Abstract
Background
Dopaminergic brain circuits participate in emotional processing and impulsivity. The dopamine transporter (DAT1) modulates dopamine reuptake. A variable number tandem repeat (VNTR) in the DAT1 gene affects DAT1 expression. The influence of DAT1 genotype on neural activation during emotional processing and impulse inhibition has not been examined.
Methods
Forty-two healthy subjects were classified as 9DAT (n=17) or 10DAT (n=25) based on DAT1 genotype (9DAT=9R/9R and 9R/10R; 10DAT=10R/10R). Subjects underwent fMRI during non-emotional and emotional go/no-go tasks. Subjects were instructed to inhibit responses to letters, happy faces, or sad faces in separate blocks.
Results
Accuracy and reaction time did not differ between groups. Within group results showed activation in regions previously implicated in emotional processing and response inhibition. Between groups results showed increased activation in 9DAT individuals during inhibition. During letter inhibition, 9DAT individuals exhibited greater activation in right inferior parietal regions. During sad inhibition, 9DAT Individuals exhibited greater activation in frontal, posterior cingulate, precuneus, right cerebellar, left paracentral, and right occipital brain regions. The interaction between DAT genotype and response type in sad versus letter stimuli showed increased activation in 9DAT individuals during sad no-go responses in the anterior cingulate cortex, extending into frontal-orbital regions.
Discussion
9DAT individuals have greater activation than 10DAT individuals during neutral and sad inhibition, showing that genotypic variation influencing basal dopamine levels can alter the neural basis of emotional processing and response inhibition. This may indicate that 9R carriers exert more effort to overcome increased basal dopamine activation when inhibiting responses in emotional contexts.
Keywords: dopamine transporter, response inhibition, emotion
INTRODUCTION
The dopamine transporter (DAT) is one determinant of dopamine availability in the brain. DAT participates in dopamine reuptake from the synaptic cleft and helps terminate the action of dopamine at neural synapses. Genetic variations in DAT availability alter the amount of dopamine available for neurotransmission. The DAT gene, DAT1, is located on chromosome 5p15 and has a variable number tandem repeat (VNTR) at the 3′ end. Differences in VNTR length affect expression of the transporter and synaptic dopamine levels (Fuke et al. 2001). The most commonly studied alleles are the 9-repeat and 10-repeat alleles. 9-repeat carriers (9R/9R or 9R/10R genotypes) have been shown to have decreased DAT1 expression relative to the 10-repeat homozygotes, especially in cortico-striatal pathways (Fuke et al. 2001; Mill et al. 2002). Decreased DAT expression leads to increased transporters at the synapse, and a consequent increase in dopamine availability for neurotransmission. The effect of DAT expression on dopamine availability has been demonstrated in mouse studies showing that DAT knock-out mice exhibit hyperdopaminergia (Gainetdinov et al. 1999).
DAT is expressed abundantly and densely in the striatum at dopaminergic synapses, and more sparsely in cortical regions (Staley et al. 1995). Bertolino et al. (2006) postulated that the distribution of DAT in cortical regions—at a distance from dopaminergic synapse—could indicate its importance in modulating the signal-to-noise ratio during activities of cognitive control and working memory (Bertolino et al. 2006). In this study, an emotional go/no-go paradigm combining the cognitive processes of emotional procession, impulse control, and working memory is used to examine the influence of DAT expression on activation in these areas.
Dopamine modulates many aspects of cognition, emotional processing, and impulse control (Garcia-Garcia et al. 2010; Badgaiyan et al. 2009; Sevy et al. 2006; Ramdani et al. 2015; Takahashi et al. 2005). DAT1 genotype has been implicated as a risk factor for psychiatric disorders including attention deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and bipolar disorder (BD). In clinical populations, the 10R/10R genotype has been associated with cognitive endophenotypes including reduced selective attention and inhibition, depressive disposition, and decreased cognitive flexibility (Cornish et al. 2005; Felten et al. 2011; Mata et al. 2012; Garcia-Garcia et al. 2010). Many studies have examined the influence of DAT1 genotype on cognitive task performance and neural activation in ADHD and other clinical populations, but few have examined how DAT1 genotype modulates cognition and emotional processing in healthy individuals. Because DAT1 may signal cognitive endophenotypes associated with psychiatric disorders, it is important to understand how dopamine activity modulates the neural circuits underlying these processes in healthy populations.
