Abstract
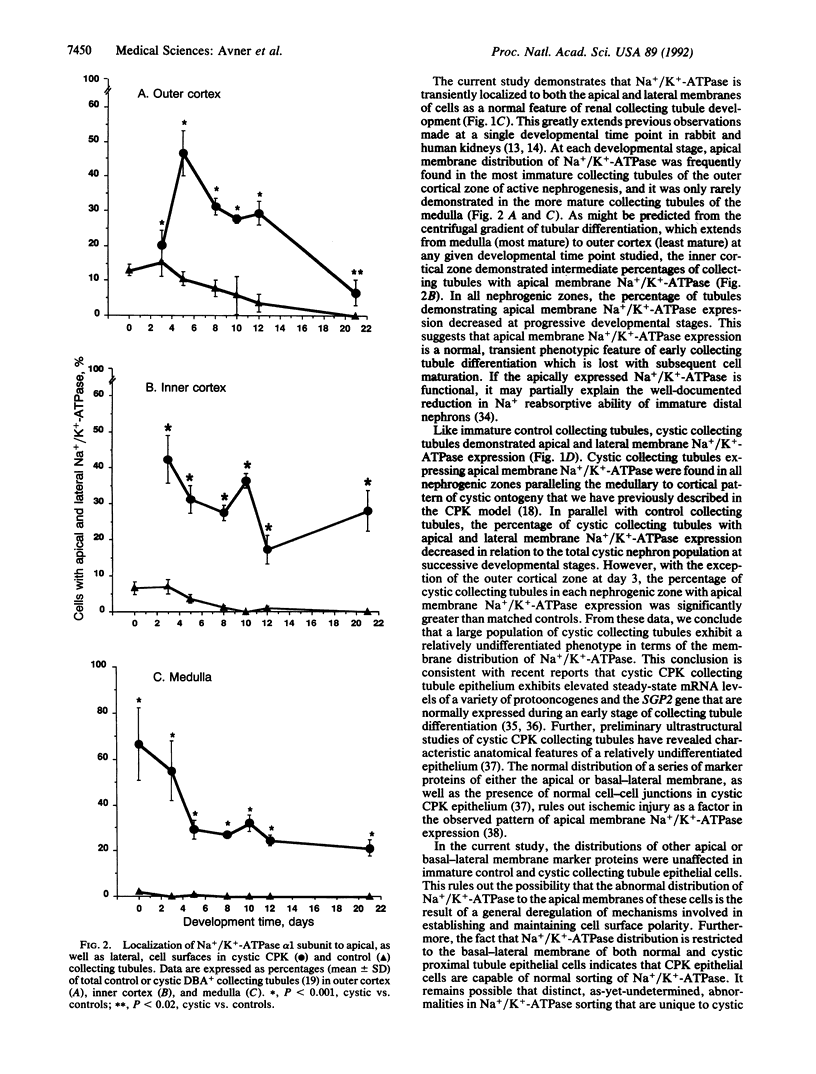
Congenital polycystic kidney disease is characterized by the formation of large fluid-filled cysts in kidney tubules. It has been postulated that increased epithelial cell proliferation and altered transtubular fluid transport are necessary for cyst formation. To address the latter problem, we have studied the plasma membrane distribution of the alpha 1 and beta 1 subunits of Na+/K(+)-ATPase during progressive stages of proximal and collecting tubular cyst formation in the CPK mouse, a murine model of autosomal recessive polycystic kidney disease. In both control and cystic proximal tubules, Na+/K(+)-ATPase distribution was restricted to the basal-lateral membrane of cells. However, in newborn through day 5 kidney tissue, 16% of control vs. 47% of cystic outer cortical, 6% of control vs. 46% of cystic inner cortical, and 2% of control vs. 63% of cystic medullary collecting tubules demonstrated apical and lateral membrane distribution of Na+/K(+)-ATPase. In all nephrogenic zones, the percentage of control or cystic collecting tubules demonstrating apical membrane distribution of Na+/K(+)-ATPase decreased over time, but the percentage of cystic collecting tubules with apical membrane Na+/K(+)-ATPase remained significantly greater than in developmentally matched controls. No alterations in the normal distributions of other apical or basal-lateral membrane marker proteins were noted at any stage of control or cystic proximal or collecting tubule development. We conclude that apical-lateral membrane Na+/K(+)-ATPase expression is a normal transient feature of early collecting tubule development. However, apical membrane Na+/K(+)-ATPase persists in cystic kidneys, suggesting that such expression may be a manifestation of the relatively undifferentiated phenotype of epithelial cells lining collecting tubule cysts. The persistence of apical membrane Na+/K(+)-ATPase, if the enzyme is functional, may have pathogenic important in abnormal transtubular fluid transport in polycystic kidney disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avner E. D. Renal cystic disease. Insights from recent experimental investigations. Nephron. 1988;48(2):89–93. doi: 10.1159/000184884. [DOI] [PubMed] [Google Scholar]
- Avner E. D., Studnicki F. E., Young M. C., Sweeney W. E., Jr, Piesco N. P., Ellis D., Fettermann G. H. Congenital murine polycystic kidney disease. I. The ontogeny of tubular cyst formation. Pediatr Nephrol. 1987 Oct;1(4):587–596. doi: 10.1007/BF00853593. [DOI] [PubMed] [Google Scholar]
- Avner E. D., Sweeney W. E., Jr, Ellis D. In vitro modulation of tubular cyst regression in murine polycystic kidney disease. Kidney Int. 1989 Dec;36(6):960–968. doi: 10.1038/ki.1989.288. [DOI] [PubMed] [Google Scholar]
- Avner E. D., Sweeney W. E., Jr, Finegold D. N., Piesco N. P., Ellis D. Sodium-potassium ATPase activity mediates cyst formation in metanephric organ culture. Kidney Int. 1985 Sep;28(3):447–455. doi: 10.1038/ki.1985.151. [DOI] [PubMed] [Google Scholar]
- Avner E. D., Sweeney W. E., Jr, Piesco N. P., Ellis D. Triiodothyronine-induced cyst formation in metanephric organ culture: the role of increased Na-K-adenosine triphosphatase activity. J Lab Clin Med. 1987 Apr;109(4):441–453. [PubMed] [Google Scholar]
