on mental health–related QOL (SF-36 MCS). However, there was a significant Group × Time × Baseline PSQI interaction effect on SF-36 MCS (F(6, 318) = 3.40, P < .01). We decomposed the interaction according to high and low (mean ± ½SD) baseline PSQI scores. There were no significant differences in SF-36 MCS scores between groups for those with low baseline sleep disturbances at any assessment point (Figure 1B). However, among the women with high baseline sleep disturbances, there was a significant Group × Time effect (F(6, 83) = 3.52; P = .003). Specifically, women with high sleep disturbances in YG reported higher 3-month SF-36 MCS scores than their counterparts in WL (t(83) = 3.15; P = .002) and higher 6-month SF-36 MCS scores than their counterparts in ST (t(83) = 2.56; P = .012), and their SF-36 MCS scores at each time point were no different from that of women reporting low sleep disturbance at baseline. There were no significant Group × Baseline sleep disturbance (PSQI) or Group × Time × Sleep disturbance (PSQI) interaction effects on physical health–related QOL (SF-36 PCS).
Hypothesis 2: Mediators of Intervention Effect on QOL
Posttraumatic Stress Symptoms
Results revealed no significant Group × Time interaction effect on IES total scale, intrusive thoughts, or avoidance behaviors subscales scores (Ps > .5). Additionally, there were no main effects of group or time on the IES total scale or intrusive thoughts subscale (Ps > .2). There was a main effect of group (F(2,132) = 3.17; P = .05), but not time (P = .6), for the IES avoidance subscale. Specifically, YG was associated with greater IES avoidance scores (LSM = 11.31; SE = 0.82) compared with WL (LSM = 8.52; SE = 0.76; P = .01). IES avoidance scores did not differ between ST (LSM = 10.16; SE = 0.79) and YG or WL (Ps > .1). Figure 2A presents LSMs of IES avoidance for groups across time.
Figure 2.
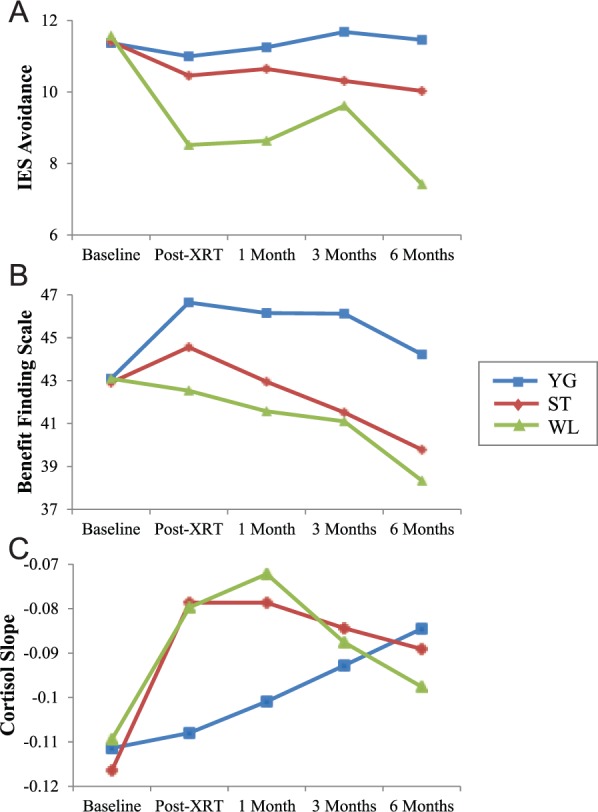
Group differences on posttraumatic response across time: these figures represent the least-squared means (adjusted for the baseline level of the outcome variable, baseline SF-36 general health subscale, and randomization factors) of a multilevel modeling analysis for each of the mediators over time.
Abbreviations: SF-36, 36-item Short Form; IES, Impact of Event Scale; XRT, radiotherapy treatment.