Dopamine has been shown to influence neural activation during both emotional processing and impulse control in healthy individuals. Takahashi et al. (2005) demonstrated that dopamine depletion attenuated activation in components of the limbic-cortico-striatal-pallidal-thalamic circuit and amygdala in response to unpleasant stimuli (Takahashi et al. 2005). Ramdani et al. (2015) showed that dopamine precursor depletion led to less proficiency during a Simon task, without affecting basal impulse activation during the task (Ramdani et al. 2015). Based on these results, it is unclear whether 9R carriers are at a disadvantage during emotional inhibition. They may exhibit greater activation in response to emotional stimuli, but also be better able to inhibit that activation due to greater dopamine availability.
Previous studies additionally show some evidence that DAT1 genotype influences cognition and behavior in healthy adults. Increased dopamine availability in 9R carriers has been associated with better working memory plasticity, decreased impulsivity, and decreased risk-taking behavior (Mata et al. 2012; Brehmer et al. 2009; Bertolino et al. 2006). These results are contrary to studies showing that artificially elevated dopamine levels (such as during amphetamine use or dopamine replacement therapy) impair impulse inhibition and enhance responses to emotional stimuli. For example, Ondo et al. (2008) surveyed a group of 300 patients taking dopamine for either Parkinson’s disease or restless legs syndrome and found that 19.7% of subjects experienced more impulsivity while on dopamine therapy (Ondo et al. 2008). Additionally, gene deletion studies show that DAT knockout mice exhibit hyperactivity and impaired sensorimotor gating in prepulse inhibition of the startle response (Gainetdinov et al. 1999; Ralph et al. 2001).
One possibility is that greater baseline activation in dopamine pathways would necessitate a larger inhibitory response during impulse inhibition to achieve equal or better performance in impulse inhibition paradigms. If greater inhibitory activation is achieved, performance is not impaired. Caldu et al. (2007) demonstrated that healthy subjects who were homozygous for the 9R allele, as well as homozygous for the Val allele of the catechol-o-methyltransferase (COMT) gene, showed the highest activation to achieve the same level of performance as 9R heterozygotes and 10R homozygotes during an N-back working memory task (Caldu et al. 2007). This could mean that 9R carriers have basal dopamine levels that are too high for optimal performance without appropriately increased activation to modulate cognitive processes. The increased activation reflects the possibility that 9R carriers have a harder time modulating their response to stimuli in cognitive tasks.
Given the role of dopamine in impulsivity and emotional response, it is important to assess the influence of dopamine availability on behavioral inhibition and emotional processing. The go/no-go task measures impulse control by assessing the ability to inhibit a prepotent response. Subjects are asked to respond rapidly to “go” cues and withhold a response to “no-go” cues. Cues are typically neutral. Replacement of neutral cues with faces conveying positive (happy) or negative (sad) emotion creates an emotional go/no-go task that allows the study of response inhibition in a contrasting emotional context. Shafritz et al. (2006) demonstrated that response inhibition in an emotional context recruits different brain areas than response inhibition in a non-emotional context. A non-emotional go/no-go task, in which the stimuli were letters, recruited brain areas associated with motor control and inhibition including the basal ganglia (BG), anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), and pre-supplementary motor area (pSMA). The addition of emotional valence to the task recruited additional limbic and paralimbic brain regions including the left inferior posterior parietal cortex (PPC), posterior inferior frontal cortex (PFC), and subgenual anterior cingulate cortex (sACC) (Shafritz et al. 2006).
In this study, we use an emotional go/no-go task (Shafritz et al. 2006) among healthy individuals to examine the effects of DAT1 genotype on performance and neural activation. We predict that during emotional inhibition, 9R carriers will have stronger BOLD signals in regions implicated in impulse inhibition, including the DLPFC, basal ganglia, and sensorimotor regions. Additionally, we predict that 9R carriers will have greater activation in limbic and paralimbic regions related to emotional processing during emotional inhibition than during letter inhibition.