- Avner E. D., Sweeney W. E., Jr Polypeptide growth factors in metanephric growth and segmental nephron differentiation. Pediatr Nephrol. 1990 Jul;4(4):372–377. doi: 10.1007/BF00862522. [DOI] [PubMed] [Google Scholar]
- Avner E. D., Sweeney W. E., Jr, Young M. C., Ellis D. Congenital murine polycystic kidney disease. II. Pathogenesis of tubular cyst formation. Pediatr Nephrol. 1988 Apr;2(2):210–218. doi: 10.1007/BF00862593. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Anderson H. C., Palade G. E., Jamieson J. D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986 Aug 15;46(4):623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Cowley B. D., Jr, Chadwick L. J., Grantham J. J., Calvet J. P. Elevated proto-oncogene expression in polycystic kidneys of the C57BL/6J (cpk) mouse. J Am Soc Nephrol. 1991 Feb;1(8):1048–1053. doi: 10.1681/ASN.V181048. [DOI] [PubMed] [Google Scholar]
- Curto K. A., Sweeney W. E., Avner E. D., Piesco N. P., Curthoys N. P. Immunocytochemical localization of gamma-glutamyltranspeptidase during fetal development of mouse kidney. J Histochem Cytochem. 1988 Feb;36(2):159–166. doi: 10.1177/36.2.2891746. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Geiser J. L., Evan A. P. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987 May;31(5):1145–1152. doi: 10.1038/ki.1987.121. [DOI] [PubMed] [Google Scholar]
- Gundersen D., Orlowski J., Rodriguez-Boulan E. Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton. J Cell Biol. 1991 Mar;112(5):863–872. doi: 10.1083/jcb.112.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerton R. W., Krzeminski K. A., Mays R. W., Ryan T. A., Wollner D. A., Nelson W. J. Mechanism for regulating cell surface distribution of Na+,K(+)-ATPase in polarized epithelial cells. Science. 1991 Nov 8;254(5033):847–850. doi: 10.1126/science.1658934. [DOI] [PubMed] [Google Scholar]
- Harding M. A., Chadwick L. J., Gattone V. H., 2nd, Calvet J. P. The SGP-2 gene is developmentally regulated in the mouse kidney and abnormally expressed in collecting duct cysts in polycystic kidney disease. Dev Biol. 1991 Aug;146(2):483–490. doi: 10.1016/0012-1606(91)90249-3. [DOI] [PubMed] [Google Scholar]
- Holthöfer H. Ontogeny of cell type-specific enzyme reactivities in kidney collecting ducts. Pediatr Res. 1987 Nov;22(5):504–508. doi: 10.1203/00006450-198711000-00005. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L. Sodium and potassium ion pump in kidney tubules. Physiol Rev. 1980 Jul;60(3):864–917. doi: 10.1152/physrev.1980.60.3.864. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- Linser P. J., Sorrentino M., Moscona A. A. Cellular compartmentalization of carbonic anhydrase-C and glutamine synthetase in developing and mature mouse neural retina. Brain Res. 1984 Mar;315(1):65–71. doi: 10.1016/0165-3806(84)90077-4. [DOI] [PubMed] [Google Scholar]
- Mays R. W., Nelson W. J. Mechanisms for regulating the cell surface distribution of Na/K-ATPase in polarized epithelial cells. Chest. 1992 Mar;101(3 Suppl):50S–52S. doi: 10.1378/chest.101.3_supplement.50s. [DOI] [PubMed] [Google Scholar]