The time point at which groups differed most on IES avoidance was at 6 months (F(2, 112) = 3.23; P = .04; Cohen’s d = 0.40), with women in YG (LSM = 11.29; SE = 1.18; P = .02) and ST (LSM = 10.14; SE = 1.19; P = .09) reporting greater IES avoidance scores compared with women in WL (LSM = 7.34; SE = 1.69). Using the 6-month time point for the mediator does not enable examining a temporal relationship between the mediator and outcome, and there were no significant group differences at earlier time points, precluding examination of IES avoidance as an intervention mediator.
Benefit Finding
Results revealed no significant Group × Time interaction effect on BFS scores (P = .9). However, there was a main effect of group (F(2,132) = 4.60; P = .01) and a main effect of time (F(3,335) = 3.12; P = .03) on benefit finding. Specifically, YG was associated with greater benefit finding (LSM = 46.21; SE = 1.28) compared with ST (LSM = 42.24; SE = 1.23; P = .03) and WL (LSM = 41.05; SE = 1.19; P < .01). Benefit finding did not differ between ST and WL (P = .5). Figure 2B presents LSMs of benefit finding for groups across time.
Women in YG reported higher levels on benefit finding relative to WL at 1, 3, and 6 months and relative to ST at 3 and 6 months (Ps < .05), with no differences between ST and WL. Groups differed most on benefit finding at 3 months (F(2, 103) = 3.12; P = .05; Cohen’s d = 0.38), with women in YG reporting greater benefit finding scores (LSM = 46.32; SE = 1.85) compared with ST (LSM = 40.90; SE = 1.78; P = .04) and WL (LSM = 40.57; SE = 1.69; P = .02). Therefore, 3-month benefit finding was examined as a mediator of group’s effects on physical health–related QOL (SF-36 PCS) at 6 months because the physical health subscale of the SF-36 PCS was the only outcome associated with group differences assessed after 3 months.
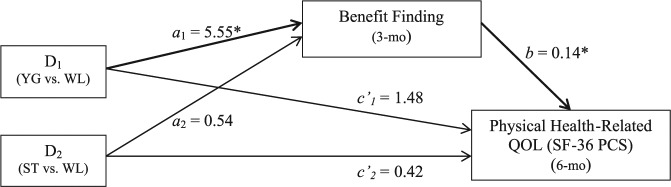
Hayes and Preacher’s bias-corrected bootstrap test of indirect effect57 revealed that group did indirectly affect physical health–related QOL (SF-36 PCS) at 6 months via benefit finding at 3 months (Figure 3). First, YG resulted in higher 3-month benefit finding compared with WL (the a1 pathway; P = .04), whereas ST and WL did not differ in 3-month benefit finding (the a2 pathway; P = .84). Second, holding group constant, those who reported higher benefit finding at 3 months reported higher 6-month physical health–related QOL (the b pathway, P = .04). Third, relative to WL, women in YG reported higher 6-month physical health–related QOL in part because of the positive effect of YG on 3-month benefit finding. Indeed, a bootstrap estimate of the indirect effect of YG compared with WL on 6-month physical health–related QOL via 3-month benefit finding revealed a 95% bias-corrected and accelerated (BCa) confidence interval that did not cross zero (B = 0.77; SE = 0.58; CI = 0.01 and 2.58). Thus, 3-month benefit finding partially mediated the effect of YG on 6-month physical health–related QOL. Conversely, the BCa confidence interval for the indirect effect of ST compared with WL did cross zero and was, therefore, not significant (B = 0.07; SE = 0.43; CI = −0.84 and 0.88). Thus, the effect of ST on 6-month physical health–related QOL was not mediated by 3-month benefit finding.
Figure 3.