METHODS
Subjects
46 healthy subjects (24 women) age 18–60 years were recruited via advertisement. Subjects had no self-reported personal or family history of psychiatric illness, neurologic illness, or substance abuse. Subjects denied alcohol use within a week of the scan session and did not have a positive urinary toxicology screening at baseline. All subjects signed an informed consent form approved by the local Institutional Review Board and were paid $75 for screening and $75 for the magnetic resonance imaging scan. Subjects were classified as 9DAT or 10DAT individuals based on their DAT1 genotype. 9DAT individuals were homozygous or heterozygous for the 9-repeat allele (9R/9R or 9R/10R). 10DAT individuals were homozygous for the 10-repeat allele (10R/10R).
Functional Magnetic Resonance Imaging Procedure
Images were acquired using a 3T Tim Trio scanner (Siemens, Erlangen, Germany). Participants underwent a high-resolution three-dimensional magnetization prepared rapid acquisition gradient-echo scan. The high-resolution (1.0 × 1.0 × 1.2 mm3 voxels) anatomical volume was comprised of 160 sagittal slices. Functional images were acquired with T2*-weighted gradient-echo planar imaging sequences sensitive to blood oxygen level-dependent (BOLD) contrast (129 measurements, repetition/echo time 2250/29 msec, 39 slices to cover whole brain, field of view 220 × 220 mm; 2.5 × 2.5 × 3.5 mm3 voxels). An integrated parallel acquisition technique reduction factor of 2 was implemented with a generalized autocalibrating partially parallel acquisition to improve spatial resolution and to reduce geometric distortion and scan time.
Task Description
Participants were trained on the go/no-go task before their scan. Task design was identical to that described by Shafritz et al. using the Ekman and Friesen Pictures of Facial Affect (Shafritz et al. 2006). Go and no-go blocks were alternated between beginning and ending fixation blocks. There were two go and no-go blocks each for sad faces, happy faces, and letters. During go blocks, participants were directed to press a button for each face or letter presented. Go blocks consisted of only a single stimulus type (happy face, sad face, or letter). During no-go blocks, subjects were instructed to press a button for go trials (targets) but to withhold a response for no-go trials (nontargets). No-go blocks consisted of 6 no-go and 12 go stimuli. For the inhibition of sad faces (sad no-go), subjects were instructed to press only for happy faces and withhold pressing for sad faces. For the inhibition of happy faces (happy no-go), subjects were instructed to press only for sad faces and withhold pressing for happy faces. For the non-emotional condition (letter no-go), subjects were instructed to withhold a response only for the letter X. Each block had 12 trials. Stimuli were presented for 500 msec, followed by a black screen for 1000 msec to record the response. An instruction screen was shown for 2.25 seconds before each condition.
Genotyping
Genomic DNA was extracted from fresh blood samples using a standard protocol (Lahiri and Nurnberger 1991). Genotyping of the SLC6A3 40 bp repeat polymorphism (rs28363170) was performed as previously described by Vandenberge et al. (1992). Amplification products were separated by agarose gel electrophoresis. Product sizes were determined by comparison to molecular weight standards and known sequenced samples.
Behavioral Analysis
Go and no-go accuracy scores and go reaction times were compared via a one-way analysis of variance (ANOVA) with IBM SPSS Statistics, Release 21.0 (IBM, Armonk, New York) to examine potential performance differences between groups.
Image Analysis
Imaging data were preprocessed and analyzed with standard procedures using Statistical Parametric Mapping software (SPM, Wellcome Trust Centre for Neuroimaging, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm). No subject included had head movement exceeding 2 mm of translation of 2° rotation in any direction. The six motion parameters were also used as covariates in the analysis to account for residual motion confounds.
Functional images were aligned and co-registered to the structural magnetization prepared rapid acquisition gradient-echo volume. Functional images were normalized to a standard Montreal Neurological Institute (MNI) template with 2 mm isotropic voxels then smoothed with a 8 mm full-width at half maximum Gaussian kernel. Individual analyses were performed using a delayed boxcar function convolved with each stimulus type (fixation, instructions, and conditions) to create a general linear model to model the BOLD response. A beta coefficient was calculated for each condition of interest (happy go, happy no-go, sad go, sad no-go, letter go, letter no-go, rest) that reflected its contribution to the BOLD time series.