- Minuth W. W., Gross P., Gilbert P., Kashgarian M. Expression of the alpha-subunit of Na/K-ATPase in renal collecting duct epithelium during development. Kidney Int. 1987 May;31(5):1104–1112. doi: 10.1038/ki.1987.115. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A. Ischemia-induced loss of epithelial polarity: potential role of the actin cytoskeleton. Am J Physiol. 1991 Jun;260(6 Pt 2):F769–F778. doi: 10.1152/ajprenal.1991.260.6.F769. [DOI] [PubMed] [Google Scholar]
- Morris S. M., Jr, Sweeney W. E., Jr, Kepka D. M., O'Brien W. E., Avner E. D. Localization of arginine biosynthetic enzymes in renal proximal tubules and abundance of mRNA during development. Pediatr Res. 1991 Feb;29(2):151–154. doi: 10.1203/00006450-199102000-00010. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Hammerton R. W. A membrane-cytoskeletal complex containing Na+,K+-ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: implications for the biogenesis of epithelial cell polarity. J Cell Biol. 1989 Mar;108(3):893–902. doi: 10.1083/jcb.108.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Hammerton R. W., Wang A. Z., Shore E. M. Involvement of the membrane-cytoskeleton in development of epithelial cell polarity. Semin Cell Biol. 1990 Oct;1(5):359–371. [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987 Aug 6;328(6130):533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Ojakian G. K., Schwimmer R. The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2377–2387. doi: 10.1083/jcb.107.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascher I. Molecular arrangements in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability. Biochim Biophys Acta. 1976 Dec 2;455(2):433–451. doi: 10.1016/0005-2736(76)90316-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F., Bonting S. L. Transport adenosine triphosphatases: properties and functions. Physiol Rev. 1981 Jan;61(1):1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- Shyjan A. W., Levenson R. Antisera specific for the alpha 1, alpha 2, alpha 3, and beta subunits of the Na,K-ATPase: differential expression of alpha and beta subunits in rat tissue membranes. Biochemistry. 1989 May 30;28(11):4531–4535. doi: 10.1021/bi00437a002. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Spector R., Johanson C. E. The mammalian choroid plexus. Sci Am. 1989 Nov;261(5):68–74. doi: 10.1038/scientificamerican1189-68. [DOI] [PubMed] [Google Scholar]
- Spitzer A. The role of the kidney in sodium homeostasis during maturation. Kidney Int. 1982 Apr;21(4):539–545. doi: 10.1038/ki.1982.60. [DOI] [PubMed] [Google Scholar]
- Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986 Sep;103(3):755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao B., Curthoys N. P. The absolute asymmetry of orientation of gamma-glutamyltranspeptidase and aminopeptidase on the external surface of the rat renal brush border membrane. J Biol Chem. 1980 Aug 25;255(16):7708–7711. [PubMed] [Google Scholar]
- Wilson P. D., Sherwood A. C., Palla K., Du J., Watson R., Norman J. T. Reversed polarity of Na(+) -K(+) -ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am J Physiol. 1991 Mar;260(3 Pt 2):F420–F430. doi: 10.1152/ajprenal.1991.260.3.F420. [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Sherwood A. C. Tubulocystic epithelium. Kidney Int. 1991 Mar;39(3):450–463. doi: 10.1038/ki.1991.56. [DOI] [PubMed] [Google Scholar]