Yoga indirectly affects physical health–related QOL (SF-36 PCS) at the 6-month follow-up via increased benefit finding (BFS) at 3 months. Values on each path are unstandardized path coefficients taken from bootstrapping analyses controlling for age, stage of disease, time since diagnosis, type of surgery, chemotherapy type, and baseline benefit finding (BFS), physical health–related QOL (SF-36 PCS), and the SF-36 general health subscale. The a1 and a2 paths correspond to the mean differences in 3-month BFS between YG relative to WL and ST relative to WL, respectively. Thus, YG resulted in 3-month BFS scores that were a1 = 5.55 units higher than WL (P = .04), and ST resulted in BFS that were a2 = 0.54 points higher than WL (P = .84). The b pathway corresponds to the relation between 3-month BFS and 6-month SF-36 PCS when group is held constant. Thus, for every 1 point increase in 3-month BFS, individuals reported an average b = 0.14-point increase in 6-month SF-36 PCS (P = .04). The relative indirect effects of group can be determined by multiplying the a and b paths. Thus, relative to WL, YG resulted in SF-36 PCS scores that were a1b = 0.77 units higher as a result of the positive effect of YG on BFS, which corresponds to a significant indirect effect of YG versus WL on 6-month SF-36 PCS via 3-month BFS (B = 0.77; SE = 0.58; 95% bias-corrected and accelerated [BCa] CI = 0.01 and 2.58). Conversely, there was no significant indirect effect of ST versus WL on 6-month SF-36 PCS via 3-month BFS (B = 0.07; SE = 0.43; BCa CI = −0.84 and 0.88).
Abbreviations: QOL, quality of life; SF-36, 36-item Short Form; PCS, physical component scale; BFS, Benefit Finding Scale; YG, yoga group; ST, stretching control group; WL, or waitlist control group.
*P < .05.
Cortisol
Results revealed no significant group or time effect on cortisol slope (P > .3). There was a trend for a Group × Time interaction effect (F(6,189) = 1.83; P = .096). Specifically, YG was associated with a steeper cortisol slope compared with ST and WL (Ps < .03) immediately after radiotherapy (post-XRT), and YG was associated with a steeper cortisol slope compared with WL at 1 month (P = .02). Figure 2C represents LSMs of cortisol slope for groups at each time point. Because the effects of group and Group × Time on cortisol slope did not reach significance, it was not examined as a mediator.
Exploratory Analyses: Changes in IES and Outcomes
Because women in WL reported unexpectedly lower IES avoidance scores over time compared with women in YG, we explored the association between changes in IES avoidance and QOL outcomes by regressing group, change in IES avoidance at 6 months (the time point associated with significant group differences), and the interaction on QOL outcome variables (physical and mental health–related QOL (SF-36 PCS and MCS), depressive symptoms (CES-D), and sleep disturbance (PSQI)) at the final follow-up time point (ie, 6 months). There were significant interaction effects for mental health–related QOL (P = .05) and depressive symptoms (P = .04). Pearson correlations within each group revealed no association between change in IES avoidance scores and 6-month mental health–related QOL or depressive symptoms for YG or ST, whereas a greater increase in IES avoidance scores was associated with worse 6-month mental health–related QOL (r = −0.31; P = .03) and higher depressive symptoms (r = 0.42; P < .01) for the WL group.
Discussion
The present study hypothesized that participating in a yoga intervention during radiotherapy would be particularly beneficial for women with high baseline depressive symptoms and sleep disturbances on posttreatment QOL compared with their counterparts participating in stretching or waitlist control groups. We also hypothesized that trauma responses (ie, change in posttraumatic stress symptoms and increased benefit finding) and better stress hormone regulation (ie, steeper cortisol slope) would mediate the effect of yoga on primary outcomes. Results partially supported each of the hypotheses.
Consistent with previous research suggesting that cancer patients with higher distress derive greater benefit from psychosocial interventions,18,58-61 the yoga intervention provided the greatest mental health–related benefits for women with elevated sleep disturbance and, to a lesser extent, depressive symptoms prior to the start of radiotherapy. This effect varied by time, with differences emerging especially 3 and 6 months after radiotherapy. Thus, yoga was especially helpful for those women with disturbed sleep and depressive symptoms at the start of radiotherapy. In fact, the women in the yoga group who had sleep disturbances at study entry had mental health scores at 3 and 6 months after radiotherapy equivalent to those of women who did not have sleep disturbances at study entry. A similar pattern was seen for the benefits of yoga for those with high depressive symptoms at baseline.