To analyze brain activation during response inhibition, no-go responses were contrasted with go responses for each subject. Only correct responses were included. The contrast for each emotional no-go condition was the go condition of the same valence (e.g., happy no-go versus. happy go). For example, in happy no-go – happy go, both blocks present emotional stimuli to the subject. However, because happy no-go has the additional requirement of inhibiting responses to happy faces, the contrast of happy no-go – happy go is assumed to reflect the emotional inhibition process.
Using the contrasts form responses inhibition, two sample t-tests controlling for age and gender were conducted to look at the group differences between 10DAT and 9DAT in each response inhibition. Full factorial analysis with age and gender as covariates was run to analyze the interaction between DAT group and condition (emotional inhibition versus letter inhibition) separately for happy inhibition versus letter inhibition and sad inhibition versus letter inhibition. From these t-tests and full factorial models, significant clusters were identified using the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al. 2002). A whole-brain gray matter mask was used in the second level analysis. Monte Carlo simulations using AFNI (Cox 1996) software program 3dClustSim (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) was utilized to determine the culsterwise significance. At least 219 contiguous voxels significant at p=0.01 were required for identifying a cluster as significant at p=0.05(corrected) cluster-wise significance level. To visualize the between group differences, the beta coefficients were extracted from the significant clusters of the second level analysis (see Table 2) and were used to make the boxplots in SPSS 21.
Table 2.
Between Groups Areas of Activation
| Between Group | Condition | Target Area | Cluster | Peak Voxel Wise P(uncorr) | MNI Peak | Z |
|---|---|---|---|---|---|---|
|
| ||||||
| 9DAT>10DAT | LetterNogo>LetterGo | R inf parietal | 334 | 0 | 44 −48 46 | 3.56 |
|
| ||||||
| 9DAT>10DAT | SadNogo>SadGo | L sup frontal, L & R sup medial frontal | 1183 | 0 | −14 52 26 | 3.63 |
| R cerebellum | 365 | 0 | 26 −74 −44 | 3.57 | ||
| L paracentral lobule | 267 | .001 | −8 −30 62 | 3.44 | ||
| L & R PCC, precuneus | 290 | .001 | −2 −50 26 | 3.10 | ||
| R mid and inf occipital | 291 | .001 | 28 −96 −4 | 3.09 | ||
|
| ||||||
| Group X Condition Interaction | Sad/Letter Interaction | L & R ACC, extending into sup medial frontal and medial orbital | 1789 | 0 | −14 42 4 | 3.49 |
Table 2 shows the between group differences in brain activation. The statistical significance threshold for examining this effect was set at p=0.01 and a cluster size of 219 voxels corresponded to a corrected p=0.05. Clusters of significant activity are represented with voxel of peak activation, peak Z-score, and MNI coordinates. MNI, Montreal Neurological Institute; L, left; R, right; sup, superior; inf, inferior; ant, anterior. LetterNogo>LetterGo (Letter No-go – Letter Go); HappyNogo>HappyGo (Happy No-go – Happy Go); SadNogo>SadGo (Sad No-go – Sad go); Group X Sad Emotion X Go/No-go Interaction (Interaction between group, sad stimuli, and response type). The statistical significance threshold for examining this effect was set at p=0.01 and a cluster size of 219 voxels corresponded to corrected p =0.05, except for the interaction between groups (10DAT & 9DAT) and condition (sad no-go>sad go, letter no-go > letter go) the statistical significance threshold for examining this effect was set at p=0.05 and a cluster size of 935 voxels corresponded to corrected p=0.05.
RESULTS
Subjects
42 subjects were included in the analysis. Four subjects were eliminated due to unknown genotype (1) and a family history of psychiatric disorder (3). Subjects were between the ages of 21 and 58 (mean: 31.5, SD: 10.3). Age did not differ between 9DAT and 10DAT groups. Subjects identified as Caucasian (38), Asian (1) or African-American (3). Ethnic distribution did not differ significantly between 9DAT and 10DAT groups. The 10DAT group had 25 subjects. The 9DAT group had 17 subjects.
Behavioral Results
Groups did not differ on response time and accuracy measures. See supplemental Table 1 for performance measures.
Imaging Within-Group Results
See Supplemental Table 1 for within-group results. Both 9DAT and 10DAT groups exhibited brain activation primarily in frontal, temporal, and occipital brain regions, in addition to limbic and motor areas. These results are in line with previous studies examining activation during emotional and inhibitory tasks (Shafritz et al. 2006; Britton et al. 2006).
Imaging Between-Group Results
Letter Inhibition
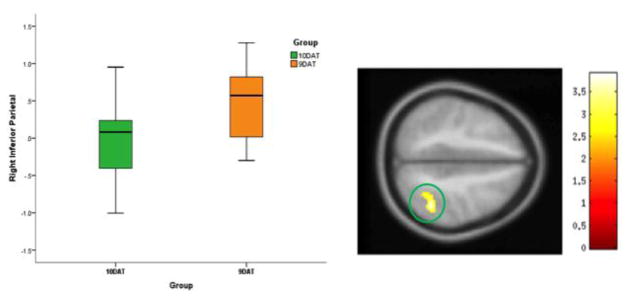
A main effect of DAT group was present in the right inferior parietal cortex during the letter no-go condition. The 9DAT group exhibited higher parietal cortex activation compared to the 10DAT group (Figure 1, Table 2).
Figure 1. Difference between 10DAT and 9DAT Group Activation for the Letter No-go>Letter Go Condition.
Figure 1 shows the differences between 10DAT (homozygous for 10-repeat allele, 10R/10R) and 9DAT (homozygous or heterozygous for 9-repeat allele, 9R/9R or 9R/10R) in the letter no-go > letter go condition. The statistical significance threshold for examining this effect was set at p=0.01 and a cluster size of 219 voxels corresponded to a corrected p=0.05. Here, the 9DAT group shows more activation in the right inferior parietal area than the 10DAT group.
Happy Face Inhibition
There was no difference in activation between 9DAT and 10DAT groups during happy inhibition.
Sad Face Inhibition
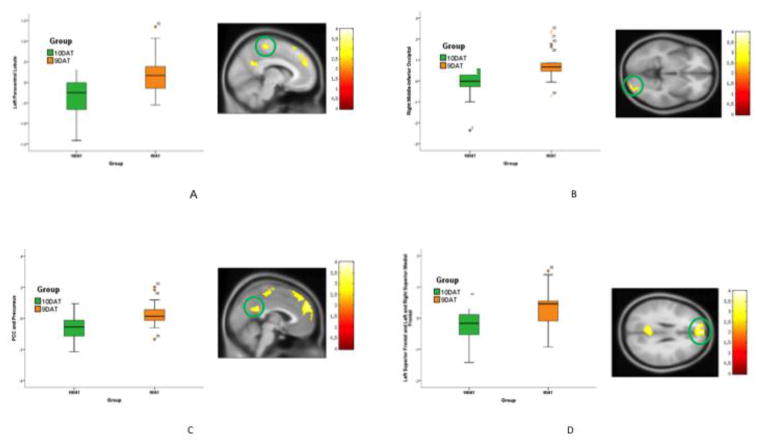
A main effect of DAT group was present in the left and right PCC and precuneus, right cerebellum, right inferior and middle occipital, left paracentral lobule, and superior frontal regions. Post hoc results showed increased activation throughout these areas in the 9DAT group compared to the 10DAT group (Figure 2, Table 2).
Figure 2. Difference between 10DAT and 9DAT Group Activation for the Sad No-go>Sad Go Condition.
Figure 2 shows the difference between 10DAT and 9DAT groups activation for sad no-go > sad go condition. The statistical significance threshold for examining this effect was set at p=0.01 and a cluster size of 219 voxels corresponded to a corrected p=0.05. (A) Left paracentral lobule, 9DAT>10DAT. (B) Right middle-inferior occipital, 9DAT>10DAT. (C) Left & Right PCC-precuneus, 9DAT>10DAT. (D) Left superior frontal and left & right superior medial frontal, 9DAT>10DAT.
Happy and Letter Interaction
There was no interaction between DAT group and response type on neural activation during happy versus letter inhibition.