Regarding our mediation hypotheses, yoga led to increased benefit finding across the follow-up period relative to the stretching and waitlist control groups, where there was consistent decrease over time. Importantly, we found that yoga indirectly affected physical health–related QOL assessed 6 months after radiotherapy via increased benefit finding reported 3 months after radiotherapy. In other words, part of the effect of yoga on physical health–related QOL at the long-term follow-up can be attributed to the increased benefit finding experienced by yoga participants midway through the follow-up period. Of note, the longitudinal nature of the data enabled the time-lagged mediation analyses, which are critical for determining mechanisms of effect. This is a particular strength of the present article because such mediation analyses are often lacking in intervention study designs.
Surprisingly, women in the waitlist group reported less avoidance behaviors (eg, “I tried not to think about it;” “I stayed away from reminders about it”) 6 months following radiotherapy compared with women in the yoga group, who reported little change in avoidance behaviors from baseline, and there was no evidence of a decrease in intrusive thoughts over time for any group. This finding was counter to our hypotheses and that of other studies, which have found mind-body practices in general, and yoga in particular, to be associated with reductions in avoidance-related coping.62,63 However, exploratory analyses suggested that the typical deleterious effect of avoidance behaviors on QOL64,65 was not found for those in either of the active groups (yoga or stretching) but remained for those in the waitlist group. This finding was somewhat consistent with our previous pilot trial, in which the yoga group reported short-term increases in intrusive thoughts relative to the waitlist control group.10 Furthermore, a meta-analysis by Helgeson et al66 found that increased intrusive or avoidant thoughts about a stressor were associated with increase in benefit finding, which was in turn associated with greater well-being. Thus, it could be that yoga may not reduce posttraumatic stress symptoms (ie, cognitive interference or avoidance) in the acute phase, but this in turn may facilitate improved long-term adjustment.
There are several limitations to recognize in this study. The majority of participants were white, non-Hispanic, married, and highly educated. Thus, future research is needed to test the generalizability of these findings to more diverse populations. Participants also were not blinded to study condition, and no measure of treatment expectation was collected, which could have biased the findings because of the subjective nature of the outcomes. In addition, although these data suggest that women with elevated levels of depressive symptoms and sleep disturbances show a greater treatment response, these findings need to be interpreted with caution because the patients were not selected based on pretreatment symptomatology. The study may have also been underpowered for the mediation analyses. In a study of empirical power simulations, Fritz and MacKinnon67 indicated that very large sample sizes are required for tests of mediation to be conducted with at least 80% power. Thus, the sample size of the present study likely limits our ability to determine mediators with full power, and the same limitation may be true for moderation analyses. Additionally, the reduced reliability of cortisol slopes assessed at later follow-up points (because of a smaller sample size) may have limited our power to detect the effects of cortisol slopes as a mediator in particular. Finally, we followed participants for only a 6-month period, so the long-term effectiveness of yoga in patients with breast cancer remains to be determined. To address these limitations, we are conducting an ongoing yoga trial using a quasi-double-blinded design, with patients not knowing the details of the intervention groups at baseline and then only knowing the specifics of their assigned group. Additionally, assessors are blind to group assignment. Treatment expectations are also being measured, and patients complete a 1-year follow-up assessment.