Sad and Letter Interaction
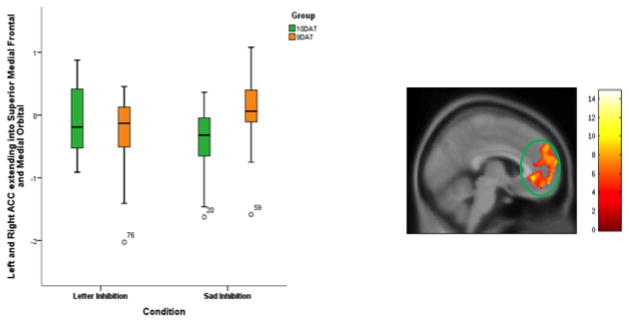
There was an interaction between DAT group and condition on neural activation during response inhibition. During inhibition of emotional stimuli, the 9DAT group exhibited greater activation in the left rostral ACC (pregenual and subgenual), extending into superior medial frontal and medial orbital regions compared to the 10DAT group. This effect was not seen during inhibition of neutral stimuli (Figure 3, Table 2).
Figure 3. Interaction between Groups (10DAT, 9DAT) and Conditions (Sad No-go > Sad Go, Letter No-Go > Letter Go).
Figure 3 shows the interaction between the sad emotion and non-emotional conditions between the 9DAT and 10DAT groups, the significance thershold for examining this this interaction was set at p=0.05 and a cluster size of 935 voxels corresponded to cluster-wise corrected p=0.05. The resultant area was the left ACC extending into the superior medial frontal and medial orbital areas.
DISCUSSION
This investigation is the first to study neural activation during an emotional response inhibition task across different DAT1 genotypes in healthy individuals. This study attempted to examine the differences in neural activation during emotional inhibition that result from genetically determined differences in dopamine availability.
Overall, 9DAT individuals exhibited greater activation across all contrasts measured in this study. Increased neural activation during emotional response inhibition could indicate that 9DAT individuals must put forth more effort to inhibit emotional responses. In this study, 9DAT individuals had the same accuracy and reaction time as 10DAT individuals, indicating that they required more activation to achieve the same performance in an emotional response inhibition task. This has implications for the effect of increased dopamine availability on impulsive behavior. The present results show that increased dopamine availability—classically associated with increased impulsivity—does not necessarily impair response inhibition if inhibitory brain circuits are sufficiently active. Previous studies examining the influence of Levodopa infusion on go/no-go performance in subjects exhibiting symptoms of Tourette’s syndrome support this finding. Hershey et al. (2004) showed that infusion of the drug Levodopa resulted in greater activation during a neutral go/no-go task (Hershey et al. 2004). The Levodopa did not alter the performance of subjects, just as DAT genotype did not alter performance in the present study. This could have implications in examining the threshold at which dopaminergic disorders, such as Parkinson’s disorder, begin to impair cognition and emotional processing.
Further studies examining the effects of dopamine levels on performance in more complex emotional inhibition tasks must be performed to determine whether 9DAT individuals have increased dopamine availability and, in turn, whether increased dopamine availability makes impulse inhibition more difficult. It could be useful to measure dopamine availability directly instead of DAT1 expression, using a joint PET and fMRI study. Alternatively, increasing dopamine release in healthy populations, as previously done in bipolar populations, could help determine whether increased dopamine availability impairs performance on impulse inhibition tasks (Anand et al. 2000). In addition, COMT genotype has been shown to interact with DAT genotype to influence performance in cognitive tasks (Caldu et al. 2007). Caldu et al. (2007) found significant interactions between DAT and COMT genotype in working memory function and activation in cortical regions implicated in working memory function, but did not find an independent effect of DAT genotype on these measures. COMT genotype was not recorded in this study, making it difficult to isolate the effects of DAT genotype on performance in the emotional go/no-go task. In future studies, COMT and DAT genotype should be recorded and interactions analyzed to isolate the effects of genotypic variation in COMT and DAT expression.
9DAT individuals had increased activation in the parietal cortex during inhibition of non-emotional stimuli. The parietal cortex has been previously implicated in go/no-go tasks as a region that is active during inhibition of prepotent responses (Goldstein et al. 2007; Watanabe et al. 2002). These findings support the role of the parietal cortex in response inhibition.
No group differences were observed during happy face inhibition. This is contrary to previous studies showing that 9R carriers have more robust activation in response to reward-inducing stimuli, which would, in turn, require greater activation for adequate inhibition (Dreher et al. 2009).