In conclusion, the current study provides a greater understanding of who will benefit most from a yoga intervention and how a yoga intervention produces change. These results suggest that future studies of the effect of yoga on cancer patients may benefit from screening for participants who report poor sleep or depressive symptoms because yoga may buffer the negative effect of poor sleep or low mood on mental health–related QOL indices in the months following treatment for cancer. Additionally, these findings imply that yoga may improve physical health–related QOL by increasing one’s ability to find benefit in the cancer experience. Finally, yoga appears to increase women’s endorsement of symptoms typically associated with posttraumatic stress (ie, intrusive thoughts and/or avoidance behaviors) but disassociates the typically harmful link between these symptoms and QOL. Based on these results, we recommend that future research continues to identify pretreatment psychosocial factors that predict intervention response, seeks mechanisms by which interventions work, and begins implementing targeted, tailored, evidence-based mind-body interventions to optimize recovery and QOL in patients affected by cancer.
Footnotes
Authors’ Note: Chelsea G. Ratcliff and Kathrin Milbury contributed equally to the article and are both considered first authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Cancer Institute (Grant Numbers R21CA102385, R01CA138800, R25CA10618), the National Cancer Institute Cancer Center Support (Grant Number A016672), the National Center for Complementary and Integrative Health (Grant Number 5 K01 AT007559-02), a National Cancer Institute cancer prevention fellowship for Chelsea G. Ratcliff (Grant Number R25T CA057730), the South Central Mental Illness, Research, Education, and Clinical Center, and philanthropic support.
References
- 1. Moyer A, Knapp-Oliver SK, Sohl SJ, Schnieder S, Floyd AH. Lessons to be learned from 25 years of research investigating psychosocial interventions for cancer patients. Cancer J. 2009;15:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moyer A, Sohl SJ, Knapp-Oliver SK, Schneider S. Characteristics and methodological quality of 25 years of research investigating psychosocial interventions for cancer patients. Cancer Treat Rev. 2009;35:475-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stefanek ME, Jacobsen PB, Christensen AJ. The society of behavioral medicine’s “great debate”: an introduction. Ann Behav Med. 2006;32:83-84. [Google Scholar]
- 4. Coyne JC, Lepore SJ, Palmer SC. Efficacy of psychosocial interventions in cancer care: evidence is weaker than it first looks. Ann Behav Med. 2006;32:104-110. [DOI] [PubMed] [Google Scholar]
- 5. Lepore SJ, Coyne JC. Psychological interventions for distress in cancer patients: a review of reviews. Ann Behav Med. 2006;32:85-92. [DOI] [PubMed] [Google Scholar]
- 6. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71. [DOI] [PubMed] [Google Scholar]
- 7. Schneider S, Moyer A, Knapp-Oliver S, Sohl S, Cannella D, Targhetta V. Pre-intervention distress moderates the efficacy of psychosocial treatment for cancer patients: a meta-analysis. J Behav Med. 2010;33:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hart SL, Hoyt MA, Diefenbach M, et al. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J Natl Cancer Inst. 2012;104:990-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43-55. [PubMed] [Google Scholar]
- 11. Carlson LE, Doll R, Stephen J, et al. Randomized controlled trial of Mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. J Clin Oncol. 2013;31:3119-3126. [DOI] [PubMed] [Google Scholar]
- 12. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25:4387-4395. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30:1335-1342. [DOI] [PubMed] [Google Scholar]
- 14. Lin KY, Hu YT, Chang KJ, Lin HF, Tsauo JY. Effects of yoga on psychological health, quality of life, and physical health of patients with cancer: a meta-analysis. Evid Based Complement Alternat Med. 2011;2011:659876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cramer H, Lange S, Klose P, Paul A, Dobos G. Yoga for breast cancer patients and survivors: a systematic review and meta-analysis. BMC Cancer. 2012;12:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harder H, Parlour L, Jenkins V. Randomised controlled trials of yoga interventions for women with breast cancer: a systematic literature review. Support Care Cancer. 2012;20:3055-3064. [DOI] [PubMed] [Google Scholar]