During sad face inhibition, the 9DAT carriers had increased activation in the left and right posterior cingulate cortex (PCC) and precuneus, superior frontal and medial frontal areas, cerebellum, temporal pole, occipital areas, and the left paracentral and lobule. The cerebellum and occipital areas have well-established roles in coordination of motor activity and emotional picture processing, respectively (Phan et al. 2002; Lotze et al. 1999). The emotional go/no-go task requires motor and visual processing, and it is expected that these areas would show activation. The PCC has been implicated in evaluative processing of emotional stimuli, specifically in the identification of and response generation to emotional stimuli (Bush et al. 2000). This finding has not been previously demonstrated during an emotional go/no-go paradigm, and suggests an important role of the PCC in intrinsic control during emotional tasks. The precuneus has not previously been shown to be active during emotional impulse inhibition and warrants further investigation. Though the inferior frontal gyrus has been shown to be crucial for stop-signal inhibition in previous studies, the superior frontal and mid-area have not been shown to influence inhibition (Aron et al. 2003). Further studies are needed to confirm this finding and delineate the function of these areas.
When combining sad and letter inhibition, the 9DAT group had increased activation in the ACC during inhibition of emotional faces relative to 10DAT individuals. The ACC has been previously implicated both in behavioral inhibition as an area that is activated when a prepotent response must be updated to avoid making a mistake, and in the emotional processing of sad faces (Shafritz et al. 2006; Hester et al. 2007). The results presented corroborate both of these potential roles for the ACC. Activation spread into superior medial frontal and medial orbital areas, approaching orbitofrontal areas. This supports previous results showing that the OFC is active during impulse inhibition.
One limitation of this study is the small sample size of healthy individuals used, and the uneven distribution of 9DAT and 10DAT individuals. Future investigations should attract a larger number of participants and compare equal numbers of 9DAT and 10DAT individuals.
Another limitation of this study is the simplicity and design of the task administered. The go/no-go task was analyzed as a block-design, but may be better characterized as an event-related design. The go/no-go task has been considered to be a mixed block- and event-related design (Rubia et al. 2001). Additionally, the comparison of faces to letters may not be adequate in distinguishing emotional responses. Future studies could compare inhibition during exposure to emotional faces and neutral faces to control for the difference between letters and human faces. This could better isolate the influence of emotional processing on behavioral inhibition.
Despite these limitations, this study provides evidence for DAT1 genotype-modulated differences on emotional response inhibition in healthy individuals. We conclude that 9DAT individuals, as a result of greater basal dopamine availability, exhibit more activation in response to emotional stimuli and, as a result, more effortful impulse inhibition. These findings need to be replicated and investigated further in future studies.
Supplementary Material
Table 1.
Performance and Accuracy Measures (Correct Responses)
| Block | Group | Mean Accuracy (%) | Reaction Time (ms) |
|---|---|---|---|
| Letter Go | 10DAT | 100 | 331.9 |
| 9DAT | 99.5 | 335.0 | |
|
| |||
| Letter No-go | |||
| Go Targets | 10DAT | 100 | 398.0 |
| 9DAT | 99.5 | 411.5 | |
| No-go Targets | 10DAT | 93.4 | N/A |
| 9DAT | 95.2 | ||
|
| |||
| Happy Go | 10DAT | 100 | 330.8 |
| 9DAT | 99.8 | 339.8 | |
|
| |||
| Happy No-go | |||
| Go Targets | 10DAT | 95.4 | 473.0 |
| 9DAT | 94.1 | 484.4 | |
| No-go Targets | 10DAT | 93.4 | N/A |
| 9DAT | 98.1 | ||
|
| |||
| Sad Go | 10DAT | 99.4 | 354.9 |
| 9DAT | 99.5 | 362.6 | |
|
| |||
| Sad No-go | |||
| Go Targets | 10DAT | 99.4 | 453.7 |
| 9DAT | 98.1 | 462.1 | |
| No-go Targets | 10DAT | 95.4 | N/A |
| 9DAT | 97.1 | ||
Table 1 shows the mean accuracy and reaction times during each block. 10DAT and 9DAT groups did not differ significantly in accuracy and reaction time.
Acknowledgments
Funding: This project was funded by the NIMH to AA (R01MH075025)
Footnotes
None of the authors have any financial conflicts to declare.