- 17. Buffart LM, van Uffelen JG, Riphagen II, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18:360-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanford SD, Beaumont JL, Butt Z, Sweet JJ, Cella D, Wagner LI. Prospective longitudinal evaluation of a symptom cluster in breast cancer. J Pain Symptom Manage. 2014;47:721-730. [DOI] [PubMed] [Google Scholar]
- 20. Sanford SD, Wagner LI, Beaumont JL, Butt Z, Sweet JJ, Cella D. Longitudinal prospective assessment of sleep quality: before, during, and after adjuvant chemotherapy for breast cancer. Support Care Cancer. 2013;21:959-967. [DOI] [PubMed] [Google Scholar]
- 21. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L, Fiorentino L, Rissling M, et al. Decreased health-related quality of life in women with breast cancer is associated with poor sleep. Behav Sleep Med. 2013;11:189-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Phillips KM, Jim HS, Donovan KA, Pinder-Schenck MC, Jacobsen PB. Characteristics and correlates of sleep disturbances in cancer patients. Support Care Cancer. 2012;20:357-365. [DOI] [PubMed] [Google Scholar]
- 24. Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253-2260. [DOI] [PubMed] [Google Scholar]
- 25. Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1-27. [DOI] [PubMed] [Google Scholar]
- 27. Stanton AL, Luecken LJ, MacKinnon DP, Thompson EH. Mechanisms in psychosocial interventions for adults living with cancer: opportunity for integration of theory, research, and practice. J Consult Clin Psychol. 2013;81:318-335. [DOI] [PubMed] [Google Scholar]
- 28. Riley KE, Park CL. How does yoga reduce stress? A systematic review of mechanisms of change and guide to future inquiry [published online April 15, 2015]. Health Psychol Rev. doi: 10.1080/17437199.2014.981778. [DOI] [PubMed] [Google Scholar]
- 29. Moyer A, Goldenberg M, Hall MA, et al. Mediators of change in psychosocial interventions for cancer patients: a systematic review. Behav Med. 2012;38:90-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Branstrom R, Kvillemo P, Brandberg Y, Moskowitz JT. Self-report mindfulness as a mediator of psychological well-being in a stress reduction intervention for cancer patients: a randomized study. Ann Behav Med. 2010;39:151-161. [DOI] [PubMed] [Google Scholar]
- 31. Thekdi SM, Spelman K, Wei A, et al. Posttraumatic stress and depressive symptoms in renal cell carcinoma: association with quality of life and utility of single item distress screening. Psychooncology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parker PA, Swartz R, Fellman B, et al. Comprehensive assessment of quality of life and psychosocial adjustment in patients with renal tumors undergoing open, laparoscopic and nephron sparing surgery. J Urol. 2012;187:822-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Devine D, Parker PA, Fouladi RT, Cohen L. The association between social support, intrusive thoughts, avoidance, and adjustment following an experimental cancer treatment. Psychooncology. 2003;12:453-462. [DOI] [PubMed] [Google Scholar]
- 34. Manne S, Glassman M, Du Hamel K. Intrusion, avoidance, and psychological distress among individuals with cancer. Psychosom Med. 2001;63:658-667. [DOI] [PubMed] [Google Scholar]
- 35. Dupont A, Bower JE, Stanton AL, Ganz PA. Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Health Psychol. 2014;33:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arpawong TE, Richeimer SH, Weinstein F, Elghamrawy A, Milam JE. Posttraumatic growth, quality of life, and treatment symptoms among cancer chemotherapy outpatients. Health Psychol. 2013;32:397-408. [DOI] [PubMed] [Google Scholar]
- 37. Wang AW, Chang CS, Chen ST, Chen DR, Hsu WY. Identification of posttraumatic growth trajectories in the first year after breast cancer surgery. Psychooncology. 2014;23:1399-1405. [DOI] [PubMed] [Google Scholar]
- 38. Danhauer SC, Case LD, Tedeschi R, et al. Predictors of posttraumatic growth in women with breast cancer. Psychooncology. 2013;22:2676-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tomich PL, Helgeson VS. Posttraumatic growth following cancer: links to quality of life. J Trauma Stress. 2012;25:567-573. [DOI] [PubMed] [Google Scholar]