Conflict of Interest: None of the authors have any conflicts of interest related to this report.
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, Innis RB. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157(7):1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Dopamine release during human emotional processing. Neuroimage. 2009;47(4):2041–2045. doi: 10.1016/j.neuroimage.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, Nardini M, Weinberger DR, Dallapiccola B. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26(15):3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Bellander M, Furth D, Karlsson S, Backman L. Working memory plasticity modulated by dopamine transporter genotype. Neurosci Lett. 2009;467(2):117–120. doi: 10.1016/j.neulet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage. 2006;31(1):397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Caldu X, Vendrell P, Bartres-Faz D, Clemente I, Bargallo N, Jurado MA, Sierra Grabulosa JM, Junque C. Impact of the f Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuroimage. 2007;37(4):1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, Grant C, Cross G, Bentley L, Hollis CP. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10(7):686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, An International Journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci U S A. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felten A, Montag C, Markett S, Walter NT, Reuter M. Genetically determined dopamine availability predicts disposition for depression. Brain Behav. 2011;1(2):109–118. doi: 10.1002/brb3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1(2):152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Gainetdinov R, Jones S, Caron M. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol Psych. 1999;46(3):303–311. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia M, Clemente I, Dominguez-Borras J, Escera C. Dopamine transporter regulates the enhancement of novelty processing by a negative emotional context. Neuropsychologia. 2010;48(5):1483–1488. doi: 10.1016/j.neuropsychologia.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia M, Barcelo F, Clemente IC, Escera C. The role of the dopamine transporter DAT1 genotype on the neural correlates of cognitive flexibility. Eur J Neurosci. 2010;31(4):754–760. doi: 10.1111/j.1460-9568.2010.07102.x. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Brendel G, Tuescher O, Pan H, Epstein J, Beutel M, Yang Y, Thomas K, Levy K, Silverman M, Clarkin J, Posner M, Kernberg O, Stern E, Silbersweig D. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage. 2007;36(3):1026–1040. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Hershey T, Black KJ, Hartlein J, Braver TS, Barch DM, Carl JL, Perlmutter JS. Dopaminergic modulation of response inhibition: an fMRI study. Brain Res Cogn Brain Res. 2004;20(3):438–448. doi: 10.1016/j.cogbrainres.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Hester R, Barre N, Mattingley JB, Foxe JJ, Garavan H. Avoiding another mistake: error and posterror neural activity associated with adaptive posterror behavior change. Cogn Affect Behav Neurosci. 2007;7(4):317–326. doi: 10.3758/cabn.7.4.317. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19(19):5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Montoya P, Erb M, Hulsmann E, Flor H, Klose U, Birbaumer N, Grodd W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999;11(5):491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- Mata R, Hau R, Papassotiropoulos A, Hertwig R. DAT1 polymorphism is associated with risk taking in the Balloon Analogue Risk Task (BART) PLoS One. 2012;7(6):e39135. doi: 10.1371/journal.pone.0039135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′UTR VNTR: Evidence from brain and lymphocytes using quantitave RT-PCR. Am J of Med Gen. 2002;114(8):975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Ondo WG, Lai D. Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism Relat Disord. 2008;14(1):28–32. doi: 10.1016/j.parkreldis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Ramdani C, Carbonnell L, Vidal F, Beranger C, Dagher A, Hasbroucq T. Dopamine precursors depletion impairs impulse control in healthy volunteers. Psychopharmacology (Berl) 2015;232(2):477–487. doi: 10.1007/s00213-014-3686-z. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13(2):250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rush R, Paulus M, Fumagalli F, Caron M, Geyer M. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21(1):305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, Delman H, Malhotra A. Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology (Berl) 2006;188(2):228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31(1):468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Staley J, Boja J, Carroll F, Seltzman H, Wyrick C, Lewin A, Abraham P, Mash D. Mapping dopamine transporters in the human brain with novel selective cocaine analog. Synapse. 1995;21(4):364–372. doi: 10.1002/syn.890210412. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Takano A, Asai K, Suhara T, Okubo Y. Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological fMRI study. Neuroimage. 2005;27(4):991–1001. doi: 10.1016/j.neuroimage.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage. 2002;17(3):1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.