- 40. Taylor SE. Adjustment to threatening events: a theory of cognitive adaptation. Am Psychol. 1983;38:1161-1173. [Google Scholar]
- 41. Diaz M, Aldridge-Gerry A, Spiegel D. Posttraumatic growth and diurnal cortisol slope among women with metastatic breast cancer. Psychoneuroendocrinology. 2014;44:83-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weinrib AZ, Sephton SE, Degeest K, et al. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. 2010;116:4410-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23:1148-1155. [DOI] [PubMed] [Google Scholar]
- 44. Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chandwani KD, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32:1058-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pocock SJ. Clinical Trials: A Practical Approach. New York, NY: John Wiley; 1983. [Google Scholar]
- 47. Davis SL. Thriving After Breast Cancer: Essential Healing Exercises for Body and Mind. New York, NY: Broadway Books; 2002. [Google Scholar]
- 48. Halverstadt A, Leonard A. Essential Exercises for Breast Cancer Survivors. Boston, MA: Harvard Common Press; 2000. [Google Scholar]
- 49. Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33:AS264-AS279. [PubMed] [Google Scholar]
- 50. Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center Hospitals; 1993. [Google Scholar]
- 51. Radloff LS. The CES-D scale: a new self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385-401. [Google Scholar]
- 52. Buysse DJ, Reynolds CF, Monk TH, Berman SR. Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 53. Horowitz M, Wilner N, Alvarez W. Impact of Events Scale: measure of subjective stress. Psychosom Med. 1979;41:209-218. [DOI] [PubMed] [Google Scholar]
- 54. Alferi SM, Antoni MH, Ironson G, Kilbourn KM, Carver CS. Factors predicting the use of complementary therapies in a multi-ethnic sample of early-stage breast cancer patients. J Am Med Womens Assoc. 2001;56:120-123. [PubMed] [Google Scholar]
- 55. Cruess DG, Antoni MH, McGregor BA, et al. Cognitive-behavioral stress management reduces serum cortisol by enhancing benefit finding among women being treated for early stage breast cancer. Psychosom Med. 2000;62:304-308. [DOI] [PubMed] [Google Scholar]
- 56. Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27(2, suppl):S101-S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014;67:451-470. [DOI] [PubMed] [Google Scholar]
- 58. Schneider S, Moyer A, Knapp-Oliver S, Sohl S, Cannella D, Targhetta V. Pre-intervention distress moderates the efficacy of psychosocial treatment for cancer patients: a meta-analysis. J Behav Med. 2010;33:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Andersen BL, Thornton LM, Shapiro CL, et al. Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants. Clin Cancer Res. 2010;16:3270-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Antoni MH, Lehman JM, Kilbourn KM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20:20-32. [DOI] [PubMed] [Google Scholar]
- 61. Chen Z, Meng Z, Milbury K, et al. Qigong improves quality of life in women undergoing radiotherapy for breast cancer: results of a randomized controlled trial. Cancer. 2013;119:1690-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Branstrom R, Kvillemo P, Moskowitz JT. A randomized study of the effects of mindfulness training on psychological well-being and symptoms of stress in patients treated for cancer at 6-month follow-up. Int J Behav Med. 2012;19:535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim SH, Schneider SM, Kravitz L, Mermier C, Burge MR. Mind-body practices for posttraumatic stress disorder. J Investig Med. 2013;61:827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bigatti SM, Steiner JL, Miller KD. Cognitive appraisals, coping and depressive symptoms in breast cancer patients. Stress Health. 2012;28:355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sorato DB, Osorio FL. Coping, psychopathology, and quality of life in cancer patients under palliative care. Palliat Support Care. 2015;13:517-525. [DOI] [PubMed] [Google Scholar]
- 66. Helgeson VS, Reynolds KA, Tomich PL. A meta-analytic review of benefit finding and growth. J Consult Clin Psychol. 2006;74:797-816. [DOI] [PubMed] [Google Scholar]
- 67. Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]